Abstract
The activity-regulated cytoskeleton-associated protein (Arc, also known as Arg3.1), an immediate early gene and synaptic regulator, is upregulated following a single cocaine exposure. However, there is not much known regarding Arc/Arg3.1’s potential contribution to addiction-relevant behaviors. Despite known learning and memory deficits in contextual fear and water-maze reversal learning tasks, we find that mice lacking Arc/Arg3.1 perform conditioned place preference and operant conditioning involving positive reinforcers (food and cocaine) with little-to-no impairment. However, following normal saline-extinction, wild type (WT) mice show a classic inverted-U dose-response function, while Arc/Arg3.1 knockout (KO) mice fail to adjust their intake across multiple doses. Importantly, Arc/Arg3.1 KO and WT mice behave comparably on an increasing cost task (FR1-FR3; acquisition dose), providing evidence that both groups find cocaine reinforcing. Differences in individuals that drive variations in use patterns and particularly, drug intake levels, are critical as they influence the likelihood of developing dependence. Our data suggest that Arc/Arg3.1 may contribute to addiction as a regulator of drug-taking vulnerability under different drug availability conditions.
Keywords: Cocaine, Intravenous self-administration, Immediate early gene, Dose-response
1. Introduction
Activity-regulated cytoskeleton-associated protein (Arc, also known as Arg3.1) is an immediate early gene linked to multiple forms of glutamatergic plasticity. Arc/Arg3.1 is a known regulator of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) endocytosis via its interactions with members of the endocytic machinery (Chowdhury et al., 2006), with roles described in long-term potentiation (LTP) (Wang et al., 2016), long-term depression (LTD) (Park et al., 2008; Jakkamsetti et al., 2013; Waung et al., 2008), and homeostatic scaling (Shepherd et al., 2006). Arc/Arg3.1 mRNA expression is induced by multiple forms of synaptic and cellular activity (Kawashima et al., 2009; Moga et al., 2004). Once transcribed, Arc/Arg3.1 mRNA is localized to neuronal dendrites via targeting sequences in its 3′ untranslated region (Ninomiya et al., 2016), and whole brain (Zalfa et al., 2003) and hippocampal studies (Niere et al., 2012) suggest that Arc/Arg3.1 translation is negatively-regulated by interaction with the RNA binding protein, FMRP (fragile X mental retardation protein). Neuronal activity, via Group I metabotropic glutamate receptor (mGluR) activation, causes the activity-dependent local translation of Arc/Arg3.1 (Waung et al., 2008).
Arc/Arg3.1 expression is induced robustly following a number of experiences involving learning (Montag-Sallaz and Montag, 2003), including fear learning (Gouty-Colomer et al., 2016) and exposure to novel environments (Ons et al., 2004; Guzowski et al., 1999). Its expression is also induced in multiple brain regions, such as hippocampus, cortex and/or striatum, following exposure to psychostimulants (Fosnaugh et al., 1995) and other drugs of abuse, as well as following re-exposure to drug-paired contexts (Hearing et al., 2008a; Hearing et al., 2010a; Hearing et al., 2008b; Lv et al., 2015), drug self-administration training (Fumagalli et al., 2009) and reinstatement of drug seeking (Zavala et al., 2008; Ziolkowska et al., 2011; Kuntz et al., 2008; Fanous et al., 2012). However, while critical roles for Arc/Arg3.1 in fear conditioning (Ploski et al., 2008) and extinction (Onoue et al., 2014), hippocampal-dependent long-term memory (Plath et al., 2006), and activity-dependent LTD (Waung et al., 2008) have been demonstrated, its role in drug-induced behaviors is still unclear. Using knockdown techniques, a requirement for Arc/Arg3.1 in various striatal subregions has been suggested for drug-related learning, including extinction of intravenous cocaine-seeking (Hearing et al., 2011), and acquisition, expression, and reinstatement of morphine conditioned place preference (CPP) (Lv et al., 2011). Arc/Arg3.1 has also previously been implicated in the negative regulation of drug sensitivity in non-contingent drug behavior paradigms, with reports of enhanced psychostimulant-induced locomotion (Managò et al., 2016; Salery et al., 2016; Penrod-Martin et al., 2017), and reward (Salery et al., 2016) in Arc/Arg3.1 knockout (KO) mice. Despite these observations, and its known role in classical conditioning and other learning-related tasks, the role of Arc/Arg3.1 in operant conditioning and drug self-administration behavior remains relatively unexplored.
Given that Arc/Arg3.1 expression is induced in key mesocorticolimbic brain regions by cocaine exposure, we sought to test its role in volitional cocaine-taking in the intravenous self-administration (IVSA) assay. Because previous reports have demonstrated impaired memory consolidation and long-term memory in Arc/Arg3.1 KO mice, we assessed Arc/Arg3.1 KO performance on aversive (fear conditioning) and appetitive (cocaine CPP) Pavlovian conditioning tasks. We then examined Arc/Arg3.1 KO mouse performance on an appetitive instrumental conditioning task, food-reinforced operant responding. Finally, experimentally naïve Arc/Arg3.1 KO mice were examined for their behavior in cocaine IVSA, including acquisition, extinction, dose-response, and increasing cost conditions.
2. Methods and materials
2.1. Animals and drugs
Arc-green fluorescent protein (GFP) knock-in mice (The Jackson Laboratory; stock no. 007662) (Wang et al., 2006), in which a destabilized form of GFP (d2EGFP) replaced the Arc locus, were backcrossed to congenicity on the C57BL/6N strain. Lack of detectible Arc mRNA and protein in this mouse line has been confirmed in brain (Wang et al., 2006); also see Fig. 2A); thus they are referred to here as Arc/Arg3.1 KO mice. Homozygous mutants and wild type (WT) littermates were generated from Arc-GFP heterozygous x heterozygous crosses, and adult (10- to 20-week-old) male littermates were used for all testing. Ages of tested mice were within 6 weeks of one another in each assay. All experimental procedures were approved by the Institutional Animal Care and Use Committee at McLean Hospital and/or at Texas A&M University.
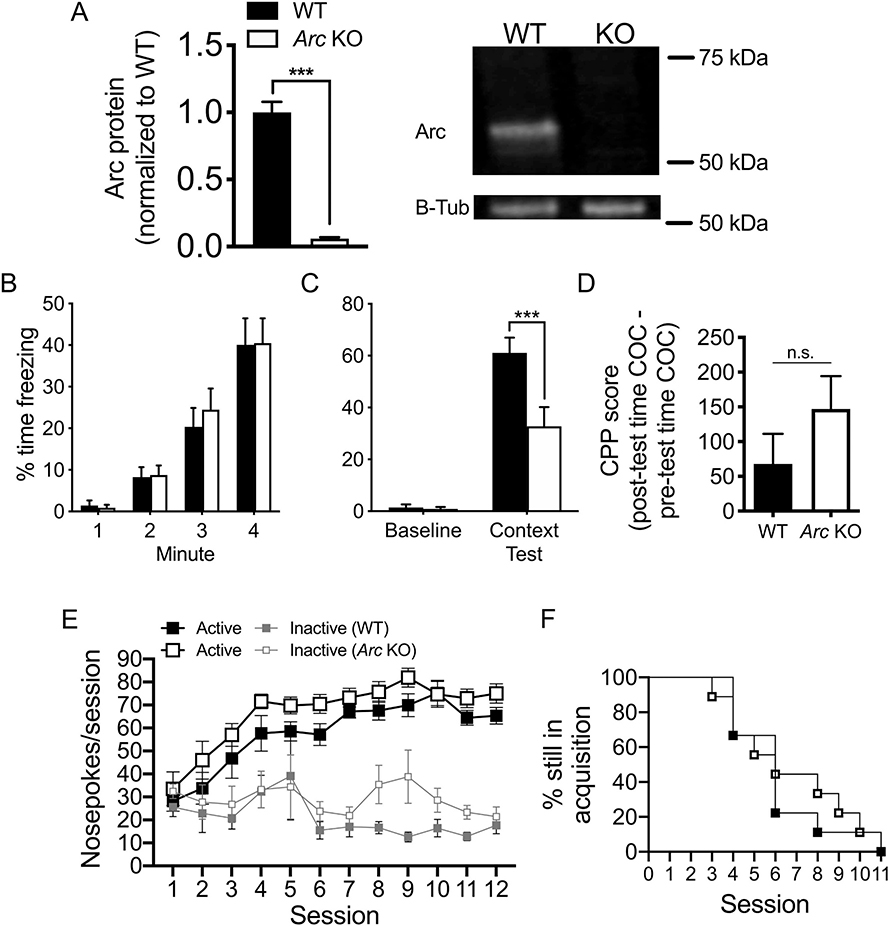
Fig. 2.
Arc/Arg 3.1 KO mice show expected fear-related memory deficits, but normal appetitive classical and operant conditioning behavior.
(A) Homozygous Arc/Arg3.1 KO mice (Arc-GFP knock-in mice) produce no detectible Arc/Arg3.1 protein in NAc. (B) During fear conditioning, KO and WT mice spend a comparable amount of time freezing over the 4 min training session (n = 10–12 per group). (C) Despite spending significantly more time freezing during the contextual fear conditioning test compared to their own baseline (0–58 s of training session), KO mice showed significant impairment in context-cued freezing compared to WT mice. (D) Arc/Arg3.1 KO mice appeared normal during low-moderate dose cocaine conditioned place preference (10 mg/kg; n = 15–16 per group). (E) They also showed normal food operant conditioning (active = reinforcers earned, large boxes; inactive, small boxes) under an FR1 schedule in 2 h sessions over 12 days and (F) showed similar survival distributions when meeting acquisition criteria (n = 9 animals per group). Time out responses are excluded. Animals meeting criteria remained in the test through Session 12. *** p < .001; data shown are mean ± S.E.M.
2.2. Tissue collection and processing
To confirm absence of Arc expression in Arc-GFP mice, brains from homozygous mutant (KO) and WT mice were removed following rapid decapitation, and coronal slices (1 mm) were prepared in ice-cold phosphate buffered saline, as previously described (Taniguchi et al., 2012). Bilateral tissue punches were taken from 1 to 2 (1 mm) slices containing the nucleus accumbens (NAc; 16 gauge for rostral, starting Bregma ~1.94 mm, and either 14 or 16 gauge for caudal, starting Bregma ~1.10 mm) using the anterior commissure as a guide. Samples were then snap frozen in a dry ice/ethanol bath and stored at −80 °C for subsequent analysis by western blotting. Tissues were sonicated (30% amplitude) in a small amount of sucrose lysis buffer containing inhibitors (11% sucrose, 0.005 M HEPES, 1% SDS, 1 mM NaF, 1 mM Na3VO4, 0.1 μM cyclosporin A, 0.1 μM okadaic acid, 1 mM PMSF, 1 mM EDTA, 1× Roche or Pierce EDTA-free protease inhibitor tablet), boiled at 98 °C for 10 min, centrifuged briefly and frozen at −80 °C until protein quantification and SDS-PAGE.
2.3. Western blotting
Protein quantification was assessed by modified-Lowry using the DC Protein Assay Kit (Biorad, Hercules, CA). SDS-PAGE gels (4–15%) were loaded with equal amounts of total protein/well (20–30 μg/well). Proteins were transferred to PVDF membrane using the mixed molecular weight setting (7 min; 2.5 A) on BioRad Trans-Blot Turbo system. Membranes were blocked in Odyssey Blocking Buffer (PBS: 1:1 in 1× PBS), rinsed in 1× TBS-T, and incubated overnight at 4 °C or ~2 h at room temperature in primary antibodies diluted in Odyssey Blocking Buffer (diluted 1:1 in 1× PBS; LI-COR) plus 0.02% NaN3. Primary antibodies: Arc 1:1000 (156–002, Synaptic Systems) and beta tubulin 1:10,000 (05–661, Millipore; 8226, Abcam). Following primary antibody exposure, membranes were rinsed in 1× TBS-T and exposed to secondary antibodies for 40–60 min at room temperature. Secondary antibodies: IRDye 800CW goat anti-rabbit and IRDye 680RD goat antimouse IgG; 1:20,000 in LI-COR Blocking Buffer with 0.01% Tween-20 and 0.1% SDS. After final rinses, membranes were scanned and analyzed using Odyssey CLX and ImageStudio (LI-COR).
2.4. Contextual fear conditioning
The procedure for fear conditioning was performed as previously described (Ramamoorthi et al., 2011). Prior to contextual fear conditioning (Day 1) and testing (Day 2), mice were allowed to acclimate to the behavior room in their home cages for a minimum of 1 h. On Day 1, mice were placed in conditioning chambers (Med Associates; metal bar flooring, house light) housed inside noise-attenuated boxes for 4 min. At 58 s, 1 min 58 s, and 2 min 58 s, mice received a 2-s footshock (0.55 mA); there were no tone presentations. On Day 2, mice were returned to the same chambers (same contextual details) and allowed to explore for 4 min. Freezing behavior, defined as the absence of movement aside from that required for respiration, was measured throughout all trials. Percent time spent freezing during the context test was compared to percent time spent freezing during the first 58 s of the training session (baseline). Fear conditioned animals were previously tested for sucrose preference, the results of which we have reported (Penrod et al., 2019).
2.5. Conditioned place preference
CPP was conducted essentially as described (Smith et al., 2016). Mice were acclimated in home cages each day to the behavioral anteroom for ≥1 h. Three-chambered CPP apparatuses (Med-Associates, St. Albans, VT) were used under dim white lighting. Two large conditioning chambers (black with bar flooring vs. white with wire grid flooring) were connected by a smaller chamber (gray with plexiglass flooring). At pretest (Day 1), mice were placed into the center and allowed to explore all three chambers for 20 min. Groups were balanced so that they had a similar pre-existing preference score for the cocaine-paired chamber and so that cocaine was paired with each chamber similarly across groups. On all 4 conditioning days, mice were given an injection (i.p.) before confinement in one of the large chambers for 30 min. Cocaine (10 mg/kg) conditioning occurred on Days 2 and 4, and saline was paired with the opposite chamber on Days 3 and 5. On Day 6 (posttest), the pretest protocol was repeated. Data are expressed as time spent in the cocaine-paired chamber during the posttest minus time spent in the cocaine-paired chamber during the pretest (CPP or preference score).
2.6. Operant conditioning and cocaine IVSA
Operant conditioning chambers and methodological details of food training, jugular vein catheter implantation and cocaine IVSA in mice were performed essentially as previously described (Thomsen and Caine, 2005; Thomsen et al., 2005). In brief, food and drug self-administration were performed in separate, naïve groups of animals (i.e., animals were not food-deprived and cocaine IVSA mice did not receive food training). Indwelling back-mounted catheters were inserted under oxygen/sevoflurane vapor anesthesia. Anchored catheters extended 1.2 cm into the jugular vein and ran subcutaneously to the base seated above the midscapular region, where the cannula guide was kept capped outside of self-administration sessions. Following surgery, mice were given one day off before daily administration of anti-clotting/antibiotic solution (0.02 mL of 0.9% saline containing 30 USP units/mL heparin and 67 mg/mL cefazolin) during their 7-day recovery period. Thereafter, catheter patency was verified periodically and at the end of the experiment by complete loss of righting reflex within 3 s of flushing 0.03 mL ketamine-midazolam solution (15 mg/mL /0.75 mg/mL).
Food and cocaine self-administration (Fig. 1A) took place in operant conditioning chambers equipped with two nose-poke ports on either side of a fixed receptacle for liquid food delivery. A beam break of the active port resulted in delivery of a reinforcer and presentation of a cue light. Beam breaks in the inactive port were counted but had no scheduled consequence. Sessions lasted either two (food reinforcer) or three (cocaine IVSA) hours per day, five to six days per week. In food-reinforced sessions, active nose-pokes were reinforced with liquid food (25 μL; Ensure® nutritional drink, vanilla flavor, 100%; Abbott Laboratories, Abbott Park, IL) delivered to the receptacle via syringe pump under a fixed-ratio 1 (FR1) schedule of reinforcement for 12 days. Criteria for acquisition in the food-reinforced operant experiment included receiving at least 20 reinforcers per session for two consecutive days, with ≤20% variation in responding between the two sessions and ≥70% active/(active + inactive) response ratio.
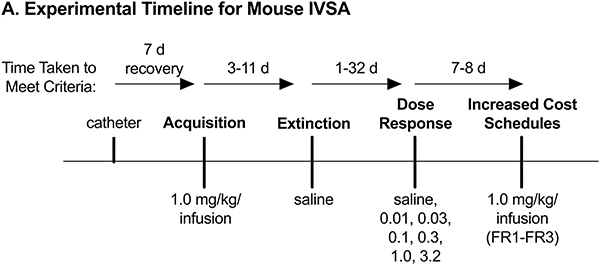
Fig. 1.
IVSA timeline.
(A) Diagram showing progression of phases in intravenous self-administration.
During cocaine IVSA acquisition, active nose-pokes were reinforced with intravenous delivery of cocaine (1.0 mg/kg/infusion in sterile 0.9% (w/v) NaCl) via a syringe pump under an FR1 schedule and copresentation of a cue light. Infusion volume was 0.56 mL/kg, and drug concentration was adjusted according to desired dose (e.g., for a 32 g mouse, each infusion equaled 18 μL, and to achieve a dose of 1.0 mg/kg/infusion, a concentration of 1.8 mg/mL was used). The length of each infusion was calculated as weight (kg)/0.01 s (i.e., a 32 g mouse receives 3.2 s infusions). Acquisition criteria for the cocaine self-administration experiment were a minimum number of reinforcers (15/day), a ≥ 70% preference for the active over the inactive port, and stable taking (≤20% variation) for two consecutive sessions. During extinction, saline was substituted for cocaine, and all other parameters remained the same (including cue light presentation). Extinction criteria were met when responding dropped to 50% or less of acquisition criteria levels. Extinction was followed by reacquisition (1.0 mg/kg/infusion cocaine; 1–2 days) and dose-response determinations (0.00, 0.01, 0.032, 0.1, 0.32, 1.0, 3.2 mg/kg/infusion cocaine; 1 day each). For the latter, doses were presented in sequential order, with the starting dose counterbalanced by Latin Square design. For increased cost, cocaine (1.0 mg/kg/infusion) was available under FR1 for one day, followed by FR2 and FR3 schedules of reinforcement for 2–3 consecutive sessions each until stable (i.e., < 20% variation over two sessions).
2.7. Statistics
Statistical analyses, variables, and results are listed in Table S1. Fear conditioning was analyzed by Two-Way ANOVAs with genotype as a between-subjects factor and time/session as a repeated measures factor. All operant tasks were initially analyzed by Three-Way ANOVAs (genotype x port x session, with session as a repeated-measure factor). CPP, days to criteria and first/last single session group comparisons, were analyzed by unpaired t-tests. Significant interactions were followed by additional ANOVAS (i.e., one- and/or two-way), paired t-tests, and/or Bonferroni or Tukey post hoc analyses, as appropriate, to determine simple main effects (SMEs). When Mauchley’s test of sphericity was significant, either Greenhouse-Geisser (G-G; when Epsilon ≤ 0.75) or Huynh-Feldt (H-F; when Epsilon > 0.75) corrections were used (see Table S1). Comparisons of group survival distributions to reaching criteria (see Methods) were performed for food operant acquisition, IVSA acquisition, and IVSA extinction using Kaplan-Meier log rank tests (Mantel-Cox); mice that failed to reach criteria were included in log rank analyses only, and are not shown in any graphs. All statistics were performed using GraphPad Prism, except SPSS software was used to handle complex data sets/analyses (e.g., three-way and MV ANOVAs, missing values). Significance was set at alpha = 0.05.
3. Results
3.1. Arc/Arg3.1 KO mice show deficits in fear conditioning, but normal cocaine conditioned place preference and operant food reward conditioning
Given the importance of striatal function in reward-related behaviors, which are the focus of this manuscript, we confirmed lack of Arc protein in striatal tissue (nucleus accumbens) of our Arc/Arg3.1 KO mice (Fig. 2A; unpaired t-test, t4 = 11.83, p < .001). As reported previously, Arc/Arg3.1 KO mice displayed significant long-term memory deficits in the Pavlovian fear conditioning assay. Following normal training performance (Fig. 2B; time main effect only, F3,60 = 53.91, p < .0001), mice were reintroduced to the training context 24 h after conditioning, and the Arc/Arg3.1 KO group showed significantly less freezing behavior than the WT group (Fig. 2C: Two-Way RM ANOVA, genotype x session interaction, F1,20 = 8.566, p < .01; SMEs of genotype at the context test session, p < .001 and of session at WT, p < .0001, and KO, p < .001 levels; Fig. S1A; Two-Way RM ANOVA, main effect of genotype, F1,20 = 8.5, p < .01). Interestingly, a deficit was not observed in memory for a cocaine-paired context. Specifically, in the CPP test (an appetitive Pavlovian conditioning assay), Arc/Arg3.1 KO mice exposed to cocaine (10 mg/kg; i.p. ×2 pairings) showed a preference for the drug-paired chamber similar to WT littermates on the drug-free posttest (Fig. 2D), suggesting intact contextual reward learning and memory. For food operant conditioning, we observed an overall group difference when nose-pokes from both ports were included in the analysis (Three-Way RM ANOVA, main effect of genotype, F1,15 = 4.829, p < .05; port x session interaction, F3.7,55.8 = 10.705, p < .00001); however, it is notable that 1) the Arc/Arg3.1 KO mice showed slightly greater nose-pokes than WT, and 2) when we did follow-up analyses on each port to interpret this finding, there were no significant effects involving genotype for either. Importantly, both genotypes showed a significant discrimination for the active over inactive port at Session 3 (WT: One-Way RM ANOVA, main effect of port, F1,8 = 14.581, p < .01; KO: One-Way RM ANOVA, main effect of port, F1,8 = 12.094, p < .01), which was not significant at Session 1 or 2. In addition, a similar number of WT (1) and KO (2)() mice failed to meet acquisition criteria for food operant conditioning within 12 days, and a log rank analysis comparing survival distributions of groups to meeting criteria did not show any significant difference (Fig. 2F). Thus, it appears that Arc/Arg3.1 KO mice display normal acquisition of an operant task reinforced by palatable food (Fig. 2E). Together, these findings suggest that, at least for appetitive tasks, contextual and operant reward-related learning and memory are not impacted by the loss of Arc expression.
3.2. Arc/Arg3.1 influences cocaine self-administration behavior
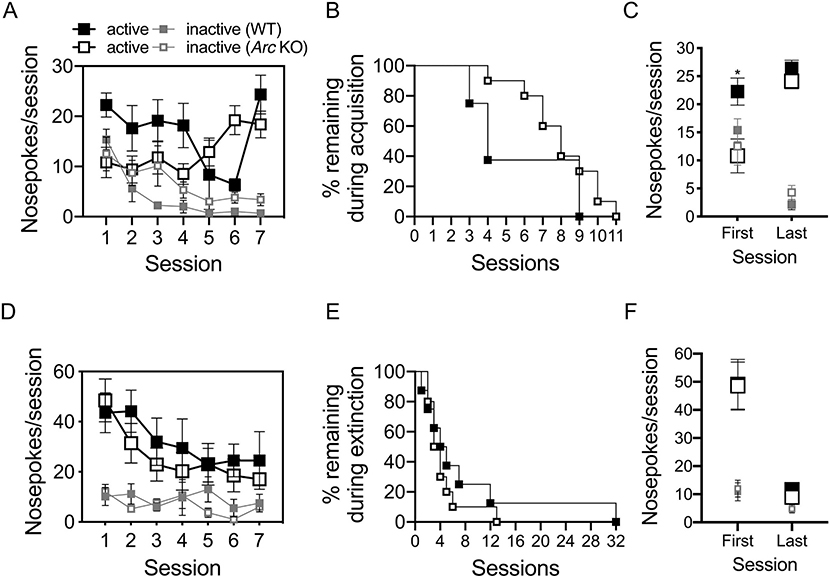
We next examined Arc/Arg3.1 KO and WT mice in the cocaine IVSA assay for acquisition, extinction, and dose-response, followed by performance under increased response requirement (cost). To limit contributions of overtraining to group differences, acquisition and extinction phases were ran to a set of criteria selected to indicate learning (see Methods); thus individual animals differed in the number of sessions during each phase (Fig. S2A, B & S2D, E). For the acquisition phase (Fig. 3A–C), a log rank test showed that the survival distributions of Arc/Arg3.1 KO and WT mice for reaching criteria were not different over sessions (Fig. 3B). Arc/Arg3.1 KO mice did earn significantly fewer reinforcers compared to WT mice on the first session (Session 1: Two-Way RM ANOVA, genotype x port interaction, F1,16 = 6.323, p < .05; active port, SME of genotype, t16 = 2.851,p < .05). Also, while the genotypes did not differ in responses on the inactive port during the first session, and both groups showed high responding in both ports, WT, in contrast to KO, mice already demonstrated preference for the active port at completion of Session 1 (WT, One-Way RM ANOVA, SME of port, F1,7 = 6.806, p < .05). However, by the last training session, Arc/Arg3.1 KO and WT mice earned a similar number of reinforcers (Fig. 3C; Last Session: Two-Way RM ANOVA, main effect of port only, F1,16 = 109.955, p < .0000001), and each group individually showed an active port preference during the last acquisition session (WT, One-Way RM ANOVA, SME of port, F1,7 = 134.141, p < .00001; KO, One-Way RM ANOVA, SME of port, F1,9 = 31.091, p < .001). Importantly, all mice met acquisition criteria, and total cocaine intake over the entire acquisition phase did not differ by group (Fig. S2C). In the extinction phase (Fig. 3D–F), a log rank test showed no difference in the distribution of survival for Arc/Arg3.1 KO and WT mice as they met extinction criteria over sessions (Fig. 3E) and the groups did not differ from one another in active or inactive port nose-pokes, as measured on their first and last days (Fig. 3F). One mouse with verified catheter patency from each genotype failed to meet extinction criteria within 35 sessions.
Fig. 3.
Arc/Arg 3.1 KO mice show early impairments in cocaine IVSA acquisition but normal extinction.
Average nose-pokes (active, large boxes; inactive, small boxes) for WT versus Arc/Arg3.1 KO mice, during the first 7 days of (A) acquisition (1.0 mg/kg/inf, cocaine) and (D) extinction (saline with cue light) (n = 8 WT, 10 KO). Time out responses are excluded. For acquisition, active nose-pokes equal reinforcers earned. As animals met criteria, they were moved to the next phase. Percent of animals remaining in each group over (B) acquisition and (E) extinction sessions were not different. Statistical comparisons of active and inactive nose-pokes were made between groups using each animal’s first and last sessions for (C) acquisition and (F) extinction. *p < .05; except for (B,E), data shown are mean ± S.E.M.
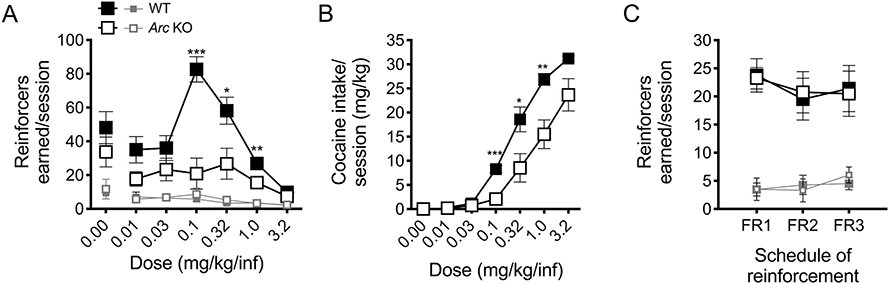
During the dose-response phase, the expected inverted U-shaped curve was observed for WT mice, with peak average responding at the 0.1 mg/kg/infusion dose; however, this “curve” was distinctly flattened in Arc/Arg3.1 KO mice. Arc/Arg3.1 KO mice continued to self-administer cocaine at all doses, with active port responses significantly above those in the inactive port, but earned significantly fewer reinforcers than WT mice at 0.1, 0.32 and 1.0 mg/kg/infusion doses (Fig. 4A; genotype x port x dose interaction, F2.3,34.9 = 6.55, p < .01; genotype x dose interaction for active port responses, F2.2,30.3 = 4.92, p < .01, but not inactive port responses; SME of genotype at the 0.1 dose, F1,15 = 21.87, p < .001; 0.32 dose, F1,15 = 6.68, p < .05; 1.0 dose, F1,15 = 10.20, p < .01). When actual cocaine intake was calculated for the dose-response phase, Arc/Arg3.1 KO mice consistently took less cocaine at each dose than their WT littermates, which reached significance at the 0.1, 0.32, and 1.0 mg/kg/infusion doses (Fig. 4B; genotype x dose interaction, F(2.6,38.6) = 4.27, p < .05; SME of genotype: 0.1, p < .001; 0.32, p < .05; 1.0 dose, p < .01; additional results listed in Table S1). When the acquisition dose (1.0 mg/kg/infusion) was again made available, with increasing response requirements between sessions (FR1, FR2, then FR3), Arc/Arg3.1 KO and WT mice responded identically (Fig. 4C; main effect of port only, F1,9 = 62.9, p < .0001).
Fig. 4.
Arc/Arg3.1 KO mice show reduced dose-response behavior and cocaine intake but sustained responding for cocaine at higher cost schedules.
(A) Average nose-pokes (active, large boxes = reinforcers earned) and inactive (small boxes) per 3 h session for WT and KO animals during dose-response testing. KO mice earned significantly fewer reinforcers at the 0.1, 0.32, and 1.0 mg/kg/infusion doses than WT mice (n = 8 WT, 9–10 KO). (B) Lower cocaine taking amongst KO mice translated to lower drug intake compared to WT mice at these same unit doses. (C) Average nose-pokes for WT and Arc/Arg3.1 KO mice per 3 h session under schedules of increasing response requirement (cost) (1.0 mg/kg/infusion; n = 7 WT, 6 KO). Time out responses are excluded. For (A & C), active nose-pokes equal reinforcers earned. *p < .05, **p < .01, *** p < .001; data shown are mean ± S.E.M.
4. Discussion
Arc/Arg3.1 is an immediate early gene rapidly induced by drug exposure in multiple brain regions and capable of regulating multiple forms of glutamatergic plasticity known to be important to reward-related behaviors. In this study, we demonstrate that despite having contextual fear conditioning deficits, mice lacking Arc/Arg3.1 expression show normal acquisition of food operant conditioning and cocaine conditioned place preference behaviors. Arc/Arg3.1 KO mice do show mild delays in their acquisition of the cocaine self-administration task, but ultimately perform comparably to WT mice and demonstrate normal extinction of the task. Subsequent dose-response testing, however, reveals a flattened curve in Arc/Arg3.1 KO mice, with significantly reduced responding and less cocaine intake than WT mice on the descending limb of the dose curve. The temporal patterns of responses in the KO mice are not typical of extinction conditions (extinction burst early in the session followed by no to very low responding). When mice are returned to the acquisition dose and given the opportunity to self-administer on increased (FR2, FR3) cost schedules, Arc/Arg3.1 KO mice perform comparably to WT mice, and indeed maintain their cocaine intake at the same level in spite of increasing cost. Together, those observations suggest that cocaine, at least at higher doses, functions as a positive reinforcer in Arc/Arg3.1 KO mice. This interpretation is also supported by the fact that the Arc/Arg3.1 KO mice showed cocaine-conditioned CPP, indicating cocaine reward.
Our findings with regard to operant behavior in Arc/Arg3.1 KO mice are intriguing for several reasons. First, Arc/Arg3.1 KO mouse performance in food and cocaine operant self-administration largely suggest normal cognitive abilities pertaining to these tasks, despite a widely reported role for Arc/Arg3.1 in memory consolidation (Lv et al., 2015; Ploski et al., 2008; Maddox and Schafe, 2011; Guzowski et al., 2000), as well as deficits in reversal learning when Arc/Arg3.1 is either knocked out (Plath et al., 2006) or prevented from normal degradation (i.e., overexpressed) (Wall and Correa, 2018). Interestingly, these memory deficits do not appear to strongly influence appetitive Pavlovian or operant conditioning. Whether this differential performance is related to divergent roles for Arc/Arg3.1 in the neurobiological substrates for reward and aversion is unclear. It is possible in our current study that the continued exposure to the operant conditioning chamber and continued training (be it under reinforcement conditions or under extinction conditions) helps to maintain performance and obscures any deficits in consolidation/reconsolidation. Indeed, previous work has similarly suggested deficits are restricted to long-term, but not shortterm, memory (Thomsen and Caine, 2005). Likewise, our data do not support the possibility that differences in exploratory behavior at session one influenced rate of acquisition. While relatively little work has been done concerning the role of Arc/Arg3.1 in the IVSA assay, knockdown of Arc/Arg3.1 levels in adult rat dorsolateral striatum delayed extinction of cocaine seeking (Hearing et al., 2010b), a deficit that we do not observe in our global Arc/Arg3.1 KO mice (Fig. 3D–F). In any case, it appears that memory and performance deficits in Arc/Arg3.1 KO mice are task- and, possibly, circuit-dependent, and that Arc/Arg3.1 likely affects such behaviors via roles in different brain regions.
Secondly, despite relatively minor impairments in acquisition and normal extinction, Arc/Arg3.1 KO mice have markedly reduced responding for multiple cocaine doses in the dose-response test. These results are in sharp contrast to the exaggerated behavioral responses (e.g., locomotor activation) to cocaine that we (Penrod-Martin et al., 2017) and others (Managò et al., 2016; Salery et al., 2016) have reported for Arc/Arg3.1 KO mice elsewhere. Importantly, loss of Arc did not impede increased nose-poke frequency, since multiple Arc/Arg3.1 KO mice produced rapid or “burst” responding at other times in the dose-response test, particularly at the 0.01 and 0.032 mg/kg/infusion doses (Fig. S3). One interpretation of the observed dose-response patterns of the KO mice, alongside their willingness to meet increased cost requirements for a higher dose, could be reduced sensitivity to cocaine’s reinforcing effects. Assuming that the rate-limiting effects of cocaine were similarly affected, we would expect to observe a rightward shift in the dose-response curve. Instead, average KO intake remained at or significantly below WT levels across doses, up to and including a relatively high dose (3.2 mg/kg/infusion), suggesting instead a vertical shift.
The interpretation of vertical dose-response shifts has been much debated (Zernig et al., 2004; Piazza et al., 2000; Katz and Higgins, 2004; Schenk and Partridge, 1997), reflecting the complexity of dosedependent responses to reinforcing substances (Calabrese, 2008). Down-shifted or flattened cocaine IVSA dose-response curves have been interpreted as a decrease in hedonic set point and/or decreased reinforcing effects of the drug (Piazza et al., 2000; Ahmed and Koob, 1998; Graham et al., 2007a; Graham et al., 2007b). Piazza et al. (2000) reported that an animal’s locomotor response to a novel environment predicts a vertically shifted dose-response function; high responders show upward shifted IVSA dose-response curves and appear more vulnerable to acquisition of drug taking at lower doses. While a relationship between response to novelty and cocaine IVSA behavior has not always been supported (Thomsen and Caine, 2011), it is notable that we have previously reported hyperactivity in three novel behavior tasks in Arc/Arg3.1 KO compared to WT mice, whereas activity in a context similar to their home cage was normal (Penrod et al., 2019). Together, these findings suggest the possibility that mice lacking Arc/Arg3.1 may be “low responders” to cocaine, a phenotype associated with impaired dopamine release in the nucleus accumbens. While the effect of cocaine exposure on Arc/Arg3.1 KO dopamine efflux is unknown, Managò et al. (2016) did find basal striatal dopamine levels in these mice to be normal, while NAc shell surface dopamine D2-type receptors (drd2s) were increased significantly compared to WT mice. Studies of natural variation in drd2 expression levels in non-human primates, and genetic manipulations (e.g., overexpression, KO) in mice, have shown higher levels of striatal drd2 to be associated with lower cocaine intake (Morgan et al., 2002; Caine et al., 2002; Nader et al., 2006; however, see Jupp et al., 2016), a possibility to be further explored.
Our findings here indicate that Arc/Arg3.1 may be dispensable for proper learning under certain conditions, and we find it to play a critical role in regulating self-administration of a strong reinforcer, cocaine. Like work concerning other neuropsychiatric disorders, our findings suggest that Arc/Arg3.1 variants (Chuang et al., 2016; Huentelman et al., 2015; Landgren et al., 2012), and other mutations in pathways regulating Arc/Arg3.1 expression and function, should be examined as potential contributing factors in addiction susceptibility. While interesting observations regarding the role of Arc/Arg3.1 in cocaine self-administration have been made by examining global Arc/Arg3.1 KO mice, future studies examining brain region-specific roles for Arc/Arg3.1 will be critical to elucidate its role in cocaine reinforcement, motivation, satiety, and craving.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Simon Barak Caine, Peter Kalivas, David Self, and Jack Bergman for helpful discussions. We thank Christopher Adam, Rachel Hart, Jonathan Krieger, and Jaswinder Kumar for behavioral assistance, and Kevin Stoll for surgical expertise. Benjamin Zirlin and Emilia Pulver provided excellent animal care.
Funding
This work was supported by the National Institutes of Health [grant numbers R01DA027664 (C.W.C), F32DA036319 (R.D.P.), R00DA027825 (M. Thomsen)]; the Brain & Behavior Research Foundation (M. Taniguchi), the Phyllis and Jerome Lyle Rappaport Foundation(L.N.S.); and Texas A&M University (L.N.S.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pbb.2019.172818.
References
- Ahmed SH, Koob GF, 1998. Transition from moderate to excessive drug intake:change in hedonic set point. Science 282 (5387), 298–300. [DOI] [PubMed] [Google Scholar]
- Caine SB, Negus SS, Mello NK, Patel S, Bristow L, Kulagowski J, Vallone D, Saiardi A, Borrelli E, 2002. Role of dopamine D2-like receptors in cocaine self-administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J. Neurosci 22 (7), 2977–2988 (Epub 2002/03/30. doi: 20026264). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, 2008. U-shaped dose response in behavioral pharmacology: historical foundations. Crit. Rev. Toxicol 38 (7), 591–598. Epub 2008/08/19. 10.1080/10408440802026307. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF, 2006. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 52 (3), 445–459. Epub 2006/11/08. 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang YA, Hu TM, Chen CH, Hsu SH, Tsai HY, Cheng MC, 2016. Rare mutations and hypermethylation of the ARC gene associated with schizophrenia. Schizophr. Res 176 (2–3), 106–113. Epub 2016/07/29. 10.1016/j.schres.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Fanous S, Guez-Barber DH, Goldart EM, Schrama R, Theberge FRM, Shaham Y, Hope BT, 2012. Unique gene alterations are induced in FACS-purified Fos-positive neurons activated during cue-induced relapse to heroin seeking. J. Neurochem 124 (1), 100–108. 10.1111/jnc.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosnaugh JS, Bhat RV, Yamagata K, Worley PF, Baraban JM, 1995. Activation of arc, a putative “effector” immediate early gene, by cocaine in rat brain. J. Neurochem 64 (5), 2377–2380 Epub 1995/05/01. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Franchi C, Caffino L, Racagni G, Riva MA, Cervo L, 2009. Single session of cocaine intravenous self-administration shapes goal-oriented behaviours and up-regulates arc mRNA levels in rat medial prefrontal cortex. Int. J. Neuropsychopharmacol 12 (3), 423–429. Epub 2008/11/26. 10.1017/S1461145708009681. [DOI] [PubMed] [Google Scholar]
- Gouty-Colomer LA, Hosseini B, Marcelo IM, Schreiber J, Slump DE, Yamaguchi S, Houweling AR, Jaarsma D, Elgersma Y, Kushner SA. Arc expression identifies the lateral amygdala fear memory trace. Mol. Psychiatry 2016;21(3):364–75. doi: 10.1038/mp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW, 2007a. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat. Neurosci 10 (8), 1029–1037. Epub 2007/07/10. 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat. Neurosci 2007b;10(8):1029–37. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci 1999;2(12):1120–4. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA, 2000. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J. Neurosci 20 (11), 3993–4001 Epub 2000/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Miller SW, See RE, McGinty JF, 2008a. Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology 198 (1), 77–91. Epub 2008/03/04. 10.1007/s00213-008-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, See RE, McGinty JF. Relapse to cocaine-seeking increases activity-regulated gene expression differentially in the striatum and cerebral cortex of rats following short or long periods of abstinence. Brain Struct. Funct 2008b;213(1–2):215–27. doi: 10.1007/s00429-008-0182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Schochet TL, See RE, McGinty JF, 2010a. Context-driven cocaine-seeking in abstinent rats increases activity-regulated gene expression in the basolateral amygdala and dorsal hippocampus differentially following short and long periods of abstinence. Neuroscience. 170 (2), 570–579. Epub 2010/07/27. 10.1016/j.neuroscience.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Schwendt M, McGinty JF, 2010b. Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. Int. J. Neuropsychopharmacol 14 (6), 784–795. Epub 2010/10/15. 10.1017/S1461145710001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Schwendt M, McGinty JF, 2011. Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. Int. J. Neuropsychopharmacol 14 (6), 784–795. Epub 2010/10/15. 10.1017/S1461145710001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huentelman MJ, Muppana L, Corneveaux JJ, Dinu V, Pruzin JJ, Reiman R, Borish CN, De Both M, Ahmed A, Todorov A, Cloninger CR, Zhang R, Ma J, Gallitano AL, 2015. Association of SNPs in EGR3 and ARC with schizophrenia supports a biological pathway for schizophrenia risk. PLoS One 10 (10), e0135076 Epub 2015/10/17. 10.1371/journal.pone.0135076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakkamsetti V, Tsai NP, Gross C, Molinaro G, Collins KA, Nicoletti F, Wang KH, Osten P, Bassell GJ, Gibson JR, Huber KM, 2013. Experience-induced Arc/Arg3.1 primes CA1 pyramidal neurons for metabotropic glutamate receptor-dependent long-term synaptic depression. Neuron. 80 (1), 72–79. Epub 2013/10/08. 10.1016/j.neuron.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Murray JE, Jordan ER, Xia J, Fluharty M, Shrestha S, Robbins TW, Dalley JW, 2016. Social dominance in rats: effects on cocaine self-administration, novelty reactivity and dopamine receptor binding and content in the striatum. Psychopharmacology 233 (4), 579–589. Epub 2015/11/12. 10.1007/s00213-015-4122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Higgins ST, 2004. What is represented by vertical shifts in self-administration dose–response curves? Psychopharmacology 171 (3), 360–361. 10.1007/s00213-003-1605-9. [DOI] [Google Scholar]
- Kawashima T, Okuno H, Nonaka M, Adachi-Morishima A, Kyo N, Okamura M, Takemoto-Kimura S, Worley PF, Bito H, 2009. Synaptic activity-responsive element in the Arc/Arg3.1 promoter essential for synapse-to-nucleus signaling in activated neurons. Proc. Natl. Acad. Sci 106 (1), 316–321. 10.1073/pnas.0806518106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz KL, Patel KM, Grigson PS, Freeman WM, Vrana KE, 2008. Heroin self-administration: II. CNS gene expression following withdrawal and cue-induced drugseeking behavior. Pharmacol. Biochem. Behav 90 (3), 349–356. Epub 2008/05/10. 10.1016/j.pbb.2008.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren S, von Otter M, Palmer MS, Zetterstrom C, Nilsson S, Skoog I, Gustafson DR, Minthon L, Wallin A, Andreasen N, Bogdanovic N, Marcusson J, Blennow K, Zetterberg H, Kettunen P. A novel ARC gene polymorphism is associated with reduced risk of Alzheimer’s disease. J. Neural Transm. (Vienna) 2012;119(7):833–42. Epub 2012/05/25. doi: 10.1007/s00702-012-0823-x. [DOI] [PubMed] [Google Scholar]
- Lv X-F, Xu Y, Han J-S, Cui C-L. Expression of activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) in the nucleus accumbens is critical for the acquisition, expression and reinstatement of morphine-induced conditioned place preference. Behav. Brain Res 2011;223(1):182–91. doi: 10.1016/j.bbr.2011.04.029. [DOI] [PubMed] [Google Scholar]
- Lv X-F, Sun L-L, Cui C-L, Han J-S, 2015. NAc shell Arc/Arg3.1 protein mediates reconsolidation of morphine CPP by increased GluR1 cell surface expression: activation of ERK-coupled CREB is required. Int. J. Neuropsychopharmacol 18 (9), pyv030 10.1093/ijnp/pyv030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox SA, Schafe GE, 2011. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for reconsolidation of a Pavlovian fear memory. J. Neurosci 31 (19), 7073–7082. Epub 2011/05/13. 10.1523/JNEUROSCI.112011.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Managò F, Mereu M, Mastwal S, Mastrogiacomo R, Scheggia D, Emanuele M, De Luca MA, Weinberger DR, Wang KH, Papaleo F, 2016. Genetic disruption of Arc/Arg3.1 in mice causes alterations in dopamine and neurobehavioral phenotypes related to schizophrenia. Cell Rep. 16 (8), 2116–2128. 10.1016/j.celrep.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga DE, Calhoun ME, Chowdhury A, Worley P, Morrison JH, Shapiro ML, 2004. Activity-regulated cytoskeletal-associated protein is localized to recently activated excitatory synapses. Neuroscience 125 (1), 7–11. Epub 2004/03/31. 10.1016/j.neuroscience.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M, Montag D, 2003. Learning-induced arg 3.1/arc mRNA expression in the mouse brain. Learn. Mem 10 (2), 99–107. Epub 2003/03/29. 10.1101/lm.53403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA, 2002. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat. Neurosci 5 (2), 169–174. Epub 2002/01/22. 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH, 2006. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat. Neurosci 9 (8), 1050–1056. Epub 2006/07/11. 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Niere F, Wilkerson JR, Huber KM, 2012. Evidence for a fragile X mental retardation protein-mediated translational switch in metabotropic glutamate receptor-triggered Arc translation and long-term depression. J. Neurosci 32 (17), 5924–5936. 10.1523/JNEUROSCI.4650-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya K, Ohno M, Kataoka N, 2016. Dendritic transport element of human arc mRNA confers RNA degradation activity in a translation-dependent manner. Genes Cells 21 (11), 1263–1269. Epub 2016/09/24. 10.1111/gtc.12439. [DOI] [PubMed] [Google Scholar]
- Onoue K, Nakayama D, Ikegaya Y, Matsuki N, Nomura H, 2014. Fear extinction requires arc/Arg3.1 expression in the basolateral amygdala. Mol Brain. 7, 30 Epub 2014/04/25. 10.1186/1756-6606-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ons S, Martí O, Armario A. Stress-induced activation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: relationship to c-fos mRNA. J. Neurochem 2004;89(5):1111–8. doi: 10.1111/j.1471-4159.2004.02396.x. [DOI] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim J-A, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF, 2008. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 59 (1), 70–83. 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penrod RD, Kumar J, Smith LN, McCalley D, Nentwig TB, Hughes BW, Barry GM, Glover K, Taniguchi M, Cowan CW, 2019. Activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) regulates anxiety- and novelty-related behaviors. Genes Brain Behav. e12561 Epub 2019/02/15. 10.1111/gbb.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viral-mediated rescue of Arc/Arg3.1 knock-out demonstrates a requirement for function in the NAc in regulating mood and drug-related behaviors In: Penrod-Martin RD, Smith LN, Kumar J, Hughes BW, Thomsen M, Jedynak JP, Barry G, Wood D, Taniguchi M, Cowan CW (Eds.), Neuropsychopharmacology, (Palm Springs, CA: ). [Google Scholar]
- Piazza PV, Deroche-Gamonent V, Rouge-Pont F, Le Moal M. Vertical shifts in self-administration dose-response functions predict a drug-vulnerable phenotype predisposed to addiction. J. Neurosci 2000;20(11):4226–32.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bosl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D, 2006. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 52 (3), 437–444. Epub 2006/11/08. 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Ploski JE, Pierre VJ, Smucny J, Park K, Monsey MS, Overeem KA, Schafe GE, 2008. The activity-regulated cytoskeletal-associated protein (Arc/Arg3.1) is required for memory consolidation of pavlovian fear conditioning in the lateral amygdala. J. Neurosci 28 (47), 12383–12395. Epub 2008/11/21. 10.1523/JNEUROSCI.1662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthi K, Fropf R, Belfort GM, Fitzmaurice HL, McKinney RM, Neve RL, Otto T, Lin Y, 2011. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science 334 (6063), 1669–1675. Epub 2011/12/24. 10.1126/science.1208049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Partridge B, 1997. Sensitization and tolerance in psychostimulant self-administration. Pharmacol. Biochem. Behav 57 (3), 543–550. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF, 2006. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 52 (3), 475–484. 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LN, Penrod RD, Taniguchi M, Cowan CW, 2016. Assessment of cocaine-induced behavioral sensitization and conditioned place preference in mice. J. Vis. Exp 108, 53107 Epub 2016/03/12. 10.3791/53107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Carreira MB, Smith LN, Zirlin BC, Neve RL, Cowan CW, 2012. Histone deacetylase 5 limits cocaine reward through cAMP-induced nuclear import. Neuron 73 (1), 108–120. 10.1016/j.neuron.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Caine SB, 2005. Chronic intravenous drug self-administration in rats and mice. Curr. Protoc. Neurosci 20 Chapter 9:Unit 9. Epub 2008/04/23. 10.1002/0471142301.ns0920s32. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Caine SB, 2011. Psychomotor stimulant effects of cocaine in rats and 15 mouse strains. Exp. Clin. Psychopharmacol 19 (5), 321–341. Epub 2011/08/17. 10.1037/a0024798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DPD, Wörtwein G, Fink-Jensen A, Wess J, Caine SB, 2005. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J. Neurosci 25 (36), 8141–8149. 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Correa SAL, 2018. The mechanistic link between Arc/Arg3.1 expression and AMPA receptor endocytosis. Semin. Cell Dev. Biol 77, 17–24. Epub 2017/09/12. 10.1016/j.semcdb.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Wang H, Ardiles AO, Yang S, Tran T, Posada-Duque R, Valdivia G, Baek M, Chuang YA, Palacios AG, Gallagher M, Worley P, Kirkwood A, 2016. Metabotropic glutamate receptors induce a form of LTP controlled by translation and Arc signaling in the hippocampus. J. Neurosci 36 (5), 1723–1729. Epub 2016/02/05. 10.1523/JNEUROSCI.0878-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salery M, Dos Santos M, Saint-Jour E, Moumné L, Pagès C, Kappès V, Parnaudeau S, Caboche J, Vanhoutte P. Activity-regulated cytoskeleton-associated protein accumulates in the nucleus in response to cocaine and acts as a brake on chromatin remodeling and long-term behavioral alterations. Biol. Psychiatry 2016;1–13. doi: 10.1016/j.biopsych.2016.05.025. [DOI] [PubMed] [Google Scholar]
- Wang KH, Majewska A, Schummers J, Farley B, Hu C, Sur M, Tonegawa S. In vivo twophoton imaging reveals a role of arc in enhancing orientation specificity in visual cortex. Cell. 2006;126(2):389–402. doi: 10.1016/j.cell.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM, 2008. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 59 (1), 84–97. 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112(3):317–27.. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Osredkar T, Joyce JN, Neisewander JL, 2008. Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse 62 (6), 421–431. Epub 2008/03/26. 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernig G, Wakonigg G, Madlung E, Haring C, Saria A, 2004. Do vertical shifts in dose-response rate-relationships in operant conditioning procedures indicate “sensitization” to “drug wanting”? Psychopharmacology 171 (3), 349–351. author reply 52–63. Epub 2003/10/08. 10.1007/s00213-003-1601-0. [DOI] [PubMed] [Google Scholar]
- Ziolkowska B, Kielbinski M, Gieryk A, Soria G, Maldonado R, Przewlocki R, 2011. Regulation of the immediate-early genes arc and zif268 in a mouse operant model of cocaine seeking reinstatement. J. Neural Transm. (Vienna) 118 (6), 877–887. Epub 2011/02/15. 10.1007/s00702-011-0583-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.