Abstract
Low back pain has become more prevalent in recent years, causing enormous economic burden for society and government. Common therapies used in clinics including conservative treatment and surgery can only relieve pain. Subsequent cell-based treatment such as mesenchymal stem cell transplantation poses problems such as short duration of therapeutic effect and tumorigenesis. Recently, the discovery and identification of stem cell niche and stem/progenitor cells in intervertebral disc bring increased attention to endogenous repair strategy. Therefore, we review the studies involving endogenous repair strategy and present the characteristics and current status of this treatment. Meanwhile, we also discuss the strategy and perspective of endogenous repair strategy in future.
Keywords: Low back pain, Intervertebral disc degeneration, Stem cell niche, Stem/progenitor cell, Endogenous repair strategy, Stem cell treatment
Core tip: Low back pain has become more prevalent and brought enormous economic burden in recent years. However, therapies including conservative treatment, surgery, and cell-based treatment still have several defects. Endogenous repair is a novel therapeutic strategy for intervertebral disc degenerative disease that draws increased attention. We review the research regarding endogenous repair strategy using stem/progenitor cells as main cell resource, concluding and analyzing the status at present and perspective of endogenous repair strategy in future.
INTRODUCTION
Low back pain (LBP) has become one of the most frequent causes for hospital visits and the leading reason of disability with population aging in the worldwide today[1]. In addition, about 80% of adults will suffer from LBP at some point in their lives, which brings frequent sick leave and enormous economic burden for society and government[2]. Especially in the United States, the estimated total expenses including direct and indirect costs of LBP exceed $100 billion per year[3]. Despite the complex and dim pathogeny and pathology of LBP, it is widely reported that 40% of LBP cases are associated with intervertebral disc (IVD) degeneration[4-6].
The IVD is fibrocartilaginous tissue located between the vertebral bodies and composed of outer annulus fibrosus (AF), inner nucleus pulposus (NP), and cartilaginous endplates (CEP)[7]. The structure of AF is formed by types I and II collagen fibers and elastin fibers arranged in concentric circles, which can withstand the tension produced by vertebral motion to maintain the position of NP[8]. Meanwhile, the NP tissue is constituted by the extracellular matrix (ECM) including collagen type II and aggrecan synthesized and secreted by NP cells[9]. Therefore, NP cells are so vitally important in IVD degeneration that we should pay more attention to them.
However, clinical therapies such as pharmacological treatments and surgeries are mainly focused on removing NP tissue and thus can only deal with relief of symptoms rather than restoration[10]. Although many studies based on mesenchymal stem cell (MSC) transplantation have obtained positive results in attenuating or even reversing IVD degeneration in pre-clinical and clinical studies[11-13], there are still some obstacles such as survival and differentiation of transplanted MSC caused by a specifically harsh environment in the degenerated IVD[14,15]. Moreover, various complications including oncogenicity, ectopic ossification, and immune reactions indicated that more therapies should be explored[16,17]. Thus, searching a new candidate for degenerative IVD treatment is especially important.
Niche, first proposed by Schofield[18], is a specific anatomic localization composed of ECM and other noncellular components[18,19]. Since this concept was introduced, many niches were found in a variety of tissues and organs including the skin, bone marrow, and the neural and digestive systems. The niche in the IVD defined as the perichondrium region adjacent to the epiphyseal plate (EP) and outer zone of the AF (AFo), raises a growing interest in boosting endogenous repair strategy (ERS) in degenerative IVD[20-24]. And the novel ERS focuses on improving proliferation and promoting differentiation of stem cells derived from the IVD niche.
Thus, this review concentrates on the retrospect and assessment of research regarding the conception of endogenous repair through activating and mobilizing reparative MSCs located in specific anatomical niches of the IVD. Besides that, the review will also analyse the obstacles and difficulties needed to be conquered, which may help to accelerate the process of endogenous repair.
ENDOGENOUS REPAIR STRATEGY
Cell resource of ERS
It has been well accepted that NP cells consist of notochordal cells (NTC) and nucleopulpocytes (NPCy; another known name is chondrocyte-like cells). Among them, NTC are responsible for maintaining tissue homeostasis and promoting growth while NPCy play a vital role in ECM synthesis[25]. However, the NTC usually coexist with NPCy in the young and health IVD and decline with aging, thus other endogenous stem/progenitor cells may help to boost the progress of endogenous repair in IVD degeneration by differentiating into various damaged IVD cells or excreting intercellular signaling molecules such as exosomes.
The cells first isolated from degenerative human NP and AF cells were characteristic of marrow MSCs and showed capacity of osteogenic, adipogenic, and chondrogenic differentiation[26]. The following research studies illustrated that MSCs derived from NP can better withstand the terrible environment in degenerative IVD with improved proliferation and vitality compared with other MSCs[27-29]. And the results of our previous study showed that the vitality and characteristics of such cells would be affected with aggravative degeneration[30]. Besides that, previous studies have not only proved the presence of stem/progenitor cells in the NP, but also isolated stem/progenitor cells from the AF and CEP[31-33]. Above all, these results show that stem/progenitor cells may migrate from the IVD niche to NP, AF, and CEP tissues. Thus, the key point of endogenous repair strategy is to increase the vitality of stem/progenitor cells in NP tissue or motivate their migration from the niche (Figure 1).
Figure 1.
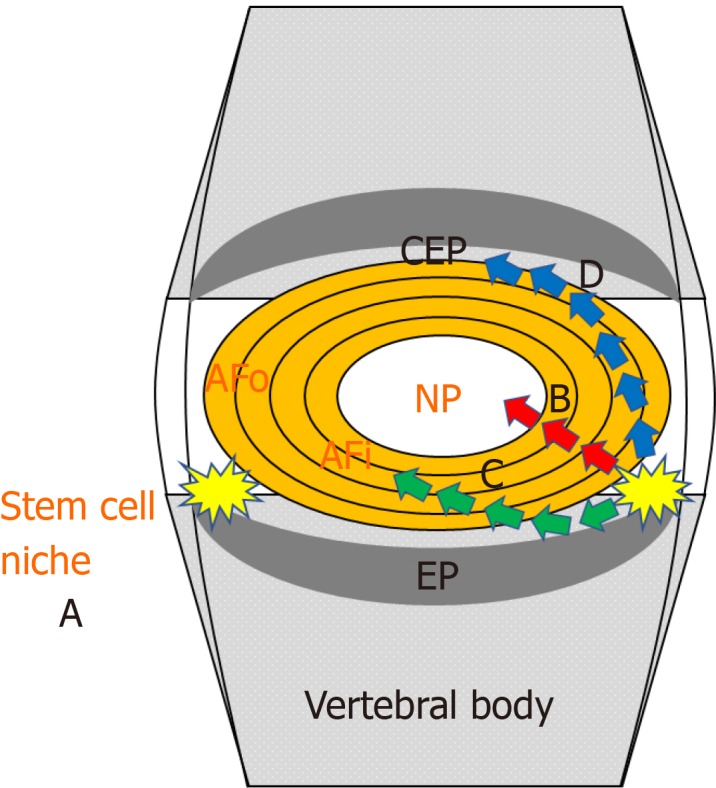
Cell resources of endogenous repair strategy. A: Stem cell niche, defined as the perichondrium region adjacent to the epiphyseal plate and outer zone of the annulus fibrosus (AF); B-D: Stem/progenitor cells could be isolated from the nucleus pulposus, AF, and cartilaginous endplates or migrate from the stem cell niche toward the nucleus pulposus (B), AF (C), and cartilaginous endplates (D). NP: Nucleus pulposus; EP: Epiphyseal plate; AF: Annulus fibrosus; AFo: Outer zone of the AF; AFi: Inner zone of the AF; CEP: Cartilaginous endplates.
Characteristics of stem/progenitor cells in the NP, AF, and CEP
Stem/progenitor cell immunophenotypes: Those kinds of cells, also called MSCs derived from the NP, AF, and CEP, grow adherently in spindle shape after passage[30] and mostly express MSC-like marker including CD73, CD90, and CD105 but not CD11b, CD14, CD19, CD34, CD45, or HLA-DR according to criteria of International Society for Cellular Therapy (ISCT) (Table 1)[35]. Various research studies also found that NP-MSCs can be isolated from human[40], rat[39,56,58], rabbit[54], and dog samples, which were positive (> 95%) for marker proteins CD29 and CD44 except in dogs. Furthermore, CD13 expressed frequently in granulocyte and CD24 related to proliferation and differentiation of B cells are membrane glycoprotein and detected in NP-MSCs from degenerative and normal IVD, respectively[50,57]. Interestingly, a study by Jia et al[54] indicated that NP-MSCs derived from rabbits are negative not only for CD14 but also for CD4 and CD8, which has not been proposed before.
Table 1.
Expression of stem/progenitor immunophenotypes and genes of the intervertebral disc
| Ref. | Type of stem cells | Expression of stem cell/progenitor immunophenotypes and genes |
| Human | ||
| Liu et al[35], 2016 | NP-MSCs | CD73+, CD90+, CD105+, Oct4+, Nanog+, Jagged+ and Notch1+, CD34-, CD45-,HLA-DR- |
| Li et al[36], 2017 | NP-SCs | GD2+, Tie2+ |
| Li et al[37], 2018 | NP-MSCs | CD73+, CD90+, CD105+, CD34-, CD45-, HLA-DR- |
| Jia et al[38], 2017 | D-NP-MSCs/ND-NP-MSCs | CD73+, CD90+, CD105+, Oct4+ and Nanog+, CD34-, CD45-, HLA-DR- |
| Wu et al[40], 2017 | NP-SCs/NPPCs | CD29+, CD44+, CD 73+, CD90+, CD105+, Oct4+ and Nanog+, CD11b-, CD14-, CD34-, CD45-, HLA-DR- |
| Wang et al[41], 2016 | NP-SCs/AF-SCs/CEP-SCs | CD73+, CD90+, CD105+, CD19-, CD34-, CD45-, HLA-DR- |
| Liang et al[42], 2018 | NP-MSCs | CD73+, CD90+, CD105+, Sox2+ and Oct4+, CD14-, CD19-, CD34-, HLA-DR- |
| Daisuke et al[43], 2012 | NPPCs | Tie2+, GD2+ |
| Chen et al[44], 2018 | NP-MSCs | CD73+, CD90+, CD105+, CD34-, HLA-DR- |
| Quan et al[48], 2015 | NP-MSCs | CD29+, CD44+, CD105+, CD14-, CD34-, CD45-, HLA-DR- |
| Liu et al[49], 2017 | AF-MSCs, NP-MSCs, CEP-MSCs | CD73+, CD90+, CD105+, CD34-, CD45-, HLA-DR- |
| Blanco et al[27], 2010 | NP-MSCs | CD73+, CD90+, CD105+, CD106+, CD166+, CD19-, CD34-, CD45-, HLA-DR- |
| Lazzarini et al[50], 2018 | NP-MSCs | CD13+, CD73+, CD90+, CD105+, CD11b-, CD14-, CD19-, CD45-, HLA-DR- |
| Pereira et al[51], 2016 | CEP-MSCs | Not shown |
| Qi et al[57], 2018 | NP-MSCs | CD24+, CD73+, CD90+, CD105+, CD29-, CD45- |
| Rat | ||
| Zhao et al[34], 2017 | NP-MSCs | CD73+, CD90+, CD105+, CD34-, CD45- |
| Li et al[39], 2019 | NP-MSCs | CD44+, CD73+, CD90+, CD105+, Oct4+, Nanog+ and Sox2+, CD34-, HLA-DR- |
| Li et al[45], 2013 | NP-MSCs | CD73+, CD90+, CD105+, CD34-, CD45- |
| Wang et al[46], 2019 | NP-MSCs | CD73+, CD90+, CD105, Tie2+, CD34-, CD45- |
| Nan et al[52], 2019 | NP-MSCs | CD73+, CD90+, CD105+, CD34-, CD45- |
| Han et al[29], 2014 | NP-MSCs | CD73+, CD90+, CD105+, CD34-, CD45- |
| Tao et al[28], 2013 | NP-MSCs | CD73+, CD90+, CD105+, Nanog+, Sox2+, Rex1+ and Oct4+, CD34-, CD45- |
| Tao et al[53], 2015 | NP-MSCs | CD73+, CD90+, CD105+, CD34-, CD45- |
| Liu et al[30], 2019 | N-NP-MSCs/D-NP-MSCs | CD73+, CD90+, CD105+, CD166+, Sox2+, Nanog+, Oct4+, LIF+, PCNA+ and C-KIT+, CD34-, CD45- |
| Li et al[55], 2018 | NP-MSCs | CD73+, CD90+, CD105+, CD34-, CD45- |
| Cheng et al[56], 2019 | NP-MSCs | CD29+, CD44+, CD90+, CD34-, CD45- |
| Lin et al[58], 2017 | NP-MSCs/NPPCs | CD29+, CD44+, CD90+, Nanog+, Oct4+ and Sox2+, CD34-, CD45- |
| Liu et al[68], 2019 | NP-MSCs | CD73+, CD90+, CD105+, Sox2+, Nanog+ and Oct4+, CD34-, CD45- |
| Zhang et al[69], 2015 | NP-MSCs | CD44+, CD73+, CD90+, CD105+, Sox2+, Nanog+ and Oct4+, CD14-, CD34-, CD45-, HLA-DR- |
| Dog | ||
| Erwin et al[47], 2013 | NPPCs | Oct3/4+, Sox2+, CD133+, Nanog+ and Nestin+ |
| Bovine | ||
| Tekari et al[63], 2016 | NPPCs | Tie2+ |
| Rabbit | ||
| Jia et al[54], 2018 | NP-MSCs | CD29+, CD44+, CD166+, CD4-, CD8-, CD14- |
MSC: Mesenchymal stem cell; NP: Nucleus pulposus; AF: Annulus fibrosus; CEP: Cartilaginous endplates; NPPC: NP-derived progenitor cell.
Stem/progenitor genes: Besides cellular markers, stem genes are considered as another criterion for identification of MSCs derived from the IVD, especially in NP tissue. Among them, Nanog (homeobox-containing), Oct4 (the POU domain-containing), and Sox2 (the HMG domain-containing) are transcription factors that play an essential role in the development and maintenance of normal pluripotent cells and are often used to assess the stemness of NP-MSCs (Table 1)[28,30,59]. In addition, Notches and their ligand named Jagged show a crucial effect on the function and differentiation of human bone marrow MSCs (hBMMSC) and NP-MSCs[35,60,61]. Moreover, our previous study provided further evidence that PCNA, CD166, and C-KIT can be chosen as stem/progenitor markers for NP-MSCs and decline with aging[30,62]. Notably, a recent study by Tekari et al[63] demonstrated that Tie2+ NP-derived progenitor cells could be maintained in subsequent monolayer culture for up to 7 d by addition of fibroblast growth factor 2 or hypoxic conditions. Thus, it may be better to isolate NP-MSCs by Tie sorting method rather than just by plastic-adherent method[36,63].
Multi-differentiation: Many studies confirmed that MSCs derived from the NP, AF, and CEP have the ability to differentiate into osteogenic, adipogenic, and chondrogenic lineages[35-44]. Liu et al[49] compared the characteristics of three types of MSCs derived from human IVD including NP-MSCs, AF-MSCs, and CEP-MSCs and found that they showed similar multilineage differentiation capacities, whereas CEP-MSCs showed the best migration and invasion potency. Moreover, a stronger capacity of osteogenic and chondrogenic differentiation was confirmed by Wang et al[41] in CEP-MSCs. Above all, CEP-MSCs may be a new useful candidate for cell-based therapy and ERS.
NP-MSCs, another essential cell resource, show different advantages and drawbacks in differentiated capacity compared with other MSCs. Several reports have shown that NP-MSCs have the regeneration ability similar to BM-MSCs and adipose tissue-derived MSCs with same or superior capacity of chondrogenesis[27,36,45,69]. But in the studies by Blanco et al[27] and Wang et al[41], NP-MSCs displayed weaker multilineage differentiation potentials and were even not able to differentiate into adipocytes. Besides the multilineage differentiation potential, such stem/progenitor cells are also capable of differentiation along neurogenic lineages both in vitro and in vivo, which needs to be further compared with other MSCs[47,50]. Furthermore, Wu et al[40] discovered that MSCs derived from degenerative NP tissue show lower differentiation potentials compared with umbilical cord derived MSCs (UC-MSCs). These results demonstrated the differentiation potentials of NP-MSCs may be affected and impaired by the degeneration status of the IVD.
Obstacles of endogenous repair
Since the existence of stem cell niche and stem/progenitor cells in the IVD has been proved, there may be three reasons for the degeneration of the IVD and failure of restoration still occurring. First, with increasing age and degeneration of the IVD, the stem/progenitor cells in the IVD or stem cell niche are possible to exhaust with aging, which cannot support the requirement of endogenous repair. Second, the degeneration of the IVD is prone to destroy the potential cellular migration pathways from the specific location of stem cell niche defined as the perichondrium region adjacent to the EP and AFo[24]. Lastly, the harsh environment such as low pH condition[35], inflammation[37,56], compression loading[42], high glucose[57,68], oxidative stress[64], hypoxia[45], and hyperosmolarity[55] may impair the biological function and arrest the proliferation of stem/progenitor cells in the IVD. Thus, searching solutions to resolve these questions may be our first priority to overcome the obstacles of endogenous repair.
Strategies and outcomes of endogenous repair
One simply effective strategy is to reduce the apoptosis and senescence of stem/progenitor cells in the IVD during IVD degeneration induced by various factors or increase the vitality and differentiation of stem/progenitor cells directly. It is well accepted that a normal pH is necessary to maintain normal cell function, whereas an excessively acidic environment induces increased cell apoptosis, reduced cell proliferation, and disordered matrix metabolism in the degenerated IVD[67]. Amiloride, an acid-sensing ion channel, may meliorate IVD degeneration by improving the biological characteristics of NP-MSCs[35]. Besides acid condition, inflammation is also a vitally important factor to induce IVD degeneration via some cytokines such as tumor necrosis factor (TNF)-α[37]. Cheng et al[56] found that TNF-α at low concentrations (0.1-10 ng/mL) promote the proliferation and migration ability of NP-MSCs, but inhibit their differentiation toward NP cells, indicating that the function of inflammatory cytokines may be a double-edged sword. In addition, pure/leukocyte-containing platelet-rich plasma (P/L-PRP) and modified notochordal cell-rich NP explants were confirmed to attenuate cell apoptosis and dysfunction of NP-MSCs induced by inflammation in the IVD[37,54]. Moreover, oxidative stress caused by mitochondrial dysfunction plays a vitally important role in IVD degeneration[68]. Our pervious research and other studies illustrated that some medicines such as cyclosporine and naringin are capable of alleviating mitochondrial dysfunction and oxidative stress[64,52]. Tao et al[53] found that synergy between transforming growth factor beta 3 and insulin-like growth factor 1 could enhance NP-MSC viability, ECM biosynthesis, and differentiation towards NPCs by the MAPK/ERK signaling pathway.
The other strategy of endogenous repair is to replenish the stem/progenitor cells straightly. Various pre-clinical and clinical studies claimed that injection of MSC or MSC-like cells with or without biomaterial could significantly relieve degeneration of the IVD[25,65]. The results of our recent study demonstrated that injectable hydrogel loaded NP-MSCs transplantation could delay the degeneration of the IVD and promote IVD regeneration in a rat model[46]. This kind of strategy involved the expansion and reservation of NP-MSCs in vitro, which is the foundation of endogenous repair. Therefore, searching techniques that can facilitate culturing and preservation seems to be especially crucial. A study by Lin et al[58] indicated that NP-MSCs at a low plated density (L-PD) (5 cells/cm2) show better biological characteristics, stronger multilineage differentiation, and higher expression of stem cell biomarkers compared with those at an M-PD (100 cells/cm2) and H-PD (10000 cells/cm2), suggesting that the limiting dilution method is a better method to isolate NP-MSCs[58]. Moreover, the importance of cryopreservation cannot be ignored as it could prolong the application of NP-MSCs. The conventional cryopreservation methods were classified into slow freezing and vitrification (rapid freezing). The most often used dimethyl sulfoxide is regarded as the standard cryoprotectant which may cause cytotoxicity to MSCs[69,70]. A recent study by Chen et al[44] showed that the addition of Icariin known as antioxidant to the conventional freezing medium could improve the viability and function of cryopreserved human NP-MSCs, which may be a new method of preserving stem/progenitor cells in the IVD for ERS (Table 2).
Table 2.
Highlights and strategy of endogenous repair
| Ref. | Cells, biomaterial, and medicine | Highlights and strategy |
| Human | ||
| Liu et al[35], 2016 | Amiloride | The biological behavior of NP-MSCs could be inhibited by acidic conditions, and amiloride may meliorate IVD degeneration by improving the activities of NP-MSCs. |
| Li et al[36], 2017 | NP-SCs | NP-SCs keep the regeneration ability similar to BMSCs with superior capacity in chondrogenesis. |
| Li et al[37], 2018 | Modified notochordal cell-rich NP explants | Modified notochordal cell-rich NP explants can attenuate degeneration and senescence of NP-MSC induced by TNF-α. |
| Jia et al[38], 2017 | D-NP-MSCs/ND-NP-MSCs | D-NP-MSCs displayed decreased biological characteristics compared with NP-MSCs. |
| Wu et al[40], 2017 | D-NP-MSCs/UCMSCs | D-NP-MSCs had lower expression of phenotype markers and exhibited reduced proliferation capability and differentiation potentials compared with UCMSCs. |
| Wang et al[41], 2016 | NPSCs/AFSCs/CESCs | A comparison of the osteogenic capacities: CESCs > AFSCs > BM-MSCs > NPSCs; for adipogenesis: BM-MSCs > NPSCs > CESCs > AFSCs; in chondrogenesis: CESCs > AFSCs > BMSCs > NPSCs. |
| Liang et al[42], 2018 | NP-MSCs | The biological behavior of NP-MSCs could be inhibited by compression loading. |
| Daisuke et al[43], 2012 | NPPCs | The frequency of Tie2+ cells decreases markedly in tissue with age and degeneration of the IVD, suggesting exhaustion of their capacity for regeneration. |
| Chen et al[44], 2018 | ICA | The addition of ICA to the conventional freezing medium could improve the viability and function of cryopreserved human NP-MSCs. |
| Quan et al[48], 2015 | MSC-like cells from NP | NP tissue contains MSC-like cells which could be isolated and proliferate in vitro. |
| Liu et al[49], 2017 | AF-MSCs, NP-MSCs, CEP-MSCs | AF-MSCs, NP-MSCs, and CEP-MSCs showed similar multilineage differentiation abilities; CEP-MSCs have the most powerful properties of migration and invasion when compared with AF-MSCs and NP-MSCs. |
| Blanco et al[27], 2010 | NP-MSCs | NP-MSCs were quite similar to BM-MSCs, with the exception that NP-MSCs are not able to differentiate into adipocytes. |
| Lazzarini et al[50], 2018 | NP-MSCs | NP-MSCs have the capacity of neuronal differentiation and could express neural markers without any electric functional properties. |
| Pereira et al[51], 2016 | CEP-MSCs | MSCs from CEP promote IVD regeneration by remodeling ECM. |
| Qi et al[57], 2018 | MSC-CM | MSC-CM has potential to alleviate HG induced cell cycle arrest and ECM degradation of NP-MSCs via p38 MAPK pathway. |
| Li et al[64], 2018 | CsA | CsA efficiently inhibited compression-induced NP-MSCs apoptosis by alleviating mitochondrial dysfunction and oxidative stress. |
| Rat | ||
| Zhao et al[34], 2017 | NP-MSCs | The efficacy of NP-MSCs is compromised by age, and old NP-MSCs displayed senescent features. |
| Li et al[39], 2019 | NP-MSCs | The MSC-CM + CC method (MSC complete medium culture + cloning cylinder) is a more reliable and efficient way for isolating and purifying NP-MSCs. |
| Li et al[45], 2013 | NP-MSCs | Compared to AD-MSCs, NP-MSCs showed greater viability, proliferation, and chondrocytic differentiation under hypoxia. |
| Wang et al[46], 2019 | Injectable hydrogel-loaded NP-MSCs | Injectable hydrogel-loaded NP-MSCs transplantation can delay the level of IVD degeneration and promote the regeneration of the degenerative IVD in a rat model. |
| Nan et al[52], 2019 | Nar | Nar efficiently attenuated H2O2-induced NP-MSCs apoptosis and mitochondrial dysfunction through PI3/AKT pathway. |
| Han et al[29], 2014 | NP-MSCs | An acidic environment is a major obstacle for IVD regeneration by AD-MSCs or NP-MSCs; NP-MSCs appeared less sensitive to inhibition by acidic PH. |
| Tao et al[28], 2013 | NPCs-NP-MSCs co-culture | NP-MSCs could tolerate IVD-like high osmolarity and NPCs-NP-MSCs co-culture increased cell proliferation and the expression of SOX-9, aggrecan, and collagen-II. |
| Tao et al[53], 2015 | TGF-β3/IGF-1 | The synergy between TGF-β3 and IGF-1 enhanced NP-MSCs viability, ECM biosynthesis, and differentiation towards NPCs by activating the MAPK/ERK signaling pathway. |
| Liu et al[30], 2019 | N-NP-MSCs/D-NP-MSCs | N-NP-MSCs showed a significantly higher proliferation rate, better stemness maintenance ability, but reduced cell apoptosis rate compared with D-NP-MSCs. |
| Li et al[55], 2018 | NP-MSCs | Hyperosmolarity of the IVD significantly inhibited the proliferation and chondrogenic differentiation of NP-MSCs by activating the ERK pathway. |
| Cheng et al[56], 2019 | TNF-α | Treatment with a high concentration of TNF-α (50-200 ng/mL) could induce apoptosis of NP-MSCs, whereas a relatively low TNF-α concentration (0.1-10 ng/mL) promoted the proliferation and migration of NP-MSCs, but inhibited their differentiation toward NP cells. |
| Lin et al[58], 2017 | L-PD of NP-MSCs | NP-MSCs at a L-PD (5 cells/cm2) have better biological characteristic, stronger multilineage differentiation, and higher expression of stem cell biomarkers compared with those at an M-PD (100 cells/cm2) and H-PD (10000 cells/cm2). |
| Yang et al[65], 2009 | BMSCs | BMSCs could arrest the degeneration of the murine notochordal NP and contribute to the augmentation of the ECM in the NP by both autonomous differentiation and stimulatory action on endogenous cells. |
| Liu et al[68], 2019 | NP-MSCs | High glucose concentration significantly decrease vitality, migration, and stemness of NP-MSCs. |
| Zhang et al[69], 2015 | NP-MSCs | The chondrogenic ability of NP-MSCs and BM-MSCs was similar under induction in vitro. |
| Dog | ||
| Erwin et al[47], 2013 | NPPCs | NPPCs have higher expression of the Nanog gene compared to MSCs and are capable of differentiation along chondrogenic, adipogenic, and neurogenic lineages in vitro and into oligodendrocyte, neuron, and astroglial specific precursor cells in vivo in the myelin-deficient shiverer mouse. |
| Bovine | ||
| Tekari et al[63], 2016 | NPPCs | The Tie2+ cells (NPPC) were spheroid in shape with capacity of multi-differentiation and may decline fast, which was partially reversed by FGF2 and hypoxic conditions. |
| Rabbit | ||
| Jia et al[54], 2018 | P-PRP/L-PRP | Both P-PRP and L-PRP could induce the proliferation and NP-differentiation of NP-MSCs; P-PRP could reduce the inflammatory and catabolic responses by avoiding the activation of the NF-κB pathway. |
| Rhesus macaque | ||
| Huang et al[66], 2013 | DPCs, SLRP | SLRP could reduce the susceptibility of DPCs to hypoxia-induced apoptosis via promoting the activation/stabilization of HIF-1α and HIF-2α. |
MSC: Mesenchymal stem cell; IVD: Intervertebral disc; NP: Nucleus pulposus; AF: Annulus fibrosus; CEP: Cartilaginous endplates; UCMSCs: Umbilical cord MSCs; AD: Adipose tissue; ECM: Extracellular matrix; ICA: Icariin; CM: Conditioned medium; HG: High glucose; CsA: Cyclosporine; Nar: Naringin; TGF-β3: Transforming growth factor beta 3; IGF: Insulin-like growth factor; TNF: Tumor necrosis factor; L-PD: Low plating density; NPPC: NP-derived progenitor cell; BM-MSC: Bone marrow mesenchymal stem cell; P-PRP: Pure platelet-rich plasma; L-PRP: Leukocyte-containing platelet-rich plasma; DPCs: Intervertebral disc progenitor cells; SLRP: Leucine-rich proteoglycans.
CONCLUSION
Although IVD cell-based therapies have achieved some accomplishment in pre-clinical and clinical studies, there are still some defects such as short duration and tumorigenicity. The discovery of stem cell niche and stem/progenitor cells in the IVD inspired a novel treatment for degenerative IVD. These stem/progenitor cells, isolated from the NP, AF, and CEP, express MSC markers proposed by the ISCT. In addition, stemness related genes including Nanog, Oct4, and Sox2 are proved to be expressed in these stem/progenitor cells. Moreover, such cells are similar to other MSCs as not only be capable of osteogenic, chondrogenic, and adipogenic differentiation, but also differentiate into the neurogenic lineages.
However, the ERS still faces some challenges such as exhaustion of stem/progenitor cells, broken migration pathway, and harsh microenvironment such as acid condition, hypoxia, compression loading, hyperosmolarity, high glucose, inflammation, and oxidative stress in degenerative IVD. Therefore, it is essential to look for methods which are able to overcome these obstacles and boost the process of ERS. These methods including reduced apoptosis and senescence caused by degenerative microenvironment or supplying stem/progenitor cells directly have been confirmed to be beneficial to treat degenerative IVD. Nevertheless, the ERS is still in pre-clinical studies and needs to be further investigated in future. In addition, seeking factors or medicines that are able to promote mobilization and migration of stem/progenitor cells in stem cell niche may become another novel direction of ERS.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
Manuscript source: Invited Manuscript
Peer-review started: February 1, 2020
First decision: March 5, 2020
Article in press: April 4, 2020
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Das U, Koumantakis GA, Tanabe S S-Editor: Wang YQ L-Editor: Wang TQ E-Editor: Liu MY
Contributor Information
Yang Liu, Department of Orthopedics, West China Hospital of Sichuan University, Chengdu 610000, Sichuan Province, China; Department of Orthopedics, Dalian Medical University, Dalian 116000, Liaoning Province, China.
Yan Li, Department of Oncology, The Affiliated Cancer Hospital, School of Medicine, UESTC, Chengdu 610000, Sichuan Province, China.
Li-Ping Nan, Department of Orthopedics, Dalian Medical University, Dalian 116000, Liaoning Province, China.
Feng Wang, Department of Orthopedics, Dalian Medical University, Dalian 116000, Liaoning Province, China.
Shi-Feng Zhou, Department of Orthopedics, Clinical Medical College of Yangzhou University, Yangzhou 225000, Jiangsu Province, China.
Xin-Min Feng, Department of Orthopedics, Clinical Medical College of Yangzhou University, Yangzhou 225000, Jiangsu Province, China.
Hao Liu, Department of Orthopedics, West China Hospital of Sichuan University, Chengdu 610000, Sichuan Province, China.
Liang Zhang, Department of Orthopedics, Clinical Medical College of Yangzhou University, Yangzhou 225000, Jiangsu Province, China. zhangliang6320@sina.com.
References
- 1.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Violante FS, Mattioli S, Bonfiglioli R. Low-back pain. Handb Clin Neurol. 2015;131:397–410. doi: 10.1016/B978-0-444-62627-1.00020-2. [DOI] [PubMed] [Google Scholar]
- 3.Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8–20. doi: 10.1016/j.spinee.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Goo B, Kim SJ, Kim EJ, Nam D, Lee HJ, Kim JS, Park YC, Baek YH, Nam SS, Seo BK. Clinical research on the efficacy and safety of Bosinji for low back pain with radiculopathy caused by herniated intervertebral disc of the lumbar spine: A protocol for a multicenter, randomized, controlled equivalence trial. Medicine (Baltimore) 2018;97:e13684. doi: 10.1097/MD.0000000000013684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng CJ, Chen J. Disc degeneration implies low back pain. Theor Biol Med Model. 2015;12:24. doi: 10.1186/s12976-015-0020-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golob AL, Wipf JE. Low back pain. Med Clin North Am. 2014;98:405–428. doi: 10.1016/j.mcna.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Bowles RD, Setton LA. Biomaterials for intervertebral disc regeneration and repair. Biomaterials. 2017;129:54–67. doi: 10.1016/j.biomaterials.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampton D, Laros G, McCarron R, Franks D. Healing potential of the anulus fibrosus. Spine (Phila Pa 1976) 1989;14:398–401. doi: 10.1097/00007632-198904000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Chen D, Xia D, Pan Z, Xu D, Zhou Y, Wu Y, Cai N, Tang Q, Wang C, Yan M, Zhang JJ, Zhou K, Wang Q, Feng Y, Wang X, Xu H, Zhang X, Tian N. Metformin protects against apoptosis and senescence in nucleus pulposus cells and ameliorates disc degeneration in vivo. Cell Death Dis. 2016;7:e2441. doi: 10.1038/cddis.2016.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makanji H, Schoenfeld AJ, Bhalla A, Bono CM. Critical analysis of trends in lumbar fusion for degenerative disorders revisited: influence of technique on fusion rate and clinical outcomes. Eur Spine J. 2018;27:1868–1876. doi: 10.1007/s00586-018-5544-x. [DOI] [PubMed] [Google Scholar]
- 11.Cai F, Wu XT, Xie XH, Wang F, Hong X, Zhuang SY, Zhu L, Rui YF, Shi R. Evaluation of intervertebral disc regeneration with implantation of bone marrow mesenchymal stem cells (BMSCs) using quantitative T2 mapping: a study in rabbits. Int Orthop. 2015;39:149–159. doi: 10.1007/s00264-014-2481-0. [DOI] [PubMed] [Google Scholar]
- 12.Noriega DC, Ardura F, Hernández-Ramajo R, Martín-Ferrero MÁ, Sánchez-Lite I, Toribio B, Alberca M, García V, Moraleda JM, Sánchez A, García-Sancho J. Intervertebral Disc Repair by Allogeneic Mesenchymal Bone Marrow Cells: A Randomized Controlled Trial. Transplantation. 2017;101:1945–1951. doi: 10.1097/TP.0000000000001484. [DOI] [PubMed] [Google Scholar]
- 13.Oehme D, Goldschlager T, Ghosh P, Rosenfeld JV, Jenkin G. Cell-Based Therapies Used to Treat Lumbar Degenerative Disc Disease: A Systematic Review of Animal Studies and Human Clinical Trials. Stem Cells Int. 2015;2015:946031. doi: 10.1155/2015/946031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wuertz K, Godburn K, Neidlinger-Wilke C, Urban J, Iatridis JC. Behavior of mesenchymal stem cells in the chemical microenvironment of the intervertebral disc. Spine (Phila Pa 1976) 2008;33:1843–1849. doi: 10.1097/BRS.0b013e31817b8f53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang C, Li H, Tao Y, Zhou X, Li F, Chen G, Chen Q. Responses of human adipose-derived mesenchymal stem cells to chemical microenvironment of the intervertebral disc. J Transl Med. 2012;10:49. doi: 10.1186/1479-5876-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Han ZB, Song YP, Han ZC. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012;2012:652034. doi: 10.1155/2012/652034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vadalà G, Sowa G, Hubert M, Gilbertson LG, Denaro V, Kang JD. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6:348–355. doi: 10.1002/term.433. [DOI] [PubMed] [Google Scholar]
- 18.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 19.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 20.Ruddy RM, Morshead CM. Home sweet home: the neural stem cell niche throughout development and after injury. Cell Tissue Res. 2018;371:125–141. doi: 10.1007/s00441-017-2658-0. [DOI] [PubMed] [Google Scholar]
- 21.Seike M, Omatsu Y, Watanabe H, Kondoh G, Nagasawa T. Stem cell niche-specific Ebf3 maintains the bone marrow cavity. Genes Dev. 2018;32:359–372. doi: 10.1101/gad.311068.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong M, Zhang P, Li C, Ma X, Yang D. Protective Mechanism of Adipose-Derived Stem Cells in Remodelling of the Skin Stem Cell Niche During Photoaging. Cell Physiol Biochem. 2018;51:2456–2471. doi: 10.1159/000495902. [DOI] [PubMed] [Google Scholar]
- 23.Bartfeld S, Koo BK. Adult gastric stem cells and their niches. Wiley Interdiscip Rev Dev Biol. 2017:6. doi: 10.1002/wdev.261. [DOI] [PubMed] [Google Scholar]
- 24.Shi R, Wang F, Hong X, Wang YT, Bao JP, Cai F, Wu XT. The presence of stem cells in potential stem cell niches of the intervertebral disc region: an in vitro study on rats. Eur Spine J. 2015;24:2411–2424. doi: 10.1007/s00586-015-4168-7. [DOI] [PubMed] [Google Scholar]
- 25.Clouet J, Fusellier M, Camus A, Le Visage C, Guicheux J. Intervertebral disc regeneration: From cell therapy to the development of novel bioinspired endogenous repair strategies. Adv Drug Deliv Rev. 2019;146:306–324. doi: 10.1016/j.addr.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Risbud MV, Guttapalli A, Tsai TT, Lee JY, Danielson KG, Vaccaro AR, Albert TJ, Gazit Z, Gazit D, Shapiro IM. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine (Phila Pa 1976) 2007;32:2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- 27.Blanco JF, Graciani IF, Sanchez-Guijo FM, Muntión S, Hernandez-Campo P, Santamaria C, Carrancio S, Barbado MV, Cruz G, Gutierrez-Cosío S, Herrero C, San Miguel JF, Briñon JG, del Cañizo MC. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine (Phila Pa 1976) 2010;35:2259–2265. doi: 10.1097/BRS.0b013e3181cb8828. [DOI] [PubMed] [Google Scholar]
- 28.Tao YQ, Liang CZ, Li H, Zhang YJ, Li FC, Chen G, Chen QX. Potential of co-culture of nucleus pulposus mesenchymal stem cells and nucleus pulposus cells in hyperosmotic microenvironment for intervertebral disc regeneration. Cell Biol Int. 2013;37:826–834. doi: 10.1002/cbin.10110. [DOI] [PubMed] [Google Scholar]
- 29.Han B, Wang HC, Li H, Tao YQ, Liang CZ, Li FC, Chen G, Chen QX. Nucleus pulposus mesenchymal stem cells in acidic conditions mimicking degenerative intervertebral discs give better performance than adipose tissue-derived mesenchymal stem cells. Cells Tissues Organs. 2014;199:342–352. doi: 10.1159/000369452. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Li Y, Huang ZN, Wang ZY, Nan LP, Wang F, Zhou SF, Wang JC, Feng XM, Zhang L. The effect of intervertebral disc degenerative change on biological characteristics of nucleus pulposus mesenchymal stem cell: an in vitro study in rats. Connect Tissue Res. 2019;60:376–388. doi: 10.1080/03008207.2019.1570168. [DOI] [PubMed] [Google Scholar]
- 31.Yu H, Vu TH, Cho KS, Guo C, Chen DF. Mobilizing endogenous stem cells for retinal repair. Transl Res. 2014;163:387–398. doi: 10.1016/j.trsl.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyu FJ, Cheung KM, Zheng Z, Wang H, Sakai D, Leung VY. IVD progenitor cells: a new horizon for understanding disc homeostasis and repair. Nat Rev Rheumatol. 2019;15:102–112. doi: 10.1038/s41584-018-0154-x. [DOI] [PubMed] [Google Scholar]
- 33.Liu LT, Huang B, Li CQ, Zhuang Y, Wang J, Zhou Y. Characteristics of stem cells derived from the degenerated human intervertebral disc cartilage endplate. PLoS One. 2011;6:e26285. doi: 10.1371/journal.pone.0026285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Jia Z, Huang S, Wu Y, Liu L, Lin L, Wang D, He Q, Ruan D. Age-Related Changes in Nucleus Pulposus Mesenchymal Stem Cells: An In Vitro Study in Rats. Stem Cells Int. 2017;2017:6761572. doi: 10.1155/2017/6761572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J, Tao H, Wang H, Dong F, Zhang R, Li J, Ge P, Song P, Zhang H, Xu P, Liu X, Shen C. Biological Behavior of Human Nucleus Pulposus Mesenchymal Stem Cells in Response to Changes in the Acidic Environment During Intervertebral Disc Degeneration. Stem Cells Dev. 2017;26:901–911. doi: 10.1089/scd.2016.0314. [DOI] [PubMed] [Google Scholar]
- 36.Li XC, Tang Y, Wu JH, Yang PS, Wang DL, Ruan DK. Characteristics and potentials of stem cells derived from human degenerated nucleus pulposus: potential for regeneration of the intervertebral disc. BMC Musculoskelet Disord. 2017;18:242. doi: 10.1186/s12891-017-1567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li XC, Wang MS, Liu W, Zhong CF, Deng GB, Luo SJ, Huang CM. Co-culturing nucleus pulposus mesenchymal stem cells with notochordal cell-rich nucleus pulposus explants attenuates tumor necrosis factor-α-induced senescence. Stem Cell Res Ther. 2018;9:171. doi: 10.1186/s13287-018-0919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia Z, Yang P, Wu Y, Tang Y, Zhao Y, Wu J, Wang D, He Q, Ruan D. Comparison of biological characteristics of nucleus pulposus mesenchymal stem cells derived from non-degenerative and degenerative human nucleus pulposus. Exp Ther Med. 2017;13:3574–3580. doi: 10.3892/etm.2017.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Z, Chen S, Ma K, He R, Xiong L, Hu Y, Deng X, Yang A, Ma X, Shao Z. Comparison of different methods for the isolation and purification of rat nucleus pulposus-derived mesenchymal stem cells. Connect Tissue Res. 2019:1–9. doi: 10.1080/03008207.2019.1611793. [DOI] [PubMed] [Google Scholar]
- 40.Wu H, Zeng X, Yu J, Shang Y, Tu M, Cheang LH, Zhang J. Comparison of nucleus pulposus stem/progenitor cells isolated from degenerated intervertebral discs with umbilical cord derived mesenchymal stem cells. Exp Cell Res. 2017;361:324–332. doi: 10.1016/j.yexcr.2017.10.034. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Zhou Y, Chu TW, Li CQ, Wang J, Zhang ZF, Huang B. Distinguishing characteristics of stem cells derived from different anatomical regions of human degenerated intervertebral discs. Eur Spine J. 2016;25:2691–2704. doi: 10.1007/s00586-016-4522-4. [DOI] [PubMed] [Google Scholar]
- 42.Liang H, Chen S, Huang D, Deng X, Ma K, Shao Z. Effect of Compression Loading on Human Nucleus Pulposus-Derived Mesenchymal Stem Cells. Stem Cells Int. 2018;2018:1481243. doi: 10.1155/2018/1481243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, Alini M, Risbud MV, Chan D, Cheah KS, Yamamura K, Masuda K, Okano H, Ando K, Mochida J. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen S, Deng X, Ma K, Zhao L, Huang D, Li Z, Shao Z. Icariin Improves the Viability and Function of Cryopreserved Human Nucleus Pulposus-Derived Mesenchymal Stem Cells. Oxid Med Cell Longev. 2018;2018:3459612. doi: 10.1155/2018/3459612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H, Tao Y, Liang C, Han B, Li F, Chen G, Chen Q. Influence of hypoxia in the intervertebral disc on the biological behaviors of rat adipose- and nucleus pulposus-derived mesenchymal stem cells. Cells Tissues Organs. 2013;198:266–277. doi: 10.1159/000356505. [DOI] [PubMed] [Google Scholar]
- 46.Wang F, Nan LP, Zhou SF, Liu Y, Wang ZY, Wang JC, Feng XM, Zhang L. Injectable Hydrogel Combined with Nucleus Pulposus-Derived Mesenchymal Stem Cells for the Treatment of Degenerative Intervertebral Disc in Rats. Stem Cells Int. 2019;2019:8496025. doi: 10.1155/2019/8496025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erwin WM, Islam D, Eftekarpour E, Inman RD, Karim MZ, Fehlings MG. Intervertebral disc-derived stem cells: implications for regenerative medicine and neural repair. Spine (Phila Pa 1976) 2013;38:211–216. doi: 10.1097/BRS.0b013e318266a80d. [DOI] [PubMed] [Google Scholar]
- 48.Shen Q, Zhang L, Chai B, Ma X. Isolation and characterization of mesenchymal stem-like cells from human nucleus pulposus tissue. Sci China Life Sci. 2015;58:509–511. doi: 10.1007/s11427-015-4839-y. [DOI] [PubMed] [Google Scholar]
- 49.Liu S, Liang H, Lee SM, Li Z, Zhang J, Fei Q. Isolation and identification of stem cells from degenerated human intervertebral discs and their migration characteristics. Acta Biochim Biophys Sin (Shanghai) 2017;49:101–109. doi: 10.1093/abbs/gmw121. [DOI] [PubMed] [Google Scholar]
- 50.Lazzarini R, Guarnieri S, Fulgenzi G, Mariggiò MA, Graciotti L, Martiniani M, Orciani M, Specchia N, Di Primio R. Mesenchymal Stem Cells from Nucleus Pulposus and Neural Differentiation Potential: a Continuous Challenge. J Mol Neurosci. 2019;67:111–124. doi: 10.1007/s12031-018-1216-x. [DOI] [PubMed] [Google Scholar]
- 51.Pereira CL, Teixeira GQ, Ribeiro-Machado C, Caldeira J, Costa M, Figueiredo F, Fernandes R, Aguiar P, Grad S, Barbosa MA, Gonçalves RM. Mesenchymal Stem/Stromal Cells seeded on cartilaginous endplates promote Intervertebral Disc Regeneration through Extracellular Matrix Remodeling. Sci Rep. 2016;6:33836. doi: 10.1038/srep33836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nan LP, Wang F, Ran D, Zhou SF, Liu Y, Zhang Z, Huang ZN, Wang ZY, Wang JC, Feng XM, Zhang L. Naringin alleviates H2O2-induced apoptosis via the PI3K/Akt pathway in rat nucleus pulposus-derived mesenchymal stem cells. Connect Tissue Res. 2019:1–14. doi: 10.1080/03008207.2019.1631299. [DOI] [PubMed] [Google Scholar]
- 53.Tao Y, Zhou X, Liang C, Li H, Han B, Li F, Chen Q. TGF-β3 and IGF-1 synergy ameliorates nucleus pulposus mesenchymal stem cell differentiation towards the nucleus pulposus cell type through MAPK/ERK signaling. Growth Factors. 2015;33:326–336. doi: 10.3109/08977194.2015.1088532. [DOI] [PubMed] [Google Scholar]
- 54.Jia J, Wang SZ, Ma LY, Yu JB, Guo YD, Wang C. The Differential Effects of Leukocyte-Containing and Pure Platelet-Rich Plasma on Nucleus Pulposus-Derived Mesenchymal Stem Cells: Implications for the Clinical Treatment of Intervertebral Disc Degeneration. Stem Cells Int. 2018;2018:7162084. doi: 10.1155/2018/7162084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li H, Wang J, Li F, Chen G, Chen Q. The Influence of Hyperosmolarity in the Intervertebral Disc on the Proliferation and Chondrogenic Differentiation of Nucleus Pulposus-Derived Mesenchymal Stem Cells. Cells Tissues Organs. 2018;205:178–188. doi: 10.1159/000490760. [DOI] [PubMed] [Google Scholar]
- 56.Cheng S, Li X, Jia Z, Lin L, Ying J, Wen T, Zhao Y, Guo Z, Zhao X, Li D, Ji W, Wang D, Ruan D. The inflammatory cytokine TNF-α regulates the biological behavior of rat nucleus pulposus mesenchymal stem cells through the NF-κB signaling pathway in vitro. J Cell Biochem. 2019;120:13664–13679. doi: 10.1002/jcb.28640. [DOI] [PubMed] [Google Scholar]
- 57.Qi L, Wang R, Shi Q, Yuan M, Jin M, Li D. Umbilical cord mesenchymal stem cell conditioned medium restored the expression of collagen II and aggrecan in nucleus pulposus mesenchymal stem cells exposed to high glucose. J Bone Miner Metab. 2019;37:455–466. doi: 10.1007/s00774-018-0953-9. [DOI] [PubMed] [Google Scholar]
- 58.Lin L, Jia Z, Zhao Y, Wu Y, Zhao X, Li Y, Guo Z, Chen J, Cheng S, Wang D, Ruan D. Use of Limiting Dilution Method for Isolation of Nucleus Pulposus Mesenchymal Stem/Progenitor Cells and Effects of Plating Density on Biological Characteristics and Plasticity. Biomed Res Int. 2017;2017:9765843. doi: 10.1155/2017/9765843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang KH, Kao AP, Chang CC, Lin TC, Kuo TC. Upregulation of Nanog and Sox-2 genes following ectopic expression of Oct-4 in amniotic fluid mesenchymal stem cells. Biotechnol Appl Biochem. 2015;62:591–597. doi: 10.1002/bab.1315. [DOI] [PubMed] [Google Scholar]
- 60.Zanotti S, Canalis E. Notch Signaling and the Skeleton. Endocr Rev. 2016;37:223–253. doi: 10.1210/er.2016-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ciria M, García NA, Ontoria-Oviedo I, González-King H, Carrero R, De La Pompa JL, Montero JA, Sepúlveda P. Mesenchymal Stem Cell Migration and Proliferation Are Mediated by Hypoxia-Inducible Factor-1α Upstream of Notch and SUMO Pathways. Stem Cells Dev. 2017;26:973–985. doi: 10.1089/scd.2016.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yasen M, Fei Q, Hutton WC, Zhang J, Dong J, Jiang X, Zhang F. Changes of number of cells expressing proliferation and progenitor cell markers with age in rabbit intervertebral discs. Acta Biochim Biophys Sin (Shanghai) 2013;45:368–376. doi: 10.1093/abbs/gmt019. [DOI] [PubMed] [Google Scholar]
- 63.Tekari A, Chan SCW, Sakai D, Grad S, Gantenbein B. Angiopoietin-1 receptor Tie2 distinguishes multipotent differentiation capability in bovine coccygeal nucleus pulposus cells. Stem Cell Res Ther. 2016;7:75. doi: 10.1186/s13287-016-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Z, Chen S, Ma K, Lv X, Lin H, Hu B, He R, Shao Z. CsA attenuates compression-induced nucleus pulposus mesenchymal stem cells apoptosis via alleviating mitochondrial dysfunction and oxidative stress. Life Sci. 2018;205:26–37. doi: 10.1016/j.lfs.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 65.Yang F, Leung VY, Luk KD, Chan D, Cheung KM. Mesenchymal stem cells arrest intervertebral disc degeneration through chondrocytic differentiation and stimulation of endogenous cells. Mol Ther. 2009;17:1959–1966. doi: 10.1038/mt.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang S, Leung VY, Long D, Chan D, Lu WW, Cheung KM, Zhou G. Coupling of small leucine-rich proteoglycans to hypoxic survival of a progenitor cell-like subpopulation in Rhesus Macaque intervertebral disc. Biomaterials. 2013;34:6548–6558. doi: 10.1016/j.biomaterials.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 67.Gilbert HTJ, Hodson N, Baird P, Richardson SM, Hoyland JA. Acidic pH promotes intervertebral disc degeneration: Acid-sensing ion channel -3 as a potential therapeutic target. Sci Rep. 2016;6:37360. doi: 10.1038/srep37360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feng C, Yang M, Lan M, Liu C, Zhang Y, Huang B, Liu H, Zhou Y. ROS: Crucial Intermediators in the Pathogenesis of Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2017;2017:5601593. doi: 10.1155/2017/5601593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yong KW, Wan Safwani WK, Xu F, Wan Abas WA, Choi JR, Pingguan-Murphy B. Cryopreservation of Human Mesenchymal Stem Cells for Clinical Applications: Current Methods and Challenges. Biopreserv Biobank. 2015;13:231–239. doi: 10.1089/bio.2014.0104. [DOI] [PubMed] [Google Scholar]
- 70.Morris TJ, Picken A, Sharp DMC, Slater NKH, Hewitt CJ, Coopman K. The effect of Me2SO overexposure during cryopreservation on HOS TE85 and hMSC viability, growth and quality. Cryobiology. 2016;73:367–375. doi: 10.1016/j.cryobiol.2016.09.004. [DOI] [PubMed] [Google Scholar]


