Abstract
BACKGROUND
Peripheral nerve injury can occur as a result of trauma or disease and carries significant morbidity including sensory and motor loss. The body has limited ability for nerve regeneration and functional recovery. Left untreated, nerve lesions can cause lifelong disability. Traditional treatment options such as neurorrhaphy and neurolysis have high failure rates. Surgical reconstruction with autograft carries donor site morbidity and often provide suboptimal results. Mesenchymal stem cells (MSCs) are known to have promising regenerative potential and have gained attention as a treatment option for nerve lesions. It is however, unclear whether it can be effectively used for nerve regeneration.
AIM
To evaluate the evidence for the use of human umbilical cord derived MSCs (UCMSCs) in peripheral nerve regeneration.
METHODS
We carried out a systematic literature review in accordance with the PRISMA protocol. A literature search was performed from conception to September 2019 using PubMed, EMBASE and Web of Science. The results of eligible studies were appraised. A risk of bias analysis was carried out using Cochrane’s RoB 2.0 tool.
RESULTS
Fourteen studies were included in this review. A total of 279 subjects, including both human and animal were treated with UCMSCs. Four studies obtained UCMSCs from a third-party source and the remainder were harvested by the investigators. Out of the 14 studies, thirteen conducted xenogenic transplantation into nerve injury models. All studies reported significant improvement in nerve regeneration in the UCMSC treated groups compared with the various different controls and untreated groups.
CONCLUSION
The evidence summarised in this PRISMA systematic review of in vivo studies supports the notion that human UCMSC transplantation is an effective treatment option for peripheral nerve injury.
Keywords: Umbilical cord, Mesenchymal stem cells, Transplantation, Peripheral nerve regeneration
Core tip: While human umbilical cord derived mesenchymal stem cells hold promise as a treatment option for peripheral nerve lesions, robust in vivo models are required in order to determine the best method of delivering mesenchymal stem cells to sites of injury.
INTRODUCTION
Peripheral nerve injuries can occur as a result of trauma or disease and can lead to significant morbidity including sensory loss, motor loss and chronic pain[1]. These injuries cause life-long disability in up to 2.8% of all trauma patients[2]. Damage to peripheral nerves most commonly occurs as a result of laceration, compression, ischaemia or traction[1]. As classified by Seddon in 1943, nerve injury can range from focal demyelination termed neurapraxia, to total nerve transection termed neurotmesis[3,4]. The mechanism of recovery post-injury occurs by either branching of collateral axons or by regeneration of the damaged neuron[4,5]. In order for full neuronal recovery to occur, Wallerian degeneration, axonal regeneration and end-organ reinnervation must take place. This is driven by an array of neurotropic factors[4]. However, recovery in function following peripheral nerve injury is hindered by complex pathological mechanisms such as poor nerve regeneration, neuromuscular atrophy, and end-plate degeneration which can lead to suboptimal neuron function[6-9].
Traditionally, peripheral nerve injury can be managed conservatively or surgically with neurolysis, neural suturing, end-to-side neurorrhaphy and nerve autograft[10-12]. Even with optimum surgical repair, most methods will attain partial but not full return of nerve function[10]. Certain peripheral nerve injuries, such as severe brachial plexus or long traction injuries remain inoperable[10]. Autografts have several disadvantages, including donor site morbidity, mismatch in nerve and graft size resulting in poor engraftment, and the potential for development of painful neuromas[11,13,14]. Alternative methods of treating peripheral nerve injuries may be through cell-based regenerative therapies[15].
Transplantation of mesenchymal stem cells (MSCs), given their regenerative properties and highly proliferative capacity, has been proposed as a promising therapeutic option for peripheral nerve regeneration[16,17]. MSCs are plastic-adherent, undifferentiated, multipotent cells that can be harvested from numerous sites of the body including bone marrow, adipose tissue, dental pulp, amniotic fluid and umbilical cord[17-19]. MSCs from different tissue origins can have distinct cytokine expression profiles, and thus may enable different MSCs to be particularly suited to certain clinical applications[20,21]. Owing to low immunogenicity, MSCs may be transplanted allogenically with minimal consequence[22]. The particular mechanisms through which MSCs aid nerve repair have not yet been fully characterised. MSCs from various sources such as adipose tissue and bone marrow are able to differentiate into Schwann cells[23,24]. While some in vitro experiments suggest that transplanted MSCs may be stimulated by peripheral nerves to differentiate into Schwann cells[25], alternative findings have instead shown that transplanted MSCs encourage endogenous cells to express regenerative phenotypes[26]. Increasingly, MCSs are believed to mediate their regenerative properties predominantly through paracrine effects[27,28]. Aside from acting through soluble factors[29], MSCs have also demonstrated the ability to secrete extracellular vesicles that contain bioactive components such as miRNA and cytokines[30]. Indeed, native Schwann cells have been shown to facilitate axonal regeneration following injury through secretion of exosomes that decrease GTPase RhoA activity[31]. Similarly, human MSCs may act to achieve the same result through exosomes by upregulation of the PI3 kinase and Akt signalling cascades[32].
MSCs from umbilical cord are convenient to harvest from post-natal tissue in a non-invasive manner and possess a high capacity to expand ex vivo[33]. They express low levels of HLA-DR compared to MSCs from other cell sources and therefore pose low risk of immunogenic complications following allogenic transplantation[34]. Through sequential treatment with β-mercaptoethanol and various cytokines, umbilical cord derived MSCs (UCMSCs) can adopt a Schwann-like phenotype[35]. In addition, UCMSCs have been shown to possess greater paracrine effects than those of bone marrow-derived MSCs (BMMSC) and adipose-derived MSCs[17,29], and are able to potentiate axonal regeneration and peripheral nerve functional regeneration through these effects[11,17,29,36]. UCMSCs have been proposed to exert neuroprotective effects through secretion of Brain Derived Neurotrophic Factor (BDNF)[37], angiopoietin-2 and CXCL-16[38,39]. Other studies have suggested that they indirectly promote neurogenesis[40,41]. UCMSCs are also able to indirectly enhance expression of neurotransmitters such as BDNF and neurotrophin-3 (NTF3) which are postulated to aid neuro-regeneration[42,43].
To date, there have been over 400 clinical trials that explore the use of MSCs in transplantation; UCMSCs follow BMMSCs as the second most commonly used cell source[44]. In this PRISMA systematic review, we analyse the evidence for the use of human UCMSCs in peripheral nerve regeneration by examining in vivo studies.
MATERIALS AND METHODS
A literature search was performed from conception to September 2019 using PubMed, EMBASE and Web of Science. The following search terms were used: ((((((((Mesenchymal stem cells) OR mesenchymal stem cell) OR MSC) OR MSCs) OR Mesenchymal stromal cell) OR Mesenchymal cell)) AND (((((Nerve) OR Peripheral nerve) OR Peripheral nerve injury) OR damaged nerve) OR nerve injury)) AND ((((((repair) OR regeneration) OR regrowth) OR regenerate) OR renew) OR restore). We adhered to the recommendations as stipulated by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines[45].
We included case series, case control, cohort studies and randomised controlled trials. We enrolled studies that examined peripheral nerve lesions treated with human UCMSCs in in vivo human and animal subjects. Studies that only conducted in vitro experiments were excluded. Studies that investigated central nervous system regeneration using UCMSCs were excluded. All included studies were published in the English language. We excluded all unpublished and retracted literature.
CB and KT carried out the search independently. RoB2 tool was used by CM and BZ to assess the risk of bias in the studies, all discrepancy in results were resolved by discussion.
RESULTS
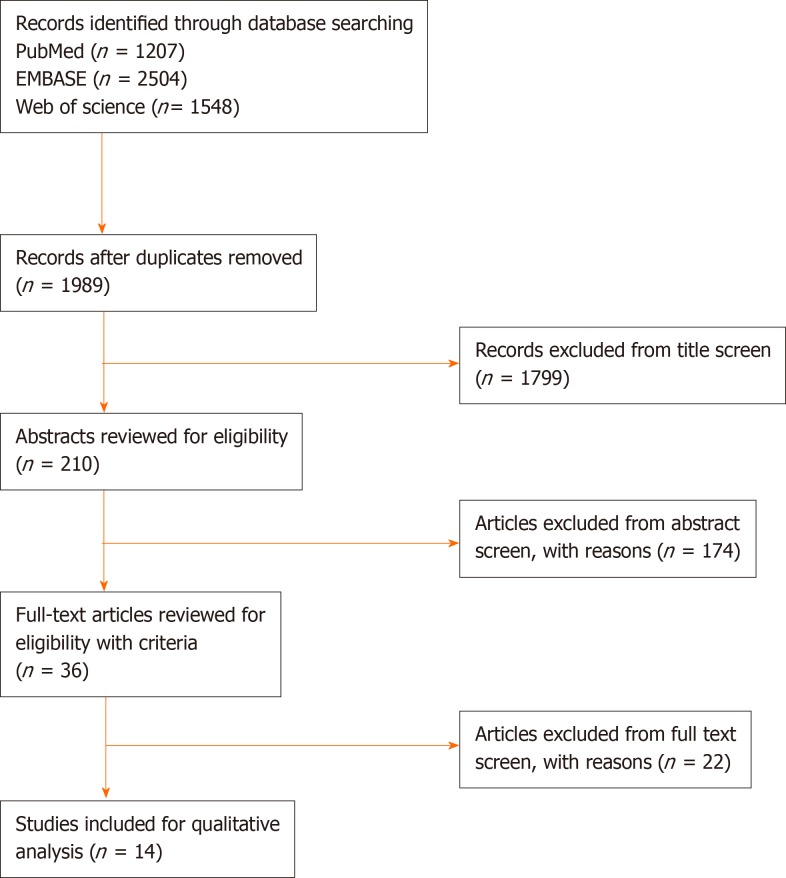
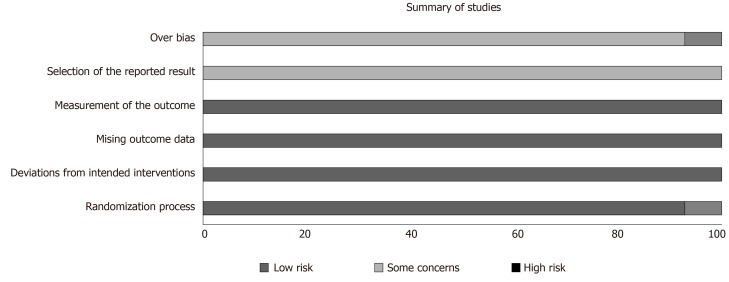
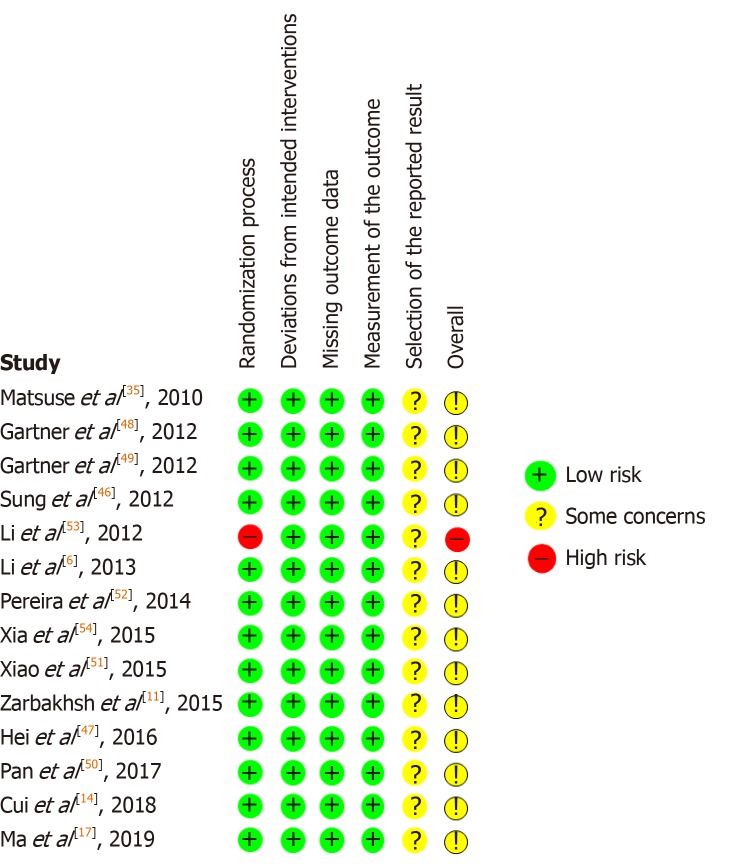
A total of 210 studies were screened for title, abstract and the inclusion/exclusion criteria were applied (Figure 1). One retracted study was excluded. Fourteen studies were reviewed in full text. The overall bias of studies is shown in Figure 2. The summary of results is shown in Figure 3. All 14 studies were of a case control design (Table 1). Four studies obtained UCMSCs from a third-party source and the remainder were harvested directly from human subjects. Out of the 14 studies, ten involved xenogenic transplantation into sciatic nerve injury specimens that were either crushed or transected. The studies were grouped according to Seddon’s seminal nerve injury classification system, which includes axonotmesis (injury to nerve sheath alone) and neurotmesis (injury to the entire nerve)[3]. A total of 279 subjects were treated with UCMSCs. All studies reported significant improvement in UCMSC treated groups compared with the various different controls and untreated groups. The studies did not report any significant complications.
Figure 1.
PRISMA Flow diagram.
Figure 2.
Summary of overall bias.
Figure 3.
Risk of bias in individual studies.
Table 1.
Studies of umbilical cord derived mesenchymal stem cells in peripheral nerve axonotmesis and diabetic neuropathy in vivo
| Ref. | Study design | Cell source | Subject | Treatment group | Control group | Extraction method | Cell treatment | Delivery method | Follow-up duration (wk) | Results |
| Hei et al[47], 2016 | Case Control | Human | Murine | 20 BDNF-transfected UCMSCs; 20 UCMSCs only | 20 PBS | Human umbilical vein obtained immediately after delivery | UCMSCs were expanded in keratinocyte-serum free medium with various growth factors. Passage 5 UCMSCs were transfected with adenovirus vector containing BDNF | Xenogenic transplantation into crushed left sciatic nerve | 4 | Significant improvement in SFI, axon count, axon density, and nerve regeneration in both treated groups. BDNF-loaded UCMSCs showed greater improvements in the above metrics than the UCMSC group |
| Sung et al[46], 2012 | Case Control | Human | Murine | 18 UCMSCs | 18 PBS | Human umbilical vein obtained immediately after delivery | UCMSCs were culture-expanded in growth factors. Passage 5 UCMSCs were labelled with PKH26 fluorescent cell linker | Xenogenic transplantation into crushed sciatic nerve | 4 | Significant improvement in SFI, axon density and axon regeneration in UCMSCs group compared to control. Increased BDNF and tyrosine kinase receptor B mRNA compared to control |
| Gartner et al[48], 2012 | Case Control | Human | Murine | 6 undifferentiated UCMSCs + PLC; 7 differentiated UCMSCs + PLC; 7 UCMSCs only | 6 injury only; 7 injury repaired with PLC only; 6 without injury | UCMSCs from human umbilical cord Wharton’s jelly matrix purchased from third-party source (PromoCell GmbH) | Passage 5 UCMSCs were supplemented with bovine foetal serum. UCMSCs were treated with neurogenic media and differentiated into neuroglial-like cells | Xenogenic transplantation into right sciatic nerve lesion (3 mm) crushed with non-serrated clamp | 12 | Significant improvement in both undifferentiated and differentiated UCMSCs groups in terms of SFI, EPT, WRL as well as myelin sheath thickness compared to all controls |
| Gartner et al[49], 2012 | Case Control | Human | Murine | 6 UCMSCs only; 6 undifferentiated USMSCs + Chitosan type III | 6 negative control; 6 wrapped in Chitosan type III | UCMSCs from human umbilical cord Wharton’s jelly matrix purchased from third-party source (PromoCell GmbH) | Passage 5 UCMSCs were loaded on Chitosan type III biomaterial scaffold | Xenogenic transplantation into crushed right sciatic nerve lesion | 12 | Significant improvement in muscle force deficit and axonal regrowth in UCMSC Chitosan type III group compared to controls |
| Xia et al[54], 2015 | Case Control | Human | Murine | 40 UCMSCs | 40 saline solution; 40 untreated rats | Human umbilical cord blood plasma obtained from different individuals with identical blood type | UCMSCs were culture-expanded in normal MSC media. Number of passage was not specified | Intravascular injection into left femoral artery of rat with streptozotocin induced diabetic foot ulcer | 2 | Significant improvement in restoring femoral nerve conduction in UCMSCs group compared to control groups at 3 days, 1 wk and 2 wk |
UCMSCs: Umbilical cord derived mesenchymal stem cells; BDNF: Brain-derived neurotrophic factor; SFI: Sciatic function index; PBS: Phosphate buffered saline; PLC: Poly (DL-lactide-ε-caprolactone); WRL: Withdrawal reflex latency; EPT: Extensor postural thrust.
UCMSCs in peripheral nerve axonotmesis
Four studies that included a total of 90 treated subjects assessed the use of UCMSCs in peripheral nerve axonotmesis models of sciatic nerve crush injury (Table 1). All four studies harvested UCMSCs from human subjects and transplanted the UCMSCs into murine subjects. The methods of UCMSC delivery to the crush injury varied among studies.
Studies, by Sung et al[46] (2012) and Hei et al[47] (2016) examined the effect of direct intralesional UCMSC injections on murine subjects with sciatic nerve crush injuries. Both studies monitored subjects up to 4 wk post-intervention. Sung et al[46] (2012) found that expression of brain-derived neurotrophic factor (BDNF) and tyrosine kinase receptor B mRNA increased at 4 wk following UCMSC injection. Functional recovery was measured in terms of the sciatic function index (SFI), which showed a dramatic improvement at 4 wk in UCMSC treated groups compared to untreated groups. Retrograde axonal transport was estimated through fluoro Gold-labelled neuron counts and the UCMSC group was found to have a significantly higher neuron count. It was found that axon density was significantly greater in the UCMSC group. Hei et al[47] (2016) transfected UCMSCs with a BDNF-adenovirus vector. The authors found that both UCMSC and BDNF-UCMSC groups had significant improvements in SFI, axon count and axon density at 4 wk after treatment. The BDNF-UCMSC group displayed increased peripheral nerve regeneration compared with UCMSC alone.
Gartner et al[48,49] conducted two studies published in the same year. In one study, Chitosan type III membrane was used to aid UCMSC infiltration in murine sciatic crush models[48]. The authors evaluated motor and sensory functional recovery up to 12 wk following transplantation with and without Chitosan type III wrapping. Both treatment groups showed improvement in SFI, extensor postural thrust (EPT) and withdrawal reflex latency (WRL). The control group with Chitosan type III membrane alone showed a significant improvement in post-traumatic axonal regrowth compared to the untreated control group. In a separate study, the same group examined the effect of using poly (DL-lactide-e-caprolactone) (PLC) membranes to deliver UCMSCs into sciatic nerve crush injuries[49]. Peripheral nerve regeneration was assessed in terms of SFI, EPT, and WRL at 12 wk. Undifferentiated and differentiated UCMSCs were used in different groups. Both groups showed an increase in myelin sheath thickness compared to control groups. The SFI was severely affected at week-2 post-crush injury in all experimental groups and improved gradually up to week 12 when values were indistinguishable from controls.
Studies of UCMSCs in peripheral nerve neurotmesis
Nine of the fourteen studies assessed the use of UCMSCs in peripheral nerve neurotmesis models (Table 2). All nine were case control studies. Five studies had murine subjects, two had rabbit subjects, one had canine subjects, and one had human subjects. Six studies transplanted UCMSCs into a sciatic nerve gap model. Two studies transplanted UCMSCs into tibial nerve and recurrent laryngeal nerve crush models. One study conducted allogenic transplantation in humans. A total of 151 subjects were treated. Methods of MSC delivery and transplantation varied among studies.
Table 2.
Studies of umbilical cord derived mesenchymal stem cells in Peripheral Nerve Neurotmesis in vivo
| Ref. | Study Design | Cell Source | Subject | Treatment Group | Control Group | Extraction Method | Cell Treatment | Delivery Method | Follow-up length (wk) | Results |
| Ma et al[17], 2019 | Case Control | Human | Murine | 24 UCMSC-extracellular vesicles injections | 24 PBS | Human umbilical cords obtained from full-term deliveries | UCMSCs were expanded ex vivo. Passage 3 UCMSCs were used | UCMSC-EV were injected into the tail veins | 8 | Significant improvement in SFI, axon regeneration, recovery of motor function and reduced muscle atrophy. Regenerated nerve fibre diameter was larger in USMSC-EV injection groups compared to control |
| Zarbakhsh et al[11], 2015 | Case Control | Human | Murine | 8 silicone tubes filled with fibrin glue seeded with 500000 UCMSCs | 8 silicone tubes filled with fibrin glue seeded with 500000 rat BMMSCs; 8 control rats with nerve gaps filled with fibrin glue | Human umbilical cords obtained from full-term deliveries | Passage 3 UCMSCs were loaded on a 12 mm silicone tube interposed into a 10 mm nerve gap | Xenogenic transplantation into sciatic nerve gap specimens | 12 | Significant improvement in nerve histomorphology in UCMSC and BMMSC groups compared to controls. BMMSC showed the greater improvement |
| Cui et al[14], 2018 | Case Control | Human | Canine | 5 LOCC with UCMSCs | 5 negative control; 5 positive control (autografted nerve segment reversed); 5 LOCC only | Human umbilical cords obtained from full-term deliveries. | UCMSCs were expanded. Passage 3 UCMSCs were cultured and embedded into a LOCC | Xenogenic transplantation into transected sciatic nerve of 15 months adult Beagles. | 39 | Significant improvement in CMAP and conduction latency in LOCC embedded with UCMSC compared to LOCC alone |
| Pan et al[50], 2017 | Case Control | Human | Rabbit | 12 NGF loaded HC- scaffold with UCMSCs; 12 HC-scaffold with UCMSCs | 12 negative control (no grafting into nerve gap); 12 HC-scaffold with PBS; 12 collagen (C)-scaffold | Human UCMSCs obtained from third party source (Stem Cell Bank of Guangdong Province) | Passage 4 UCMSCs were embedded into NGF- loaded HC-scaffold or C-scaffold | Xenogenic transplantation into transected recurrent laryngeal nerve tissue specimens with daily penicillin injection until day 5 post-intervention | 8 | Significant improvement in transected nerve repair in UCMSC NGF-loaded HC-scaffold as compared to all other groups |
| Li et al[53], 2012 | Case Control | Human | Murine | 40 amnion tube with UCMSCs | 40 amnion tube with saline implant | Human umbilical cords obtained from full-term deliveries | Passage 3-4 UCMSCs were cultured and loaded on an amniotic scaffold | Xenogenic transplantation into transected sciatic nerve tissue specimens | 20 | Significant improvement in SFI and CMAP in UCMSC group compared to control. Gradual improvement in threshold stimulus and maximum stimulus intensity in UCMSC group compared to control |
| Li et al[6], 2013 | Case Control | Human | Human | 12 neurolysis followed by 10 mL UCMSCs injection of 1.75 × 107 cells | 20 neurolysis only | Human umbilical cords obtained from full-term deliveries | Passage 2 UCMSCs were loaded on an amniotic membrane scaffold. Both groups received 3 days of oral cephalosporin | Allogenic transplantation into radial nerve injury following radial shaft fracture | 12 | Significant improvement in muscular strength, touch and pain sensations in UCMSC group compared to control. Improved electrophysiological function in UCMSC group as compared to control |
| Matsuse et al[35], 2010 | Case Control | Human | Murine | 6 UCMSCs; 10 Induced UCMSC | 6 negative control; 5 induced UCMSC | Wharton’s Jelly extracted from umbilical cords of full-term caesarean deliveries | Passage 3 UCMSCs were induced into Schwann-like cells | Xenogenic transplantation into transected sciatic nerve tissue specimens. | 3 | Significant improvement in SFI in all treated as compared to control with the greatest improvement in UCMSC group |
| Xiao et al[51], 2015 | Case Control | Human | Rabbit | 10 chitosan conduit anastomosis bridge filled with UCMSCs | 10 chitosan conduit anastomosis only; 10 untreated | Not specified | UCMSCs were loaded into a chitosan conduit | Xenogenic transplantation into tibial-common peroneal nerve end-to-side anastomosis | 12 | Significant improvement in myelin sheath thickness, Schwann cell growth, growth of axis bud and growth velocity of regenerated fibre in UCMSC group compared to controls. No significant difference observed between either control groups |
| Pereira et al[52], 2014 | Case Control | Human | Murine | 6 undifferentiated UCMSCs + PLC; 6 differentiated UCMSCs into neural-glial-like cells + PLC | 6 untreated; 6 treated with suture; 6 without nerve gap | Human Wharton’s Jelly UCMSCs obtained from third-party source (PromoCell GmbH) | Passage 5 UCMSCs were fixed onto PLC scaffold | Xenogenic transplantation into sciatic nerve gap specimens | 20 | Both UCMSC treated groups showed increased myelin sheath thickness, enhanced recovery in motor and sensory function. No significant difference was noted between differentiated and undifferentiated groups. PLC use did not significantly improve nerve regeneration |
UCMSCs: Umbilical cord derived mesenchymal stem cells; LOCC: Longitudinally orientated collagen conduit; SFI: Sciatic function index; NGF: Nerve growth factor; PBS: Phosphate buffered saline; HC: Heparinized collagen; PLC: Poly (DL-lactide-ε-caprolactone); EV: Extracellular vesicle.
Several groups sought to improve nerve regeneration with UCMSCs combined with longitudinal scaffolds. Zarbakhsh et al[11] (2015) loaded UCMSCs on a silicone tube and interposed it into a murine sciatic nerve gap model. The authors attempted to compare the histological outcomes of human UCMSCs and rat BMMSCs in regenerating sciatic nerve gap in rats. While the author showed favourable results in nerve regeneration for both UCMSCs and BMMSC, the latter was found to produce superior results at the end point of 12 wk. The BMMSC group showed greater axon number and thicker myelin sheath diameter than the UCMSC group.
Ma et al[17] (2019) injected UCMSC-derived extracellular vesicles (EVs) into the tail veins of rats and sutured a silicone rubber tube into the sciatic nerve gaps of 24 rats. The authors found that UCMSC-EVs promoted motor function recovery and regeneration of axons and attenuated muscle atrophy. SFI analysis was used to assess the functional improvements. At 8 wk, UCMSC-EV group had similar SFI values to normal rats.
Matsuse et al[35] (2010) combined UCMSCs and Matrigel into transpermeable tubes and transplanted it into transected murine sciatic nerve tissue specimens. The authors induced UCMSCs into cells with Schwann cell properties by using β-mercaptoethanol, all-trans-retinoic acid and various cytokines. Subsequently, Matsuse et al[35] examined the effect of these induced UCMSCs and used two control groups; a positive control of human Schwann cells and a negative control of Matrigel alone. The group assessed SFI values and compared immunoelectron micrographs. They concluded that the treatment group with Schwann Cell-UCMSCs group was equivalent to treatment with Schwann cells based on histological criteria and functional recovery.
Cui et al[14] (2018) and Pan et al[50] (2017) delivered UCMSCs using a collagen conduit. Cui et al[14] (2018) transplanted human UCMSCs into canine sciatic nerve gap models via a longitudinally orientated collagen conduit embedded with UCMSCs. Compound muscle action potential (CMAP) was found to be statistically greater in the UCMSC treated group compared with the collagen conduit only group. Pan et al[50] (2017) appraised the use of UCMSCs with a heparinised collagen conduits in transected rabbit recurrent laryngeal nerves. The authors assessed the effectiveness of passage-4 UCMSCs loaded on heparinised scaffold that released Nerve growth factor (NGF). Electromyograms at 8 wk revealed that treated lesions recovered normal nerve function. Biological markers of neurogenesis, including calcium-binding protein S100, neurofilament and AChE, were expressed at a greater level following treatment. Xiao et al[51] (2015) undertook a study exploring the effect of UCMSCs in a chitosan conduit interposed into the tibial nerve of a rabbit model. Xiao et al[51] found that nerve conduction velocity was significantly higher in the treatment group. The myelin sheath thickness and the growth of axis bud were both increased in the UCMSC group. Pereira et al[52] (2014) used PLC as a conduit for UCMSCs in murine sciatic nerve crush models. The group compared differentiated and undifferentiated UCMSCs. They established no difference in the degree of nerve regeneration between UCMSC that were differentiated into neural-glial-like cells and undifferentiated UCMSC groups. Both UCMSC groups showed increased myelin sheath thickness and enhanced recovery in motor and sensory function.
Two groups sought to investigate the use of UCMSCs embedded on a human amniotic membrane scaffold[5,53]. Li et al[53] (2012) found significant improvements in SFI, CMAP and gastrocnemius muscle diameter in UCMSC-loaded scaffolds group compared to cell-free scaffolds. Li et al[6] (2013) analysed how UCMSCs loaded on a human amniotic membrane scaffold affected the repair of a transected radial nerve in human subjects. Thirty-two patients with radial nerve injuries from radial shaft fractures were included in the study; twelve patients received neurolysis to remove neural scar tissue, and transplantation of UCMSCs on an amniotic membrane. The remainder 18 patients received neurolysis only. At 12 wk, the electrophysiological function of the UCMSC-treated group had improved electromyography readings. The muscular power, touch sensation and pain sensation were also significantly improved as compared to the neurolysis group.
Studies of UCMSCs in diabetic neuropathy
One study explored the role of UCMSCs in femoral nerve neuropathy[54] (Table 1). The authors modelled diabetic neuropathy in murine subjects by inducing diabetes with streptozotocin and created a dorsal hind foot ulcer through empyrosis. UCMSCs were delivered intravascularly through the femoral artery in the treatment group. Saline injections were used in the control group. Serum NGF and neurofilament 200 (NF-200) were measured by Enzyme Linked Immunosorbent Assay (ELISA). The results demonstrated that serum NGF and NF-200 increased in the UCMSC treated rats. Additionally, functional studies using electroneurogram showed that femoral nerve conduction was improved in the UCMSC subjects.
DISCUSSION
The studies in this review reported compelling positive outcomes for the use of human UCMSC to repair peripheral nerve lesions. None of the studies reported immunogenic nor significant complications. While the source cell utilised was consistent among the studies, there were significant variability in cell treatment and methods of transplantation with variable effectiveness as determined by several different outcome measures. There was also moderate heterogeneity in the in vivo models used. It is therefore difficult to draw conclusions on the optimal method of cell delivery to nerve lesions. Nevertheless, it does imply that UCMSCs are a useful cell source.
The process of cell harvest did not vary greatly between the studies. Human umbilical cord and umbilical cord blood are generally considered medical waste and so there are minimal ethical barriers to tissue sampling[55]. This provides a practical advantage for the use of UCMSCs and may explain why it is commonly used in tissue engineering experiments. The biochemical properties of UCMSCs may also be of advantage as studies comparing different source cells for MSCs have found UCMSCs to possess a greater ability to proliferate ex vivo and express a higher level of Vascular Endothelial Growth Factor (VEG-F) and Human Growth Factor (HGF) at late passages[56,57]. One study in this review compared UCMSCs to BMMSCs in sciatic nerve regeneration and found BMMSCs to produce superior results. The authors however, evaluated cell architecture on microscopy but did not carry out nerve conduction studies or functional analysis which may better inform clinical relevance[11]. UCMSCs also appear to have a different multilineage differentiation profiles to other MSC, and is able to be induced into neuron-like cells in vitro, which may favour applications in nerve regeneration[58]. There is some evidence to suggest that characteristics of the donor affect the ability of UCMSCs to differentiate. For example, undifferentiated UCMSCs obtained from patients with pre-eclampsia may produce greater levels of neuronal markers[59]. Therefore, exploration of different patient and gestational characteristics, such as age could help determine optimal source conditions.
There is no set protocol for the ex vivo expansion of UCMSCs in the literature. In our article, UCMSCs were generally transplanted between the third and fifth passage. Indeed, the gene expression profile of UCMSC is known to vary according to the number of passages, with some studies showing that UCMSCs do not express CD105, a defining marker for MSCs, until passage-5[60]. In view of this, it would be meaningful to identify and investigate the expression of important neurogenic markers as a function of stages of passage in future experiments. Some of the studies in this review pre-treated UCMSCs in order to induce them into particular cellular phenotypes prior to transplantation. Pereira et al[52] and Gartner et al[49] utilised a similar culture protocol and pre-treated UCMSCs with neurogenic media. They observed a neuroglial-like morphology on microscopy and a transcriptomic profile showing upregulation of neuroglial genes including Glial Fibrillary Acidic Protein (GFAP), Growth Associated Protein 43 (GAP43) and Neuronal Specific Nuclear Protein (NeuN). Both studies found differentiated MSCs to be more effective than undifferentiated UCMSCs. Aside from gene expression, it would be important to clarify the exact mechanism through which these differentiated cells act to promote nerve regeneration. The optimal protocol for differentiation is also ill-defined as Matsuse et al[35] used a different set of culture condition to induce UCMSCs into Schwann-like cells and found the latter to be more effective. Furthermore, there is a lack of agreement on the primary mode of action of MSCs in promoting nerve regeneration. It is unclear whether transplanted cells directly replicate and replace cells in the lesion. Some experiments of optic nerve lesions suggest that transplanted MSCs remain local and replicate[61]. Emerging in vitro evidence points towards paracrine effects as the predominant mechanism of action. It appears that pre-treatment of Schwann cells with UCMSC conditioned media increases BDNF and NGF expression which are surrogate measures of neurogenic potential[29]. In our review, Mak et al[17] examined the effectiveness of UCMSC-derived exosomes in nerve repair. Through peripheral intravenous injection of UCMSC EVs, they demonstrated that it could act systemically to encourage nerve regeneration at a nerve gap without off-site complications. As the use of EVs in this endeavour gains attention, further studies would be required to establish a dose-response relationship and the best method for delivering EVs to lesions.
It is difficult to determine the best UCMSC implantation method. Our review has captured studies that directly implanted UCMSCs and reported good outcomes. Studies investigating the use of conduits to guide nerve regeneration suggest that this is superior to direct implantation of MSCs alone[14,50]. The use of conduit that elude growth factors such as NGF along with UCMSC implantation appear to confer additional benefit[50]. Additionally, intravenous injection of UCMSC-EVs at a peripheral site also produce positive outcomes[17]. Interestingly, a comparison of local and intravenous BMMSC administration in sciatic nerve injury models suggest that systemic treatment provides a more significant improvement in nerve conduction, whereas local treatment improved neuronal fibre counts[62]. Other experiments have shown that peripherally injected MSCs localise to nerve lesions in murine models of sciatic nerve injury[63]. It could be inferred from these findings that there are differing mechanisms and sites of action for the two methods of implantation, suggesting that a treatment regime including both delivery methods concomitantly may produce the best outcome.
There are several issues pertaining to translating the findings derived from in vivo animal models for therapeutic application in humans. The majority of studies in our review employed a murine surgical sciatic nerve defect model to assess nerve regeneration. The critical nerve gap length, defined as a gap across which regeneration would not occur without nerve grafting or bridging is considered to be greater in humans than murine subjects[64,65]. Therefore, studies assessing murine nerve gaps may overestimate the therapeutic potential of treatments. Furthermore, it may be difficult to scale-up effective concentrations of transplanted UCMSCs to humans. In a study of rat nerve defects treated with tacrolimus, functional recovery tapers off at 9 wk following treatment and becomes indistinguishable from untreated rats at 10 wk[66]. Therefore, at later time points, which are more relevant to clinical presentations of nerve injury, the regenerative biology of murine nerve appears to differ from that of humans. Our interpretation from the in vivo animal studies in this review is complicated by the use of the sciatic nerve, which possesses a sensory and motor component, and thus renders functional analysis difficult. It is conceivable for sensory loss to mask a post-surgical motor defect on gait analysis, similarly, it may be possible for loss in motor function to cause underestimation of sensory recovery. Owing to the heterogeneity in starting points for different functional measures, a pooled analysis of quantitative outcomes could not be performed in this review. Therefore, clinically relevant and robust quantifiable outcome measures remain a significant barrier to the reliability of animal studies. One study in this review assessed UCMSC transplantation in human radial nerve defects and reported improved motor and sensory function and electrophysiological measures[53]. The group however, delivered the MSCs through a scaffold, and did not compare outcomes with a control group of the scaffold alone.
According to the results of our risk of bias analysis, 13 of 14 studies had a moderate risk of bias, and one study had a high risk of bias (Figure 1). The reporting of outcome measures contributed to an increased risk of bias in all studies, as most of the studies reported improvement in some but not all outcomes yet concluded that UCMSCs were effective overall. This could be owing to the significant heterogeneity in cell treatment and delivery methods which as the literature suggests, could contribute to different aspects of nerve regeneration.
In conclusion, while there are homeostatic responses that promote nerve regeneration following injury, the body’s natural capacity is inadequate for the recovery of satisfactory nerve function. The evidence summarised in this systematic review supports the notion that UCMSC transplantation is an effective treatment option for nerve injury. Several barriers must be overcome before these findings can be translated into the clinical setting. Importantly, development of a reliable in vivo animal model, and a standardised method of assessing nerve regeneration would allow the optimal method of cell transplantation to be determined.
ARTICLE HIGHLIGHTS
Research background
Peripheral nerve injury can be a debilitating condition. Traditional treatment options are often ineffective. There is an urgent need for new treatment modalities. Mesenchymal stem cell (MSC) transplantation holds promise as a cell-based regenerative approach in treating nerve lesions. MSCs can be sourced from various tissues, and this may affect their regenerative capacity. Here, we appraise the in vivo evidence for the use of human umbilical cord-derived MSCs (UCMSCs) in peripheral nerve regeneration.
Research motivation
There is contention regarding the optimal cell-source for the harvest of MSCs. Some evidence suggests that MSCs from certain tissue types have superior neurogenic capacity. It is critical that we determine the best cell-source for nerve repair, in order to facilitate an efficient production protocol and maximise clinical benefit.
Research objectives
To investigate whether UCMSCs are effective in nerve regeneration in in vivo models of nerve injury.
Research methods
We performed a systematic literature review according to the PRISMA statement. A search was conducted on three databases (PubMed, EMBASE and Web of Science) by two independent investigators from inception to September 2019 for studies examining the use of UCMSCs in in vivo models of nerve injury. The evidence was appraised using Cochrane’s RoB 2.0 Tool.
Research results
A total of 14 studies were included in the review, with a total of 279 subjects. The studies reported that transplantation of human umbilical cord MSCs were effective in regenerating nerve lesions. There were general improvements in histological and functional outcomes. The studies did not report significant complications.
Research conclusions
Human umbilical cord-derived MSCs were effective in repairing nerve lesions in both animal and human models of nerve injury. Additional studies are required to correlate histological outcomes with functional improvements, as not all studies assessed both. More human studies are necessary to inform the efficacy in humans. High quality randomized controlled trials would be instructive in this case. Long-term follow up in these types of study will help inform the safety of MSC transplantation.
Research perspectives
There is limited evidence examining the use of MSCs derived from other tissues in their capacity to regenerate nerve lesions. Further studies comparing different tissue cell-source directly would be highly informative. In vitro studies of MSC-biomaterial scaffolds may aid the development of more efficient MSC delivery methods. As the nature of nerve injury can vary significantly, the approach to transplantation, such as dose delivery may need to be catered to the individual lesion. Studies comparing the effect of MSCs on different in vivo models could help delineate this.
Footnotes
Conflict-of-interest statement: All authors declare that they have no conflict of interest.
PRISMA 2009 Checklist statement: The authors declare that the manuscript satisfies the PRISMA 2009 Checklist and the manuscript has been prepared and revised according to the checklist requirements.
Manuscript source: Invited manuscript
Peer-review started: December 16, 2019
First decision: February 20, 2020
Article in press: March 23, 2020
Specialty type: Cell and tissue engineering
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chivu-Economescu M, Huang YC S-Editor: Wang YQ L-Editor: A E-Editor: Xing YX
Contributor Information
Christine Bojanic, Department of Plastic and Reconstructive Surgery, Cambridge University Hospitals NHS Trust, Cambridge CB2 0QQ, United Kingdom.
Kendrick To, Division of Trauma and Orthopaedic Surgery, Addenbrooke's Hospital, University of Cambridge, Cambridge CB2 0QQ, United Kingdom.
Bridget Zhang, School of Clinical Medicine, University of Cambridge, Cambridge CB2 0QQ, United Kingdom.
Christopher Mak, School of Clinical Medicine, University of Cambridge, Cambridge CB2 0QQ, United Kingdom.
Wasim S Khan, Division of Trauma and Orthopaedic Surgery, Addenbrooke's Hospital, University of Cambridge, Cambridge CB2 0QQ, United Kingdom. wasimkhan@doctors.org.uk.
References
- 1.Sullivan R, Dailey T, Duncan K, Abel N, Borlongan CV. Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26:151–160. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- 3.Seddon HJ. Three types of nerve injury. Brain. 1943;66:237–288. [Google Scholar]
- 4.Menorca RM, Fussell TS, Elfar JC. Nerve physiology: mechanisms of injury and recovery. Hand Clin. 2013;29:317–330. doi: 10.1016/j.hcl.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguayo AJ, Peyronnard JM, Bray GM. A quantitative ultrastructural study of regeneration from isolated proximal stumps of transected unmyelinated nerves. J Neuropathol Exp Neurol. 1973;32:256–270. doi: 10.1097/00005072-197304000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Qin H, Feng Z, Liu W, Zhou Y, Yang L, Zhao W, Li Y. Human umbilical cord mesenchymal stem cell-loaded amniotic membrane for the repair of radial nerve injury. Neural Regen Res. 2013;8:3441–3448. doi: 10.3969/j.issn.1673-5374.2013.36.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niver GE, Ilyas AM. Management of radial nerve palsy following fractures of the humerus. Orthop Clin North Am. 2013;44:419–424, x. doi: 10.1016/j.ocl.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Caldwell JM, Kim HM, Levine WN. Radial nerve injury associated with application of a hinged elbow external fixator: a report of 2 cases. J Shoulder Elbow Surg. 2013;22:e12–e16. doi: 10.1016/j.jse.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Ma L, Feng XY, Cui BL, Law F, Jiang XW, Yang LY, Xie QD, Huang TH. Human umbilical cord Wharton's Jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chin Med J (Engl) 2005;118:1987–1993. [PubMed] [Google Scholar]
- 10.Kaiser R, Ullas G, Havránek P, Homolková H, Miletín J, Tichá P, Sukop A. Current Concepts in Peripheral Nerve Injury Repair. Acta Chir Plast. 2017;59:85–91. [PubMed] [Google Scholar]
- 11.Zarbakhsh S, Goudarzi N, Shirmohammadi M, Safari M. Histological Study of Bone Marrow and Umbilical Cord Stromal Cell Transplantation in Regenerating Rat Peripheral Nerve. Cell J. 2016;17:668–677. doi: 10.22074/cellj.2016.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bellamkonda RV. Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy. Biomaterials. 2006;27:3515–3518. doi: 10.1016/j.biomaterials.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Lundborg G, Dahlin L, Dohi D, Kanje M, Terada N. A new type of "bioartificial" nerve graft for bridging extended defects in nerves. J Hand Surg Br. 1997;22:299–303. doi: 10.1016/s0266-7681(97)80390-7. [DOI] [PubMed] [Google Scholar]
- 14.Cui Y, Yao Y, Zhao Y, Xiao Z, Cao Z, Han S, Li X, Huan Y, Pan J, Dai J. Functional collagen conduits combined with human mesenchymal stem cells promote regeneration after sciatic nerve transection in dogs. J Tissue Eng Regen Med. 2018;12:1285–1296. doi: 10.1002/term.2660. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu S, Kitada M, Ishikawa H, Itokazu Y, Wakao S, Dezawa M. Peripheral nerve regeneration by the in vitro differentiated-human bone marrow stromal cells with Schwann cell property. Biochem Biophys Res Commun. 2007;359:915–920. doi: 10.1016/j.bbrc.2007.05.212. [DOI] [PubMed] [Google Scholar]
- 16.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y, Dong L, Zhou D, Li L, Zhang W, Zhen Y, Wang T, Su J, Chen D, Mao C, Wang X. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J Cell Mol Med. 2019;23:2822–2835. doi: 10.1111/jcmm.14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trohatou O, Roubelakis MG. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Past, Present, and Future. Cell Reprogram. 2017;19:217–224. doi: 10.1089/cell.2016.0062. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 20.Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 22.Gu LH, Zhang TT, Li Y, Yan HJ, Qi H, Li FR. Immunogenicity of allogeneic mesenchymal stem cells transplanted via different routes in diabetic rats. Cell Mol Immunol. 2015;12:444–455. doi: 10.1038/cmi.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X, Zhu Y, Yin HY, Guo ZY, Xu F, Xiao B, Jiang WL, Guo WM, Meng HY, Lu SB, Wang Y, Peng J. Differentiation of adipose-derived stem cells into Schwann cell-like cells through intermittent induction: potential advantage of cellular transient memory function. Stem Cell Res Ther. 2018;9:133. doi: 10.1186/s13287-018-0884-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zaminy A, Shokrgozar MA, Sadeghi Y, Noroozian M, Heidari MH, Piryaei A. Mesenchymal stem cells as an alternative for Schwann cells in rat spinal cord injury. Iran Biomed J. 2013;17:113–122. doi: 10.6091/ibj.1121.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Zhang H, Liu M, Wang N. Distal segment extracts of the degenerated rat sciatic nerve induce bone marrow stromal cells to express Schwann cell markers in vitro. Neurosci Lett. 2013;544:89–93. doi: 10.1016/j.neulet.2013.03.048. [DOI] [PubMed] [Google Scholar]
- 26.Sowa Y, Kishida T, Imura T, Numajiri T, Nishino K, Tabata Y, Mazda O. Adipose-Derived Stem Cells Promote Peripheral Nerve Regeneration In Vivo without Differentiation into Schwann-Like Lineage. Plast Reconstr Surg. 2016;137:318e–330e. doi: 10.1097/01.prs.0000475762.86580.36. [DOI] [PubMed] [Google Scholar]
- 27.Rani S, Ryan AE, Griffin MD, Ritter T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol Ther. 2015;23:812–823. doi: 10.1038/mt.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horwitz EM, Dominici M. How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy. 2008;10:771–774. doi: 10.1080/14653240802618085. [DOI] [PubMed] [Google Scholar]
- 29.Guo ZY, Sun X, Xu XL, Zhao Q, Peng J, Wang Y. Human umbilical cord mesenchymal stem cells promote peripheral nerve repair via paracrine mechanisms. Neural Regen Res. 2015;10:651–658. doi: 10.4103/1673-5374.155442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61:1795–1806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- 32.Wei JJ, Chen YF, Xue CL, Ma BT, Shen YM, Guan J, Bao XJ, Wu H, Han Q, Wang RZ, Zhao CH. Protection of Nerve Injury with Exosome Extracted from Mesenchymal Stem Cell. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38:33–36. doi: 10.3881/j.issn.1000-503X.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Fong CY, Tam K, Cheyyatraivendran S, Gan SU, Gauthaman K, Armugam A, Jeyaseelan K, Choolani M, Biswas A, Bongso A. Human Wharton's jelly stem cells and its conditioned medium enhance healing of excisional and diabetic wounds. J Cell Biochem. 2014;115:290–302. doi: 10.1002/jcb.24661. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Jo CH, Kim HR, Hwang YI. Comparison of Immunological Characteristics of Mesenchymal Stem Cells from the Periodontal Ligament, Umbilical Cord, and Adipose Tissue. Stem Cells Int. 2018;2018:8429042. doi: 10.1155/2018/8429042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuse D, Kitada M, Kohama M, Nishikawa K, Makinoshima H, Wakao S, Fujiyoshi Y, Heike T, Nakahata T, Akutsu H, Umezawa A, Harigae H, Kira J, Dezawa M. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration. J Neuropathol Exp Neurol. 2010;69:973–985. doi: 10.1097/NEN.0b013e3181eff6dc. [DOI] [PubMed] [Google Scholar]
- 36.Wakao S, Hayashi T, Kitada M, Kohama M, Matsue D, Teramoto N, Ose T, Itokazu Y, Koshino K, Watabe H, Iida H, Takamoto T, Tabata Y, Dezawa M. Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Exp Neurol. 2010;223:537–547. doi: 10.1016/j.expneurol.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Mukai T, Tojo A, Nagamura-Inoue T. Umbilical Cord-Derived Mesenchymal Stromal Cells Contribute to Neuroprotection in Neonatal Cortical Neurons Damaged by Oxygen-Glucose Deprivation. Front Neurol. 2018;9:466. doi: 10.3389/fneur.2018.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ribeiro CA, Fraga JS, Grãos M, Neves NM, Reis RL, Gimble JM, Sousa N, Salgado AJ. The secretome of stem cells isolated from the adipose tissue and Wharton jelly acts differently on central nervous system derived cell populations. Stem Cell Res Ther. 2012;3:18. doi: 10.1186/scrt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin YC, Ko TL, Shih YH, Lin MY, Fu TW, Hsiao HS, Hsu JY, Fu YS. Human umbilical mesenchymal stem cells promote recovery after ischemic stroke. Stroke. 2011;42:2045–2053. doi: 10.1161/STROKEAHA.110.603621. [DOI] [PubMed] [Google Scholar]
- 40.Cui B, Li E, Yang B, Wang B. Human umbilical cord blood-derived mesenchymal stem cell transplantation for the treatment of spinal cord injury. Exp Ther Med. 2014;7:1233–1236. doi: 10.3892/etm.2014.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton's jelly of the human umbilical cord. Stem Cell Rev Rep. 2013;9:226–240. doi: 10.1007/s12015-012-9418-z. [DOI] [PubMed] [Google Scholar]
- 42.Dasari VR, Spomar DG, Gondi CS, Sloffer CA, Saving KL, Gujrati M, Rao JS, Dinh DH. Axonal remyelination by cord blood stem cells after spinal cord injury. J Neurotrauma. 2007;24:391–410. doi: 10.1089/neu.2006.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee IH, Lin WS, Wu CH, Lin WY, Cheng SM. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS One. 2013;8:e72604. doi: 10.1371/journal.pone.0072604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fung M, Yuan Y, Atkins H, Shi Q, Bubela T. Responsible Translation of Stem Cell Research: An Assessment of Clinical Trial Registration and Publications. Stem Cell Reports. 2017;8:1190–1201. doi: 10.1016/j.stemcr.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sung MA, Jung HJ, Lee JW, Lee JY, Pang KM, Yoo SB, Alrashdan MS, Kim SM, Jahng JW, Lee JH. Human umbilical cord blood-derived mesenchymal stem cells promote regeneration of crush-injured rat sciatic nerves. Neural Regen Res. 2012;7:2018–2027. doi: 10.3969/j.issn.1673-5374.2012.26.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hei WH, Almansoori AA, Sung MA, Ju KW, Seo N, Lee SH, Kim BJ, Kim SM, Jahng JW, He H, Lee JH. Adenovirus vector-mediated ex vivo gene transfer of brain-derived neurotrophic factor (BDNF) tohuman umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) promotescrush-injured rat sciatic nerve regeneration. Neurosci Lett. 2017;643:111–120. doi: 10.1016/j.neulet.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 48.Gärtner A, Pereira T, Simões MJ, Armada-da-Silva PA, França ML, Sousa R, Bompasso S, Raimondo S, Shirosaki Y, Nakamura Y, Hayakawa S, Osakah A, Porto B, Luís AL, Varejão AS, Maurício AC. Use of hybrid chitosan membranes and human mesenchymal stem cells from the Wharton jelly of umbilical cord for promoting nerve regeneration in an axonotmesis rat model. Neural Regen Res. 2012;7:2247–2258. doi: 10.3969/j.issn.1673-5374.2012.29.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gärtner A, Pereira T, Alves MG, Armada-da-Silva PA, Amorim I, Gomes R, Ribeiro J, França ML, Lopes C, Carvalho RA, Socorro S, Oliveira PF, Porto B, Sousa R, Bombaci A, Ronchi G, Fregnan F, Varejão AS, Luís AL, Geuna S, Maurício AC. Use of poly(DL-lactide-ε-caprolactone) membranes and mesenchymal stem cells from the Wharton's jelly of the umbilical cord for promoting nerve regeneration in axonotmesis: in vitro and in vivo analysis. Differentiation. 2012;84:355–365. doi: 10.1016/j.diff.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Pan Y, Jiao G, Yang J, Guo R, Li J, Wang C. Insights into the Therapeutic Potential of Heparinized Collagen Scaffolds Loading Human Umbilical Cord Mesenchymal Stem Cells and Nerve Growth Factor for the Repair of Recurrent Laryngeal Nerve Injury. Tissue Eng Regen Med. 2017;14:317–326. doi: 10.1007/s13770-017-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao Q, Zhang X, Wu Y. Experimental Research on Differentiation-Inducing Growth of Nerve Lateral Bud by HUC-MSCs Chitosan Composite Conduit. Cell Biochem Biophys. 2015;73:305–311. doi: 10.1007/s12013-015-0578-8. [DOI] [PubMed] [Google Scholar]
- 52.Pereira T, Gärtner A, Amorim I, Almeida A, Caseiro AR, Armada-da-Silva PA, Amado S, Fregnan F, Varejão AS, Santos JD, Bartolo PJ, Geuna S, Luís AL, Mauricio AC. Promoting nerve regeneration in a neurotmesis rat model using poly(DL-lactide-ε-caprolactone) membranes and mesenchymal stem cells from the Wharton's jelly: in vitro and in vivo analysis. Biomed Res Int. 2014;2014:302659. doi: 10.1155/2014/302659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li D, Wang C, Shan W, Zeng R, Fang Y, Wang P. Human amnion tissue injected with human umbilical cord mesenchymal stem cells repairs damaged sciatic nerves in rats. Neural Regen Res. 2012;7:1771–1778. doi: 10.3969/j.issn.1673-5374.2012.23.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia N, Xu JM, Zhao N, Zhao QS, Li M, Cheng ZF. Human mesenchymal stem cells improve the neurodegeneration of femoral nerve in a diabetic foot ulceration rats. Neurosci Lett. 2015;597:84–89. doi: 10.1016/j.neulet.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 55.Stewart C, Kerridge I. Umbilical cord blood banking and the next generation of human tissue regulation: an agenda for research. J Law Med. 2012;19:423–429. [PubMed] [Google Scholar]
- 56.Ren H, Sang Y, Zhang F, Liu Z, Qi N, Chen Y. Comparative Analysis of Human Mesenchymal Stem Cells from Umbilical Cord, Dental Pulp, and Menstrual Blood as Sources for Cell Therapy. Stem Cells Int. 2016;2016:3516574. doi: 10.1155/2016/3516574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, Han ZB, Xu ZS, Lu YX, Liu D, Chen ZZ, Han ZC. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–1026. [PubMed] [Google Scholar]
- 58.Li JF, Yin HL, Shuboy A, Duan HF, Lou JY, Li J, Wang HW, Wang YL. Differentiation of hUC-MSC into dopaminergic-like cells after transduction with hepatocyte growth factor. Mol Cell Biochem. 2013;381:183–190. doi: 10.1007/s11010-013-1701-z. [DOI] [PubMed] [Google Scholar]
- 59.Joerger-Messerli M, Brühlmann E, Bessire A, Wagner A, Mueller M, Surbek DV, Schoeberlein A. Preeclampsia enhances neuroglial marker expression in umbilical cord Wharton's jelly-derived mesenchymal stem cells. J Matern Fetal Neonatal Med. 2015;28:464–469. doi: 10.3109/14767058.2014.921671. [DOI] [PubMed] [Google Scholar]
- 60.Bakhshi T, Zabriskie RC, Bodie S, Kidd S, Ramin S, Paganessi LA, Gregory SA, Fung HC, Christopherson KW 2nd. Mesenchymal stem cells from the Wharton's jelly of umbilical cord segments provide stromal support for the maintenance of cord blood hematopoietic stem cells during long-term ex vivo culture. Transfusion. 2008;48:2638–2644. doi: 10.1111/j.1537-2995.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mesentier-Louro LA, Zaverucha-do-Valle C, da Silva-Junior AJ, Nascimento-Dos-Santos G, Gubert F, de Figueirêdo AB, Torres AL, Paredes BD, Teixeira C, Tovar-Moll F, Mendez-Otero R, Santiago MF. Distribution of mesenchymal stem cells and effects on neuronal survival and axon regeneration after optic nerve crush and cell therapy. PLoS One. 2014;9:e110722. doi: 10.1371/journal.pone.0110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooney DS, Wimmers EG, Ibrahim Z, Grahammer J, Christensen JM, Brat GA, Wu LW, Sarhane KA, Lopez J, Wallner C, Furtmüller GJ, Yuan N, Pang J, Sarkar K, Lee WP, Brandacher G. Mesenchymal Stem Cells Enhance Nerve Regeneration in a Rat Sciatic Nerve Repair and Hindlimb Transplant Model. Sci Rep. 2016;6:31306. doi: 10.1038/srep31306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matthes SM, Reimers K, Janssen I, Liebsch C, Kocsis JD, Vogt PM, Radtke C. Intravenous transplantation of mesenchymal stromal cells to enhance peripheral nerve regeneration. Biomed Res Int. 2013;2013:573169. doi: 10.1155/2013/573169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Angius D, Wang H, Spinner RJ, Gutierrez-Cotto Y, Yaszemski MJ, Windebank AJ. A systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds. Biomaterials. 2012;33:8034–8039. doi: 10.1016/j.biomaterials.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daly W, Yao L, Zeugolis D, Windebank A, Pandit A. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9:202–221. doi: 10.1098/rsif.2011.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brenner MJ, Moradzadeh A, Myckatyn TM, Tung TH, Mendez AB, Hunter DA, Mackinnon SE. Role of timing in assessment of nerve regeneration. Microsurgery. 2008;28:265–272. doi: 10.1002/micr.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]