Abstract
Aims
Externalizing psychopathology in early adolescence is a highly heritable risk factor for drug use, yet how it relates to marijuana use development is not well-characterized. We evaluate this issue in independent twin samples from Colorado (N=2,608) and Minnesota (N=3,630), assessed from adolescence to early adulthood.
Methods
We used a biometric latent growth model of marijuana use frequency with data from up to five waves of assessment from ages 14–30, to examine change in marijuana use and its relationship with a factor model of adolescent externalizing psychopathology.
Results
The factor structure of adolescent externalizing psychopathology was similar across samples, as was the association between that common factor and early marijuana use (Minnesota r = .67 [.60, .75]; Colorado r = .69 [.59, .78]), and increase in use (Minnesota r = .18 [.10, .26]; Colorado r = .20 [.07, .34]). Early use was moderately heritable in both samples (Minnesota h2 = .57 [.37, .79]; Colorado h2 = .42 [.14, .73]). Increase in use was highly heritable in Minnesota (h2 =.82 [.72, .88]), less so in Colorado (h2 =.22 [.01, .66]), and shared environmental effects were larger in Colorado (c2=.55 [.14, .83]) than Minnesota (c2=0 [0, .06]). We found moderate genetic correlations between externalizing psychopathology and early use in both samples. Finally, additional analyses in the Minnesota sample indicated that marijuana use decreased during the late 20s. This decline is strongly heritable (h2=.73 [.49, .91]) and moderately negatively correlated with adolescent externalizing psychopathology (r=−.41 [−.54, −.28]).
Conclusions
Adolescent externalizing psychopathology is genetically correlated with change in late adolescent marijuana use (late teens, early 20s), as well as maintenance of use in early adulthood (late 20s) even after controlling for the effects of early use.
Keywords: marijuana, heritability, externalizing psychopathology, measurement invariance, latent growth model
Most individuals who use marijuana begin in mid- to late-adolescence, on average around age 16 (Richmond-Rakerd et al. 2017). Average use increases until the mid-20s and declines thereafter. Marijuana use initiation, frequency of use, and problematic use are heritable (e.g., h2 ~.44-.55) and genetically correlated (rg~.62 ) (Gillespie et al. 2009; Verweij et al. 2010; Hines et al. 2018). These behaviors also show substantial genetic correlations with other substance use behaviors and with disruptive behavioral disorders (Han et al. 1999; Kendler et al. 2003a, 2008; Vrieze et al. 2012; Grant et al. 2015; Tielbeek et al. 2018). One theoretical account for such observed genetic correlation is that various manifestations of substance use are each influenced by a more general genetic predisposition (Young et al. 2000; Krueger et al. 2002; Iacono et al. 2008; Vanyukov et al. 2012), termed the externalizing spectrum.
The externalizing spectrum is a highly heritable liability to engage in disinhibitory, rule-breaking behaviors across a range of severities. Individuals high on the spectrum are characterized by the tendency to act impulsively and seek reward without considering consequences (Vanyukov et al. 2012). Confirmatory factor models support this notion, where a single highly heritable (h2~80%) latent factor loads strongly and relatively uniformly on various measures of psychiatric symptoms and diagnoses, including ADHD, conduct disorder, adult antisocial behavior, and personality measures of low impulse control (Young et al. 2000; Krueger et al. 2002; Nadder et al. 2002; Kendler et al. 2003a, b; Hicks et al. 2004, 2011; Dick et al. 2005; Iacono et al. 2008; Tuvblad et al. 2009).
Substance use and dependence are also considered indicators of the externalizing spectrum (Krueger et al. 2002; Hicks et al. 2004; Dick et al. 2008; Palmer et al. 2013; McGue et al. 2014; Derringer et al. 2015), and early manifestations of externalizing psychopathology (e.g., indexed by childhood disorders such as conduct disorder, ADHD) predict substance use and dependence years later (Iacono et al. 1999; King et al. 2004; Sousa et al. 2011; Prisciandaro et al. 2012; Palmer et al. 2013; Tully et al. 2014; McGue et al. 2014; Sibley et al. 2014; Colder et al. 2018; Tielbeek et al. 2018). Given these robust cross-sectional and prospective associations among indicators of externalizing, here we expand on this literature by evaluating specifically the relationship between externalizing psychopathology as measured in mid-adolescence, and its relationship with the development of marijuana use through early adulthood.
Genetically informative studies are important in understanding the nature of the observed comorbidity between normative substance use, drug abuse, and externalizing psychopathology. Twin studies indicate that there are genetic and shared environmental correlations between substance use phenotypes such as initiation and progression across substances (Fowler et al. 2007; Shelton et al. 2007; Huizink et al. 2010; Korhonen et al. 2012). Furthermore, the shared environment was more influential for initiation compared to progression (Fowler et al. 2007; Shelton et al. 2007; Huizink et al. 2010; Korhonen et al. 2012). In addition to genetic and environmental covariation between substance use phenotypes, previous work indicates that the covariation between substance use and other indicators of externalizing reflects additive genetic and shared environmental influences (Chang et al. 2012; Korhonen et al. 2012; Elkins et al. 2018). There may also exist age-specific and reciprocal relationships between early externalizing psychopathology, such as ADHD or conduct disorder, and later substance use, but conclusions about the nature of these relationships is less clear, as these studies were not genetically informative (Loeber and Keenan 1994; Tarter et al. 1999; Molina et al. 2007; Storr et al. 2012; Tully et al. 2014; Thompson et al. 2015).
While this literature has informed our understanding of the association between substance use and externalizing across the course of development, many of these studies are limited in developmental timeframe. Analyses primarily focus on the early-to-mid teen years as a period of particular importance to substance use development, reasonably so given the average age of initiation and average age of onset of substance problems. Additional genetically informative studies across longer developmental periods are necessary to fully understand these transitions and change in substance use across major developmental periods spanning decades.
We aim to address these gaps in the literature by evaluating the relationship between adolescent externalizing psychopathology and development of marijuana use in a genetically informative sample across ages 14–30. We first sought to establish evidence of measurement invariance of an externalizing psychopathology factor across independent samples which, to our knowledge, has not previously been conducted. Following this, we had three research questions: 1) what is the typical trajectory of marijuana use (estimated as a latent growth model); 2) to what extent does externalizing psychopathology covary with marijuana use development and what is the nature of this covariation (genetic or environmental); and 3) how do trajectories and their relationship with externalizing psychopathology differ between samples?
To evaluate these questions, we leveraged two large twin samples, one from Colorado and one from Minnesota. These samples were selected due to similarities in assessment content, wave structure, and birth years. We used phenotypes we could measure with approximate equivalence across studies to derive a model of the externalizing spectrum similar to that which has been reported previously (conduct disorder, alcohol dependence, and ADHD), and used the derived latent externalizing construct to predict development of a more deviant aspect of externalizing, illicit drug use, as represented by marijuana involvement, which typically occurs later in development than the other three disorders.
Methods
Participants
Participants from both states were initially assessed in adolescence and were followed longitudinally through early adulthood. Minnesota participants were recruited and assessed through the Minnesota Twin Family Study in three intake cohorts. There were 3630 participants from 1815 twin pairs: 1163 monozygotic (MZ) pairs and 652 same-sex dizygotic (DZ) pairs. For a full description of sample size by cohort, sample size by wave, and years of data collection see Supplemental Table 1. Ethnicity in Minnesota was determined via parent-report or birth-record report. About 8% more twins were reported as white in Minnesota (94.4%) versus Colorado (86.4%). 1.3% of Minnesota twins reported as Hispanic and the remaining 4.3% as other. Most Minnesota twins were evaluated with genome-wide genotyping (Miller et al. 2012).
Colorado participants were recruited through the Colorado Twin Registry and assessed as part of the Center for Antisocial Drug Dependence in two intake cohorts (Rhea et al. 2006, 2013). There were 2608 individuals from 1442 twin pairs: 699 monozygotic (MZ) pairs, 468 same-sex dizygotic (DZ) pairs, and 275 opposite-sex dizygotic pairs. For a full description of sample size by cohort, sample size by wave, and years of data collection see Supplemental Table 1. To maintain similarity between samples, one twin from each opposite-sex pair was randomly selected for inclusion and their co-twin’s data were set to missing, as Minnesota did not recruit opposite-sex pairs. Opposite-sex twins therefore contributed information to estimates of means and variances, but not biometric variance decompositions. 84.7% of Colorado twins self-reported as white, 9.5% as Hispanic, and the remaining 5.8% as other. DNA was collected from twins seen in-person and zygosity confirmed by DNA testing (Rhea et al. 2006).
We used data from six waves of assessment in Minnesota and three in Colorado. In Minnesota, participants were assessed at target ages of 11, 14, 17, 20, 24, and 29; exact ages of assessment are clustered around the target age. Participation in particular assessments depends on recruitment cohort (Iacono and McGue 2002; Keyes et al. 2009; Miller et al. 2012). In Colorado, participants were assessed approximately every five years, with age at intake ranging from 11–19 years. See Table 1 for wave structure, sample sizes, and age descriptives for both samples.
Table 1.
Wave Structure and Sample Size, Split by Cohort
| Minnesota | |||
| Wave | N (Total) | Age Mean (SD) | Age Range |
| 14 | 2,232 | 14.9 (0.5) | 13.6 – 17.0 |
| 17 | 3,363 | 17.8 (0.6) | 16.5 – 20.3 |
| 20 | 2,618 | 21.1 (0.8) | 19.4 – 24.3 |
| 24 | 3,052 | 24.9 (0.9) | 22.6 – 29.3 |
| 29 | 1,999 | 29.4 (0.7) | 28.2 – 33.2 |
| Colorado | |||
| Wave | N (Total) | Age Mean (SD) | Age Range |
| 1 | 2,307 | 15.0 (2.2) | 11.3 – 19.0 |
| 2 | 2,214 | 20.1 (2.7) | 16.1 – 29.1 |
| 3 | 2,244 | 25.4 (2.7) | 21.1 – 34.4 |
Note: N reflects number of individuals with non-missing marijuana frequency at a given wave.
Measures
We selected three indicators of adolescent externalizing psychopathology based on diagnostic information that was similarly assessed in the two samples. Among externalizing disorders, only three were comparably assessed and these yielded symptom counts for alcohol dependence, conduct disorder, and ADHD. Additional substances were not included due to differences in measurement between samples. We chose the assessment closest to age 17, excluding assessments before age 15 and after 21, for alcohol dependence and conduct disorder. This maximized sample size and helped ensure similarity in age distribution between states, but also avoided age effects from the inclusion of individuals who have had different opportunities to use alcohol (participants younger than 15 may have had limited opportunity, participants over 21 have legal access). This age window also ensured that participants were past the reporting period for conduct disorder and minimizes recall bias, as diagnostic criteria require that symptoms must be present by age 15. For ADHD, we used the youngest available assessment in both Colorado and Minnesota to minimize the effects of recall bias, as diagnostic criteria in both DSM-III-R and DSM-IV require that at least some ADHD symptoms must appear before age 7.
To evaluate alcohol dependence, twins in both states were administered an adapted version of the CIDI-SAM (Cottler 2000). Lifetime conduct disorder symptoms were assessed through self-report in Minnesota using a modified section from the SCID for DSM-III-R Personality Disorders (Spitzer et al. 1987). In Colorado, conduct disorder was assessed through self-report using the DIS or DISC depending on participant age (Robins et al. 2000; Shaffer et al. 2000). In Minnesota, ADHD was assessed using the DICA-R (Reich 1997). In Colorado, ADHD was assessed using the DISC.
The measures used to evaluate disorder symptoms were based on DSM-IIIR in Minnesota, and DSM-IV in Colorado, which are highly similar, but required some harmonization between samples. Prior to data analysis, we reviewed the disorder symptoms in each DSM version, removing symptoms specific to either system. Retained and excluded symptoms are presented in Supplemental Table 2. Retained symptoms were used to calculate symptom counts for analysis (13 symptoms for ADHD, 9 for alcohol dependence, and 12 for conduct disorder).
Marijuana use frequency was available at all waves of assessment in each state. Only assessments where the individual was older than 13 were used, as there was no use prior to that age in either sample. Marijuana use frequency was assessed in Colorado as the number of days, in the last 180 days, on which a participant used marijuana. In Minnesota, use was assessed as average frequency of use in the last 12 months as: never or less than once a year, less than once a month but at least once a year, about once a month, 2 or 3 times a month, 1 or 2 times a week, 3 to 4 times a week, nearly every day, and daily. Colorado responses were converted to the Minnesota scale for comparability. Category mappings are presented in Supplemental Table 3. The correlation between raw and rescaled scores in Colorado was .892 (95% CI=[.885, .899]).
Tests of mean differences accounted for the nested structure of the twin data. We used univariate twin models in which group means were free to vary and models in which group means were constrained to be equal. We compared the goodness of fit between free and restricted models, indexed by twice the difference in log likelihood, which under the null hypothesis of equal means is distributed as χ2. We compared means for all behavioral and drug measures between gender within states and within gender between states. Heritability estimates for the externalizing indicators were estimated with a biometric variance decomposition for each trait in each state (Martin and Eaves 1977).
Analyses
Externalizing psychopathology was modeled in a confirmatory factor model on which loaded the symptom counts of alcohol dependence, ADHD, and conduct disorder. This model is consistent with prior work (Young et al. 2000; Krueger et al. 2002) but does not use identical indicators because the present indicators were selected to maximize content similarity between samples, as described above. Symptom counts were log transformed and residualized on covariates within each sample. Covariates include age, age2, age × cohort, cohort, and sex, where cohort refers to two distinct recruitment efforts within the Colorado sample and three within the Minnesota sample (Iacono and McGue 2002; Rhea et al. 2006, 2013; Keyes et al. 2009; Miller et al. 2012; Wilson et al. 2019). Log transformation was chosen as it can help address positive skew, results in approximately unbiased parameter estimates, and lognormal distributions outperform normal distributions in terms of variance around estimates in simulation studies (Kirkpatrick and Neale 2016).
We used OpenMx v2.6.9 for factor and growth modelling (Boker et al. 2011; Neale et al. 2016).We used a standard biometric variance decomposition within a common pathway model to estimate the genetic (A), shared environmental (C), and unique environmental (E) variance components of the common and specific factors extracted from the externalizing indicators. To evaluate fit, the common pathway model was compared to a saturated model using likelihood ratio tests, the Akaike/Bayesian Information Criteria (AIC/BIC), and the root mean square error of approximation (RMSEA) (Hu and Bentler 1999; Vrieze 2012).
We conducted tests of measurement invariance across the two samples including weak, strong, and strict factorial invariance (Meredith 1993). For any invariance test passed, we further restricted the variance decomposition of the common factor to be equivalent across the two samples, something we term “biometric” invariance.
We used latent growth curve modeling to examine marijuana use development. The number of available waves of assessment across the two studies allowed for modeling initiation of use and the linear increase in use across adolescence between ages 14 to 24; all assessments between these ages were included in the model. To determine a maximum age cutoff, a generalized additive mixed model was used to evaluate evidence for nonlinear trends (Lin and Zhang 1999) and this predicted curve indicates decline in use in the mid 20s. In these samples use peaks at age 22, but there are few observations taken at exactly this age. To maximize sample size yet maintain close proximity to the age of maximum use, we chose to use assessments up to age 24.
We estimated a latent intercept and a latent slope. Interpretation of the latent intercept depends on how the age of participants is centered. We chose to center them at age 16.5, as that is the mean age of marijuana use initiation in our samples, and therefore we interpret the intercept as the expected frequency of use at the average age of initiation in this sample. The latent slope is invariant to centering, and we take it here to represent average increase in use across adolescence. Participants’ exact ages at assessment were used to allow for individually-varying factor loadings from the latent slope to each observed variable. Both the latent intercept and slope were regressed on sex. The latent intercept and slope were allowed to correlate with each other and with the latent externalizing psychopathology factor in order to examine the relationship between risk and marijuana use trajectory. Residual correlations between alcohol dependence and marijuana indicators were freely estimated to avoid inflation in the latent correlations between externalizing and the growth model due to substance use-specific effects. Missing data were accommodated with full information maximum likelihood.
We used a standard biometric variance decomposition within the latent growth model to estimate the genetic (A), shared environmental (C), and unique environmental (E) variance components of the latent intercept and slope as well as the specific factors extracted from the marijuana variables. Global fit indices are not available when evaluating models with individually-varying time metrics (Sterba 2014; Grimm et al. 2016), therefore, to evaluate relative fit the latent growth model was compared to an intercept-only model using a likelihood ratio test, AIC, and BIC. We conducted tests of equivalence between samples of the mean and variance of the slope and intercept, as well as correlations between all latent factors.
In an additional analysis, we modeled decline in marijuana use in the Minnesota sample. We consider this analysis somewhat exploratory because it could not be replicated in the Colorado sample, which did not have sufficient numbers of assessment waves to support non-linear growth. To model decline in use we employed a piecewise model with biometric variance decomposition, in which the intercept and ascending slope were modeled from 14–24 as described above, and the decline in use was modeled from 25 through early 30s.The descending slope was free to correlate with the externalizing factor, intercept, and adolescent slope. We evaluated relative fit by comparing the piecewise latent growth model to intercept-only and linear models using log-likelihood, AIC, and BIC.
Results
Table 2 contains descriptive statistics for the externalizing psychopathology indicators, including average age at assessment, sample size, and mean and standard deviation of all symptom counts. Results of mean comparisons are also presented in Table 2. Symptom counts did not differ significantly between samples for ADHD in males and females or for conduct disorder in males. All other comparisons were significantly different between samples, with rates being higher in CO. When comparing males to females within each sample, males scored higher than females on all externalizing indicators.
Table 2:
Sample Size and Age Descriptives by Measure, Measure Descriptives, and Mean Comparisons
| Measure | Sex | Sample | Mean age at assessment (SD) | Sample Size | Mean Symptom Count (SD) [residualized SD] | Test of between sample differences χ2 (p) | Test of between gender differences χ2 (p) | ||
|---|---|---|---|---|---|---|---|---|---|
| Total | MZ | DZ | |||||||
| ADHD | M | MN | 13.6 (2.7) | 1730 | 1118 | 612 | 1.39 (1.98) [0.67] | 2.3 (.13) | 57.2 (< .001) |
| CO | 14.9 (2.2) | 1167 | 594 | 573 | 1.25 (2.38) [0.71] | 10.7 (.001) | |||
| F | MN | 13.8 (2.8) | 1867 | 1185 | 682 | 0.87 (1.59) [0.58] | 0.5 (.48) | ||
| CO | 15.0 (2.2) | 1310 | 723 | 587 | 0.94 (1.99) [0.63] | ||||
| Alcohol Dependence | M | MN | 17.5 (0.6) | 1628 | 1042 | 586 | 0.65 (1.44) [0.54] | 6.3 (.01) | 42.6 (< .001) |
| CO | 18.0 (1.6) | 1012 | 521 | 491 | 0.83 (1.53) [0.56] | 10.6 (.001) | |||
| F | MN | 17.6 (0.7) | 1771 | 1137 | 634 | 0.31 (1.05) [0.40] | 28.4 (< .001) | ||
| CO | 18.1 (1.7) | 1175 | 649 | 526 | 0.60 (1.30) [0.52] | ||||
| Conduct Disorder | M | MN | 17.5 (0.6) | 1165 | 776 | 389 | 1.12 (1.58) [0.61] | 1.4 (.24) | 180.4 (< .001) |
| CO | 18.1 (1.5) | 1012 | 522 | 490 | 1.21 (1.43) [0.57] | 74.3 (.001) | |||
| F | MN | 17.6 (0.7) | 1308 | 842 | 466 | 0.27 (0.73) [0.36] | 74.7 (< .001) | ||
| CO | 18.3 (1.6) | 1177 | 650 | 527 | 0.63 (0.96) [0.47] | ||||
Note: Descriptives are split by measure and sex, including age, number of individuals, and symptom counts (raw mean and standard deviation and residualized standard deviation). Sample size columns refer to number of individuals. ADHD=Attention Deficit Hyperactivity Disorder; M=Male, F=Female; MZ=Monozygotic; DZ=Dizygotic; MN=Minnesota; CO=Colorado; SD=Standard Deviation. Residualized SD refers to the standard deviation of the residuals which were analyzed in the externalizing psychopathology factor model, symptom counts were regressed on age, age2, age × cohort, cohort, and sex. Test of between state differences refers to testing equivalence of symptom count means in Colorado and Minnesota within each gender. Test of between gender differences refers to testing equivalence of symptom count means in males and females within each state.
Table 3 presents the cross-twin cross-trait correlation matrix of the residualized symptom counts in Minnesota and Colorado. In every case, the MZ cross-twin within trait correlations are larger than the corresponding DZ correlations, which suggests genetic influence on these phenotypes. Results from the regressions used to residualize the symptom counts are provided in Supplemental Table 4. Heritability was .34 (95% CI=[.19, .41]) in Colorado and .35 [.27, .40] in Minnesota for ADHD, .29 [.13, .48] in Colorado and .56 [.48, .60] in Minnesota for alcohol dependence, .30 [0, .51] in Colorado and .15 [0, .35] in Minnesota for conduct disorder.
Table 3:
Cross-Twin Cross-Trait Correlation Matrix
| ADHDA | Alcohol Dep.A | Conduct DisorderA | |
|---|---|---|---|
| Minnesota MZ | |||
| ADHDB | .35* [.29, .40] | .15* [.09, .21] | .18* [.12, .25] |
| Alcohol Dep.B | .17* [.11, .23] | .53* [.49, .57] | .28* [.22, .35] |
| Conduct DisorderB | .21* [.14, .27] | .31* [.25, .37] | .36* [.30, .42] |
| Minnesota DZ | |||
| ADHDB | .12* [.05, .20] | .16* [.08, .24] | .19* [.10, .28] |
| Alcohol Dep.B | .06 [−.02, .14] | .26* [.19, .34] | .15* [.06, .24] |
| Conduct DisorderB | .10* [.01, .20] | .22* [.13, .31] | .33* [.24, .41] |
| Colorado MZ | |||
| ADHDB | .35* [.28, .42] | .14* [.06, .22] | .23* [.15, .31] |
| Alcohol Dep.B | .18* [.10, .26] | .53* [.47, .59] | .30* [23, .38] |
| Conduct DisorderB | .25* [.17, .32] | .24* [.16, .32] | .48* [.41, .54] |
| Colorado DZ | |||
| ADHDB | .14* [.05, .23] | .07 [−.03, .16] | .06 [−.04, .16] |
| Alcohol Dep.B | .11* [.01, .21] | .38* [.29, .46] | .27* [.17, .36] |
| Conduct DisorderB | .17* [.07, .26] | .21* [.11, .30] | .32* [.23, .41] |
Note: Cross-twin within-trait correlations are bolded on the diagonal. Subscript of A refers to twin A and subscript of B refers to twin B within a twin pair. All abbreviations same as in Table 1.
p<.05, values in square brackets represent the 95% confidence interval.
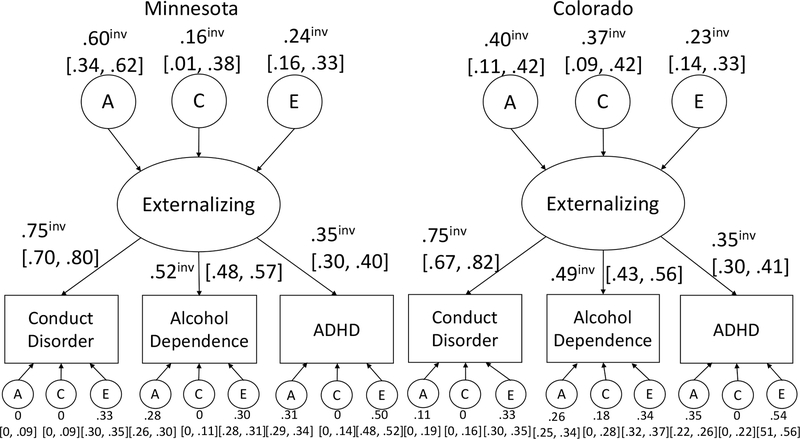
Figure 1 shows the parameter estimates for the base common factor model in each sample. The model fit well as indexed by RMSEA, which may be a more appropriate index than chi-square as chi-square is very sensitive to sample size (χ2=131.1, df=74, p=4.8×10−5; RMSEA=.015). Standardized loadings of the latent phenotype on each measure are similar between samples and the pattern of loadings is the same.
Figure 1.
Loadings and biometric estimates for the common factor models fit to the Minnesota and Colorado behavioral data. All freely estimated parameters are shown. Those that could be set as invariant are denoted with a superscript “inv”. Numbers in brackets represent 95% confidence intervals around the point estimates.
Measurement invariance results are presented in Table 4; all models presented are compared to the unconstrained two-group model. Only strict invariance was not met (χ2=60.4, df=12, p=1.9×10−8). Factor loadings (standardized and unstandardized) and variance decomposition of the latent factor (biometric) are invariant across samples.
Table 4:
Results of Measurement Invariance Tests with Externalizing Psychopathology Model
| Model | −2LL | df | est. par. | AIC | BIC | χ2 | LRT | diff df | p |
|---|---|---|---|---|---|---|---|---|---|
| Base | 24,504.2 | 16,288 | 36 | −8,071.8 | −107,242.3 | 131.1 | - | - | - |
| Weak | 25,505.1 | 16,291 | 36 | −8,076.9 | −107,265.7 | 132.0 | 0.89 | 3 | .83 |
| Strong | 24,506.3 | 16,291 | 33 | −8,075.7 | −107.264.5 | 133.2 | 2.03 | 3 | .57 |
| Biometric | 24,505.8 | 16,291 | 33 | −8.076.2 | −107,264.9 | 132.8 | 1.61 | 3 | .66 |
| Biometric and Strong | 24,507.7 | 16,294 | 30 | −8080.3 | −107,287.3 | 134.7 | 3.51 | 6 | .74 |
| Strict | 24,564.6 | 16,300 | 24 | −8.035.4 | −107,278.9 | 191.6 | 60.40 | 12 | 1.9 × 10−8 |
Note: Base model is the single factor model. Biometric invariance constrains the factor variance components to be equivalent between states. −2LL = log likelihood; df = degrees of freedom for model where the number of estimated parameters is subtracted from the total number of observations (N individuals multiplied by n non-missing symptom counts); est. par. = Estimated Parameters; AIC = Akaike Information Criterion; BIC = Bayesian Information Criterion; LRT = Likelihood Ratio Test, diff df= degrees of freedom for LRT. Best fitting model (by AIC and BIC) is bolded.
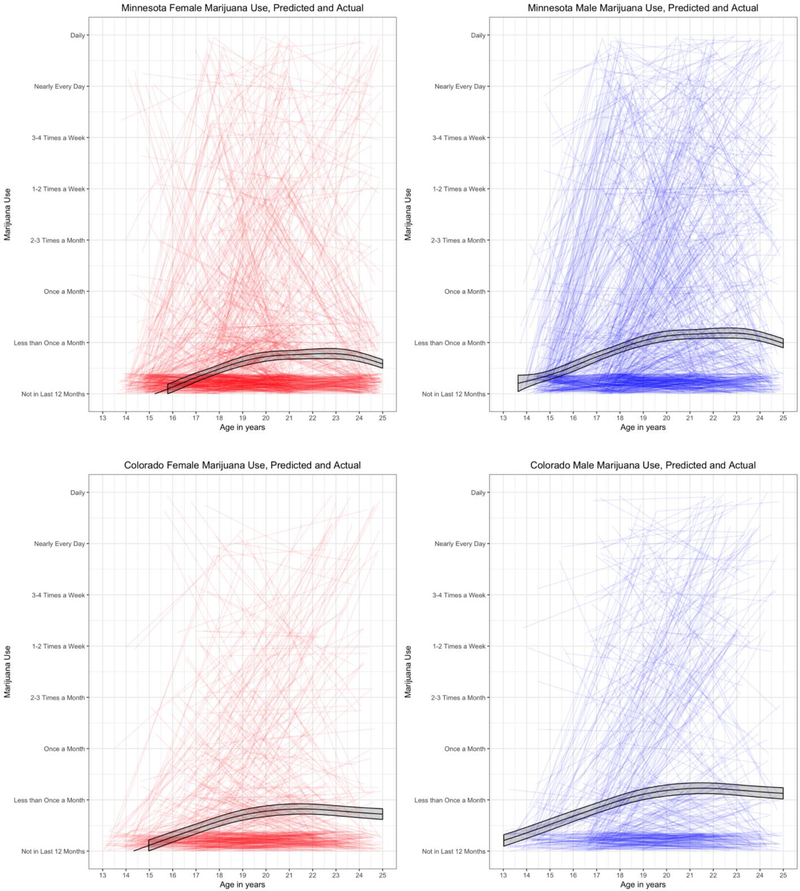
Figure 2 depicts the raw individual trajectories in each state as well as the generalized additive mixed model-predicted mean trajectory (with 95% confidence interval), split by gender. We found the linear growth model adequately described change in marijuana use frequency from age ~14 to 24 as it fit better than the alternative intercept-only model (difference in AIC=1476.7; difference in BIC=1787.2; difference in log-likelihood=1,374.7 [df=22]).
Figure 2.
Raw change in marijuana use (substantially jittered to prevent overplotting), split by sex (red/left is female and blue/right is male) and by state. Black lines are predicted mean marijuana use over time predicted via a generalized additive mixture model in each state. The shaded ribbon represents the 95% confidence interval around the predicted mean trajectory. Data from the Minnesota sample is truncated at age 25 in order to provide a more direct comparison to the Colorado sample.
The latent intercept mean in Minnesota was .63 (95% CI=[.51, .75]) and in Colorado it was .83 [.65, 1.01]; this represents the expected marijuana use at age 16.5, which corresponds approximately to less than once a month on the ordinal frequency scale. The variance of the intercept was .45 [.40, .50] in Minnesota and .41 [.33, .49] in Colorado. The latent slope mean was .20 [.17, .24] in Minnesota and .23 [.19, .28] in Colorado. The variance of the slope was .032 [.029, .036] in Minnesota and .02 [.013, .025] in Colorado.
The mean and variance of the intercept was not significantly different between samples (Mean χ2=3.3, df=1, p=.06; variance χ2=1.01, df=1, p=.60). The mean of the latent slope was also not significantly different (χ2=1.1, df=1, p=.29), but the variance of the slope in the Minnesota sample was larger than the Colorado sample (χ2=12.7, df=1, p=.0003). Table 5 contains the biometric variance decomposition estimates for each latent factor as well as the corresponding significance test evaluating if each estimate is significantly different from 0. One difference between samples was the biometric decomposition of the slope; change in marijuana use was more heritable in Minnesota (χ2=6.9, df=1, p=.008), and the shared environmental component of the slope was larger in Colorado (χ2=8.0, df=1, p=.004), at nominal levels of significance.
Table 5:
Variance Decomposition of Externalizing Psychopathology and the Growth Model
| Parameter | Sample | Proportion of variance | [95% CI] | LRT | p |
|---|---|---|---|---|---|
| Externalizing A2 | MN | .54 | [.30, .74] | 28.8 | 8.1× 10−8 |
| CO | .45 | [.17, .76] | 15.1 | 1.0× 10−4 | |
| Externalizing C2 | MN | .20 | [.04, .41] | 5.1 | .02 |
| CO | .33 | [.04, .57] | 6.7 | .01 | |
| Externalizing E2 | MN | .26 | [.17, .34] | 42.4 | 7.3× 10−11 |
| CO | .23 | [.14, .32] | 30.3 | 3.6× 10−8 | |
| Intercept A2 | MN | .57 | [.37, .79] | 66.3 | 3.8× 10−16 |
| CO | .42 | [.14, .73] | 14.4 | 1.0× 10−4 | |
| Intercept C2 | MN | .31 | [.09, .48] | 10.1 | .001 |
| CO | .47 | [.17, .73] | 12.1 | 5.0× 10−4 | |
| Intercept E2 | MN | .13 | [.08, .19] | 90.1 | 2.3× 10−21 |
| CO | .11 | [.04, .21] | 12.8 | 3.0 × 10−4 | |
| Slope A2 | MN | .82 | [.72, .88] | 97.5 | 5.3× 10−23 |
| CO | .22 | [.01, .66] | 3.9 | .049 | |
| Slope C2 | MN | 0 | [0, .06] | .003 | .96 |
| CO | .55 | [.14, .83] | 8.9 | .003 | |
| Slope E2 | MN | .18 | [.11, .25] | 59.7 | 1.1× 10−14 |
| CO | .23 | [.07, .45] | 10.5 | .001 |
Note: Parameter significance was obtained by estimating a base model in which all parameters were free to vary, then setting each parameter in turn to 0 and comparing the constrained model to the base model. LRT = Likelihood Ratio Test. All tests were 1 degree of freedom tests. 95% CI refers to the 95% confidence interval around the estimate.
All three latent factors were free to correlate, and we expected a correlation between externalizing psychopathology and the growth factors, as previous literature has established a relationship between externalizing psychopathology and substance use. Phenotypic correlations are presented in Table 6. We found externalizing was strongly correlated with use at age ~16 and weakly correlated with increase in use over time, meaning that individuals higher on adolescent externalizing tend to use more marijuana at age 16.5 and increase their use at a faster rate. The phenotypic correlations were not significantly different across samples (externalizing-intercept χ2=.12, df=1, p=.73; externalizing-slope χ2=.10, df=1, p=.74). Use at age ~16 and increase in use were also strongly correlated, meaning that individuals who use more at 16.5 tend to increase at faster rates. The magnitude of this correlation was larger in Colorado than Minnesota (χ2=8.46, df=1, p=.003).
Table 6:
Correlations between Marijuana Use Frequency Intercept, Slope, and Externalizing Psychopathology
| Ext-Intercept (p) | Ext- Slope (p) | Intercept-Slope (p) | ||
|---|---|---|---|---|
| Phenotypic | MN | .67 (1.5× 10−66) | .18 (2.3× 10−5) | .57 (7.5× 10−33) |
| CO | .69 (5.2× 10−33) | .20 (.01) | .84 (2.4× 10−15) | |
| Genetic | MN | .64 (1.4× 10−5) | .26 (.12) | .63 (1.9× 10−7) |
| CO | .86 (6.8× 10−4) | .49 (.24) | .86 (.049) | |
| Shared | MN | 1 (.003) | -- | -- |
| Environment | CO | .69 (.018) | .22 (.46) | .86 (.003) |
| Unique | MN | .37 (.02) | .03 (.83) | .94 (2.7× 10−7) |
| Environment | CO | .31 (.12) | −.20 (.31) | .87 (.004) |
Note: Ext=Externalizing psychopathology. In Minnesota, the C component of the slope was estimated to be 0, so those correlations are not estimated. Note that genetic and environmental correlations are scaled according to the genetic and environmental variances.
We also decomposed the phenotypic correlations into genetic, shared environmental, and unique environmental components, see Table 6. We found significant genetic correlations between adolescent externalizing and the intercept in both samples, indicating shared genetic liability between externalizing psychopathology and marijuana use at 16.5. We also found a significant genetic correlation between the intercept and slope in Minnesota, indicating shared genetic liability to use at 16.5 and escalation of use.
In the Minnesota sample we evaluated a piecewise model of decline in marijuana use frequency in the participants’ late 20s. The piecewise decline model fit better than the alternative intercept-only and linear models (see Table 7). The latent intercept and first slope were highly similar to the results from the linear growth model. The mean of the second slope (representing decline in use in the late 20s) was −.15 [−.19, −.11] with variance .018 [.013, .024]. This indicates that on average, people decrease their marijuana use after age 25 into their late twenties and early thirties.
Table 7:
Relative Fit Comparisons of Piecewise Model to Two Alternative Models
| Model | AIC | BIC | Log-Likelihood |
|---|---|---|---|
| Piecewise | 9,746.1 | −115,119.5 | 54,985.1 |
| Intercept Only | 11,980.0 | −112,924.2 | 57,368.0 |
| Linear | 10,569.8 | −114,273.9 | 55,935.8 |
Note: Smaller values of AIC and BIC indicate better relative fit.
Heritability of the descending slope was .73 [.49, .91]. The shared environment accounted for 10% of the variance, though the confidence interval includes 0 [0, .31]. The nonshared environment accounted for 16% of the variance in decline in use [.05, .33]. The correlation between externalizing psychopathology and the second slope was −.41 [−.54, −.28]; higher externalizing psychopathology is associated with smaller decreases in use in early adulthood. The correlation between the intercept and the second slope was −.66 [−.88, −.53] meaning greater use at age 16.5 is associated with smaller decreases in use in early adulthood. The correlation between the first and second slopes was −.84 [−.92, −.72] meaning that greater increase in use across adolescence is associated with less decrease across young adulthood.
Discussion
The present study evaluated measurement invariance in adolescent externalizing psychopathology as expressed by the covariation of ADHD, conduct disorder, and alcohol dependence symptoms and its prospective relationship with marijuana use development across adolescence through young adulthood. These relationships were evaluated in two large, independent longitudinal twin studies spanning ages 11 to 30, with up to 5 waves of assessment. Our results indicate that adolescent externalizing can largely be similarly measured across independent samples, allowing us to compare associations with marijuana use in both samples. Overall, there were few differences between the two samples, whether in externalizing, change in marijuana use, or in the heritable or environmental influences on either.
In agreement with previous research, early marijuana use was heritable; heritability of predicted use at age 16 was .57 in Minnesota and .42 in Colorado. Shared environment contributions were .31 and .47 in Minnesota and Colorado, respectively, reflecting a complex etiology consisting of genetic and environmental effects on adolescent marijuana use consistent with previous estimates (Kendler et al. 2008). Change in marijuana use was also heritable and use increased in an approximately linear fashion between ages 14 and 24. Moreover, using additional waves of assessment available in the Minnesota sample indicated that marijuana use decreased during late 20s and this change was also highly heritable.
Early marijuana use is strongly correlated (~.7) with adolescent externalizing psychopathology, consistent with the expectation that early drug use is a strong indicator of externalizing behavior. The relationship was primarily attributable to shared genetic influences, reflected in a high genetic correlation. However, shared environment also contributed to the correlation between adolescent externalizing psychopathology and use at age 16.5. In a more novel result, increases in use were moderately positively correlated (~.3) with adolescent externalizing in both samples. In the Minnesota sample, the young-adult decline was moderately inversely correlated with adolescent externalizing psychopathology and strongly negatively correlated with average marijuana use at age 16.5 and increases in marijuana use from 14–24. The results suggest that change in marijuana use may be considered a weak to moderate indicator of externalizing psychopathology, of a magnitude similar to diagnostic symptom counts of ADHD, alcohol dependence, and conduct disorder.
One must take care in interpreting a latent intercept (here interpreted as early marijuana use) and its correlation with other latent factors (here as externalizing psychopathology) as the mean and variance of the intercept depend on its centering. Furthermore, the relationships between the intercept, externalizing, and the change in marijuana use depends on the centering of the intercept. As the centering of the intercept changes, the variance of the intercept changes accordingly, which impacts its covariance with other factors as well as its biometric decomposition. We chose the center-point based on previous research around age of initiation, but other center-points may be valid to examine as well, and choosing a different intercept would alter the results and their interpretation.
There was little difference between samples in the factor model of adolescent externalizing psychopathology, marijuana use at age 16, and increases in use from age 14 to 24. The biometric decomposition of change in use differed between samples; in Minnesota the heritability of this increase was .82 but in Colorado it was .22. Most of the variability in change in Colorado was due to the shared environment (C=.55) whereas in Minnesota the shared environment contributed very little (C=0). This was one of the few differences between the two samples, and further research will be required to explore the factors that may contribute to this difference.
These results regarding the low heritability and large shared environmental influence in adolescent increase in marijuana use, however, are not consistent with previous research. If anything, we would expect the environment surrounding marijuana use in Colorado to be more permissive than in Minnesota. Previous research indicates that behavior in more permissive environments typically show higher heritability, rather than attenuated heritability, which suggests alternative explanations besides the legal landscape such as cohort effects or within-family risk (Legrand et al. 2008; Mezquita et al. 2018). One simple explanation for our finding of lower heritability in the Colorado dataset is that the result is due to sampling error. The difference is statistically significant at nominal levels, but not after correcting for the large number of tests reported in the present article. Further work should replicate these sample differences before conclusions can be drawn.
A major strength of the present study is the replication/invariance strategy. Our sample sizes (>2000 in each sample at each wave) were powered to detect even relatively small differences, yet we show that the vast majority of effects are highly similar across the two samples, with the two exceptions described above. However, the use of two samples did introduce some limitations. Measures needed to be available in both samples, limiting the full repertoire of measures available. Future work will examine alternative conceptualizations of marijuana use, such as transitions between initiation, regular use, and problematic use, as previous research suggests there are shared and distinct genetic influences on each (Gillespie et al. 2009; Verweij et al. 2010). Furthermore, differences in waves of assessment informed and limited our model selection for sample comparisons. The Colorado sample had only been assessed three times, therefore we were unable to model non-linear growth in this sample.
On the note of model selection, one limitation is that we did not consider any of the many alternative models of developmental change. Some model choices, such as the limited representation of non-linear models, were dictated by available data. Others, such as the lack of comparison to alternative conceptualizations like mixture models or autoregression, were limited by our focus on developing and testing invariance of the externalizing psychopathology model. Another analytical limitation was the application of a log-normal likelihood to symptom counts and marijuana use frequency indicators that themselves are not normally distributed. Based on prior work, we log-transformed residualized symptom counts in an attempt to mitigate this issue, but one future direction would be to evaluate alternative models of non-normal manifest variables.
An additional limitation is the lack of longitudinal representation of externalizing psychopathology. Our indicators were intended to capture externalizing psychopathology in adolescence and its relationships with concurrent substance use and change in substance use into adulthood. While we can characterize the etiological nature of these relationships (i.e., by decomposing into genetic and environmental components), we are unable here to draw causal conclusions about the relationships between adolescent externalizing psychopathology, early marijuana use, and change in use over time. Using alternative ages of assessment, or perhaps a longitudinal representation of externalizing across the lifespan, combined with a more causally informative design (e.g., co-twin control), will help to address this issue. Lastly, since many constructs exist under the umbrella term of externalizing, our definition of externalizing psychopathology is only one of several possible (Derringer et al. 2015), and alternative definitions are again limited in that any items would need to be available in both samples.
Conclusions
Adolescent externalizing psychopathology can be robustly and replicably represented as a confirmatory factor model. Externalizing is related to model-predicted marijuana use at age ~16.5 and increase in use across adolescence and early adulthood. Exploratory analyses indicate that adolescent externalizing is also inversely related to the decline in marijuana use in adulthood, in that individuals who exhibit more externalizing symptoms taper their use at a slower rate in adulthood. Additive-genetic effects explain much of the correlation between adolescent externalizing and use at initiation. Sample differences in the biometric decomposition of the change in marijuana use highlight the importance of environmental context to heritability estimates.
Supplementary Material
Acknowledgements
This work was supported by NIH grants DA042755, DA046413, AA009367, MH066140, DA005147, DA013240, DA036216, AA023974, DA037904, DA032555, DA035804, DA011015, DA012845, and DA038065.
Footnotes
The authors declare no competing interests or financial interests.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Boker S, Neale M, Maes H, et al. (2011) OpenMx: An Open Source Extended Structural Equation Modeling Framework. Psychometrika 76:306–317. doi: 10.1007/s11336-010-9200-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z, Lichtenstein P, Larsson H (2012) The effects of childhood ADHD symptoms on early-onset substance use: A Swedish twin study. J Abnorm Child Psychol. doi: 10.1007/s10802-011-9575-6 [DOI] [PubMed] [Google Scholar]
- Colder CR, Frndak S, Lengua LJ, et al. (2018) Internalizing and Externalizing Problem Behavior: a Test of a Latent Variable Interaction Predicting a Two-Part Growth Model of Adolescent Substance Use. J Abnorm Child Psychol 46:319–330. doi: 10.1007/s10802-017-0277-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottler LB (2000) Composite International Diagnostic Interview Substance Abuse Module [Google Scholar]
- Derringer J, Corley RP, Haberstick BC, et al. (2015) Genome-Wide Association Study of Behavioral Disinhibition in a Selected Adolescent Sample. Behav Genet 45:375–381. doi: 10.1007/s10519-015-9705-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Wang JC, et al. (2008) Using dimensional models of externalizing psychopathology to aid in gene identification. Arch Gen Psychiatry. doi: 10.1001/archpsyc.65.3.310 [DOI] [PubMed] [Google Scholar]
- Dick DM, Viken RJ, Kaprio J, et al. (2005) Understanding the covariation among childhood externalizing symptoms: Genetic and environmental influences on conduct disorder, attention deficit hyperactivity Ddsorder, and oppositional defiant disorder symptoms. J Abnorm Child Psychol. doi: 10.1007/s10802-005-1829-8 [DOI] [PubMed] [Google Scholar]
- Elkins IJ, Saunders GRB, Malone SM, et al. (2018) Associations between childhood ADHD, gender, and adolescent alcohol and marijuana involvement: A causally informative design. Drug Alcohol Depend 184:33–41. doi: 10.1016/j.drugalcdep.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Ridder EM (2007) Conduct and attentional problems in childhood and adolescence and later substance use, abuse and dependence: Results of a 25-year longitudinal study. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2006.12.011 [DOI] [PubMed] [Google Scholar]
- Fowler T, Lifford K, Shelton K, et al. (2007) Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. doi: 10.1111/j.1360-0443.2006.01694.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Neale MC, Kendler KS (2009) Pathways to cannabis abuse: A multi-stage model from cannabis availability, cannabis initiation and progression to abuse. Addiction 104:430–438. doi: 10.1111/j.1360-0443.2008.02456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JD, Lynskey MT, Madden PAF, et al. (2015) The role of conduct disorder in the relationship between alcohol, nicotine and cannabis use disorders. Psychol Med 45:3505–3515. doi: 10.1017/S0033291715001518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm KJ, Ram N, Estabrook R (2016) Growth Modeling: Structural Equation and Multilevel Modeling Approaches. Guilford Press [Google Scholar]
- Han C, McGue MK, Iacono WG (1999) Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: Univariate and multivariate behavioral genetic analyses. Addiction. doi: 10.1046/j.1360-0443.1999.9479814.x [DOI] [PubMed] [Google Scholar]
- Hicks BM, Iacono WG, McGue M (2010) Consequences of an adolescent onset and persistent course of alcohol dependence in men: Adolescent risk factors and adult outcomes. Alcohol Clin Exp Res 34:819–833. doi: 10.1111/j.1530-0277.2010.01154.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Krueger RF, Iacono WG, et al. (2004) Family transmission and heritability of externalizing disorders: A Twin-Family Study. Arch Gen Psychiatry 61:922–928. doi: 10.1001/archpsyc.61.9.922 [DOI] [PubMed] [Google Scholar]
- Hicks BM, Schalet BD, Malone SM, et al. (2011) Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behav Genet 41:459–475. doi: 10.1007/s10519-010-9417-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines LA, Morley KI, Rijsdijk F, et al. (2018) Overlap of heritable influences between cannabis use disorder, frequency of use and opportunity to use cannabis: Trivariate twin modelling and implications for genetic design. Psychol Med 48:2786–2793. doi: 10.1017/S0033291718000478 [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM (1999) Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Model 6:1–55. doi: 10.1080/10705519909540118 [DOI] [Google Scholar]
- Huizink AC, Levälahti E, Korhonen T, et al. (2010) Tobacco, cannabis, and other illicit drug use among finnish adolescent twins: Causal relationship or correlated liabilities? J Stud Alcohol Drugs. doi: 10.15288/jsad.2010.71.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, et al. (1999) Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota twin family study. Dev Psychopathol 11:869–900. doi: 10.1017/S0954579499002369 [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M (2008) Behavioral Disinhibition and the Development of Early-Onset Addiction: Common and Specific Influences. Annu Rev Clin Psychol 4:325–348. doi: 10.1146/annurev.clinpsy.4.022007.141157 [DOI] [PubMed] [Google Scholar]
- Iacono WG, McGue M (2002) Minnesota twin family study. Twin Res 5:482–487. doi: 10.1375/136905202320906327 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC (2003a) Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry 160:687–695. doi: 10.1176/appi.ajp.160.4.687 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC (2003b) The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry 60:929–937. doi: 10.1001/archpsyc.60.9.929 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA (2008) Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry 65:674–682. doi: 10.1001/archpsyc.65.6.674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes MA, Malone SM, Elkins IJ, et al. (2009) The Enrichment Study of the Minnesota Twin Family Study: Increasing the Yield of Twin Families at High Risk for Externalizing Psychopathology. Twin Res Hum Genet 12:489–501. doi: 10.1375/twin.12.5.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King SM, Iacono WG, McGue M (2004) Childhood externalizing and internalizing psychopathology in the prediction of early substance use. Addiction 99:1548–1559. doi: 10.1111/j.1360-0443.2004.00893.x [DOI] [PubMed] [Google Scholar]
- Kirkpatrick RM, Neale MC (2016) Applying Multivariate Discrete Distributions to Genetically Informative Count Data. Behav Genet. doi: 10.1007/s10519-015-9757-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T, Latvala A, Dick DM, et al. (2012) Genetic and environmental influences underlying externalizing behaviors, cigarette smoking and illicit drug use across adolescence. Behav Genet. doi: 10.1007/s10519-012-9528-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, et al. (2002) Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. J Abnorm Psychol 111:411–424. doi: 10.1037/0021-843X.111.3.411 [DOI] [PubMed] [Google Scholar]
- Legrand LN, Keyes M, McGue M, et al. (2008) Rural environments reduce the genetic influence on adolescent substance use and rule-breaking behavior. Psychol Med 38:1341–1350. doi: 10.1017/S0033291707001596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Zhang D (1999) Inference in generalized additive mixed models by using smoothing splines. J R Stat Soc Ser B Stat Methodol 61:381–400. doi: 10.1111/1467-9868.00183 [DOI] [Google Scholar]
- Loeber R, Keenan K (1994) Interaction between conduct disorder and its comorbid conditions: Effects of age and gender. Clin Psychol Rev. doi: 10.1016/0272-7358(94)90015-9 [DOI] [Google Scholar]
- Martin NG, Eaves LJ (1977) The genetical analysis of covariance structure. Heredity (Edinb) 38:79–95. doi: 10.1038/hdy.1977.9 [DOI] [PubMed] [Google Scholar]
- McGue M, Irons D, Iacono WG (2014) The adolescent origins of substance use disorders: A behavioral genetic perspective. Nebraska Symp Motiv 61:31–50. doi: 10.1007/978-1-4939-0653-6_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith W (1993) Measurement invariance, factor analysis and factorial invariance. Psychometrika 58:525–543. doi: 10.1007/BF02294825 [DOI] [Google Scholar]
- Mezquita L, Sánchez-Romera JF, Ibáñez MI, et al. (2018) Effects of Social Attitude Change on Smoking Heritability. Behav Genet. doi: 10.1007/s10519-017-9871-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Basu S, Cunningham J, et al. (2012) The Minnesota Center for Twin and Family Research Genome-Wide Association Study. Twin Res Hum Genet 15:767–774. doi: 10.1017/thg.2012.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE, Gnagy EM, et al. (2007) Attention-deficit/hyperactivity disorder risk for heavy drinking and alcohol use disorder is age specific. Alcohol Clin Exp Res. doi: 10.1111/j.1530-0277.2007.00349.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadder TS, Rutter M, Silberg JL, et al. (2002) Genetic effects on the variation and covariation of attention deficit-hyperactivity disorder (ADHD) and oppositional-defiant disorder/conduct disorder (ODD/CD) symptomatologies across informant and occasion of measurement. Psychol Med. doi: 10.1017/s0033291701004792 [DOI] [PubMed] [Google Scholar]
- Neale MC, Hunter MD, Pritikin JN, et al. (2016) OpenMx 2.0: Extended Structural Equation and Statistical Modeling. Psychometrika 81:535–549. doi: 10.1007/s11336-014-9435-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RHC, Knopik VS, Rhee SH, et al. (2013) Prospective effects of adolescent indicators of behavioral disinhibition on DSM-IV alcohol, tobacco, and illicit drug dependence in young adulthood. Addict Behav 38:2415–2421. doi: 10.1016/j.addbeh.2013.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer RHC, Young SE, Stallings MC, et al. (2011) Genetics of the associations between adolescent indicators of behavioral disinhibition and young adult measures of alcohol, Tobacco and other substance use disorders. Behav. Genet. [Google Scholar]
- Prisciandaro JJ, Korte JE, McRae-Clark AL, Brady KT (2012) Associations between behavioral disinhibition and cocaine use history in individuals with cocaine dependence. Addict Behav 37:1185–1188. doi: 10.1016/j.addbeh.2012.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W (1997) Diagnostic Interview for Children and Adolescents - IV. J Am Acad Child Adolesc Psychiatry 39:59–66 [DOI] [PubMed] [Google Scholar]
- Rhea SA, Gross AA, Haberstick BC, Corley RP (2006) Colorado Twin Registry. Twin Res Hum Genet 9:941–949. doi: 10.1375/183242706779462895 [DOI] [PubMed] [Google Scholar]
- Rhea SA, Gross AA, Haberstick BC, Corley RP (2013) Colorado twin registry: An update. Twin Res Hum Genet 16:351–357. doi: 10.1017/thg.2012.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond-Rakerd LS, Slutske WS, Wood PK (2017) Age of initiation and substance use progression: A multivariate latent growth analysis. Psychol Addict Behav 31:664–675. doi: 10.1037/adb0000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Buchholz KK, et al. (2000) Diagnostic Interview Schedule for DSM-IV (DIS-IV). Washington University School of Medicine, St. Louis, MO [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, et al. (2000) NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC- IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry 39:28–38. doi: 10.1097/00004583-200001000-00014 [DOI] [PubMed] [Google Scholar]
- Shelton K, Lifford K, Fowler T, et al. (2007) The association between conduct problems and the initiation and progression of marijuana use during adolescence: A genetic analysis across time. Behav. Genet [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Molina BSG, et al. (2014) The role of early childhood ADHD and subsequent CD in the initiation and escalation of adolescent cigarette, alcohol, and marijuana use. J Abnorm Psychol 123:362–374. doi: 10.1037/a0036585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa NO, Grevet EH, Salgado CAI, et al. (2011) Smoking and ADHD: An evaluation of self medication and behavioral disinhibition models based on comorbidity and personality patterns. J Psychiatr Res 45:829–834. doi: 10.1016/j.jpsychires.2010.10.012 [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M (1987) Structured Clinical Interview for DSM-IV (SCID). New York State Psychiatric Institute Biometrics Research, New York, NY [Google Scholar]
- Sterba SK (2014) Fitting Nonlinear Latent Growth Curve Models With Individually Varying Time Points. Struct Equ Model 21:630–647. doi: 10.1080/10705511.2014.919828 [DOI] [Google Scholar]
- Storr CL, Pacek LR, Martins SS (2012) Substance Use Disorders and Adolescent Psychopathology. Public Health Rev 34:. doi: 10.1007/bf03391678 [DOI] [Google Scholar]
- Tarter R, Vanyukov M, Giancola P, et al. (1999) Etiology of early age onset substance use disorder: A maturational perspective. Dev. Psychopathol [DOI] [PubMed] [Google Scholar]
- Thompson KD, Leadbeater BJ, Ames ME (2015) Reciprocal effects of internalizing and oppositional defiance symptoms on heavy drinking and alcohol-related harms in young adulthood. Subst Abus Res Treat. doi: 10.4137/SART.S33928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielbeek JJ, Vink JM, Polderman TJC, et al. (2018) Genetic correlation of antisocial behaviour with alcohol, nicotine, and cannabis use. Drug Alcohol Depend 187:296–299. doi: 10.1016/j.drugalcdep.2018.03.020 [DOI] [PubMed] [Google Scholar]
- Tully E, Iacono WG, Tully EC (2014) An Integrative Common Liabilities Model for the Comorbidity of Substance Use Disorders with Externalizing and Internalizing Disorders
- Tuvblad C, Zheng M, Raine A, Baker LA (2009) A common genetic factor explains the covariation among ADHD ODD and CD symptoms in 9–10 year old boys and girls. J Abnorm Child Psychol. doi: 10.1007/s10802-008-9278-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanyukov MM, Tarter RE, Kirillova GP, et al. (2012) Common liability to addiction and “gateway hypothesis”: Theoretical, empirical and evolutionary perspective. Drug Alcohol Depend 123:. doi: 10.1016/j.drugalcdep.2011.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij KJH, Zietsch BP, Lynskey MT, et al. (2010) Genetic and environmental influences on cannabis use initiation and problematic use: A meta-analysis of twin studies. Addiction 105:417–430. doi: 10.1111/j.1360-0443.2009.02831.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI (2012) Model selection and psychological theory: A discussion of the differences between the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Psychol Methods 17:228–243. doi: 10.1037/a0027127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, Hicks BM, Iacono WG, McGue M (2012) Decline in genetic influence on the co-occurrence of alcohol, marijuana, and nicotine dependence symptoms from age 14 to 29. Am J Psychiatry 169:1073–1081. doi: 10.1176/appi.ajp.2012.11081268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S, Haroian K, Iacono WG, et al. (2019) Minnesota Center for Twin and Family Research. Twin Res Hum Genet [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SE, Stallings MC, Corley RP, et al. (2000) Behavioral Disinhibition. Am J Med Genet 695:684–695 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.