Abstract
Background and Objective:
To compare the 2016 Coronary Artery Disease Reporting and Data System (CAD-RADS) for coronary CT angiography (CTA) to traditional stenosis categories and the coronary artery calcium score (CACS) for predicting cardiovascular events in patients with stable chest pain and suspected CAD.
Methods:
PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) trial participants’ CTAs were assessed by a central CT core lab for CACS, traditional stenosis-based categories, and modified CAD-RADS grade including high-risk coronary plaque features (HRP). Traditional stenosis categories and CAD-RADS grade were compared for the prediction of the composite endpoint of death, myocardial infarction, or hospitalization for unstable angina over a median follow-up of 25 months. Incremental prognostic value over traditional risk factors and CACS was assessed.
Results:
In 3,840 eligible patients (mean age: 60.4±8.2 years; 49% men), 3.0% (115) experienced an event. CAD-RADS (c-statistic 0.747) had significantly higher discriminatory value than traditional stenosis-based asessments (c-statistic 0.698-0.717; all p for comparison ≤0.001). With no plaque (CAD-RADS 0) as the baseline, the hazard ratio for an event increased from HR 2.43 [95% CI: (1.16-5.08)] for CAD-RADS 1 to 21.84 [95% CI: (8.63-55.26)] for CAD-RADS 4b+5. In stepwise nested models, CAD-RADS added incremental prognostic value beyond ASCVD risk score and CACS (c-statistic 0.776 vs. 0.682, p<0.001), and added incremental value persisted in all CACS strata.
Conclusion:
These data from a large representative contemporary cohort of patients undergoing coronary CTA for stable chest pain support the prognostic value of CAD-RADS as a standard reporting system for coronary CTA.
Keywords: Coronary CT Angiography, Coronary Artery Disease, Coronary Stenosis, Coronary Artery Calcium, CAD-RADS, Prognosis, High-risk Plaque
INTRODUCTION
The current 2012 ACC/AHA guidelines give a class IIb indication for coronary computed tomography angiography (CTA) for patients with stable chest pain (1). In 2015, two large randomized controlled trials (PROMISE and SCOT-HEART), demonstrated that a diagnostic strategy including CTA has similar to superior health outcomes to functional testing in stable chest pain, and again confirmed by the 5-year follow-up data from SCOT-HEART in 2018 (2-4). In 2016, these results led the UK National Institute for Health and Clinical Excellence (NICE) to recommend coronary CTA as the first-line test for patients with atypical or typical anginal symptoms (5). Based on these developments, it seems likely that coronary CTA will take an increasingly important role in the evaluation of chest pain.
Over the last 20 years, many studies have established the high diagnostic accuracy of coronary CTA to detect obstructive coronary artery disease (CAD) on invasive coronary angiography (6-8). Likewise CTA can also identify non-obstructive plaque and high risk plaque seen on intravascular ultrasound (9-11). Obstructive plaque, non-obstructive plaque, and high-risk plaque are independent predictors of MACE, and have the potential to guide management decisions (2,4,12,13). Initially, prognostic studies were limited to assessing the value of stenosis, which remains the strongest predictor of future MACE. More recently, the value of nonobstructive CAD and high risk plaque has been established (12,14). The coronary artery calcium score (CACS) is often acquired before coronary CTA, and while the prognostic value of CACS is best established in asymptomatic populations, it also has strong prognostic value in patients with stable chest pain (15,16).
To standardize and facilitate the reporting of CAD on coronary CTA, in 2016 the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI) established the Coronary Artery Disease Reporting and Data System (CAD-RADS) (17). Compared to the prevailing traditional cut points for per lesion stenosis on CTA (none, 1-49%, 50-69%, 70-100%), CAD-RADS adds additional categories intended for risk stratification: 1) explicitly differentiating between minimal (1-29%) and mild (30-49%) stenosis, 2) adding categories for left main and multivessel stenosis, and 3) including high-risk coronary plaque (HRP) features. However the prognostic value of CAD-RADS including HRP, and whether there is incremental value beyond existing traditional stenosis categories, ASCVD risk score, or CACS is not known.
Thus, the aim of this study was to determine the prognostic value of CAD-RADS and compare to established predictors of MACE in a large contemporary population with stable chest pain.
METHODS
Study design and population
The Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial was a randomized comparative effectiveness trial in stable outpatient chest pain patients who required noninvasive cardiac testing to determine the presence of obstructive CAD or myocardial ischemia. The study population and inclusion and exclusion criteria are detailed elsewhere (2,18). In brief, 10,003 patients from 193 sites across North America with expertise in the fields of cardiology, primary care, radiology, and anesthesia were included in PROMISE between July 2010 and September 2013. Patients were randomly assigned to either anatomic coronary CTA or functional testing (exercise electrocardiography, stress echocardiography or nuclear stress testing), with interpretation of testing and subsequent decision making by the local physicians. Local institutional review boards approved the study, and all patients provided written informed consent.
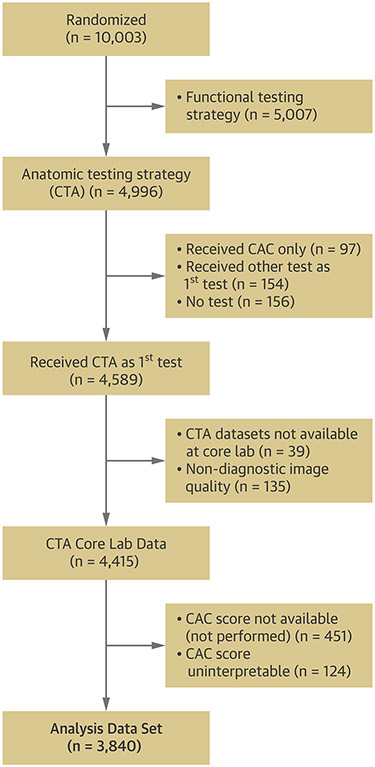
Our analysis included PROMISE patients who were randomized to the coronary CTA arm and received diagnostic non-contrast CT for calcium scoring and contrast-enhanced coronary CTA (Figure 1). In the parent PROMISE trial, the non-contrast CT prior to the coronary CTA was not mandatory but left to the discretion of site investigators.
Figure 1: Consort Diagram.
Cosort Diagram showing included and excluded patients originating from the coronary CTA-arm of the parent PROMISE trial.
Coronary Artery Calcium, CT angiography and high-risk plaque
Coronary artery calcification was assessed on all available non-contrast CT data sets by one of seven readers blinded to clinical information and outcome. We quantified the CACS using the Agatston method and dedicated software (Syngo.via, Siemens Healthcare, Forchheim, Germany). Upfront, interobserver agreement was assessed in 30 randomly selected non-contrast CT datasets with an intraclass correlation coefficient ranging from 0.99-1.0 between all readers.
Coronary CTA datasets were analyzed by one of six expert readers in coronary CTA who were also blinded to clinical information and outcome (19). Upfront, interobserver reliability was assessed using 50 randomly selected coronary CTAs between all readers (≥70% stenosis or left main ≥50% stenosis: kappa = 0.69; high-risk plaque: kappa = 0.56). Evaluable coronary artery segments were assessed for the presence of stenosis using five predefined categories: 0%, 1-29%, 30-49%, 50-69%, or ≥70% stenosis. Stenosis was categorized in three ways.
First, stenosis were categorized using CAD-RADS (17). CAD-RADS was introduced after the start of the core laboratory measurements; to translate our stenosis categories to those in CAD-RADS, we made a minor modification to CAD-RADS category 1 (stenosis 1-29% instead of 1-24%) and category 2 (stenosis 30-49% instead of 25-49%). Thus this analysis evaluates a slightly modified CAD-RADS; the term CAD-RADS is used throughout for readability.
Second, we defined traditional stenosis in two ways. Traditional definition I corresponds to the preexisting standard for coronary CTA incluing stenosis categories of no CAD (0%). mild CAD (1-49% stenosis), moderate CAD (50-69% stenosis in any major vessels/branch), severe CAD (≥50% stenosis of left main (LM) or ≥70% in any major vessel/branch). Traditional definition II was defined according to the obstructive CAD definition used in the PROMISE trial (14): normal (absence of coronary atherosclerosis), mildly abnormal (non-obstructive CAD: 1%–69% stenosis in any major vessels/branch OR <50% LM stenosis), moderately abnormal (obstructive CAD: ≥70% stenosis in one major vessel/branch) and severely abnormal (high-risk CAD: two or more vessel disease (≥70%) or ≥50% LM stenosis or ≥70% proximal left anterior descending stenosis).
Beyond stenosis, all coronary segments were assessed for high-risk plaque features (positive remodeling, spotty calcification, low CT attenuation <30 HU and napkin-ring sign) as previously defined (12). As per the CAD-RADS definition, the “V” modifier for “vulnerable plaque” was defined as two or more high-risk plaque features in at least one coronary plaque/segment (17).
Study endpoint
The endpoint was a composite of death from any cause, myocardial infarction (MI), or hospitalization for unstable angina (UAP). An independent clinical events committee adjudicated all endpoints in a blinded fashion on the basis of standard, prospectively determined definitions (2,18).
Statistical analysis
Continuous variables are presented as mean ± standard deviation. Categorical and ordinal variables are presented as frequencies and percentages. Comparisons between groups were performed using an independent sample t-test for continuous variables, the Fisher’s exact test for categorical variables, and the Wilcoxon rank-sum test for ordinal variables.
Cox proportional hazards regression models were used to calculate hazard ratios unadjusted and adjusted for ASCVD risk score with 95% confidence intervals and assess the relationship of test results to the time to the first clinical event (or censoring) (20). Cumulative event rates based on test results were computed for each testing strategy (CACS, stenosis, or CAD-RADS) using the method of Kaplan and Meier (21).
The discriminatory value of traditional and CAD-RADS grading schemes for the composite outcome was assessed using the c-statistic (22,23). Due to low individual prevalence, CAD-RADS categories 4b and 5 were combined as one composite category. A stepwise c-statistic comparison between nested models assessed the incremental prognostic value of ASCVD, CACS, and CAD-RADS over ASCVD risk score. C-statistics were compared using “somersd” and “lincom” packages in Stata. The Stata routines used to compare the C-statistics account for the nested model structure.
A two-sided p-value of less than 0.05 was considered to indicate statistical significance. All analyses were performed using Stata (SE 14.2, StataCorp LP, College Station, TX).
RESULTS
Of 4,996 PROMISE patients randomized to an anatomic testing strategy (CTA), 3,840 patients (77%) were included in the analysis. The reasons for exclusion are provided in Figure 1. Excluded patients were older, differed in risk profile and presenting symptoms, and had a higher prevalence of statin therapy as compared to included patients (supplemental Table 1).
Of those patients included in the study, the mean age was 60.4 ± 8.2 years and 49% (1,868/3,840) were men (Table 1). Over median follow-up of 25 months (interquartile range: 18-34 months), 115 patients (3.0%) experienced the composite outcome, including 53 (1.4%) all-cause deaths, 29 (0.8%) cardiovascular deaths, 18 (0.5%) myocardial infarctions, and 46 (1.2%) unstable angina admissions.
Table 1.
Baseline characteristics of PROMISE patients included in this analysis
| All Patients (n=3,840) |
|
|---|---|
| Demographics | |
| Age (yrs) | 60.4 ± 8.2 |
| Male sex | 1,868/3,840 (48.7) |
| Racial or ethnic minority † | 861/3,814 (22.6) |
| Cardiac risk factors | |
| BMI (kg/m2) ‡ | 30.3 ± 5.8 |
| Hypertension | 2,461/3,840 (64.1) |
| Diabetes | 778/3,840 (20.3) |
| Dyslipidemia | 2,588/3,840 (67.4) |
| Family history of premature CAD § | 1,272/3,829 (33.2) |
| Peripheral or cerebrovascular disease | 192/3,839 (5.0) |
| CAD equivalent ¶ | 920/3,840 (24.0) |
| History of heart failure | 150/3840 (3.9) |
| Metabolic syndrome ∥ | 1,393/3,840 (36.3) |
| Current or past tobacco use | 1,977/3,839 (51.5) |
| Sedentary lifestyle ** | 1,844/3.832 (48.1) |
| History of depression | 732/3,840 (19.1) |
| Risk factor burden and risk score †† | |
| No risk factors | 97/3,840 (2.5) |
| Risk factor burden | 2.4 ± 1.1 |
| Combined Diamond-Forrester and Coronary Artery Surgery risk score‡‡ | 53.0 ± 21.1 |
| Framingham risk score | |
| Low risk (<6%) | 253/3,834 (6.6) |
| Intermediate risk (6-20%) | 2,006/3,834 (52.3) |
| High risk (>20%) | 1,575/3,834 (41.1) |
| ASCVD pooled cohort risk prediction (2013) | |
| Low risk (<7.5%) | 1,249/3,795 (32.9) |
| Elevated risk (>=7.5%) | 2,546/3,795 (67.1) |
| Relevant medications | |
| Beta blocker | 911/3,675 (24.8) |
| ACE or ARB | 1,586/3,675 (43.2) |
| Statin | 1,670/3,675 (45.4) |
| Aspirin | 1,648/3,675 (44.8) |
| Clopidogrel | 48/3,675 (1.3) |
| Prasugrel | 1/3,675 (0.03) |
| Warfarin | 53/3,675 (1.4) |
| Primary presenting symptom and anginal type | |
| Chest pain | 2,810/3,837 (73.2) |
| Dyspnea on exertion | 547/3,837 (14.3) |
| Anginal type - site-reported ¶¶ | |
| Typical | 408/3,840 (10.6) |
| Atypical | 3,021/3,840 (78.7) |
| Non-anginal | 411/3,840 (10.7) |
ACE indicates angiotensin-converting enzyme; ARB, angiotensin-receptor blocker; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CAD, coronary artery disease.
Plus–minus values are means ± standard deviation.
Racial or ethnic minority group was self-reported, with the status of “minority” being defined by the patient.
Body-mass index is the weight in kilograms divided by the square of the height in meters.
A family history of premature CAD was defined as diagnosis of the disease in a male first-degree relative before 55 years of age or in a female first-degree relative before 65 years of age.
CAD risk equivalent was defined as diabetes, peripheral vascular disease, or cerebrovascular disease.
The metabolic syndrome was defined according to consensus criteria of the American Heart Association and the National Heart, Lung, and Blood Institute.
Sedentary lifestyle was defined by the patient as not participating in regular physical activities at least one time per week over the previous month.
Risk factors included hypertension, diabetes, dyslipidemia, family history of premature CAD, and tobacco use.
Combined Diamond and Forrester and Coronary Artery Surgery Study risk scores range from 0 to 100, with higher scores indicating a greater likelihood of obstructive CAD.
The type of angina was reported by the study-site investigators.
Prevalence of CAD
On coronary CTA, 1,303 (34%) of patients had no visible CAD, whereas 186 (4.8%) showed 70-99% stenosis (CAD-RADS 4a) and 54 (1.4%) showed ≥50% LM or ≥70% stenosis in 3 vessels (CAD-RADS 4b+5). Using the traditional definition I, 294 (7.7%) and 240 (6.3%) patients had moderate (50-69% stenosis) and severe stenosis (≥50% stenosis of LM or ≥70% in any major vessel), respectively. Using the traditional definition II, moderately abnormal (≥70% stenosis in one major vessel) and severely abnormal (≥2 vessel disease (≥70%) or ≥50% left main stenosis or ≥70% proximal left anterior descending stenosis) were detected in 145 (3.8%) and 95 (2.5%) patients, respectively.
Any HRP feature was present in 1,938 (50.5%) patients, of whom 416 (21.5%) had at least one coronary segment with two or more HRP features. The number of patients with presence of two or more HRP features per segment gradually increased across CAD-RADS categories from 9.1% (112/1,236) for CAD-RADS 1 to 37.0% (20/54) for CAD-RADS 4b+5 (p<0.001).
Downstream invasive angiography and revascularisation
Overall 437 (11.4%) patients underwent invasive coronary angiography (ICA), of whom 217 (49.7%) were revascularized. As displayed in supplemental Table 2, the rate of ICA as well as the percent leading to revascularisation increased across CAD-RADS categories (both p<0.001), with CAD-RADS 4a and 4b+5 showing the highest rates of ICA [61.8% (115/186) and 64.8% (35/54)] and revascularisation [50.5% (94/186) and 46.3% (25/54)] respectively. The percentage of ICA leading to revascularization increased from 10% (6/56) for CAD-RADS 1 to 82% (94/115) for CAD-RADS 4a and 71% (25/35) for CAD-RADS 4b+5.
Prognostic value of presence and extent of CAD
Higher CAD stenosis category by CTA was significantly associated with the composite endpoint in univariate and multivariate analysis (adjusted for ASCVD risk score) for all definitions used to categorize degree of stenosis (traditional definition I, traditional definition II and CAD-RADS categories) as displayed in Table 2. The risk for the composite endpoint increased from HR 2.43 [95% CI: (1.16–5.08)] for CAD-RADS 1 to HR 21.84 [95% CI: (8.63–55.26)] for CAD-RADS 4b+5. The presence of HRP features showed significant associations to the time to event (supplemental Figure 1) and was significantly associated with a higher hazard for the composite endpoint in univariate (HR 3.08 [95% CI: 2.04–4.65)]; p<0.001) and multivariate analysis (HR 2.61 [95% CI: (1.71–3.98)]; p<0.001 (Table 2).
Table 2.
Association of different definitions for CAD severity and HRP characteristics with adverse events in PROMISE patients
| Variables | Events N (%) |
Unadjusted | Adjusted for ASCVD risk score (continuous variable) |
||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Degree of stenosis by CTA with following definitions: | |||||
| 1. Traditional Definition I | |||||
| No CAD | 10/1,303 (0.8) | Base | --- | Base | --- |
| Mild CAD (1-49% stenosis) | 58/2,003 (2.9) | 3.82 (1.95–7.48) | <0.001 | 3.40 (1.73–6.70) | <0.001 |
| Moderate CAD (50-69% stenosis) | 18/294 (6.1) | 8.25 (3.81–17.86) | <0.001 | 6.91 (3.14–15.18) | <0.001 |
| Severe CAD (≥50% stenosis of LM or ≥70% in any major vessel) | 29/240 (12.1) | 17.61 (8.58–36.14) | <0.001 | 13.26 (6.28–28.00) | <0.001 |
| 2. Traditional Definition II | |||||
| Normal (No CAD) | 10/1,303 (0.8) | Base | --- | Base | --- |
| Mildly abnormal (1-69% stenosis in any major vessels or <50% LM stenosis) | 76/2,297 (3.3) | 4.38 (2.26–8.46) | <0.001 | 3.82 (1.96–7.44) | <0.001 |
| Moderately abnormal (≥70% in one major vessel) | 19/145 (13.1) | 19.21 (8.93–41.32) | <0.001 | 15.18 (6.92–33.29) | <0.001 |
| Severely abnormal (≥2 vessel disease (≥70%) or ≥50% left main stenosis or ≥70% proximal left anterior descending stenosis) | 10/95 (10.5) | 15.20 (6.33–36.53) | <0.001 | 9.62 (3.72–24.84) | <0.001 |
| 3. CAD-RADS Stenosis Categories | |||||
| No plaque/stenosis – CAD-RADS 0 | 10/1,303 (0.8) | Base | --- | Base | --- |
| 1-29% - CAD-RADS 1 | 25/1,236 (2.0) | 2.66 (1.28–5.54) | 0.009 | 2.43 (1.16–5.08) | 0.019 |
| 30-49% - CAD-RADS 2 | 33/767 (4.3) | 5.70 (2.81–11.57) | <0.001 | 5.02 (2.45–10.28) | <0.001 |
| 50-69% - CAD-RADS 3 | 18/294 (6.1) | 8.25 (3.81–17.87) | <0.001 | 7.03 (3.20–15.47) | <0.001 |
| 70-99% - CAD-RADS 4a | 19/186 (10.2) | 14.38 (6.69–30.93) | <0.001 | 11.39 (5.16–25.12) | <0.001 |
| ≥50% LM or ≥70% in 3 vessels or total occlusion - CAD-RADS 4b+5 | 10/54 (18.5) | 30.80 (12.81–74.07 | <0.001 | 21.84 (8.63–55.26) | <0.001 |
| High-risk plaque features* | |||||
| Absence of “Vulnerable Plaque” | 84/3,424 (2.5) | Base | --- | Base | --- |
| Presence of “Vulnerable Plaque” | 31/416 (7.5) | 3.08 (2.04–4.65) | <0.001 | 2.61 (1.71–3.98) | <0.001 |
CAC, coronary artery calcium; CAD, coronary artery disease; CTA, coronary computed tomography angiography; LAD, left ascending coronary artery; LM, left main coronary artery; Adverse events include all-cause death, hospitalization for unstable angina, and MI.
Presence of “vulnerable plaque” defined as two or more high-risk plaque features in at least one coronary plaque/segment.
Discriminatory capacity of stratification of CAD to predict events
The capacity to discriminate future events (all-cause death, MI, UAP) for the traditional definition I, traditional definition II, and CAD-RADS categories were: c-statistic 0.717 (95%CI: 0.673-0.760), c-statistic 0.698 (95%CI: 0.658-0.739) and c-statistic 0.747 (95%CI: 0.703-0.792) respectively. CAD-RADS had significantly higher discriminatory value as compared to both traditional definitions (p≤0.001).
As determined by log-rank test in Kaplan-Meier estimates, CAD-RADS categories also showed significant associations to the time to event, with an increase in risk for the composite endpoint with the next higher category (p<0.001; Figure 2a-c).
Figure 2: Kaplan-Meier estimates of the composite outcome.
(death, MI and UAP) by severity of stenosis using the a) Traditional Definition I, b) Traditional Definition II, and c) CAD-RADS.
2a) Traditional Definition I
No CAD (0%). mild CAD (1-49% stenosis), moderate CAD (50-69% stenosis in any major vessels/branch), severe CAD (≥50% stenosis of left main (LM) or ≥70% in any major vessel/branch).
2b) Traditional Definition II
Normal (absence of coronary atherosclerosis), mildly abnormal (non-obstructive CAD: 1%–69% stenosis in any major vessels/branch OR <50% LM stenosis), moderately abnormal (obstructive CAD: ≥70% stenosis in one major vessel/branch) and severely abnormal (high-risk CAD: two or more vessel disease (≥70%) or ≥50% LM stenosis or ≥70% proximal left anterior descending stenosis).
2c) CAD-RADS
CAD-RADS=Coronary Artery Disease Reporting and Data System; CAD-RADS 0: no plaque/stenosis, CAD-RADS 1: 1-29% stenosis, CAD-RADS 2: 30-49% stenosis, CAD-RADS 3: 50-69% stenosis, CAD-RADS 4a: 70-99% stenosis, CAD-RADS 4b+5: ≥50% LM stenosis or ≥70% stenosis in 3 vessels or total occlusion.
Incremental value of CAD-RADS Stenosis Categories beyond CACS
Prevalence of CAC and the association to clinical events
Out of 3,840 patients, 1,498 (39%) had a CACS of 0. The CACS was 1-100 in 1,160 (30%), 101-400 in 676 (18%) and >400 in 506 (13%) patients. Across CACS categories, the prevalence of coronary artery disease significantly increased (p<0.001) (Table 3a). In patients with CACS of zero, 87% were free of coronary artery disease (CAD-RADS 0), whereas 12.3% (184/1498) had non-obstructive disease (CAD-RADS 1-3) and 0.7% (11/1498) had obstructive disease (CAD-RADS 4-5). In 9.2% (17/184) of those patients with CACS of zero and nonobstructive disease (CAD-RADS 1-3), 2 or more HRP features were present, whereas HRP were present in 54.5% (6/11) of patients with a CAC of zero and obstructive disease (CAD-RADS 4-5).
Table 3a.
CAD-RADS categories across CACS strata
| CAD-RADS Stenosis categories |
All Patients (n=3,840) |
CACS strata | |||
|---|---|---|---|---|---|
| CACS 0 (n=1,498) |
CACS 1-100 (n=1,160) |
CACS >100- 400 (n=676) |
CACS >400 (n=506) |
||
| No plaque/stenosis – CAD-RADS 0 | 1,303 (33.9) | 1,303 (87.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| 1-29% - CAD-RADS 1 | 1,236 (32.2) | 142 (9.5) | 774 (66.7) | 254 (37.6) | 66 (13.0) |
| 30-49% - CAD-RADS 2 | 767 (20.0) | 35 (2.3) | 273 (23.5) | 261 (38.6) | 198 (39.1) |
| 50-69% - CAD-RADS 3 | 294 (7.7) | 7 (0.5) | 68 (5.9) | 93 (13.8) | 126 (24.9) |
| 70-99% - CAD-RADS 4a | 186 (4.8) | 10 (0.7) | 36 (3.1) | 54 (8.0) | 86 (17.0) |
| ≥50% LM or ≥70% in 3 vessels or total occlusion – CAD-RADS 4b+5 | 54 (1.4) | 1 (0.1) | 9 (0.8) | 14 (2.1) | 30 (5.9) |
Overall, the incidence of the composite endpoint increased across CACS categories (p<0.001) as well as CAD-RADS stenosis categories within each CAC group (p<0.001) as displayed in Table 3b. Among patients with a CAC score of zero, 1.5% (22/1498) experienced an event. Ten of these patients had no visible CAD on CT (CAD-RADS 0). Nevertheless, the incidence of the primary endpoint increased with severity of CAD, reflected by increasing hazard ratios from 5.7 (95%CI: 2.3 - 14.5) for non-obstructive CAD (CAD-RADS 1-3) to 58.0 (95%CI: 18.1 - 185.3) for obstructive CAD (CAD-RADS 4-5), with a p<0.001 for comparison to patients without plaque.
Table 3b.
Composite outcome (all-cause death, MI, UAP) by CAD-RADS category across CAC strata
| CAD-RADS Stenosis categories |
All Patients (n=3,840) |
CACS strata | |||
|---|---|---|---|---|---|
| CACS 0 (n=1,498) |
CACS 1-100 (n=1,160) |
CACS >100- 400 (n=676) |
CACS >400 (n=506) |
||
| No plaque/stenosis – CAD-RADS 0 | 10/1,303 (0.8) | 10/1,303 (0.8) | 0/0 (---) | 0/0 (---) | 0/0 (---) |
| 1-29% - CAD-RADS 1 | 25/1,236 (2.0) | 6/142 (4.2) | 12/774 (1.6) | 5/254 (2.0) | 2/66 (3.0) |
| 30-49% - CAD-RADS 2 | 33/767 (4.3) | 2/35 (5.7) | 9/273 (3.3) | 14/261 (5.4) | 8/198 (4.0) |
| 50-69% - CAD-RADS 3 | 18/294 (6.1) | 0/7 (0.0) | 2/68 (2.9) | 8/93 (8.6) | 8/126 (6.4) |
| 70-99% - CAD-RADS 4a | 19/186 (10.2) | 3/10 (30.0) | 2/36 (5.6) | 7/54 (13.0) | 7/86 (8.1) |
| ≥50% LM or ≥70% in 3 vessels or total occlusion – CAD-RADS 4b+5 | 10/54 (18.5) | 1/1 (100.0) | 2/9 (22.2) | 3/14 (21.4) | 4/30 (13.3) |
| Total | 115/3,840 (3.0) | 22/1,498 (1.5) | 27/1,160 (2.3) | 37/676 (5.5) | 29/506 (5.7) |
Incremental value of CTA using CAD-RADS categories beyond CACS to predict events
Information about the burden of CAC (strata) significantly improved the discriminatory capacity of the ASCVD pooled cohort risk calculator to predict the composite endpoint of all-cause death, MI and UAP (c-statistic 0.629 [95%CI: 0.572-0.687] vs. 0.682 [95%CI: 0.629-0.735]; p=0.008), as displayed in Table 4a. Data from CTA using CAD-RADS categories (including HRP features) further incrementally increased the prognostic value to predict the composite endpoint (c-statistic 0.776 [95%CI: 0.734-0.818)]; p<0.001). Stratified by CAC categories, the significant incremental value of coronary CTA over ASCVD risk stratification persisted as listed in Table 4b,
Table 4.
Incremental value of CT based assessment of CAD using CAD-RADS categories beyond risk factors and CACS (a) in the overall population and (b) across CAC strata
| (a) | ||||
|---|---|---|---|---|
| Univariable Model |
C statistic | Multivariable models | C statistic | p-value (Diff betw. Models)* |
| ASCVD | 0.629 (0.572-0.687) | |||
| CACS | 0.657 (0.606-0.708) | ASCVD+CACS | 0.682 (0.629-0.735) | 0.008 |
| CAD-RADS(+HRP) | 0.747 (0.703-0.792) | ASCVD+CACS+CAD-RADS(+HRP) | 0.776 (0.734-0.818) | <0.001 |
| (b) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | CACS strata | ||||||||
| CACS 0 | CACS 1-100 | CACS >100-400 | CACS >400 | ||||||
| Description | c stat | p-value | c stat | p-value | c stat | p- value |
c stat | p-value | |
| 1 | ASCVD | 0.539 | 0.607 | 0.551 | 0.572 | ||||
| 2 | ASCVD+CAD-RADS(+HRP) | 0.751 | 0.005 | 0.775 | 0.023 | 0.673 | 0.031 | 0.697 | 0.041 |
Description: ASCVD as continuous variable; CAC as categorical variable (0, 1-100, 101-400, >400 CACS), CTA per CAD-RADS definition including HRP (Vulnerable Plaque)
p-value shows difference of the stepwise c-statistic comparison between the specific model and the consecutive model
p-value calculations: Modell 2 vs 1
DISCUSSION
In a large contemporary trial of patients with stable chest pain randomized to coronary CTA, we found that the CAD-RADS reporting system had greater prognostic value and discriminatory ability for future MACE than previous traditional stenosis-based categories. This can be explained by CAD-RADS’s more granular grading of nonobstructive and obstructive CAD, inclusion of both stenosis and plaque burden components, and the inclusion of HRP features. Our second major finding is that CAD-RADS adds substantial prognostic value over the ASCVD risk score and CACS, across all CACS strata. Together, these results support the prognostic value of CAD-RADS for standardized reporting of coronary CTA.
Strengths of this study include that it was conducted within a large multicenter randomized controlled trial at 192 sites, with prospective enrollment of patients, collection of CTA, and independent adjudication of adverse events. In this analysis, coronary CT was interpreted for CAC, coronary artery stenosis, and high-risk coronary plaque features by a central core lab with expert CT readers blinded to clinical information and outcomes. These factors may explain why our results differ from that in a recent analysis of the CONFIRM registry, that found CAD-RADS did not have greater discriminatory value for myocardial infarction or death (c-statistic CAD-RADS 0.705 vs. traditional 0.710, p=0.78) (24). In the CONFIRM analysis, CTA stenosis was graded by local site physicians, who tend to call severe stenosis more often than blinded expert core lab readers (19). Furthermore the CONFIRM analysis did not include high risk plaque features, which are a part of CAD-RADS due to their known prognostic value (12,25-28).
Our results should be interpreted in the context of previous PROMISE publications that assessed the prognostic value of CTA. First, Hoffmann et al compared the prognostic value of CTA to functional testing finding that CTA had greater prognostic value (14). In contrast to the present study, this analysis used the local site interpretations of CTA and functional testing, as well as disease categories tailored to allow comparison between CTA and functional testing that may not reflect how coronary CTA is currently interpreted. A subsequent paper by Lu et al found that blinded expert central core lab interpretation of coronary CTA found 41% fewer patients with stenosis ≥50% than the site readers, yet with better accuracy using quantitative invasive coronary angiography as the reference standard (19). Ferencik et al found that high risk plaque features on coronary CTA were associated with major adverse cardiovascular events after adjustment for stenosis and ASCVD risk score; in this study high risk plaque was defined differently (any plaque with at least one high risk feature, not including spotty calcification) than in CAD-RADS (two high risk plaque features in a single segment, including spotty calcification) (29). Finally, Budoff et al compared the coronary artery calcium score to functional testing for estimating prognosis, finding that most patients having an event had a calcium score >0 compared to fewer than half with an abnormality on functional testing (15). However, whether coronary CTA adds prognostic value beyond coronary calcium was not assessed.
A second major finding of our study was that CAD-RADS had greater prognostic value than the coronary artery calcium score. While the CACS is one of the best-studied prognostic imaging biomarkers in asymptomatic populations (30,31) (32), how it relates to stenosis, high-risk plaque, and events in symptomatic chest pain populations is not as well established. Indeed, CACS had limited value for diagnosing stenosis, with only half of patients with a CACS >400 having a stenosis ≥50% (Table 3a). For those with a CACS between 101 and 400, only a quarter had a stenosis ≥50%. On the other hand, 13% (195/1498) of patients with a CAC score of 0 had detectable coronary plaque, and 6% (12/195) of patients with CACS of zero but detectable plaque on CTA experienced an event in our analysis, an event rate twice as high as the overall population. It should be noted that of the 1,498 studies with CACS zero, only 21 had an event (15), yet a majority of these (12/21) occurred in the group with CACS zero and non-obstructive CAD on CTA. This finding is complementary to CONFIRM observational data finding that a minority of symptomatic patients with CACS zero have coronary plaque on CTA, and that this noncalcified plaque is associated with increased cardiac events (33). CAD-RADS had substantial incremental prognostic value over CACS, and this was true in all CACS strata (Table 4). One mechanistic explanation could be the fact that the prevalence of HRP in patients with severe stenosis was the highest in patients without CAC (55%; 6/11) and decreased with increasing CACS (45%; 52/116), reflecting the potentially higher vulnerability of plaque in patients with less CAC. The Computed Tomography vs. Exercise Testing in Suspected Coronary Artery Disease (CRESCENT) randomized controlled trial, which used a tiered CT approach in patients with stable angina, suggested no need for CTA in patients without CAC and low pretest probability (<70%) for obstructive CAD (16). In this trial, 98 out of 242 patients (39%) without CAC and low pretest probability did not undergo downstream testing and no adverse events occurred after a follow up of 1 year. The shorter follow up in CRESCENT in comparison to PROMISE (median follow-up 25 months) might partially explain the demonstrated value of CTA in patients without calcification in our analysis, as cardiovascular events in low-risk groups might not be apparent for years. Also, it is possible that events in PROMISE patients would have been even higher without the use of CTA, as CTA was shown to be associated with a higher proportion of patients newly initiated on aspirin and statins as compared to standard of care (4). This demonstrates the need for longer follow up especially in stable chest pain cohorts with expected low incidence of events.
A recent 1,769-patient analysis of the SCOT-HEART trial (34) found that both obstructive (≥70% stenosis) coronary artery disease and adverse plaque were associated with cardiac events, however these associations were not independent of the CAC score. This contrasts with our result that CAD-RADS had significant incremental prognostic value beyond both the ASCVD risk score and CAC. Besides the differences in demographics and outcomes between the two trials, differences in how stenosis and high risk plaque were defined may explain the discrepancy. In SCOT-HEART, stenosis was categorized as normal (0%), nonobstructive (1-69%) or obstructive (≥70%). This approach provides less granular information about the degree of non-obstructive stenosis and presence of multivessel disease than CAD-RADS. Furthermore, SCOT-HEART defined adverse plaque as the presence of at least one plaque with positive remodeling or low attenuation, in contrast to the CAD-RADS “V” modifier that requires at least two high-risk plaque features in a single coronary plaque segment. Nevertheless, the unadjusted hazard ratio for adverse/high risk plaque was similar between the two trials (SCOT-HEART HR 3.01 (1.61 to 5.63); p<0.001, PROMISE HR 3.08 (2.04–4.65); p<0.001). In the end CAD-RADS is currently the preferred reporting system for coronary CTA by the Society of Cardiovascular Computed Tomography (SCCT), American College of Radiology (ACR), North American Society for Cardiovascular Imaging (NASCI) and American College of Cardiology (ACC), and so we believe it has the greatest clinical relevance.
LIMITATIONS
Limitations of this study should be considered. First, PROMISE was a pragmatic trial, where the results of CTA were shared with patients and influenced management (35). A slightly modified CAD-RADS was evaluated, with a threshold of 30% instead of 25% between CAD-RADS 1 and 2. Overall the prevalence of obstructive CAD was rather low, and CAD-RADS categories 4b and 5 were conflated, due to small numbers. This data from a contemporary cohort of stable chest pain patients reflects real world practice. Patients with known CAD or prior interventions were excluded from PROMISE; and thus, we could not investigate the prognostic value of the CAD-RADS S (stent) or G (graft) category modifiers.
CONCLUSION
In PROMISE, a large prospective trial of coronary CTA in patients with stable chest pain and suspected CAD, the CAD-RADS reporting system had greater prognostic value than ASCVD score, CAC score and traditional stenosis-based grading schemes.
Supplementary Material
Supplemental Figure 1. Kaplan-Meier estimates of the composite outcome (death, MI and UAP) by presence of CAD-RADS vulnerable plaque
HRP: high-risk plaque features (positive remodeling, spotty calcification, low CT attenuation <30 HU and napkin-ring sign), the CAD-RADS definition of vulnerable plaque was used, defined as positive if two or more high-risk plaque features were identified in one coronary plaque/segment.
Central Illustration: Prognostic value of coronary CTA using Coronary Artery Disease Reporting and Data System in PROMISE.
In this large contemporary trial of patients with stable chest pain randomized to coronary CTA, the Coronary Artery Disease Reporting and Data System (CAD-RADS) including information about presence of high-risk plaque features (HRP) had higher prognostic value and discriminatory ability for future MACE (c-statistic 0.747) as compared to traditional stenosis-based assessment (c-statistic 0.698-0.717; p for comparison ≤0.001), (Kapian-Meyer curves for CAD-RADS for the prediction of the composite endpoint of death, myocardial infarction, or hospitalization for unstable angina over a median follow-up of 25 months on the left). Moreover, CAD-RADS added prognostic value over the ASCVD risk score and CAC score (table on the right), across all CAC strata.
PERSPECTIVES.
Competency in Patient Care: For patients undergoing coronary CTA for stable chest pain, the Coronary Artery Disease Reporting and Data System (CAD-RADS) has greater prognostic value than traditional stenosis categories, the ASCVD risk score, and the coronary artery calcium score (CACS).
Translational Outlook: CAD-RADS is currently recommended by several societies for the reporting of coronary artery disease on CTA; further research is necessary to determine whether improved prognostic value translates into improved clinical decision-making and outcomes.
Acknowledgments
Funding
The parent PROMISE trial was supported by National Heart, Lung, and Blood Institute grants R01HL098237, R01HL098236, R01HL98305, and R01HL098235.
D.O.B. was supported by NIH/NHLBI 5K24HL113128.
M.F. was supported by American Heart Association Grant 13FTF16450001.
U.H. was supported by K24HL113128.
M.T.L. was supported by the American Heart Association Precision Medicine Institute 18UNPG34030172 and the Harvard University Center For AIDS Research 5P30AI060354-14.
Abbreviations:
- ASCVD
atherosclerotic cardiovascular disease
- CAC(S)
coronary artery calcium (score)
- CAD
coronary artery disease
- CAD-RADS
Coronary Artery Disease Reporting and Data System
- CTA
CT-angiography
- HRP
high risk plaque features
- MACE
major adverse cardiovascular event
- PROMISE
Prospective Multicenter Imaging Study for Evaluation of Chest Pain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
D.O.B., T.M., B.S., B.F., T.H., A.I., S.J., N.M., P.S. and S.A. have nothing to disclose.
M.B. reports grants from NIH, during the conduct of the study; grants from General Electric , outside the submitted work.
M.F. reports grants from American Heart Association, outside the submitted work.
P.S.D. reports grants from HeartFlow, outside the submitted work.
U.H. reports grants from NIH-NHLBI National Heart, Lung, and Blood Institute, during the conduct of the study; grants from Duke University/ Abbott U.S., grants from HeartFlow, Inc. , grants from Kowa Company, Ltd. , grants and non-financial support from MedImmne, LLC. , personal fees and non-financial support from Abbott U.S., outside the submitted work.
M.T.L. reports research support to his institution from the American Heart Association, Nvidia, Kowa and Medimmune, outside the submitted work.
References
- 1.Fihn SD, Gardin JM, Abrams J et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60:e44–e164. [DOI] [PubMed] [Google Scholar]
- 2.Douglas PS, Hoffmann U, Patel MR et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372:1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.investigators S-H. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. Lancet 2015;385:2383–91. [DOI] [PubMed] [Google Scholar]
- 4.Investigators S-H, Newby DE, Adamson PD et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N Engl J Med 2018;379:924–933. [DOI] [PubMed] [Google Scholar]
- 5.National Institute for Health and Clinical Excellence. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin (update). . CG95 London: National Institute for Health and Clinical Excellence; 2016. [PubMed] [Google Scholar]
- 6.Meijboom WB, Meijs MF, Schuijf JD et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135–44. [DOI] [PubMed] [Google Scholar]
- 7.Miller JM, Rochitte CE, Dewey M et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324–36. [DOI] [PubMed] [Google Scholar]
- 8.Budoff MJ, Dowe D, Jollis JG et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol 2008;52:1724–32. [DOI] [PubMed] [Google Scholar]
- 9.Marwan M, Taher MA, El Meniawy K et al. In vivo CT detection of lipid-rich coronary artery atherosclerotic plaques using quantitative histogram analysis: a head to head comparison with IVUS. Atherosclerosis 2011;215:110–5. [DOI] [PubMed] [Google Scholar]
- 10.Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol 2014;11:390–402. [DOI] [PubMed] [Google Scholar]
- 11.Schlett CL, Maurovich-Horvat P, Ferencik M et al. Histogram analysis of lipid-core plaques in coronary computed tomographic angiography: ex vivo validation against histology. Invest Radiol 2013;48:646–53. [DOI] [PubMed] [Google Scholar]
- 12.Ferencik M, Mayrhofer T, Bittner DO et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motoyama S, Ito H, Sarai M et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J Am Coll Cardiol 2015;66:337–46. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann U, Ferencik M, Udelson JE et al. Prognostic Value of Noninvasive Cardiovascular Testing in Patients With Stable Chest Pain: Insights From the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;135:2320–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budoff MJ, Mayrhofer T, Ferencik M et al. Prognostic Value of Coronary Artery Calcium in the PROMISE Study (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;136:1993–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubbers M, Dedic A, Coenen A et al. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: the multicentre, randomized CRESCENT trial. Eur Heart J 2016;37:1232–43. [DOI] [PubMed] [Google Scholar]
- 17.Cury RC, Abbara S, Achenbach S et al. CAD-RADS(TM) Coronary Artery Disease - Reporting and Data System. An expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J Cardiovasc Comput Tomogr 2016;10:269–81. [DOI] [PubMed] [Google Scholar]
- 18.Douglas PS, Hoffmann U, Lee KL et al. PROspective Multicenter Imaging Study for Evaluation of chest pain: rationale and design of the PROMISE trial. Am Heart J 2014;167:796–803.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu MT, Meyersohn NM, Mayrhofer T et al. Central Core Laboratory versus Site Interpretation of Coronary CT Angiography: Agreement and Association with Cardiovascular Events in the PROMISE Trial. Radiology 2018;287:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox DR. Regression models and life-tables. . J R Stat Soc 1972. [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. . J Am Stat Assoc 1958;53:457–481. [Google Scholar]
- 22.Harrell FEJ, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA 1982;247(18):2543–2546. [PubMed] [Google Scholar]
- 23.Harrell FEJ, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. . Stat Med 1996;15(4):361–387. [DOI] [PubMed] [Google Scholar]
- 24.Xie JX, Cury RC, Leipsic J et al. The Coronary Artery Disease-Reporting and Data System (CAD-RADS): Prognostic and Clinical Implications Associated With Standardized Coronary Computed Tomography Angiography Reporting. JACC Cardiovasc Imaging 2018;11:78–89. [DOI] [PubMed] [Google Scholar]
- 25.Motoyama S, Sarai M, Harigaya H et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 2009;54:49–57. [DOI] [PubMed] [Google Scholar]
- 26.Puchner SB, Liu T, Mayrhofer T et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 2014;64:684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomsen C, Abdulla J. Characteristics of high-risk coronary plaques identified by computed tomographic angiography and associated prognosis: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging 2016;17:120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nerlekar N, Ha FJ, Cheshire C et al. Computed Tomographic Coronary Angiography-Derived Plaque Characteristics Predict Major Adverse Cardiovascular Events: A Systematic Review and Meta-Analysis. Circ Cardiovasc Imaging 2018;11:e006973. [DOI] [PubMed] [Google Scholar]
- 29.Ferencik M, Mayrhofer T, Bittner DO et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol 2018;3:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Detrano R, Guerci AD, Carr JJ et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336–45. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann U, Massaro JM, D'Agostino RB Sr, Kathiresan S, Fox CS, O'Donnell CJ. Cardiovascular Event Prediction and Risk Reclassification by Coronary, Aortic, and Valvular Calcification in the Framingham Heart Study. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budoff MJ, Young R, Burke G et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J 2018;39:2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villines TC, Hulten EA, Shaw LJ et al. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. J Am Coll Cardiol 2011;58:2533–40. [DOI] [PubMed] [Google Scholar]
- 34.Williams MC, Moss AJ, Dweck M et al. Coronary Artery Plaque Characteristics Associated With Adverse Outcomes in the SCOT-HEART Study. J Am Coll Cardiol 2019;73:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ladapo JA, Hoffmann U, Lee KL et al. Changes in Medical Therapy and Lifestyle After Anatomical or Functional Testing for Coronary Artery Disease. J Am Heart Assoc 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Kaplan-Meier estimates of the composite outcome (death, MI and UAP) by presence of CAD-RADS vulnerable plaque
HRP: high-risk plaque features (positive remodeling, spotty calcification, low CT attenuation <30 HU and napkin-ring sign), the CAD-RADS definition of vulnerable plaque was used, defined as positive if two or more high-risk plaque features were identified in one coronary plaque/segment.