Abstract
Previous studies have shown that Pneumocystis binds to pneumocytes, but the proteins responsible for binding have not been well-defined. Mucins are the major glycoproteins present in mucus, which serves as the first line of defense during airway infection. MUC1 is the best characterized membrane-tethered mucin and is expressed on the surface of most airway epithelial cells.
Although by electron microscopy, Pneumocystis primarily binds to type I pneumocytes, it can also bind to type II pneumocytes. We hypothesized that Pneumocystis organisms can bind to MUC1 expressed by type II pneumocytes. Over-expression of MUC1 in human embryonic kidney HEK293 cells increased Pneumocystis binding, while knock-down of MUC1 expression by siRNA in A549 cells, a human adenocarcinoma-derived alveolar type II epithelial cell line, decreased Pneumocystis binding. Immunofluorescence labeling indicated that MUC1 and Pneumocystis were co-localized in infected mouse lung tissue. Incubation of A549 cells with Pneumocystis led to phosphorylation of ERK1/2 that increased with knock down of MUC1 expression by siRNA. Pneumocystis caused increased IL-6 and IL-8 secretion by A549 cells, and knock down of MUC1 further increased their secretion in A549 cells. Taken together, these results suggest that binding of Pneumocystis to MUC1 expressed by airway epithelial cells may facilitate establishment of productive infection.
Keywords: MUC1, Pneumocystis, co-localization, pneumocytes, pneumonia, adherence
Introduction
Pneumocystis is an opportunistic fungal pathogen that causes life-threatening pneumonia (PCP) in HIV patients and other immunocompromised hosts (J. A. Kovacs & Masur, 2009). Better understanding of the pathogenesis of PCP, including the early stages of infection, may provide improved methods for prevention and treatment of this life-threatening infection.
Based on electron microscopy studies, Pneumocystis appears to bind tightly to type I pneumocytes (Millard, Wakefield, & Hopkin, 1990; Pottratz & Weir, 1995; Yoneda & Walzer, 1980, 1983), but the mediators of this binding have not been well-characterized. Fibronectin and vitronectin have been reported to increase Pneumocystis binding to lung epithelial cell lines as well as alveolar macrophages in vitro (Limper, Standing, Hoffman, Castro, & Neese, 1993; Pottratz, Paulsrud, Smith, & Martin, 1994). Whether these are necessary or sufficient for in vivo binding will require further evaluation.
While Pneumocystis organisms have also been reported to bind to type II pneumocytes (Millard et al., 1990), what role these cells or their surface proteins may play in Pneumocystis infection has not been extensively explored. Certain proteins secreted by type II pneumocytes, such as surfactant proteins A and D, have been shown to bind to Pneumocystis (McCormack, Festa, Andrews, Linke, & Walzer, 1997; Vuk-Pavlovic, Standing, Crouch, & Limper, 2001) and may play a role in its adherence to alveolar macrophages in vivo (O’Riordan et al., 1995; Williams, Wright, March, & Martin, 1996). More recently, heat shock protein 5 (HSPA5) was identified by affinity chromatography, and demonstrated to mediate binding of Pneumocystis to a type II lung epithelial cell line (Kottom, Hebrink, & Limper, 2018).
Respiratory mucins are important glycoprotein components of mucus that are synthesized by type II pneumocytes and contribute to the viscoelastic properties of mucus (Lai, Wang, Wirtz, & Hanes, 2009). Mucus lining the airway lumen serves as a major protective barrier, and provides one of the first lines of defense during respiratory infections: pathogens trapped in the mucus layer are removed by the mucociliary clearance mechanism of the underlying airway epithelium. At least twenty-two mucin genes have been identified in humans, 16 of which are expressed in the respiratory tract (Li & Cozzi, 2007; Rose & Voynow, 2006). Among the respiratory membrane-tethered mucins, which primarily include MUC1, MUC4, and MUC16, MUC1 was the first to be cloned and is the best characterized (Gendler et al., 1990; Lan, Batra, Qi, Metzgar, & Hollingsworth, 1990). MUC1 is expressed on the apical surface of most airway epithelial cells, including goblet cells, ciliated cells and type II alveolar pneumocytes (Jarrard et al., 1998; Rose & Voynow, 2006). MUC1 is a transmembrane mucin consisting of a heavily O-glycosylated extracellular domain, a transmembrane domain, and a cytoplasmic tail of 72 amino acids which has a variable number of highly conserved tandem repeats of 20 amino acids. The cytoplasmic tail domain contains seven tyrosine residues, some of which can be phosphorylated, leading to activation of intracellular signal transduction cascades (Hattrup & Gendler, 2008; Thornton, Rousseau, & McGuckin, 2008). The cytoplasmic domain of MUC1 interacts with a variety of molecules such as epidermal growth factor receptor, c-Src, β-catenin, and Grb2 (Li, Kuwahara, Ren, Wen, & Kufe, 2001; Li, Ren, et al., 2001; Pandey, Kharbanda, & Kufe, 1995; Wang, Lillehoj, & Kim, 2003).
MUC1 can mediate binding of microorganisms such as Pseudomonas aeruginosa to lung epithelial cells (Kato, Lillehoj, Kai, & Kim, 2010); such binding can dampen inflammatory responses (Kato, Lillehoj, Lu, & Kim, 2017). It is unknown if MUC1 plays a role in Pneumocystis invasion and pathogenesis, although serum MUC1 levels have been evaluated for the serodiagnosis of PCP (Esteves et al., 2015), and higher levels of MUC1 (KL-6) were associated with greater mortality in a retrospective study of intensive care patients with PCP (Kotani et al., 2017). The current study was undertaken to determine if Pneumocystis binds to MUC1, potentially facilitating adherence of cysts to the alveolar lining following inhalation, and to examine the potential immunomodulatory effects of MUC1 on Pneumocystis infection.
Materials and Methods
Reagents
All reagents were purchased from Sigma (St. Louis, MO) unless noted otherwise. Anti-MUC1 mouse monoclonal antibody (GP1.4, Cat. # MA5–13168), Alexa Fluor 488-conjugated rabbit anti-mouse or goat anti-rabbit IgG, and Alexa Fluor 594-conjugated rabbit anti-mouse or goat anti-rabbit IgG were from Thermo Fisher Scientific (Waltham, MA). Polyclonal rabbit anti-mouse MUC1 antibody was from Abcam (Cat. # ab15481, Cambridge, MA). Horseradish peroxidase (HRP)-conjugated goat anti-mouse and goat anti-rabbit IgG antibodies were from Jackson ImmunoResearch Laboratories, Inc (West Grove, PA). The pCI-MUC1 expression plasmid and pCI-neo empty vector were a generous gift from Dr. Sandra J. Gendler (Mayo Clinic, Scottsdale, AZ). MUC1 smart pool siRNA and non-targeting control siRNA were purchased from Dharmacon (Lafayette, CO). Rabbit monoclonal anti-total ERK1/2, rabbit monoclonal anti-phospho-ERK1/2, and HRP-conjugated mouse monoclonal anti-beta actin antibodies were purchased from Cell Signaling (Danvers, MA). Polyclonal mouse anti-major surface glycoprotein (Msg) of Pneumocystis murina (P. murina) was generated as previously described (Bishop, Helman, & Kovacs, 2012). Human IL-6 and IL-8 ELISA kits were from R&D (Minneapolis, MN).
Cell lines
HEK293 cells (ATCC, CRL-1573), a human embryonic kidney cell line, were grown in DMEM medium supplemented with 10% fetal bovine serum, 100 unit/ml penicillin and 100 μg/ml streptomycin. A549 cells (ATCC, CCL-185), a human lung adenocarcinoma cell line characterized as type II pneumocytes, were grown in Ham’s F-12 medium, supplemented with 10% fetal bovine serum, and penicillin and streptomycin. Both cell lines were initially obtained from ATCC (Manassas, VA) and were passaged in our laboratory.
P. murina organisms
P. murina organisms were partially purified from the lungs of heavily infected CD40L knock-out mice by Ficoll-Hypaque (GE Healthcare) gradient centrifugation as previously described (J.A. Kovacs et al., 1988).
MUC1 expression plasmid and MUC1 siRNA transfection
HEK293 cells were transfected with 2 μg pCI-neo or pCI-MUC1 vector in a 12-well plate using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Over-expression of MUC1 was confirmed by immunofluorescence and immunoblot. A549 cells were transfected with 50 nM of MUC1 smart pool siRNA (4 siRNAs, each 21 bp long) or a nontargeting control siRNA using RNAi MAX (Thermo Fisher Scientific). Knock down of MUC1 was confirmed by reverse transcriptase quantitative PCR (RT-qPCR) and immunoblot.
Quantitative PCR (qPCR) and Reverse Transcriptase qPCR (RT-qPCR)
Total RNA was extracted from A549 cells transfected with MUC1 siRNA or control siRNA, and incubated with or without Pneumocystis, using QIA Shredder columns and RNeasy kits following the manufacturer’s instructions (Qiagen) and was quantified using a NanoDrop spectrophotometer (BioLabNet, Great Falls, VA). Messenger RNA expression for selected genes was measured by RT-qPCR performed on an ABI Prism 7900 sequence detection system (Applied Biosystems). We used the following commercially available probe and primers sets (Applied Biosystems): MUC1 (Hs00159357_m1 433118) and GAPDH (Hs04420566_g1 4331182) together with the High Capacity RNA-to-cDNA kit and TaqMan Universal PCR master mix (Thermo Fisher Scientific) according to the manufacturer’s instructions. Gene expression levels were presented as relative mRNA copy numbers compared with the internal control (GAPDH) expression levels and calculated as percentage of control siRNA, using the delta-delta Ct method (Livak & Schmittgen, 2001).
qPCR and RT-qPCR to quantitate P. murina organisms was performed using the single copy dihydrofolate reductase (dhfr) gene (Vestereng et al., 2004) adapted to the Taqman system. Primers were PmDHFR.f5 5’-TGGGTAGAAAGACATGGGAAAG-3’ (forward primer), PmDHFR.r6 5’- CCAAAGATTCTTCTCGACTAATAACAAC −3’ (reverse primer), and PmDHFR.p5 5’-/5HEX/ACAATTCCG/ZEN/GCCTCTTAAAGGTCGT/3IABkFQ/−3’ (Taqman probe). RNA and DNA were extracted using RNAeasy mini kit (Qiagen) and the QIAamp DNA Mini Kit (Qiagen). Amplification conditions were as follows: 50°C for 2 minutes, followed by 95°C for 10 minutes, then 50 cycles of: 95°C for 15 sec and 60°C for 1 minute.
For RT-qPCR, samples were treated with DNase prior to reverse transcription, and a no reverse transcriptase control was included to verify that RNA, and not DNA, was being amplified. All no reverse transcriptase controls had <10 copies per reaction.
Immunoblot analysis
HEK293 cells or A549 cells grown in a 6-well plate were transfected with pCI-MUC1 over-expression vector or MUC1 siRNA, respectively, or controls. Forty-eight hours after transfection, cells were lysed in RIPA buffer containing Halt Proteinase Inhibitor Cocktail and Halt Phosphatase Inhibitor Cocktail (Thermo Scientific, Rockford, IL). The cell lysates were centrifuged, and equivalent amounts of protein were separated by SDS-PAGE and transferred to nitrocellulose membranes, which were then incubated with mouse anti-MUC1 antibody followed by HRP-conjugated secondary antibody. An electrochemiluminescence (ECL) detection kit (GE Health Care, Piscataway, NJ) was used to develop the blots; the signals were visualized on a Bio-Rad Imager (Bio-Rad, Hercules, CA). The blot was then stripped and re-probed with HRP-conjugated anti-β-actin antibody to demonstrate equal loading of samples.
Phospho-ERK1/2 was detected by immunoblot in HEK293 cells or A549 cells 48 hours after transfection with pCI-MUC1 over-expression vector or MUC1 siRNA respectively, or controls, followed by serum starvation for 2 hours, then incubation with Pneumocystis organisms for 10 or 60 minutes. The blots were then stripped and re-probed for total ERK1/2 to demonstrate equal loading of samples. Antibodies utilized for immunoblots were either monoclonal rabbit anti-phospho-ERK1/2 or anti-total ERK1/2 (both from Cell Signaling, Danvers, MA), each detected by HRP-conjugated goat anti-rabbit antibodies. The intensity of immunoblot bands was quantified by densitometry analysis. The ratio of phospho-ERK1 to total ERK1 for HEK293 cells or phospho-ERK1/2 to total ERK1/2 for A549 cells was set to 1.0 for controls, and the fold change for other treatments was determined by comparing the ratio to that of the respective controls. Only 10 minute incubation results are shown for all experiments as the 60 minute results were similar.
P. murina binding assays
HEK293 cells transfected with pCI-neo or pCI-MUC1, and A549 cells transfected with control siRNA or MUC1 siRNA were incubated for 48 hours, trypsinized, and plated into wells of an 8-chamber slide. Following cell attachment, equal amounts of partially purified P. murina were added to each chamber. After 16 hours, the supernatant was removed, and the wells were washed with PBS. Cells and bound P. murina were fixed with 4% paraformaldehyde for 15 minutes, washed three times with PBS, blocked with 3% BSA in TBS, and labeled with mouse anti-Msg serum, followed by Alexa Fluor 488-conjugated rabbit anti-mouse antibody, stained with DAPI and mounted with Prolong Gold Antifade Mountant (Thermo Fisher Scientific). Attached organisms were counted in 10 high power fields (600x).
Alternatively, 48 hours after transfection, equal numbers of P. murina were added to each well and incubated for 16 hours. The wells were then washed with PBS, cells and bound organisms were scraped and pelleted, and adherent organisms were quantitated by qPCR or RT-qPCR as described above.
Immunofluorescence for MUC1 and Pneumocystis
For detection of MUC1 expression by HEK293 cells, cells were trypsinized forty-eight hours after transfection with pCI-neo or pCI-MUC1, re-plated into an 8-chamber glass slide, cultured for 16 hours, washed with PBS, fixed with 4% paraformaldehyde, labeled with mouse anti-MUC1 antibody, detected with rabbit anti-mouse Alexa Fluor 488-conjugated antibody, and stained with DAPI.
For in situ studies, a mouse lung highly infected with P. murina was fixed in HistoChoice with 20% EtOH (Sigma-Aldrich), paraffin-embedded, sectioned at 4 μm, and dual-labeled for MUC1 and Msg antigens. The antibodies were applied in the following order: rabbit anti-mouse MUC1 antibody, Alexa Fluor 594-conjugated goat anti-rabbit antibody, mouse anti-Msg serum, and Alexa Fluor 488-conjugated rabbit anti-mouse antibody, with 1x tris buffered saline between each step. To eliminate cross reactivity between the mouse serum and the murine tissue target the protein concentrate and mouse IgG blocking reagent from the Mouse on Mouse Basic Kit from Vector Laboratories (Burlingame, CA) were applied per vendor instructions. Appropriate single labeled and no primary antibody control slides were included in the assays. All slides were stained with DAPI and mounted as described above. Images were captured and deconvolved on a Nikon Eclipse 90i epifluorescence microscope with NIS Elements, version 4.0 (Nikon; Melville, NY). Further processing and analysis of images was done using Imaris version 8.3.1 (Bitplane; Zurich, Switzerland) and Photoshop CC (Adobe; San Jose, CA).
Quantitation of IL-6 and IL-8
A549 cells were transfected with MUC1 siRNA or control siRNA for 48 hours, followed by serum starvation for 24 hours, after which the cells were incubated with or without Pneumocystis for 16 hours, in serum-free medium. Cell culture supernatants were then harvested and stored at −70°C. IL-6 and IL-8 levels in supernatants were determined using human IL-6 and IL-8 ELISA kits (R&D) per the manufacturer’s instructions; the detection sensitivity is 0.7 pg/ml and 7.5 pg/ml respectively. The experiment was performed in triplicate.
Statistical analysis
Quantitative data for Pneumocystis binding were compared using Student’s t-test. IL-6 and IL-8 ELISA results were compared using two way ANOVA followed by Tukey’s multiple comparisons test. Statistical analyses were performed using Prism, version 8.3 (GraphPad Software, San Diego, CA).
Results
Expression of MUC1 by HEK293 cells increases Pneumocystis binding
HEK293 cells, which do not express MUC1, were transfected with vector pCI-MUC1 to over-express MUC1, or with the control vector pCI-neo. By immunoblot using an anti-MUC1 monoclonal antibody, no MUC1 expression was seen following transfection with the control vector, while multiple bands, presumably representing isoforms with different levels of glycosylation, were seen following transfection with pCI-MUC1 (Figure 1A). Similarly, by immunofluorescence labeling of HEK293 cells with the same antibody, MUC1 was visualized only following transfection with pCI-MUC1 and not the control vector or the secondary antibody alone (Figure 1B).
Figure 1.

Expression of MUC1 by transfected HEK293 cells. A. Immunoblot using an anti-MUC1 antibody demonstrates MUC1 expression by HEK293 cells that were transfected with pCI-MUC1 but not pCI-neo. The same blot was stripped, then re-probed with HRP-conjugated anti-β actin antibody to demonstrate equal loading of protein. The multiple bands seen in the MUC1 transfected cells presumably represent different levels of glycosylation.
B. Immunofluorescence demonstrates MUC1 expression by HEK293 cells that were transfected with pCI-MUC1 but not pCI-neo. Cells were labeled with mouse anti-MUC1 antibody, detected with rabbit anti-mouse Alexa Fluor 488-conjugated antibody (green, top panels), and stained with DAPI (blue, bottom panels). Data for A and B are representative of three independent experiments. Bar in the top left panel is 20 μm.
To determine if MUC1 expression modulated binding of Pneumocystis, organisms were incubated with HEK293 cells for 16 hours following transfection with the two plasmids (Figure 2A). The mean (±SD) number of organisms binding to cells transfected with pCI-MUC was ~75% higher compared to cells transfected with pCI-neo (51± 27 vs 29 ± 20 organisms per high power field, p = 0.045. Figure 2B).
Figure 2.


Increased binding of Pneumocystis to HEK293 cells expressing MUC1. A. Immunofluorescence of Pneumocystis attached to HEK293 cells. HEK293 cells transfected with pCI-neo or pCI-MUC1 were incubated with P. murina organisms for 16 hours, washed, fixed with 4% paraformaldehyde, then labeled with mouse anti-Msg serum, detected by Alexa Fluor 488-conjugated rabbit anti-mouse antibody (green, top panels) and stained with DAPI (blue, bottom panels). Bar in the top left panel is 20 μm. B. Bar graphs represent the mean (± SD) number of P. murina organisms per high power fields (HPF, 600x); 10 random high power fields were counted for each transfection condition. Data are representative of three independent experiments. C. Bar graphs represent the mean (± SD) dhfr copies per well using a PCR assay targeting either DNA (qPCR) or RNA (RT-qPCR) in a single experiment with triplicate wells for each. Statistical significance was determined using Student’s t-test.
Increased Pneumocystis binding was confirmed by qPCR and RT-qPCR assays utilizing the Pneumocystis dhfr gene, with 2,518 (±233) dhfr copies per well in the MUC1 overexpressing cells compared to 1,728 (±145) for the Neo control (p=0.008; Figure 2C) by qPCR, and 1,936 (± 60) dhfr copies per well in the MUC1 overexpressing cells compared to 1,255 (±166) for the Neo control (p= 0.003; Figure 2C) by RT-qPCR. Notably, the qPCR results paralleled the RT-qPCR results, suggesting that measuring Pneumocystis DNA provides an accurate assessment of viable organisms.
Knock down of MUC1 in A549 cells decreases Pneumocystis binding
To see if down-regulation of MUC1 leads to decreased binding of Pneumocystis, A549 cells, which are an immortalized human type II pneumocyte line and which constitutively express MUC1, were transfected with MUC1 siRNA or control siRNA. A549 cells were selected in part because they have previously shown some success in attempts to culture Pneumocystis over short periods (Cushion, Ruffolo, Linke, & Walzer, 1985). The efficiency of the siRNA in inhibiting MUC1 RNA and protein expression was demonstrated by immunoblot assays as well as RT-qPCR (Figure 3A and 3B). The mean (±SD) number of organisms binding to cells transfected with MUC1 siRNA was ~70% lower compared to cells transfected with the control siRNA (3.2 ± 1.5 vs 11.2 ± 4.8 organisms per high power field, p<0.0001, Figure 3C).
Figure 3.


Attachment of P. murina to A549 cells decreases following downregulation of MUC1 expression by siRNA. A. Immunoblot using an anti-MUC1 antibody demonstrates decreased MUC1 protein levels in A549 cells that were transfected with MUC1 siRNA compared to control siRNA. The relative expression, normalized to β actin, was determined by densitometry. Anti-β actin antibody demonstrates equal loading of protein. B. Similarly, mRNA levels of MUC1, as determined by RT-qPCR, were lower in A549 cells transfected with MUC1 siRNA compared to control. C and D. P. murina attachment was lower in A549 cells transfected with MUC1 siRNA compared to control, as determined by (C) immunofluorescence (as in Figure 2) or (D) qPCR and RT-qPCR (as in Figure 2). For B-D, values shown represent the mean ± SD. Data are representative of at least three independent experiments, except for the RT-qPCR data which represent results from a single experiment performed in triplicate. Statistical significance was determine using Student’s t-test.
Decreased Pneumocystis binding was confirmed by qPCR and RT-qPCR assays utilizing the Pneumocystis dhfr gene, with 13,547 (±188) dhfr copies per well in the MUC1 siRNA compared to 18,201 (±2,594) for the control by qPCR (Figure 3D, p=0.036), and 8,532 (±1,461) dhfr copy in the MUC1 siRNA compared to 12,441 (±1,010) for the control by RT-qPCR (Figure 3D, p=0.019). The results of qPCR again parallel those of RT-qPCR.
MUC1 is co-localized with Pneumocystis in mouse lungs
A mouse lung with a heavy Pneumocystis infection was labeled with rabbit anti-mouse MUC1 antibody and DAPI. As shown in Figure 4, MUC1 co-localized to some extent with clusters of Pneumocystis, which were identified by the clusters of small nuclei or by staining of organisms using an anti-Msg antibody.
Figure 4.
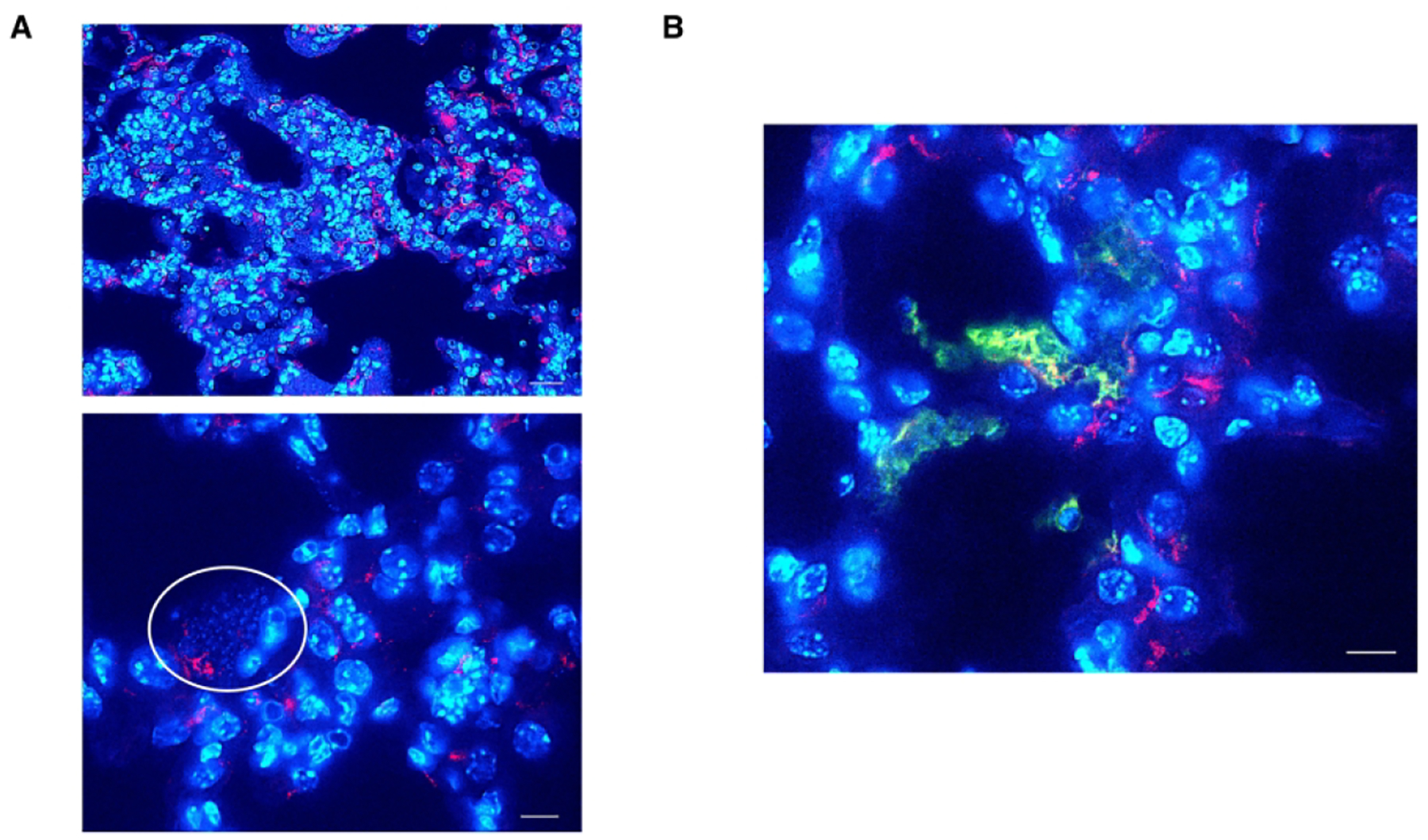
Co-localization of P. murina and MUC1. A section of a mouse lung with a heavy P. murina infection was A. labeled with rabbit anti-mouse MUC1 antibody detected with Alexa Fluor 594-conjugated goat anti-rabbit antibody and stained with DAPI. The circle highlights a cluster of P. murina, identified by multiple small nuclei (blue), proximate to MUC1 (red). The bars are 30 μm and 10 μm, top and bottom images respectively. In B. the same tissue target was dual-labeled with rabbit anti-mouse MUC1 antibody detected with Alexa Fluor 594-conjugated goat anti-rabbit antibody (red) as well as mouse anti-Msg antibody detected with Alexa Fluor 488-conjugated rabbit anti-mouse secondary antibody (green) and stained with DAPI (blue). Yellow-orange indicates colocalization of Msg and Muc1. The bar is 10 μm.
Pneumocystis binding causes phosphorylation of ERK1 in HEK293 cells and ERK1/2 in A549 cells
P. aeruginosa has been shown to phosphorylate MUC1 and ERK2 in a MUC1 transfected Chinese hamster ovary cell line (Lillehoj, Kim, Chun, & Kim, 2004). To see if Pneumocystis induced similar phosphorylation, HEK293 cells were transfected with pCI-MUC1 over-expression vector or pCI-Neo vector and incubated with Pneumocystis. No phosphorylation of MUC1 (Tyr 1229) was detected by immunoblot (data not shown). Pneumocystis binding did result in phosphorylation of ERK1, but this was independent of MUC1 expression (Figure 5A).
Figure 5.

P. murina induces phosphorylation of ERK. A. Phospho-ERK1/2 was detected in HEK293 cells transfected with pCI-neo or pCI-MUC1 after incubation with or without Pneumocystis organisms for 10 or 60 minutes (only 10 minute incubation results are shown as the 60 minute incubation results were similar). Increased phosphorylation of ERK1 was seen following Pneumocystis exposure regardless of MUC1 expression. B. Phospho-ERK1/2 was detected in A549 cells transfected with either a control siRNA or MUC1 siRNA after incubation with or without Pneumocystis organisms for 10 minutes. Either Pneumocystis or MUC1siRNA alone increased phosphorylation of both ERK1 and ERK2, while the 2 combined resulted in greater phosphorylation than either alone. In both sets of blots, the top blot demonstrates labeling of phospho-ERK1/2; the blot was then stripped and re-probed for total ERK1/2 to demonstrate equal protein loading. The table at the bottom of each panel indicates which plasmid or siRNA was used for transfection and whether cells were exposed to Pneumocystis. The relative expression refers to the relative phospho-ERK1 (for A) or phospho-ERK1/2 (for B) levels after normalization to total ERK, compared to lane 1 in each set. The intensity of phospho-ERK and total ERK bands were determined by densitometry.
To study a more biologically relevant cell line, we examined phosphorylation of ERK1/2 by Pneumocystis in A549 cells after transfection with MUC1 siRNA or control siRNA, followed by incubation with Pneumocystis organisms. Pneumocystis caused phosphorylation of ERK1/2 in A549 cells transfected with control siRNA (Figure 5B). Knock down of MUC1 increased phospho-ERK1/2 compared to that of control siRNA, and the combination of MUC1 siRNA and Pneumocystis further increased phospho-ERK1/2 compared to either condition alone (Figure 5B).
Pneumocystis causes a nonsignificant increase in MUC1 RNA expression by A549 cells
To determine whether Pneumocystis can increase MUC1 expression in A549 cells, A549 cells were incubated with or without Pneumocystis for 24 hours, and MUC1 expression was determined by RT-qPCR. There was a non-significant trend towards increased MUC1 expression, by 18%, compared to cells not incubated with Pneumocystis (P=0.087; Figure 6A).
Figure 6.

Effects of Pneumocystis on MUC1 expression and IL-6 and IL-8 secretion A. A549 cells were incubated with or without Pneumocystis for 24 hours, and MUC1 expression was determined by RT-qPCR. A nonsignificant trend towards increased MUC1 expression was seen following incubation with Pneumocystis. B. Triplicate wells of A549 cells were transfected with control siRNA or MUC1 siRNA, serum starved for 24 hours, and then incubated with Pneumocystis for 16 hours; IL-6 and IL-8 levels were measured in the culture supernatants. Incubation with Pneumocystis and transfection with MUC1 siRNA each led to significant increases in both IL-6 and IL-8 secretion, with the highest levels seen when the two were combined. Statistical significance was determined using Student’s t-test (A.) or two way ANOVA followed by Tukey’s multiple comparisons test (B.).
Pneumocystis induces IL-6 and IL-8 secretion by A549 cells
To determine the effects of Pneumocystis and MUC1 on IL-6 and IL-8 secretion, A549 cells were transfected with MUC1 siRNA or control siRNA and then incubated with or without Pneumocystis organisms. Incubation of control siRNA-transfected cells with Pneumocystis increased IL-6 and IL-8 secretion, from 11.4 ±0.4 pg/ml, and 1,456 ±142 pg/ml to 70.9 ±2.9 pg/ml and 3,911 ±214 pg/ml respectively. Knock down of MUC1 further increased IL-6 and IL-8 secretion, from 36.5 ±1.4 pg/ml and 2,639 ±61 pg/ml with no Pneumocystis to 129.8 ±5.4 pg/ml, 6,484 ±531 pg/ml with Pneumocystis, respectively (Figure 6B and 6C).
Discussion
In the current study, we have demonstrated that P. murina organisms can bind to cell-surface expressed MUC1. This was shown by increased binding of organisms to HEK293 cells that overexpressed MUC1 as well as decreased binding to A549 cells with inhibition of MUC1 expression. Further, MUC1 could be co-localized to clusters of Pneumocystis organisms in a heavily infected mouse lung. These results suggest that MUC1 may play a role in the pathogenesis of Pneumocystis infection by allowing binding of inhaled organisms, as a first step to establishing infection.
MUC1 is expressed on the surface of type II pneumocytes as well as other lung airway epithelial cells, and can also be shed from the cell surface. An important function of MUC1 in the lung is to protect underlying cells and facilitate clearance of invading microbes such as Pneumocystis (Rose & Voynow, 2006). Intriguingly, MUC1 can mediate adherence of P. aeruginosa to lung epithelial cells, and appears to facilitate infection and decrease inflammation (Kato et al., 2010; Kato et al., 2017). If it were operating in a similar manner in Pneumocystis infection, it’s possible that MUC1 expression in the alveoli may facilitate infection with Pneumocystis by allowing attachment of air-borne cysts that can subsequently release the replicating trophic forms. Intriguingly, a recent study found an increase in other mucins, including Muc5ac mRNA expression as well as an increase in the numbers of cells expressing MUC5b in the distal airways of immunocompetent infant rats infected with Pneumocystis (Mendez et al., 2019).
Similar to MUC1, HSPA5 was recently shown to mediate P. carinii adherence to a type II pneumocyte cell line (RLE-6TN). Both of these proteins appear to be expressed preferentially by type II rather than type I pneumocytes (Kottom et al., 2018) which suggests that there are redundant mechanisms for potential attachment of Pneumocystis organisms within the alveolus. However, neither is likely to be key to long-term growth and replication of this organism, given that adherence to type I pneumocytes appears to be essential for Pneumocystis growth and replication. It is unlikely MUC1 and HSPA5 represent unique species-specific attachment mechanisms, given that HSPA5 is >99% conserved at the amino acid level in rats and mice.
Factors implicated in mediating binding of Pneumocystis to type I pneumocytes include the extracellular matrix proteins fibronectin and vitronectin, as well as alpha v- and alpha 5-containing integrins expressed on the epithelial cell surface (Limper et al., 1993; Pottratz, Paulsrud, et al., 1994; Pottratz, Weir, & Wisniowski, 1994). Msg (or gp120) of Pneumocystis has been shown to interact with several host glycoproteins, including concanavalin A, fibronectin, and vitronectin and likely represents a major ligand mediating the interactions of Pneumocystis with alveolar epithelial cells as well as macrophages (Limper et al., 1993; O’Riordan et al., 1995; Pottratz, Paulsrud, et al., 1994; Williams et al., 1996). The carbohydrate recognition domain of surfactant proteins A and D also can mediate binding to Msg (McCormack et al., 1997; Vuk-Pavlovic et al., 2001).
MUC1 may also play an anti-inflammatory role in Pneumocystis infection, as it does in P. aeruginosa infection. IL-6 and IL-8 are pro-inflammatory cytokines that can be secreted by pulmonary epithelial cells. Incubation with Pneumocystis induced ERK1/2 phosphorylation and IL-6 and IL-8 secretion by A549 cells, while decreased expression of MUC1 with siRNA further increased both of these processes, suggesting MUC1 has a negative regulatory effect on both. Pneumocystis also showed a trend towards increasing MUC1 expression by A549 cells, which may facilitate infection by allowing increased organism binding and also by decreasing inflammation.
IL-8 is a chemokine that induces an activated neutrophil response (Kunkel, Standiford, Kasahara, & Strieter, 1991), and IL-6 is a multifunctional cytokine with broad immunoregulatory properties (Tanaka, Narazaki, & Kishimoto, 2014). Consistent with our results, a previous study reported that Pneumocystis cell wall beta-glucan stimulated ERK phosphorylation and IL-8 secretion in a human airway epithelial cell line (Carmona, Lamont, Xue, Wylam, & Limper, 2010). We previously demonstrated that IL-6 expression was increased in mice with a heavy Pneumocystis infection, and that treatment with caspofungin, a β-(1,3)-glucan synthase inhibitor, significantly deceased IL-6 expression (Kutty et al., 2016). Although neither IL-6 nor IL-8 appear to be critical in controlling Pneumocystis infection (Chen, Havell, Gigliotti, & Harmsen, 1993), both can exacerbate inflammation associated with Pneumocystis, and have been associated with more severe disease and death in patients with PCP (Chou, Lin, Tsai, & Chang, 2013; Tasaka et al., 2010). The mechanism and the biologic relevance of the phosphorylation of ERK by Pneumocystis need further evaluation.
Characterizing factors that mediate adherence of pathogens to host cells is critical to gain a better understanding the pathogenesis of diseases caused by these organisms. While our study has identified a potential protein involved in Pneumocystis binding, the proteins responsible for the critical attachment to type I pneumocytes need to be identified, since they likely contribute to the highly restricted host species specificity of each Pneumocystis species, and may play a role in meeting the nutritional requirements of this fastidious organism.
Supplementary Material
Supplemental Figure 1. Co-localization of Pneumocystis and MUC1. This panel demonstrates each antigen and DAPI individually, specifically A. DAPI (blue), B. Muc1 (red), C. Msg (green), as well as the merged image already shown in Figure 4B. Bar in A. is 10 μm and applies to all images.
Acknowledgements
We thank Rene Costello for providing animal care, and the staff of the Kansas State University Veterinary Diagnostic Laboratory’s Histopathology Laboratory for technical assistance. This project has been funded by the Intramural Research Program of the NIH Clinical Center, National Institutes of Health and startup funds provided to A. Sally Davis by Kansas State University. All authors declare no conflict of interest.
References
- Bishop LR, Helman D, & Kovacs JA (2012). Discordant antibody and cellular responses to Pneumocystis major surface glycoprotein variants in mice. BMC Immunology, 13, 39. doi: 10.1186/1471-2172-13-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona EM, Lamont JD, Xue A, Wylam M, & Limper AH (2010). Pneumocystis cell wall beta-glucan stimulates calcium-dependent signaling of IL-8 secretion by human airway epithelial cells. Respiratory Research, 11, 95. doi: 10.1186/1465-9921-11-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Havell EA, Gigliotti F, & Harmsen AG (1993). Interleukin-6 production in a murine model of Pneumocystis carinii pneumonia: relation to resistance and inflammatory response. Infect.Immun, 61, 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CW, Lin FC, Tsai HC, & Chang SC (2013). The importance of pro-inflammatory and anti-inflammatory cytokines in Pneumocystis jirovecii pneumonia. Medical Mycology, 51(7), 704–712. doi: 10.3109/13693786.2013.772689 [DOI] [PubMed] [Google Scholar]
- Cushion MT, Ruffolo JJ, Linke MJ, & Walzer PD (1985). Pneumocystis carinii: growth variables and estimates in the A549 and WI-38 VA13 human cell lines. Exp.Parasitol, 60, 43–54. [DOI] [PubMed] [Google Scholar]
- Esteves F, Cale SS, Badura R, de Boer MG, Maltez F, Calderon EJ, … Matos O (2015). Diagnosis of Pneumocystis pneumonia: evaluation of four serologic biomarkers. Clinical Microbiology and Infection, 21(4), 379 e371–310. doi: 10.1016/j.cmi.2014.11.025 [DOI] [PubMed] [Google Scholar]
- Gendler SJ, Lancaster CA, Taylor-Papadimitriou J, Duhig T, Peat N, Burchell J, … Wilson D (1990). Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. Journal of Biological Chemistry, 265(25), 15286–15293. [PubMed] [Google Scholar]
- Hattrup CL, & Gendler SJ (2008). Structure and function of the cell surface (tethered) mucins. Annual Review of Physiology, 70, 431–457. doi: 10.1146/annurev.physiol.70.113006.100659 [DOI] [PubMed] [Google Scholar]
- Jarrard JA, Linnoila RI, Lee H, Steinberg SM, Witschi H, & Szabo E (1998). MUC1 is a novel marker for the type II pneumocyte lineage during lung carcinogenesis. Cancer Research, 58(23), 5582–5589. [PubMed] [Google Scholar]
- Kato K, Lillehoj EP, Kai H, & Kim KC (2010). MUC1 expression by human airway epithelial cells mediates Pseudomonas aeruginosa adhesion. Front Biosci (Elite Ed), 2, 68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Lillehoj EP, Lu W, & Kim KC (2017). MUC1: The First Respiratory Mucin with an Anti-Inflammatory Function. J Clin Med, 6(12). doi: 10.3390/jcm6120110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani T, Katayama S, Miyazaki Y, Fukuda S, Sato Y, & Ohsugi K (2017). Risk Factors for the Mortality of Pneumocystis jirovecii Pneumonia in Non-HIV Patients Who Required Mechanical Ventilation: A Retrospective Case Series Study. Biomed Res Int, 2017, 7452604. doi: 10.1155/2017/7452604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottom TJ, Hebrink DM, & Limper AH (2018). Binding of Pneumocystis carinii to the lung epithelial cell receptor HSPA5 (GRP78). Journal of Medical Microbiology, 67(12), 1772–1777. doi: 10.1099/jmm.0.000864 [DOI] [PubMed] [Google Scholar]
- Kovacs JA, Halpern JL, Swan JC, Moss J, Parrillo JE, & Masur H (1988). Identification of antigens and antibodies specific for Pneumocystis carinii. Journal of Immunology, 140, 2023–20231. [PubMed] [Google Scholar]
- Kovacs JA, & Masur H (2009). Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA, 301(24), 2578–2585. doi: 10.1001/jama.2009.880 [DOI] [PubMed] [Google Scholar]
- Kunkel SL, Standiford T, Kasahara K, & Strieter RM (1991). Interleukin-8 (IL-8): the major neutrophil chemotactic factor in the lung. Experimental Lung Research, 17(1), 17–23. doi: 10.3109/01902149109063278 [DOI] [PubMed] [Google Scholar]
- Kutty G, Davis AS, Ferreyra GA, Qiu J, Huang DW, Sassi M, … Kovacs JA (2016). β-glucans are masked but contribute to pulmonary inflammation during Pneumocystis pneumonia. Journal of Infectious Diseases, 214(5), 782–791. doi: 10.1093/infdis/jiw249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Wang YY, Wirtz D, & Hanes J (2009). Micro- and macrorheology of mucus. Advanced Drug Delivery Reviews, 61(2), 86–100. doi: 10.1016/j.addr.2008.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan MS, Batra SK, Qi WN, Metzgar RS, & Hollingsworth MA (1990). Cloning and sequencing of a human pancreatic tumor mucin cDNA. Journal of Biological Chemistry, 265(25), 15294–15299. [PubMed] [Google Scholar]
- Li Y, & Cozzi PJ (2007). MUC1 is a promising therapeutic target for prostate cancer therapy. Current Cancer Drug Targets, 7(3), 259–271. [DOI] [PubMed] [Google Scholar]
- Li Y, Kuwahara H, Ren J, Wen G, & Kufe D (2001). The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3 beta and beta-catenin. Journal of Biological Chemistry, 276(9), 6061–6064. doi: 10.1074/jbc.C000754200 [DOI] [PubMed] [Google Scholar]
- Li Y, Ren J, Yu W, Li Q, Kuwahara H, Yin L, … Kufe D (2001). The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. Journal of Biological Chemistry, 276(38), 35239–35242. doi: 10.1074/jbc.C100359200 [DOI] [PubMed] [Google Scholar]
- Lillehoj EP, Kim H, Chun EY, & Kim KC (2004). Pseudomonas aeruginosa stimulates phosphorylation of the airway epithelial membrane glycoprotein Muc1 and activates MAP kinase. American Journal of Physiology. Lung Cellular and Molecular Physiology, 287(4), L809–815. doi: 10.1152/ajplung.00385.2003 [DOI] [PubMed] [Google Scholar]
- Limper AH, Standing JE, Hoffman OA, Castro M, & Neese LW (1993). Vitronectin binds to Pneumocystis carinii and mediates organism attachment to cultured lung epithelial cells. Infection and Immunity, 61, 4302–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, & Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods, 25(4), 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- McCormack FX, Festa AL, Andrews RP, Linke M, & Walzer PD (1997). The carbohydrate recognition domain of surfactant protein A mediates binding to the major surface glycoprotein of Pneumocystis carinii. Biochemistry, 36(26), 8092–8099. doi: 10.1021/bi970313f [DOI] [PubMed] [Google Scholar]
- Mendez A, Rojas DA, Ponce CA, Bustamante R, Beltran CJ, Toledo J, … Vargas SL (2019). Primary infection by Pneumocystis induces Notch-independent Clara cell mucin production in rat distal airways. PLoS ONE, 14(6), e0217684. doi: 10.1371/journal.pone.0217684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard PR, Wakefield AE, & Hopkin JM (1990). A sequential ultrastructural study of rat lungs infected with Pneumocystis carinii to investigate the appearances of the organism, its relationships and its effects on pneumocytes. International Journal of Experimental Pathology, 71, 895–904. [PMC free article] [PubMed] [Google Scholar]
- O’Riordan DM, Standing JE, Kwon KY, Chang D, Crouch EC, & Limper AH (1995). Surfactant protein D interacts with Pneumocystis carinii and mediates organism adherence to alveolar macrophages. J Clin Invest, 95, 2699–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P, Kharbanda S, & Kufe D (1995). Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Research, 55(18), 4000–4003. [PubMed] [Google Scholar]
- Pottratz ST, Paulsrud JR, Smith JS, & Martin WJ (1994). Evidence for Pneumocystis carinii binding to a cell-free substrate: role of the adhesive protein fibronectin. Journal of Laboratory and Clinical Medicine, 123, 273–281. [PubMed] [Google Scholar]
- Pottratz ST, & Weir AL (1995). Attachment of Pneumocystis carinii to primary cultures of rat alveolar epithelial cells. Experimental Cell Research, 221, 357–362. [DOI] [PubMed] [Google Scholar]
- Pottratz ST, Weir AL, & Wisniowski PE (1994). Pneumocystis carinii attachment increases expression of fibronectin-binding integrins on cultured lung cells. Infection and Immunity, 62, 5464–5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MC, & Voynow JA (2006). Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiological Reviews, 86(1), 245–278. doi: 10.1152/physrev.00010.2005 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Narazaki M, & Kishimoto T (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol, 6(10), a016295. doi: 10.1101/cshperspect.a016295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka S, Kobayashi S, Kamata H, Kimizuka Y, Fujiwara H, Funatsu Y, … Hasegawa N (2010). Cytokine profiles of bronchoalveolar lavage fluid in patients with Pneumocystis pneumonia. Microbiology and Immunology, 54(7), 425–433. doi: 10.1111/j.1348-0421.2010.00229.x [DOI] [PubMed] [Google Scholar]
- Thornton DJ, Rousseau K, & McGuckin MA (2008). Structure and function of the polymeric mucins in airways mucus. Annual Review of Physiology, 70, 459–486. doi: 10.1146/annurev.physiol.70.113006.100702 [DOI] [PubMed] [Google Scholar]
- Vestereng VH, Bishop LR, Hernandez B, Kutty G, Larsen HH, & Kovacs JA (2004). Quantitative real-time polymerase chain-reaction assay allows characterization of Pneumocystis infection in immunocompetent mice. Journal of Infectious Diseases, 189(8), 1540–1544. [DOI] [PubMed] [Google Scholar]
- Vuk-Pavlovic Z, Standing JE, Crouch EC, & Limper AH (2001). Carbohydrate recognition domain of surfactant protein D mediates interactions with Pneumocystis carinii glycoprotein A. American Journal of Respiratory Cell and Molecular Biology, 24(4), 475–484. doi: 10.1165/ajrcmb.24.4.3504 [DOI] [PubMed] [Google Scholar]
- Wang H, Lillehoj EP, & Kim KC (2003). Identification of four sites of stimulated tyrosine phosphorylation in the MUC1 cytoplasmic tail. Biochemical and Biophysical Research Communications, 310(2), 341–346. doi: 10.1016/j.bbrc.2003.09.030 [DOI] [PubMed] [Google Scholar]
- Williams MD, Wright JR, March KL, & Martin WJ 2nd. (1996). Human surfactant protein A enhances attachment of Pneumocystis carinii to rat alveolar macrophages. American Journal of Respiratory Cell and Molecular Biology, 14(3), 232–238. doi: 10.1165/ajrcmb.14.3.8845173 [DOI] [PubMed] [Google Scholar]
- Yoneda K, & Walzer PD (1980). Interaction of Pneumocystis carinii with host lungs: an ultrastructural study. Infection and Immunity, 29, 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda K, & Walzer PD (1983). Attachment of Pneumocystis carinii to type I alveolar cells studied by freeze-fracture electron microscopy. Infection and Immunity, 40, 812–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Co-localization of Pneumocystis and MUC1. This panel demonstrates each antigen and DAPI individually, specifically A. DAPI (blue), B. Muc1 (red), C. Msg (green), as well as the merged image already shown in Figure 4B. Bar in A. is 10 μm and applies to all images.


