Abstract
Since the first discovery of the lysosome and the definition of autophagy by Christian de Duve more than 60 years ago, research on autophagy, a process targeting cytoplasmic materials for lysosomal degradation and recycling, has expanded dramatically. This research has extended our understanding of the basic mechanism of autophagy as well as its role in pathophysiology. Autophagy deficiency has been reported to be involved in numerous diseases, among which cancer has been extensively studied, in part because autophagy appears to play a dual role, depending on the stage of tumorigenesis. In this review, we will briefly revisit the intriguing history of autophagy and cancer, underscoring the importance of harnessing this pathway for the benefit of human health.
Keywords: ATG, autophagy, cancer, lysosome, stress, tumor
1. Introduction
Autophagy, meaning “self-eating”, is a highly regulated cellular primarily catabolic process, conserved from yeast to more complex eukaryotes, including humans [1]. During autophagy, cytoplasmic materials are delivered into the lysosome (or the analogous organelle, the vacuole, in yeast) for degradation by resident hydrolases; the resulting breakdown products are recycled back to the cytoplasm to maintain cellular homeostasis [2]. Under normal nutrient conditions, autophagy occurs at a basal level, which can play a critical role in cell physiology by inhibiting the otherwise gradual accumulation of damaged protein aggregates and organelles; thus autophagy functions in part as a quality control system [3]. When cells face particular stimuli or stress from the intracellular or extracellular microenvironment, autophagy can be massively upregulated to handle the increased demand. The normal functions of autophagy are also fundamental in maintaining other aspects of cell physiology, such as efficient cell metabolism and genomic integrity [4].
In mammalian cells, there are various types of autophagy, which can be differentiated based on the mechanisms of cytoplasmic sequestration as well as the form of cargo delivered to the lysosome [5]. Among these types, macroautophagy is the best characterized [6]. Accordingly, we primarily focus on macroautophagy (simply referred to as autophagy hereafter) in this review.
The malfunction of autophagy is associated with various diseases, including cancer, aging, metabolic and neurodegenerative diseases [7]. Compared to other pathophysiologies, the role of autophagy is more complicated in cancer because autophagy can either inhibit or promote tumorigenesis [8]. Some of the pioneering work connecting autophagy and cancer can be traced back to the 1970s and 1980s. For example, in 1976, researchers found that deprivation of serum and amino acids can induce autophagy in HeLa cells [9]. Later, a 1977 study from Gunn and colleagues compared the proteolysis rate between non-growing rat liver hepatocytes and chemically transformed hepatoma, as well as mouse fibroblasts and virus-transformed fibroblasts. The transformed cells exhibit a lower rate of protein degradation, which became an initial indicator for analyzing autophagy in cancer cells [10]. In this review, we will briefly summarize the mechanism of autophagy, and its involvement in cancer based on a timeline of the past 20 years, coinciding with the identification of the molecular machinery involved in autophagy. The potential of autophagy as a therapeutic target for cancer treatment will be further discussed.
2. Molecular Mechanism of Autophagy
The key morphological feature of autophagy is the formation of a double-membrane vesicle termed the autophagosome, which is derived from a phagophore [11]. The mammalian phagophore is initially generated near the endoplasmic reticulum (ER), and different membrane sources can contribute to the formation of this transient double-membrane structure [12, 13]. The phagophore is the active sequestering compartment of autophagy; this structure expands and ultimately seals to form the autophagosome [14]. The completed autophagosome subsequently fuses with a lysosome; in many cell types the autophagosome may first fuse with an endosome, and the resulting amphisome then fuses with the lysosome. The fusion of the autophagosome or amphisome with the lysosome generates an autolysosome. In this compartment, the cytoplasmic cargo is exposed to the lumenal contents of the lysosome and subsequently degraded by resident hydrolases [15, 16].
Although autophagy had been studied morphologically and pharmacologically since the 1950s, detailed studies into the molecular mechanism of autophagy only started in the late 1990s, following the analysis of this pathway in yeast, and the identification of the autophagy-related (ATG) genes [17]. At present, over 40 ATG genes have been identified in yeast, and, because this process is evolutionarily conserved, many of them have homologs in all other eukaryotes. The ATG proteins participate in different stages of autophagy including induction, nucleation of the phagophore, expansion of the phagophore, autophagosome completion and fusion with the lysosome, and the final steps of degradation and efflux of the breakdown products; those components that are required to form an autophagosome are referred to as the core autophagy machinery [18–21]. Based on their roles in these different stages, the core ATG proteins can be roughly categorized into the following major groups (Fig. 1): A. The ULK kinase complex (including ULK1 or ULK2, RB1CC1/FIP200, ATG13 and ATG101) can phosphorylate downstream factors for the induction of autophagy; B. The class III phosphatidylinositol 3-kinase (PtdIns3K) complex 1, mainly composed of BECN1/beclin 1, PIK3C3/VPS34, PIK3R4/VPS15, ATG14 and NRBF2, acts in phagophore nucleation; C. The only transmembrane protein, ATG9A works together with ATG2A/ATG2B and WDR45/WIPI4 to function in phagophore membrane elongation; D. Two ubiquitin-like systems function in the completion of the autophagosome, utilizing ATG12 (along with components ATG5, ATG7, ATG10, and ATG16L1) and LC3/GABARAP (along with ATG3, ATG4A to ATG4D, ATG7 and WIPI2); there are seven isoforms of LC3/GABARAP, referred to as Atg8-family proteins because of their homology to yeast Atg8. These core ATG proteins may not only work at one stage. For example, when binding to UVRAG (UV irradiation resistance associated) instead of ATG14, BECN1 functions as part of the PtdIns3K complex 2 to help in autophagosome formation and fusion with the lysosome [22]. In addition, RB1CC1/FIP200 may interact with ATG16L1 and regulate its targeting to the phagophore [23]. ATG13, a component of the ULK kinase complex, can also interact with ATG9A for the recruitment of the latter to the site of autophagosome formation [24].
Figure 1.
A model of different stages of macroautophagy.
Autophagy can be divided into several stages from induction to final degradation and efflux. Key proteins that participate in each stage are indicated (see the text for details).
In addition to the above-mentioned core ATG proteins, the success of autophagy also relies on other key factors. The autophagy cargo receptor SQSTM1/p62 can recognize ubiquitin on cargo and deliver it to the phagophore by interacting with LC3 on the concave side of the membrane [25]. Various receptors function in different types of selective autophagy, connecting the cargo with the phagophore via binding to an Atg8-family protein. The RAB GTPase RAB7 and the HOPS complex, along with SNARE proteins (STX17, SNAP29 and VAMP8), modulate various processes related to autophagy, such as autophagosome maturation, lysosomal biogenesis and maintenance, as well as autophagosome-lysosome fusion [26] (Fig. 1).
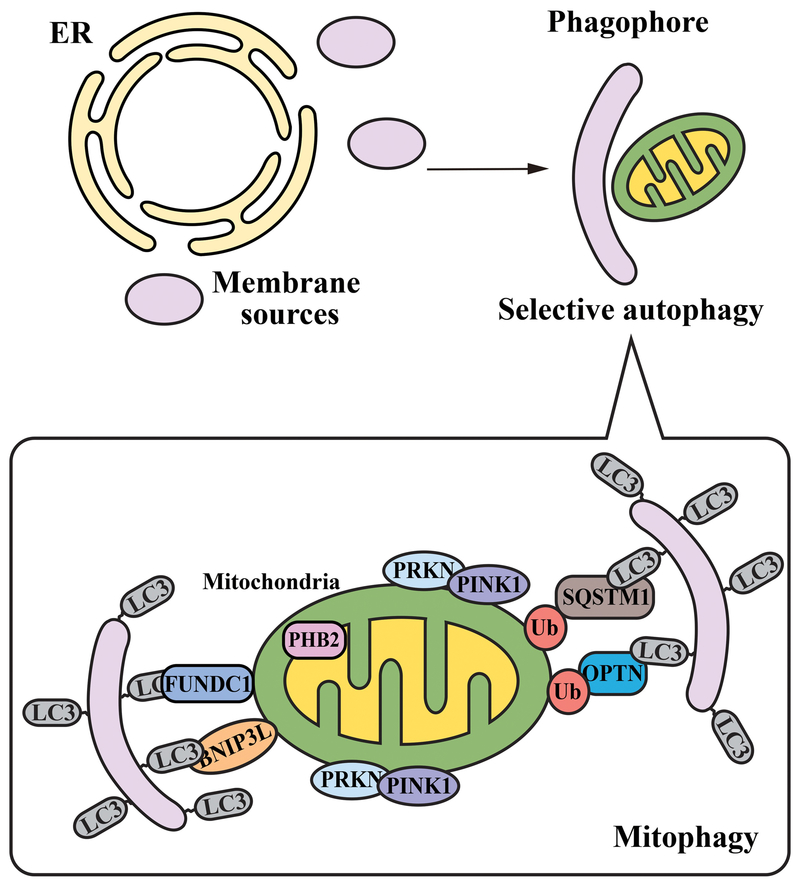
As alluded to above, autophagy can either be non-selective or selective: the non-selective mode is thought to involve degradation of random portions of cytoplasm, whereas the selective mode is highly specific for certain components [1, 27]. Mitophagy is one of the best-studied types of selective autophagy, which involves the sequestration of damaged or superfluous mitochondria and prevents the generation of reactive oxygen species (ROS) [28]. This process is essential for maintaining the integrity of mitochondria and oxygen homeostasis for cellular and organismal survival. PINK1 (PTEN induced kinase 1) and the E3 ubiquitin ligase PRKN/Parkin are two well-characterized factors that mediate mitophagy; however, mitophagy can also be independent of PINK1-PRKN, and not all mammalian cells express PRKN [29]. Mitophagy receptors include SQSTM1 and OPTN, which can bind to LC3 and recognize the polyubiquitinated mitochondria [30, 31]. Other receptors include LIR-containing outer mitochondrial membrane proteins BNIP3L/Nix and FUNDC1, as well as the inner mitochondrial membrane protein PHB2 [32] (Fig. 2). Different studies have reported selective autophagic degradation of other components including, but not limited to, protein aggregates (aggrephagy), peroxisomes (pexophagy), lysosomes (lysophagy), endoplasmic reticulum (reticulophagy), pathogen invasion (xenophagy), ferritin (ferritinophagy) and glycogen (glycophagy) [5].
Figure 2.
Mitophagy as an example of selective autophagy.
The key difference between nonselective and selective autophagy is the specificity of cargo; selective autophagy is highly specific for certain cellular components. This is a composite schematic illustrating some of the different receptors that are involved in various types of mitophagy.
3. A Brief History of Autophagy and Cancer
As made clear by various studies, autophagy is important for multiple aspects of cancer biology, and its role in tumorigenesis is context dependent [33]. That is, autophagy can be tumor-suppressive, tumor-promoting or even neutral, depending on the dynamic progression of the cancer [34]. During tumor development, cytoprotective autophagy plays an anticancer role by maintaining cellular and genomic integrity. In 2007, Eileen White’s group showed that autophagy can suppress tumor progression by restricting chromosomal instability, and a similar result has been further validated in a recently published paper from Jan Karlseder’s group [35, 36]. Alternatively, a 2015 study suggested that defective autophagy may interfere with cellular senescence, a process that can limit the proliferation of damaged cells; thereby leading to aberrant proliferation of cancer progenitor cells [37]. Once a tumor is formed, autophagy may have an opposite outcome, enabling tumor progression and proliferation [38].
In addition, the functional consequences of autophagy can expand to endothelial, stromal and immune cells of the tumor microenvironment, further reinforcing the complicated role of autophagy in cancer. For instance, the upregulation of angiogenesis, a mechanism by which tumors gain nutrients and oxygen, has been regarded as a hallmark of tumorigenesis [39]. The balance between pro- and anti-angiogenic factors governs the angiogenic switch in cancer cells. Several studies have shown that autophagy defects can either cause cerebrovascular disorders [40], or show increased angiogenic potential [41, 42]; therefore, autophagy has both both pro- and anti-angiogenic effects. The cancer-associated fibroblasts and cancer-associated adipocytes are examples of cancer-related stromal cells that can exhibit upregulation of autophagy to drive cancer progression [43, 44]. In these cells, autophagy participates in several intricate signaling pathways to influence tumor progression, and may represent a future target for tumor treatment [8]. Furthermore, the relation between autophagy and immunity under the context of cancer is also complicated. Autophagy can play a bona fide role in both innate and adaptive immunity by controlling antigen presentation and modulating lymphocyte homeostasis, while the immune system can modulate autophagy in turn [45–47].
Overall, the controversial role of autophagy in cancer leads to another question for cancer therapeutics: is it better to inhibit or to enhance autophagy? The last decade has witnessed a surge in research into the role of autophagy in cancer, and several comprehensive reviews have been published on the connection of autophagy and tumorigenesis [8, 33, 48, 49]. Here, instead of explaining the complicated relation between cancer and autophagy, we will briefly review the history of autophagy and cancer, with a focus on key events that occurred in the past 20 years (Fig. 3).
Figure 3.
A timeline of studies on autophagy and cancer.
Since the first identification of BECN1 as a tumor suppressor in 1999, the field of autophagy and cancer has expanded tremendously. The graph displays the number of papers retrieved from an EndNote PubMed search using “All fields” of “autophagy” and “cancer” for each indicated year. Some selected key findings during the past 20 years are noted.
3.1. Core ATGs in Cancer
The first landmark study to link autophagy and cancer was from Beth Levine’s group in 1999, which identified BECN1, the mammalian homolog of yeast Vps30/Atg6 (an essential component for autophagy activity), as a tumor suppressor [50]. Later, a 2003 study from the Levine group used the targeted mutant mouse model to further demonstrate that heterozygous disruption of BECN1 can promote tumorigenesis [51]. That same year, Zhenyu Yue, Shengkan Jin, Arnold Levine and Nathaniel Heintz published a paper further supporting the view that BECN1 is a haploinsufficient tumor suppressor [52]. Subsequent studies have confirmed that BECN1 is monoallelically lost in several cancer cell lines while Becn1 heterozygous mutant mice are prone to develop various malignancies [53]. Downregulation of BECN1 mRNA expression is also associated with poor prognosis in human breast cancers [54]. The importance of BECN1-mediated autophagy in tumor suppression is further strengthened by its association with UVRAG. The latter is a coiled-coil protein, which is monoallelically mutated at high frequency in human colon and breast cancers [55]. In 2006, Jae Jung’s lab found that UVRAG can interact with BECN1, activate autophagy and suppress colon cancer tumorigenicity [56]. Through UVRAG, SH3GLB1/Bif-1 (SH3 domain containing GRB2 like, endophilin B1) forms a complex with BECN1 and regulates autophagosome formation. Furthermore, SH3GLB1 ablation significantly enhances the development of spontaneous tumors in a BECN1-dependent manner in mice [57].
In addition, several studies have been carried out with the goal of revealing the direct link between other ATG proteins and cancer. For example, a 2009 study from Sug Hyung Lee’s lab observed frameshift mutations of ATG2, ATG5, ATG9 and ATG12 in gastric and colorectal cancers with microsatellite instability [58]. In 2011, Noboru Mizushima’s group generated autophagy-deficient mice with a systemic mosaic deletion of ATG5 or the liver-specific knockout of ATG7, both of which spontaneously accumulate benign liver adenomas [59]. A further study from Eileen White’s lab in 2013 showed that the knockout of Atg7 initially promotes BRAFV600E-driven lung tumor growth, but ultimately stalls tumor growth and promotes oncocytic differentiation in mice [60]. In contrast, work from Guido Kroemer, Josef Penninger and colleagues suggested that Atg5 deletion might delay the onset of lung cancer in a KRAS-driven model, but enhance the survival of tumors at a later stage [61].
3.2. Autophagy Receptors and Cancer
In response to stress, autophagy needs different receptors to target misfolded proteins and dysfunctional organelles to phagophores. As one of the best-defined autophagy receptors, SQSTM1, was first found residing in the late endosome-lysosome, binding ubiquitin-associated domains and LC3-interacting regions. Unlike other autophagy receptors, SQSTM1 is a central hub due to its ability to interact with several key signaling proteins [62]. A significant study from Eileen White’s group in 2009 demonstrated that defective autophagy could be a mechanism responsible for SQSTM1 accumulation in human tumors [63]. This finding was further confirmed by Masaaki Komatsu and colleagues, and their work suggested that upon autophagy loss there was a persistent activation of NFE2L2/Nrf2 through SQSTM1, which contributes to hepatocellular carcinoma [64]. In contrast, according to a 2014 study from the labs of Jorge Moscat and Maria Diaz-Meco, SQSTM1 is maintained at a reduced level for further tumor progression in cancer-associated fibroblasts, leading to decreased MTORC1 activity and a concomitant increase in ROS levels [65]. Therefore, SQSTM1 may also hold a controversial role in affecting cancers through autophagy.
3.3. Regulation of autophagy in Cancer
Given the essential role of autophagy in cellular homeostasis and survival, either insufficient or excessive autophagy can seriously compromise cell physiology and be associated with many pathologies including cancer [66]. Therefore, the magnitude of autophagy should be tightly regulated to ensure appropriate levels. Numerous studies have focused on understanding the complex regulatory network that controls autophagy and identified several tumor-suppressive and oncogenic autophagy regulators, many of which play a major role in the autophagic regulatory network.
3.3.1. MTOR Signaling Pathway and Cancer
The best-known negative modulator of autophagy is the mechanistic target of rapamycin kinase complex 1 (MTORC1), a serine-threonine protein kinase, which acts on the core autophagy machinery in response to growth factors and nutrients [67]. MTORC1 can inhibit autophagy initiation by phosphorylation of the ULK1-ATG13-RB1CC1 complex when nutrients are available, or repress autophagosome formation by preventing PtdIns3K complex 1 activity [68, 69]. More importantly, dysregulated MTORC1 is related with cancer progression [70, 71]. The phosphoinositide 3-kinase complex and the serine/threonine kinase AKT work upstream of MTOR, thus forming the phosphoinositide 3-kinase-AKT-MTOR signaling pathway, the activation of which is regarded as oncogenic in many human cancers [72]. Several tumor suppressive proteins can repress MTORC1 oncogenic activity in autophagy. The tuberous sclerosis tumor suppressor complex (TSC1-TSC2) is one of the primary negative regulators of MTORC1 [73]. Different studies have reported that genetic mutations in the corresponding two genes can cause tumor development in various tissues [74]. PTEN, which is a frequently mutated tumor suppressor in human cancers, also suppresses MTOR activity, subsequently activating autophagy [75, 76]. Under conditions of energy depletion, 5′-AMP-activated protein kinase (AMPK) is a central energy sensor that participates in the inhibition of MTORC1 by TSC1-TSC2, and AMPK has also been found defective in a variety of human cancers [77].
Active MTORC1 can also directly phosphorylate TFEB, a master regulator of transcription. Phosphorylated TFEB is retained in the cytoplasm in growing conditions, whereas after starvation dephosphorylated TFEB translocates to the nucleus to bind to promoter regions of numerous genes involved in protein turnover [78]. Altered TFEB expression is associated with pancreatic cancer cell proliferation and non-small cell lung cancer motility [79, 80]. Importantly, in 2015, Nabeel Bardeesy and colleagues discovered that constitutively active TFEB can escape from MTORC1 inhibition and enable pancreatic ductal adenocarcinoma cells to benefit from the activation of autophagy, which affords metabolic fine-tuning and adaptation to stress [81].
3.3.2. BCL2, DAPK and Cancer
Of note, BECN1 was originally identified as a BCL2 (BCL2 apoptosis regulator)-interacting protein [82]. The upregulated expression of BCL2 has been confirmed in many human cancers and can mediate resistance of cancers to chemotherapeutics and radiotherapy [83]. Pioneering work from Beth Levine’s lab in 2005 proposed that BCL2 is an anti-autophagy protein, due to its inhibitory interaction with BECN1 [84]. Therefore, oncogenic BCL2, together with the tumor suppressive BECN1, can play a central role in the cross-regulation between autophagy and apoptosis in determining the fate of cancer cells. Similarly, DAPK (death associated protein kinase), belonging to a family of five serine/threonine kinases that possess tumor suppressive function, also mediates a wide range of cellular processes, including apoptosis and autophagy [85]. Work from Adi Kimchi’s lab revealed that this kinase can phosphorylate BECN1, disrupt interaction between BCL2 and BECN1, and induce autophagy [86]. Other studies have also shown that DAPK can activate autophagy by interacting with TSC2 to inhibit MTOR activity or by forming a stable complex with the microtubule-associated protein MAP1B, which in turn associates with LC3-II [87, 88].
3.3.3. TP53 and Cancer
TP53/p53, which exists in both the nucleus and cytosol, is a critical component of the cellular response to acute stress. Nuclear TP53 is a well-defined transcription factor, whereas cytoplasmic TP53 works independently of its gene regulation ability [89]. The loss of TP53 has been strongly associated with an increased susceptibility to cancer, and this gene is the most frequently mutated gene in human cancers, indicating its significance as a tumor suppressor [90]. Perhaps the first piece of evidence connecting TP53 and autophagy came from Shengkan Jin’s lab in 2005, when they found that activation of TP53 can inhibit MTOR activity by transactivating AMPK and promoting AMPK-dependent phosphorylation of TSC1-TSC2, therefore leading to autophagy induction [91]. A further 2006 study from Kevin Ryan’s lab identified DRAM (DNA damage regulated autophagy modulator), a lysosomal protein, as a novel TP53 target required for autophagy induction [92]. In this study, the expression of DRAM was also found to be downregulated in a subset of epithelial cancers. Interestingly, a 2008 study from Guido Kroemer and colleagues pointed out that the inactivation of cytosolic TP53 could repress MTOR activity and induce autophagy, suggesting a negative role of this population of TP53 in autophagy, in contrast with two previous studies showing the positive role of nuclear TP53 [93]. Therefore, TP53 can exert a bidirectional control of autophagy depending on its subcellular location. Another study from Kevin Ryan’s lab in 2013 showed that TP53 can decide the role of autophagy in tumor development in a genetically-modified mouse model of pancreatic ductal adenocarcinoma [94]. Loss of autophagy in the mouse initially inhibits tumor development; however, after the knockout of TP53, this inhibition is reversed to accelerate tumor onset by enhanced glucose uptake and anabolism.
3.3.4. microRNAs and Cancer
As a class of evolutionarily conserved non-coding RNA molecules with 18-25 nucleotides in length, microRNAs (miRNAs) regulate gene expression at both post-transcriptional and translational levels [95]. It has been proposed that miRNAs are related to different aspects of cancer biology and play multifaceted roles depending on targeted genes and cancer types. The first identified miRNA tumor suppressors were MIR15A and MIR16-1, which are frequently lost in chronic lymphocytic leukemia, and can target BCL2 family members [96]. In 2009, Zhu and colleagues further identified the first autophagy-related miRNA, MIR30A, that can inhibit autophagy by downregulating BECN1 expression in cervical cancer cells [97]. At present, numerous tumor-associated miRNAs that have been identified, some of which are likely to be novel and potent regulators of autophagy in cancer.
3.4. Autophagy in Different Cancer stages
As mentioned above, during tumor progression, autophagy can be initially tumor suppressive in the initiation stage but can then be tumor promoting once a tumor is formed. It is clear that as carcinomas progresses to higher grades of malignancy, cancer cells can develop alterations to increase motility, resulting in migration to other sites [98]. This process is referred to as cancer metastasis, which is also a crucial hallmark of tumorigenesis. In addition, in recent years, several studies have demonstrated the existence of neoplastic cells that are responsible for intratumor heterogeneity, termed cancer stem cells (CSCs) [99]. Of no surprise, accumulated evidence has suggested that autophagy can play an important role in these two unique cancer stages.
3.4.1. Autophagy and Cancer Metastasis
There are several different stages of cancer metastasis, from local invasion to the final outgrowth at a second site [100]. The role of autophagy in metastasis is complicated, because it may serve both pro- and anti-metastatic functions depending on the contextual demands throughout the metastatic process [101]. Autophagy can be used to cope with the environmental and cellular stresses during metastasis. For example, a 2006 study from Eileen White and colleagues pointed out that by promoting survival during hypoxia and metabolic stress, autophagy decreased tumor cell necrosis and consequent macrophage infiltration of the primary tumor; thus attenuating the induction of metastasis [102]. However, a 2009 study from Marja Jäättelä’s group showed that autophagy is upregulated in cancer cells that are resistant to the metastasis suppressor TNFSF10/TRAIL, suggesting that autophagy upregulation might help in cancer metastasis [103]. Furthermore, the success of metastasis requires carcinoma cells to survive when the extracellular matrix contact is ablated [104]. Autophagy was first proposed to promote cell survival during extracellular matrix detachment in a 2008 study from Jayanta Debnath’s lab [105]. Later, a 2011 study used mammary tumor models to demonstrate that this detachment-induced autophagy is associated with ROS-dependent activation of EIF2AK3/PERK [106]. In addition, in 2014, the Debnath lab linked autophagy with increased tumor cell invasion by showing that autophagy is required for the production of the pro-migratory cytokine IL6 [107]. Kay Macleod’s group showed in 2015 that defects in mitophagy can promote cancer progression to metastasis [108]. Of note, during tumor dormancy, cancer cells can exist undetected while still harboring the ability to form metastases [109]. Pioneering work in 2008 from Robert Bast Jr and colleagues first established the connection between autophagy and tumor cell dormancy: the tumor suppressor DIRAS3/ARHI can induce autophagy and promote survival of dormant cells [110].
3.4.2. Autophagy and CSCs
In the past 10 years, studies on autophagy and CSCs have expanded rapidly. Like normal stem cells, CSCs hold pluripotency, which allows for the ability of self-renewal and maintenance of an undifferentiated state [111]. The first line of evidence from Javier Menendez’s lab in 2011 demonstrated that autophagy is required for the breast cancer stem-like phenotype [112]. Two separate studies from 2013 further supported the idea that breast CSCs are often characterized by increased autophagy activity: these CSCs may depend on autophagy to establish carcinoma efficiently, because tumor formation can be abolished by the genetic inhibition of BECN1 or ATG4A [113, 114]. In 2016, Jun-Lin Guan’s lab showed that ablation of RB1CC1, resulting in decreased autophagy, can impair tumorigenicity of breast CSCs by interfering with different intracellular signaling pathways [115]. Further studies have shown that autophagy is also related to various CSCs including liver, pancreatic, osteosarcoma, ovarian, and gliobastoma [116].
In addition, recent findings have revealed the connection between mitophagy and CSCs. In 2017, Jing-Hsiung James Ou’s group found that mitophagy could positively modulate hepatic CSCs by controlling the activity of TP53 and another transcription factor, NANOG [117], and a 2018 study from Craig Jordan’s and colleagues showed that AMPK-mediated mitophagy is required for a healthy mitochondrial network and leukemia CSCs self-renewal [118].
3.5. Targeting Autophagy in Cancer
Because autophagy plays a dichotomous role in cancer, researchers debate about whether to inhibit or to induce this process when regarding autophagy as a therapeutic target. As a master regulator of autophagy, MTOR has become an appealing therapeutic target. The widely-used MTOR inhibitor rapamycin and its paralogs can stimulate autophagy [119]. One of the paralogs, everolimus (RAD001), has been approved by the FDA as an anti-cancer treatment in several cancers such as advanced breast cancer, advanced renal cell carcinoma and certain types of pancreatic, lung, and gastrointestinal neuroendocrine tumors [120]. However, the effectiveness of MTOR inhibitors may depend on the specific cancer cell types, and MTOR signaling is crucial for other processes such as cell metabolism; thus a block of MTOR activity may lead to other unexpected effects.
Some FDA-approved anti-cancer drugs induce autophagy, such as the BCR-ABL tyrosine kinase inhibitor imatinib and the epidermal growth factor receptor (EGFR) antibody cetuximab [121]. Conversely, inhibition of autophagy may also increase the efficacy of these anti-neoplastic drugs. For example, a study has shown that blocking autophagy at a late stage might increase the efficiency of imatinib for malignant glioma [122]. Additionally, autophagy suppression could help enhance cetuximab treatment of metastatic colorectal cancer [123].
Chloroquine (CQ) and hydroxychloroquine (HCQ) are two of the most widely tested drugs that inhibit autophagy, which have been used for pre-clinical trials; these drugs can deacidify the lysosome and block fusion of autophagosomes with lysosomes [124]. One early line of evidence regarding these drugs came from a 2003 study, showing CQ has an anti-cancer function in patients with glioblastoma [125]. Vladimir Trajkovic’s group further reinforced this idea that CQ-mediated lysosomal dysfunction could enhance its anticancer effect [126]. Autophagy can also be a side effect of some anti-cancer therapies; thus, autophagy inhibition can be applied to enhance anti-tumor efficacy [127]. An early combinatory work from Ravi Amaravadi and Craig Thompson using mouse models was done in 2007, which revealed that genetic or pharmacological autophagy suppression together with anti-cancer drugs can improve anti-tumor results [128]. This study also suggested that at a range of doses, the anti-tumor effects of HCQ were likely to occur through autophagy inhibition [128]. After this initial study, phase I and phase II trials continued on combining HCQ with antineoplastic regimens in patients with a wide range of tumors, and some of them have shown encouraging results for future therapeutics [129]. Nonetheless, although several groups have launched clinical trials of CQ and HCQ, the biggest problem with these drugs is the lack of specificity because they can interfere with other important biological processes. For example, in 2016, an extensive examination using CQ on a large number of human cancer cell lines showed that autophagy might be dispensable for the drug’s efficacy [130]. Therefore, to date, there are no clinically available inhibitors of autophagy to treat cancer. Alternatively, developing drugs targeting other autophagy molecules for cancer treatment are promising. Along these lines, there are reports about pharmacological inhibition of the BECN1-containing PtdIns3K complex, the ULK kinase complex, ATG4B and ATG7, but specificity and efficacy of the drugs need further testing [127].
In 2013, Beth Levine’s group showed that a fusion peptide, Tat-beclin 1, could be a potent specific autophagy inducer, which has potential efficacy in treating diseases [131]. Later, in 2018, the same group verified that Tat-beclin 1 can inhibit tumor growth of ERBB2/HER2-positive breast cancers, as effectively as the clinically used ERBB2/HER2 tyrosine kinase inhibitor [132]. Concomitant with the improved understanding of autophagy and immunology in cancer, Guido Kroemer and colleagues pointed out the importance of autophagy in immunogenic cell death [133]; Tat1-beclin1, in conjunction with other agents, can improve the therapeutic outcome of immunogenic chemotherapies and enhance anti-cancer immunosurveillance [134].
4. Conclusions and Future Challenges
Looking back to the short but intriguing history connecting autophagy and cancer, we can see that tremendous work has been done to understand their relation, and to target autophagy for future therapy. However, an enigma still exists because the role of autophagy can change from constraining cancer initiation to promoting later tumorigenesis. The same autophagy-related molecules may play distinct roles under different conditions. More importantly, autophagy may function in a highly dynamic network not only restrained to intracellular homeostasis, which makes the role of autophagy more complicated.
Autophagy is a double-edged sword in cancer therapy, and it remains to be established whether autophagy should be inhibited or induced for therapeutic benefits, although various clinical trials for blocking autophagy in cancers are undergoing. Even for the inhibition of autophagy, we should consider the balance between potency and toxicity, due to the crucial role of autophagy in cell homeostasis. The increasing autophagy activity still remains an open possibility for cancer treatment. As we see, the FDA-approved anti-neoplastic drugs specifically treat certain types of cancers; thus there are also important questions that remain to be answered, including how autophagy is regulated in different types of cancers and which factors would determine the specificity of drugs on tumorigenesis. Moreover, current assays for monitoring autophagy in humans are still limited, and advanced methods will be beneficial for a better observation of autophagy activity in the context of tumorigenesis.
Acknowledgments
This work was supported by NIH grant GM131919 to DJK.
Abbreviations:
- AMPK
5′-AMP-activated protein kinase
- ATG
autophagy-related
- CQ
chloroquine
- CSC
cancer stem cell
- DAPK
death associated protein kinase
- DRAM
DNA damage regulated autophagy modulator
- EGRF
epidermal growth factor receptor
- ER
endoplasmic reticulum
- ERBB2/HER2
erb-b2 receptor tyrosine kinase 2
- HCQ
hydroxychloroquine
- miRNAs
microRNAs
- MTORC1
mechanistic target of rapamycin kinase complex 1
- PINK1
PTEN induced kinase 1
- PtdIns3K
phosphatidylinositol 3-kinase
- ROS
reactive oxygen species
- SH3GLB1
SH3 domain containing GRB2 like, endophilin B1
- TSC
tuberous sclerosis complex
- UVRAG
UV irradiation resistance associated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Uncategorized References
- 1.Yang Z and Klionsky DJ, Eaten alive: a history of macroautophagy. Nat Cell Biol, 2010. 12(9): p. 814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng Y, et al. , The machinery of macroautophagy. Cell Res, 2014. 24(1): p. 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mizushima N and Komatsu M, Autophagy: renovation of cells and tissues. Cell, 2011. 147(4): p. 728–41. [DOI] [PubMed] [Google Scholar]
- 4.Levine B and Kroemer G, Biological Functions of Autophagy Genes: A Disease Perspective. Cell, 2019. 176(1–2): p. 11–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galluzzi L, et al. , Molecular definitions of autophagy and related processes. EMBO J, 2017. 36(13): p. 1811–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi AM, Ryter SW, and Levine B, Autophagy in human health and disease. N Engl J Med, 2013. 368(7): p. 651–62. [DOI] [PubMed] [Google Scholar]
- 7.Levine B and Kroemer G, Autophagy in the pathogenesis of disease. Cell, 2008. 132(1): p. 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rybstein MD, et al. , The autophagic network and cancer. Nat Cell Biol, 2018. 20(3): p. 243–251. [DOI] [PubMed] [Google Scholar]
- 9.Mitchener JS, et al. , Cellular autophagocytosis induced by deprivation of serum and amino acids in HeLa cells. Am J Pathol, 1976. 83(3): p. 485–92. [PMC free article] [PubMed] [Google Scholar]
- 10.Gunn JM, et al. , Reduced rates of proteolysis in transformed cells. Nature, 1977. 266(5597): p. 58–60. [DOI] [PubMed] [Google Scholar]
- 11.Reggiori F and Ungermann C, Autophagosome Maturation and Fusion. J Mol Biol, 2017. 429(4): p. 486–496. [DOI] [PubMed] [Google Scholar]
- 12.Axe EL, et al. , Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol, 2008. 182(4): p. 685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamb CA, Yoshimori T, and Tooze SA, The autophagosome: origins unknown, biogenesis complex. Nat Rev Mol Cell Biol, 2013. 14(12): p. 759–74. [DOI] [PubMed] [Google Scholar]
- 14.Kraft C and Martens S, Mechanisms and regulation of autophagosome formation. Curr Opin Cell Biol, 2012. 24(4): p. 496–501. [DOI] [PubMed] [Google Scholar]
- 15.Settembre C, et al. , Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol, 2013. 14(5): p. 283–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yorimitsu T and Klionsky DJ, Autophagy: molecular machinery for self-eating. Cell Death Differ, 2005. 12 Suppl 2: p. 1542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klionsky DJ, et al. , A unified nomenclature for yeast autophagy-related genes. Dev Cell, 2003. 5(4): p. 539–45. [DOI] [PubMed] [Google Scholar]
- 18.Dikic I and Elazar Z, Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol, 2018. 19(6): p. 349–364. [DOI] [PubMed] [Google Scholar]
- 19.Mizushima N, Autophagy: process and function. Genes Dev, 2007. 21(22): p. 2861–73. [DOI] [PubMed] [Google Scholar]
- 20.Wen X and Klionsky DJ, An overview of macroautophagy in yeast. J Mol Biol, 2016. 428(9 Pt A): p. 1681–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu L, Chen Y, and Tooze SA, Autophagy pathway: Cellular and molecular mechanisms. Autophagy, 2018. 14(2): p. 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itakura E, et al. , Beclin 1 forms two distinct phosphatidylinositol 3-kinase complexes with mammalian Atg14 and UVRAG. Mol Biol Cell, 2008. 19(12): p. 5360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura T, et al. , FIP200 regulates targeting of Atg16L1 to the isolation membrane. EMBO Rep, 2013. 14(3): p. 284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki SW, et al. , Atg13 HORMA domain recruits Atg9 vesicles during autophagosome formation. Proc Natl Acad Sci U S A, 2015. 112(11): p. 3350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pankiv S, et al. , p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem, 2007. 282(33): p. 24131–45. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura S and Yoshimori T, New insights into autophagosome-lysosome fusion. J Cell Sci, 2017. 130(7): p. 1209–1216. [DOI] [PubMed] [Google Scholar]
- 27.Jin M, Liu X, and Klionsky DJ, SnapShot: Selective autophagy. Cell, 2013. 152(1-2): p. 368–368 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashrafi G and Schwarz TL, The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ, 2013. 20(1): p. 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin SM and Youle RJ, PINK1- and Parkin-mediated mitophagy at a glance. J Cell Sci, 2012. 125(Pt 4): p. 795–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geisler S, et al. , PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol, 2010. 12(2): p. 119–31. [DOI] [PubMed] [Google Scholar]
- 31.Wong YC and Holzbaur EL, Optineurin is an autophagy receptor for damaged mitochondria in parkin-mediated mitophagy that is disrupted by an ALS-linked mutation. Proc Natl Acad Sci U S A, 2014. 111(42): p. E4439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gatica D, Lahiri V, and Klionsky DJ, Cargo recognition and degradation by selective autophagy. Nat Cell Biol, 2018. 20(3): p. 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimmelman AC, The dynamic nature of autophagy in cancer. Genes Dev, 2011. 25(19): p. 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White E, The role for autophagy in cancer. J Clin Invest, 2015. 125(1): p. 42–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mathew R, et al. , Autophagy suppresses tumor progression by limiting chromosomal instability. Genes Dev, 2007. 21(11): p. 1367–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nassour J, et al. , Autophagic cell death restricts chromosomal instability during replicative crisis. Nature, 2019. 565(7741): p. 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dou Z, et al. , Autophagy mediates degradation of nuclear lamina. Nature, 2015. 527(7576): p. 105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White E, Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer, 2012. 12(6): p. 401–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanahan D and Weinberg RA, Hallmarks of cancer: the next generation. Cell, 2011. 144(5): p. 646–74. [DOI] [PubMed] [Google Scholar]
- 40.Marchi S, et al. , Defective autophagy is a key feature of cerebral cavernous malformations. EMBO Mol Med, 2015. 7(11): p. 1403–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee SJ, et al. , Beclin 1 deficiency is associated with increased hypoxia-induced angiogenesis. Autophagy, 2011. 7(8): p. 829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres A, Gubbiotti MA, and Iozzo RV, Decorin-inducible Peg3 Evokes Beclin 1-mediated Autophagy and Thrombospondin 1-mediated Angiostasis. J Biol Chem, 2017. 292(12): p. 5055–5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.New J, et al. , Secretory Autophagy in Cancer-Associated Fibroblasts Promotes Head and Neck Cancer Progression and Offers a Novel Therapeutic Target. Cancer Res, 2017. 77(23): p. 6679–6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wen YA, et al. , Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death Dis, 2017. 8(2): p. e2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deretic V and Levine B, Autophagy balances inflammation in innate immunity. Autophagy, 2018. 14(2): p. 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deretic V, Saitoh T, and Akira S, Autophagy in infection, inflammation and immunity. Nat Rev Immunol, 2013. 13(10): p. 722–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pan H, et al. , Autophagy-associated immune responses and cancer immunotherapy. Oncotarget, 2016. 7(16): p. 21235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathew R, Karantza-Wadsworth V, and White E, Role of autophagy in cancer. Nat Rev Cancer, 2007. 7(12): p. 961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amaravadi R, Kimmelman AC, and White E, Recent insights into the function of autophagy in cancer. Genes Dev, 2016. 30(17): p. 1913–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang XH, et al. , Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature, 1999. 402(6762): p. 672–6. [DOI] [PubMed] [Google Scholar]
- 51.Qu X, et al. , Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest, 2003. 112(12): p. 1809–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yue Z, et al. , Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A, 2003. 100(25): p. 15077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Towers CG and Thorburn A, Therapeutic Targeting of Autophagy. EBioMedicine, 2016. 14: p. 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang H, et al. , Decreased BECN1 mRNA Expression in Human Breast Cancer is Associated with Estrogen Receptor-Negative Subtypes and Poor Prognosis. EBioMedicine, 2015. 2(3): p. 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liang C, et al. , UVRAG: a new player in autophagy and tumor cell growth. Autophagy, 2007. 3(1): p. 69–71. [DOI] [PubMed] [Google Scholar]
- 56.Liang C, et al. , Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat Cell Biol, 2006. 8(7): p. 688–99. [DOI] [PubMed] [Google Scholar]
- 57.Takahashi Y, et al. , Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol, 2007. 9(10): p. 1142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang MR, et al. , Frameshift mutations of autophagy-related genes ATG2B, ATG5, ATG9B and ATG12 in gastric and colorectal cancers with microsatellite instability. J Pathol, 2009. 217(5): p. 702–6. [DOI] [PubMed] [Google Scholar]
- 59.Takamura A, et al. , Autophagy-deficient mice develop multiple liver tumors. Genes Dev, 2011. 25(8): p. 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strohecker AM, et al. , Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer Discov, 2013. 3(11): p. 1272–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rao S, et al. , A dual role for autophagy in a murine model of lung cancer. Nat Commun, 2014. 5: p. 3056. [DOI] [PubMed] [Google Scholar]
- 62.Moscat J and Diaz-Meco MT, p62 at the crossroads of autophagy, apoptosis, and cancer. Cell, 2009. 137(6): p. 1001–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathew R, et al. , Autophagy suppresses tumorigenesis through elimination of p62. Cell, 2009. 137(6): p. 1062–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Inami Y, et al. , Persistent activation of Nrf2 through p62 in hepatocellular carcinoma cells. J Cell Biol, 2011. 193(2): p. 275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valencia T, et al. , Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell, 2014. 26(1): p. 121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Das G, Shravage BV, and Baehrecke EH, Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb Perspect Biol, 2012. 4(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saxton RA and Sabatini DM, mTOR Signaling in Growth, Metabolism, and Disease. Cell, 2017. 168(6): p. 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hosokawa N, et al. , Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell, 2009. 20(7): p. 1981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nazio F, et al. , mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol, 2013. 15(4): p. 406–16. [DOI] [PubMed] [Google Scholar]
- 70.Guertin DA and Sabatini DM, Defining the role of mTOR in cancer. Cancer Cell, 2007. 12(1): p. 9–22. [DOI] [PubMed] [Google Scholar]
- 71.Grabiner BC, et al. , A diverse array of cancer-associated MTOR mutations are hyperactivating and can predict rapamycin sensitivity. Cancer Discov, 2014. 4(5): p. 554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vivanco I and Sawyers CL, The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer, 2002. 2(7): p. 489–501. [DOI] [PubMed] [Google Scholar]
- 73.Laplante M and Sabatini DM, Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci, 2013. 126(Pt 8): p. 1713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang J and Manning BD, The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J, 2008. 412(2): p. 179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J, et al. , PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science, 1997. 275(5308): p. 1943–7. [DOI] [PubMed] [Google Scholar]
- 76.Sansal I and Sellers WR, The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol, 2004. 22(14): p. 2954–63. [DOI] [PubMed] [Google Scholar]
- 77.Fogarty S and Hardie DG, Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta, 2010. 1804(3): p. 581–91. [DOI] [PubMed] [Google Scholar]
- 78.Napolitano G and Ballabio A, TFEB at a glance. J Cell Sci, 2016. 129(13): p. 2475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giatromanolaki A, et al. , Increased expression of transcription factor EB (TFEB) is associated with autophagy, migratory phenotype and poor prognosis in non-small cell lung cancer. Lung Cancer, 2015. 90(1): p. 98–105. [DOI] [PubMed] [Google Scholar]
- 80.Marchand B, et al. , Glycogen synthase kinase-3 (GSK3) inhibition induces prosurvival autophagic signals in human pancreatic cancer cells. J Biol Chem, 2015. 290(9): p. 5592–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perera RM, et al. , Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature, 2015. 524(7565): p. 361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sinha S and Levine B, The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene, 2008. 27 Suppl 1: p. S137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Frenzel A, et al. , Bcl2 family proteins in carcinogenesis and the treatment of cancer. Apoptosis, 2009. 14(4): p. 584–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pattingre S, et al. , Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell, 2005. 122(6): p. 927–39. [DOI] [PubMed] [Google Scholar]
- 85.Farag AK and Roh EJ, Death-associated protein kinase (DAPK) family modulators: Current and future therapeutic outcomes. Med Res Rev, 2019. 39(1): p. 349–385. [DOI] [PubMed] [Google Scholar]
- 86.Zalckvar E, et al. , DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep, 2009. 10(3): p. 285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stevens C, et al. , Peptide combinatorial libraries identify TSC2 as a death-associated protein kinase (DAPK) death domain-binding protein and reveal a stimulatory role for DAPK in mTORC1 signaling. J Biol Chem, 2009. 284(1): p. 334–44. [DOI] [PubMed] [Google Scholar]
- 88.Harrison B, et al. , DAPK-1 binding to a linear peptide motif in MAP1B stimulates autophagy and membrane blebbing. J Biol Chem, 2008. 283(15): p. 9999–10014. [DOI] [PubMed] [Google Scholar]
- 89.Kastenhuber ER and Lowe SW, Putting p53 in Context. Cell, 2017. 170(6): p. 1062–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Beroud C and Soussi T, The UMD-p53 database: new mutations and analysis tools. Hum Mutat, 2003. 21(3): p. 176–81. [DOI] [PubMed] [Google Scholar]
- 91.Feng Z, et al. , The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A, 2005. 102(23): p. 8204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Crighton D, et al. , DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell, 2006. 126(1): p. 121–34. [DOI] [PubMed] [Google Scholar]
- 93.Tasdemir E, et al. , Regulation of autophagy by cytoplasmic p53. Nat Cell Biol, 2008. 10(6): p. 676–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosenfeldt MT, et al. , p53 status determines the role of autophagy in pancreatic tumour development. Nature, 2013. 504(7479): p. 296–300. [DOI] [PubMed] [Google Scholar]
- 95.Shivdasani RA, MicroRNAs: regulators of gene expression and cell differentiation. Blood, 2006. 108(12): p. 3646–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cimmino A, et al. , miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A, 2005. 102(39): p. 13944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu H, et al. , Regulation of autophagy by a beclin 1-targeted microRNA, miR-30a, in cancer cells. Autophagy, 2009. 5(6): p. 816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Valastyan S and Weinberg RA, Tumor metastasis: molecular insights and evolving paradigms. Cell, 2011. 147(2): p. 275–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hu Y and Fu L, Targeting cancer stem cells: a new therapy to cure cancer patients. Am J Cancer Res, 2012. 2(3): p. 340–56. [PMC free article] [PubMed] [Google Scholar]
- 100.Chaffer CL and Weinberg RA, A perspective on cancer cell metastasis. Science, 2011. 331(6024): p. 1559–64. [DOI] [PubMed] [Google Scholar]
- 101.Kenific CM, Thorburn A, and Debnath J, Autophagy and metastasis: another double-edged sword. Curr Opin Cell Biol, 2010. 22(2): p. 241–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Degenhardt K, et al. , Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell, 2006. 10(1): p. 51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Herrero-Martin G, et al. , TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J, 2009. 28(6): p. 677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chambers AF, Groom AC, and MacDonald IC, Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer, 2002. 2(8): p. 563–72. [DOI] [PubMed] [Google Scholar]
- 105.Fung C, et al. , Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell, 2008. 19(3): p. 797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Avivar-Valderas A, et al. , PERK integrates autophagy and oxidative stress responses to promote survival during extracellular matrix detachment. Mol Cell Biol, 2011. 31(17): p. 3616–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lock R, et al. , Autophagy-dependent production of secreted factors facilitates oncogenic RAS-driven invasion. Cancer Discov, 2014. 4(4): p. 466–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chourasia AH, et al. , Mitophagy defects arising from BNip3 loss promote mammary tumor progression to metastasis. EMBO Rep, 2015. 16(9): p. 1145–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aguirre-Ghiso JA, Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer, 2007. 7(11): p. 834–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu Z, et al. , The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest, 2008. 118(12): p. 3917–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lobo NA, et al. , The biology of cancer stem cells. Annu Rev Cell Dev Biol, 2007. 23: p. 675–99. [DOI] [PubMed] [Google Scholar]
- 112.Cufi S, et al. , Autophagy positively regulates the CD44(+) CD24(-/low) breast cancer stem-like phenotype. Cell Cycle, 2011. 10(22): p. 3871–85. [DOI] [PubMed] [Google Scholar]
- 113.Gong C, et al. , Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene, 2013. 32(18): p. 2261–72, 2272e, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wolf J, et al. , A mammosphere formation RNAi screen reveals that ATG4A promotes a breast cancer stem-like phenotype. Breast Cancer Res, 2013. 15(6): p. R109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yeo SK, et al. , Autophagy Differentially Regulates Distinct Breast Cancer Stem-like Cells in Murine Models via EGFR/Stat3 and Tgfbeta/Smad Signaling. Cancer Res, 2016. 76(11): p. 3397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nazio F, et al. , Autophagy and cancer stem cells: molecular mechanisms and therapeutic applications. Cell Death Differ, 2019. 26(4): p. 690–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu K, et al. , Mitophagy Controls the Activities of Tumor Suppressor p53 to Regulate Hepatic Cancer Stem Cells. Mol Cell, 2017. 68(2): p. 281–292 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pei S, et al. , AMPK/FIS1-Mediated Mitophagy Is Required for Self-Renewal of Human AML Stem Cells. Cell Stem Cell, 2018. 23(1): p. 86–100 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meric-Bernstam F and Gonzalez-Angulo AM, Targeting the mTOR signaling network for cancer therapy. J Clin Oncol, 2009. 27(13): p. 2278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim YC and Guan KL, mTOR: a pharmacologic target for autophagy regulation. J Clin Invest, 2015. 125(1): p. 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang ZJ, et al. , The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther, 2011. 10(9): p. 1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shingu T, et al. , Inhibition of autophagy at a late stage enhances imatinib-induced cytotoxicity in human malignant glioma cells. Int J Cancer, 2009. 124(5): p. 1060–71. [DOI] [PubMed] [Google Scholar]
- 123.Koustas E, et al. , Co-targeting of EGFR and autophagy signaling is an emerging treatment strategy in metastatic colorectal cancer. Cancer Lett, 2017. 396: p. 94–102. [DOI] [PubMed] [Google Scholar]
- 124.Yang YP, et al. , Application and interpretation of current autophagy inhibitors and activators. Acta Pharmacol Sin, 2013. 34(5): p. 625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Briceno E, Reyes S, and Sotelo J, Therapy of glioblastoma multiforme improved by the antimutagenic chloroquine. Neurosurg Focus, 2003. 14(2): p. e3. [DOI] [PubMed] [Google Scholar]
- 126.Harhaji-Trajkovic L, et al. , Chloroquine-mediated lysosomal dysfunction enhances the anticancer effect of nutrient deprivation. Pharm Res, 2012. 29(8): p. 2249–63. [DOI] [PubMed] [Google Scholar]
- 127.Santana-Codina N, Mancias JD, and Kimmelman AC, The Role of Autophagy in Cancer. Annu Rev Cancer Biol, 2017. 1: p. 19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Amaravadi RK, et al. , Autophagy inhibition enhances therapy-induced apoptosis in a Myc-induced model of lymphoma. J Clin Invest, 2007. 117(2): p. 326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Levy JMM, Towers CG, and Thorburn A, Targeting autophagy in cancer. Nat Rev Cancer, 2017. 17(9): p. 528–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eng CH, et al. , Macroautophagy is dispensable for growth of KRAS mutant tumors and chloroquine efficacy. Proc Natl Acad Sci U S A, 2016. 113(1): p. 182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shoji-Kawata S, et al. , Identification of a candidate therapeutic autophagy-inducing peptide. Nature, 2013. 494(7436): p. 201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Vega-Rubin-de-Celis S, et al. , Increased autophagy blocks HER2-mediated breast tumorigenesis. Proc Natl Acad Sci U S A, 2018. 115(16): p. 4176–4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Galluzzi L, Kepp O, and Kroemer G, Immunogenic cell death in radiation therapy. Oncoimmunology, 2013. 2(10): p. e26536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pietrocola F, et al. , Caloric Restriction Mimetics Enhance Anticancer Immunosurveillance. Cancer Cell, 2016. 30(1): p. 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]