Abstract
Allogeneic hematopoietic cell transplantation (alloHCT) is a highly specialized procedure. We surveyed adult transplant centers in the United States (US) and then used data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) (2008–2010) to evaluate associations of center volume, infrastructure, and care delivery models with survival post alloHCT. Based on their 2010 alloHCT volume, centers were categorized as low-volume (≤40 alloHCTs; N=42 centers, 1,900 recipients) or high-volume (>40 alloHCTs; N=41 centers, 9,637 recipients). 100-day survival was 86% (95% CI, 85–87%) in high-volume compared to 83% (95% CI, 81–85%) in low-volume centers (difference 3%; P<0.001). One-year survival was 62% (95% CI, 61–63%) and 56% (95% CI, 54–58%), respectively (difference 6%; P < 0.001). Logistic regression analyses adjusted for patient and center characteristics; alloHCT at high-volume centers (odds ratio [OR] 1.32; P<0.001) and presence of a survivorship program dedicated to HCT recipients (OR 1.23; P=0.009) were associated with favorable 1-year survival compared to low-volume centers. Similar findings were observed in a CIBMTR validation cohort (2012–2014); high-volume centers had better 1-year survival (OR 1.24, P<0.001). Among US adult transplant centers, alloHCT at high-volume centers and at centers with survivorship programs is associated with higher 1-year survival.
Keywords: Hematopoietic stem cell transplantation, Overall survival, Center factors, Care delivery models, Provider factors, Volume
INTRODUCTION
Allogeneic hematopoietic cell transplantation (alloHCT) is a highly specialized and complex but standard medical procedure for hematologic cancers and other diseases.1 The practice of alloHCT varies among transplant centers, including variation in patient selection, transplantation regimens, supportive care practices, and the management of post-transplant complications.2–7 Additionally, infrastructure and care delivery models differ substantially among centers.8–11 This variation in center practices, experience and resources may influence recipient outcomes.
Few studies have examined the association of transplant center characteristics with survival after HCT.12–16 A 1992 study from the Center for International Blood and Marrow Transplant Research (CIBMTR), an international HCT clinical outcomes registry, showed higher risk of treatment failure in centers that transplanted fewer than six patients annually.12 A followup study in 2001 that used survey information from United States (US) transplant centers and their patient outcomes data showed that clinical severity of patients and physician case load was associated with 1-year mortality.13 Retrospective studies from Europe have also suggested an association between center volume and alloHCT survival.14, 15 We conducted a study to examine the association of center characteristics with alloHCT outcomes in a period during which substantive changes in indications and practice and advances in transplantation techniques and supportive care have occurred.1, 17, 18 The utilization of this procedure is increasing due to expanding indications, transplantation in older patients, and the routine use of alternative donors. Furthermore in the current era, all US centers must report outcome data on their alloHCT procedures to the CIBMTR. Hence, we surveyed adult HCT centers in the US and then used their patient data reported to the CIBMTR to evaluate associations of center volume, infrastructure, personnel and care delivery models with survival after alloHCT.
METHODS
Data Source
US transplant centers were identified from the CIBMTR.1 Centers contribute detailed data on consecutive HCTs to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program (NMDP) in Minneapolis. Patients are followed longitudinally with yearly follow-up. Computerized checks for errors, physician review of submitted data, and on-site audits of participating centers ensure data quality. The CIBMTR also administers the Stem Cell Therapeutic Outcomes Database, a component of the C.W. Bill Young Transplantation Program, through a contract with the Health Resources and Services Administration.19 Under the purview of this law, transplant centers in the US are required to report data for all alloHCT recipients to the CIBMTR, including complete followup through 1-year post-transplantation. The CIBMTR performs an annual center-specific survival analysis (CSA) and reports risk-adjusted 1-year survival for first alloHCT for each center.20, 21 Observational studies by the CIBMTR are performed in compliance with the Privacy Rule (HIPAA) as a Public Health Authority and with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review of the Institutional Review Board (IRB) of the NMDP.
Transplant Center Survey
The development, administration and results of the US HCT center survey were reported previously.8 Briefly, a 42-item web-based instrument directed towards transplant center medical directors was administered in 2012. The survey inquired about four broad domains of center characteristics: (1) Physician and healthcare provider characteristics (number of transplant physicians and advanced practice providers (APPs), nurse staffing ratio, and other personnel); (2) Transplant unit structure and resources (inpatient and outpatient facilities, stem cell processing facilities, Foundation for the Accreditation of Cellular Therapies (FACT) accreditation status, emergency call structure, and enrollment of patients on clinical trials); (3) Care delivery structure and models (composition of inpatient and outpatient clinical teams and models of care, critical care support, and transition of care); and (4) Medical center characteristics (center location, teaching status, hospital size, National Cancer Institute Comprehensive Cancer Center (NCI CCC) designation, and patient population treated). The survey was conducted under guidance of the NMDP IRB.
We identified 115 centers that had reported alloHCT data primarily on adult patients (age ≥18 years at transplantation) at the time of survey administration, among which seven were deemed ineligible (inactive at time of survey administration [N=1] or reported no alloHCT in the preceding three years [N=6]). Overall, 85/108 eligible centers responded (response rate 79%).
Patient Data
Patient clinical and outcomes information were obtained from the 2012 CSA dataset that includes first alloHCT recipients at US centers transplanted between January 2008 and December 2010. Two centers that responded to the survey had submitted incomplete patient data to the CIBMTR and were excluded. The remaining 83 centers included in the analysis had reported data on 11,634 first alloHCT recipients during the three-year time period. The analysis considered patients who died within the first 12 months or who were alive with ≥11 months of follow-up; 97 patients who were alive with <11 months of follow up were excluded.20 Thus, our final analysis consisted of center characteristics information on 83 transplant centers and patient data on 11,537 recipients.
Statistical Analysis
Descriptive analyses using Fisher’s or Chi-square tests for categorical variables and T-tests for continuous variables were conducted to determine the distribution of patient and center characteristics. The primary outcomes for our study were overall survival at 100-days and at 1-year after transplantation. Survival was estimated from the date of transplantation. Kaplan Meier method was used to estimate survival probabilities at 100-day and at 1-year.
Random effect logistic regression models were used to identify center and provider characteristics associated with 100-day and 1-year survival. Of note, since the primary endpoints were survival at fixed time points (100-day and 1-year post transplant, with complete follow-up on all patients out to each time point), logistic regression was used rather than a time-to-event Cox model. To minimize the confounding effects between center case mix and center- and provider-level characteristics, all regression models included patient-level characteristics using the same set of patient-level variables considered for the risk adjustment in the 2012 CIBMTR center-specific analysis (the methodology for the CSA was described previously.20) These variables included recipient age group, recipient race, Karnofsky performance score, disease group, chemotherapy sensitivity (non-Hodgkin lymphoma and Hodgkin lymphoma only), recipient cytomegalovirus status, time from diagnosis to transplant for acute leukemia, donor type, graft type, HLA match (bone marrow and peripheral blood stem cell grafts only), intensity of conditioning regimen, donor age group at transplant (unrelated donor only), donor/recipient sex match (bone marrow and peripheral blood stem cell donors only), prior autologous transplant, HCT comorbidity index score group,22 and year of transplantation. All variables used in the CSA (listed above) except for year of transplantation were deemed clinically important and were included in the models regardless of statistical significance. Transplant year was included only in the model for 100-day survival, where it reached statistical significance.
The effects of all center characteristics were evaluated one at a time as well as in the presence of other center characteristics to minimize the effect of multicollinearity due to high correlation between some center characteristics. As annual center transplant activity can vary, to provide a simple categorization for center volume, we classified transplant centers based on their overall alloHCT activity reported during the last year of the CSA dataset used for our analysis (i.e., calendar year 2010). Of note, there was good correlation between center alloHCT volume over the entire 3-year period and their volume in 2010 (R=0.96). Without knowledge of a transplant volume cut-point associated with survival, we evaluated categories of several sizes and ultimately a dichotomous categorization of 40 alloHCT/year was determined the optimal cut-point using the maximum likelihood approach based on 1-year survival.23 Coincidentally, this cut-point was close to the median number of alloHCT reported by surveyed centers in 2010 (median 39). Furthermore, we confirmed the cut-point by reviewing a spline fit of the effect of alloHCT volume on 1-year survival. During the 2008–2010 time period, 1,900 patients received HCT at centers reporting ≤40 alloHCT in 2010 (‘low-volume’; N=42 centers), while 9,637 recipients were reported by centers with >40 alloHCT (‘high-volume’; N=41 centers). All logistic regression models included random effects for transplant center to account for potential correlation of outcome among patients within the same center.
Our analysis revealed an association between center volume and survival (see Results). To specifically validate this finding, we evaluated the effect of center volume on 1-year survival in a subsequent cohort of patients reported by adult transplant centers that were included in the 2016 CSA dataset (first alloHCT reported between 2012 and 2014). Centers were considered irrespective of whether they had been included in the initial analysis (N=107 centers, 14,659 alloHCT recipients), and centers were categorized using the same volume threshold (≤40 vs. >40 alloHCT in 2014). Logistic regression analysis for 1-year overall survival was performed adjusting for patient characteristics that were considered in the 2016 CSA and including random effects for transplant center.
Analyses were conducted using the SAS statistical software (SAS Institute, Cary, NC). All P-values reported are two sided and a P-value of <0.05 was considered significant.
RESULTS
Center Characteristics
As noted in Methods, 79% of the 108 eligible centers responded to our survey. Compared to responding centers, non-responding centers reported lower total HCT activity in 2010 (median 46 vs. 101 for responding centers [P<0.01]) and had lower 1-year survival for their alloHCT recipients (56%, vs. 62% for responding centers [P<0.01]). Table 1 provides characteristics of 83 centers included in the analysis. Resources, personnel, and models of inpatient and outpatient care delivery and discharge practices addressed by the survey varied among centers in both categories.
Table 1:
Center characteristics
| Characteristic | Center volume* |
||
|---|---|---|---|
| ≤ 40 alloHCT N (%) | > 40 alloHCT N (%) | P-Value | |
| Number of centers | 42 | 41 | |
| Affiliation with teaching hospital | 0.020 | ||
| No | 12 (28.6) | 3 (7.3) | |
| Yes | 30 (71.4) | 38 (92.7) | |
| Ownership status | 0.214 | ||
| Government | 14 (33.3) | 8 (19.5) | |
| Private | 28 (66.7) | 33 (80.5) | |
| Hospital size (inpatient beds) | 0.043 | ||
| <500 | 18 (42.9) | 12 (29.3) | |
| 500–999 | 19 (45.2) | 23 (56.1) | |
| ≥1000 | 5 (11.9) | 6 (14.6) | |
| NCI Comprehensive Cancer Center | 0.001 | ||
| No | 31 (73.8) | 15 (36.6) | |
| Yes | 11 (26.2) | 26 (63.4) | |
| EHR in inpatient and/or outpatient area | 0.676 | ||
| No | 4 (9.5) | 2 (4.9) | |
| Yes | 38 (90.5) | 39 (95.1) | |
| Inpatient beds exclusively dedicated to HCT | 0.324 | ||
| No | 3 (7.1) | 3 (7.3) | |
| Yes | 39 (92.9) | 38 (92.7) | |
| Separate outpatient clinic for HCT patients | 0.359 | ||
| No | 17 (40.5) | 12 (29.3) | |
| Yes | 25 (59.5) | 29 (70.7) | |
| Stem cell processing lab on site/campus | 0.156 | ||
| No | 7 (16.7) | 2 (4.9) | |
| Yes | 35 (83.3) | 39 (95.1) | |
| FACT accreditation for allogeneic HCT | 0.055 | ||
| No | 5 (11.9) | 0 | |
| Yes | 37 (88.1) | 41 (100.0) | |
| Participation in cooperative group clinical trials | 0.007 | ||
| No | 12 (28.6) | 2 (4.9) | |
| Yes | 30 (71.4) | 39 (95.1) | |
| Patients enrolled on IRB approved protocols | 0.016 | ||
| None | 4 (9.5) | 0 | |
| <25% | 20 (47.6) | 14 (34.2) | |
| 25–49% | 7 (16.7) | 18 (43.9) | |
| ≥50% | 11 (26.2) | 9 (22.0) | |
| Affiliated with hematology-oncology fellowship program | 0.006 | ||
| No | 14 (33.3) | 3 (7.3) | |
| Yes | 28 (66.7) | 38 (92.7) | |
| Long-term follow-up or survivorship program | 0.359 | ||
| No | 30 (71.4) | 25 (61.0) | |
| Yes | 12 (28.6) | 16 (39.0) | |
| Graft-versus-host disease clinic | 0.049 | ||
| No | 38 (90.5) | 30 (73.2) | |
| Yes | 4 (9.5) | 11 (26.8) | |
| Average inpatient nurse-patient ratio | 0.048 | ||
| ≤1:2 | 15 (35.7) | 5 (12.2) | |
| 1:3 | 22 (52.4) | 29 (70.7) | |
| ≥1:4 | 5 (11.9) | 7 (17.1) | |
| FTE transplant clinical coordinators | <0.001 | ||
| ≤1 | 8 (19.1) | 1 (2.4) | |
| 2–3 | 28 (66.7) | 8 (19.5) | |
| 4–6 | 6 (14.3) | 23 (56.1) | |
| ≥7 | 0 | 9 (22.0) | |
| FTE pharmacists | <0.001 | ||
| ≤1 | 27 (64.3) | 7 (17.1) | |
| 2–3 | 14 (33.3) | 26 (63.4) | |
| ≥4 | 1 (2.4) | 8 (19.5) | |
| FTE psychosocial clinicians | <0.001 | ||
| ≤1 | 26 (61.9) | 5 (12.2) | |
| 2–3 | 16 (38.1) | 28 (68.3) | |
| ≥4 | 0 | 8 (19.5) | |
| Median number of attending physicians (IQR) | 4 (3–5) | 8 (6–12) | |
| Median number of APPs (IQR) | 2 (1–5) | 8 (5–14) | |
| Clinical effort of majority of HCT physicians | 0.007 | ||
| See HCT patients only | 7 (16.7) | 14 (34.2) | |
| See HCT and hematologic oncology patients | 28 (66.7) | 27 (65.9) | |
| See HCT and general oncology patients | 7 (16.7) | 0 | |
| Provider responsible for after hour calls | 0.652 | ||
| Attending physician | 23 (54.8) | 27 (65.9) | |
| Fellow | 14 (33.3) | 10 (24.4) | |
| Other providers (e.g., hospitalists, APPs) | 5 (11.9) | 4 (9.8) | |
| Primary team for patients on ventilator | 0.700 | ||
| HCT team | 3 (7.1) | 4 (9.8) | |
| Critical care team | 15 (35.7) | 11 (26.8) | |
| Co-managed by HCT and critical care teams | 24 (57.1) | 26 (63.4) | |
| Primary unit for ventilator patients | 0.183 | ||
| HCT unit | 6 (14.3) | 11 (26.8) | |
| Critical care unit | 36 (85.7) | 30 (73.2) | |
| Physician care model in first 100 days | 0.013 | ||
| Same physician inpatient and outpatient | 10 (23.8) | 1 (2.4) | |
| >1 physician inpatient and same outpatient | 22 (52.4) | 29 (70.7) | |
| >1 physician inpatient and outpatient | 10 (23.8) | 11 (26.8) | |
| Outpatient care model till day 100 for most patients | 0.133 | ||
| Seen by attending physician | 24 (57.1) | 23 (56.1) | |
| Seen by APPs and staffed with physician | 18 (42.9) | 14 (34.2) | |
| Seen by APPs independently | 0 | 4 (9.8) | |
| Discharge practice for most patients without complications | 0.712 | ||
| Varies from provider to provider | 25 (59.5) | 25 (61.0) | |
| Co-followed with referring oncologist | 8 (19.1) | 10 (24.4) | |
| Patients are not discharged from transplant center | 9 (21.4) | 6 (14.6) | |
Abbreviations: HCT – hematopoietic cell transplantation; NCI – National Cancer Institute; EHR – electronic health record; FACT: Foundation for the Accreditation of Cellular Therapy; IRB – Institutional Review Board; IQR – interquartile range; APPs – advanced practice providers; FTE – full time equivalent
Based on allogeneic hematopoietic cell transplant volume reported to the CIBMTR in 2010
Patient Characteristics
Table 2 describes characteristics of patients who received alloHCT from 2008–2010 at the 83 transplant centers included in the analysis. Although statistically significant differences were observed in the distribution of several recipient characteristics, in general these differences were small. The characteristics of patients treated at low- and high-volume centers were mostly similar.
Table 2.
Characteristics of adult allogeneic hematopoietic cell transplantation recipients from centers that responded to the survey
| Characteristic | Center size* |
P-value | |
|---|---|---|---|
| ≤ 40 alloHCT N (%) | > 40 alloHCT N (%) | ||
| Number of centers | 42 | 41 | |
| Number of recipients | 1900 | 9637 | |
| Recipient age group | 0.457 | ||
| < 40 years | 466 (24.6) | 2236 (23.2) | |
| 40 to 59 years | 961 (50.6) | 4946 (51.3) | |
| ≥ 60 years | 473 (24.9) | 2455 (25.5) | |
| Recipient race | <0.001 | ||
| Non-Hispanic White | 1501 (79.0) | 7828 (81.2) | |
| Hispanic | 170 (9.0) | 769 (8.0) | |
| Black/African American | 166 (8.7) | 524 (5.4) | |
| Other | 63 (3.3) | 516 (5.4) | |
| Karnofsky performance score | <0.001 | ||
| 90 to 100 | 1120 (59.0) | 5972 (62.0) | |
| <90 | 732 (38.5) | 3173 (32.9) | |
| Unknown | 48 (2.5) | 492 (5.1) | |
| Diagnosis | |||
| Acute myeloid leukemia | 762 (40.1) | 3572 (37.1) | |
| Acute lymphoblastic leukemia | 248 (13.1) | 1114 (11.6) | |
| Chronic myeloid leukemia | 85 (3.6) | 366 (3.8) | |
| Chronic lymphocytic leukemia | 86 (4.5) | 598 (6.2) | |
| Other leukemia | 12 (<1) | 96 (1.0) | |
| Myelodysplastic syndromes | 224 (11.8) | 1093 (11.3) | |
| Myeloproliferative diseases | 66 (3.5) | 292 (3.0) | |
| Non-Hodgkin lymphoma | 228 (12.0) | 1481 (15.4) | |
| Hodgkin lymphoma | 53 (2.8) | 296 (3.1) | |
| Plasma cell disorders | 67 (3.5) | 448 (4.7) | |
| Other malignancy | 1 (<1) | 12 (<1) | |
| Severe aplastic anemia | 57 (3.0) | 208 (2.2) | |
| Other non-malignant diseases | 11 (<1) | 61 (<1) | |
| Donor type | <0.001 | ||
| Unrelated donor | 1066 (56.1) | 5636 (58.5) | |
| Matched sibling | 748 (39.4) | 3331 (34.6) | |
| Syngeneic | 18 (<1) | 52 (<1) | |
| Other related | 68 (3.6) | 618 (6.4) | |
| Graft type | 0.064 | ||
| Bone marrow | 232 (12.2) | 1310 (13.6) | |
| PBSC ± bone marrow | 1533 (80.7) | 7544 (78.3) | |
| Cord blood ± others | 135 (7.1) | 783 (8.1) | |
| Prior autologous transplant | 0.210 | ||
| No | 1672 (88.0) | 8379 (87.0) | |
| Yes | 228 (12.0) | 1258 (13.1) | |
| HCT comorbidity index score | <0.001 | ||
| 0 | 834 (43.9) | 3696 (38.4) | |
| 1–2 | 501 (26.4) | 2710 (28.1) | |
| ≥ 3 | 529 (27.8) | 3010 (31.2) | |
| Data not collected | 36 (1.9) | 221 (2.3) | |
| Year of transplant | 0.040 | ||
| 2008 | 575 (30.1) | 2966 (30.8) | |
| 2009 | 609 (32.1) | 3316 (34.4) | |
| 2010 | 716 (37.8) | 3355 (34.8) | |
Abbreviations: alloHCT – allogeneic hematopoietic cell transplantation; HCT – hematopoietic cell transplantation; CR – complete remission; PIF – primary induction failure; HLA – human leukocyte antigen; PBSC – peripheral blood stem cell; IQR – interquartile range
Based on alloHCT volume reported to the CIBMTR in 2010
Center Characteristics and Patient Survival
Table 3 presents results of univariate analysis for 1-year survival for each center characteristic. Table 4 describes the results of random effect logistic regression models evaluating the associations of center characteristics with 100-day and 1-year overall survival. For the 100-day model, only center volume was found to be significantly associated with the outcome; the odds of survival were 41% higher in patients who received alloHCT at high-volume compared to low-volume centers (odds ratio 1.41; 95% CI, 1.16–1.72; P <0.001). Center volume was also associated with 1-year survival with 32% higher odds of survival among patients transplanted at high-volume centers (odds ratio 1.32; 95% CI, 1.13–1.55; P <0.001). Among other center characteristics tested in the model (see Table 1), the only factor significantly associated with 1-year survival was the presence of a dedicated survivorship program for HCT recipients (odds ratio 1.23; 95% CI, 1.05–1.43; P=0.009). The standard deviation (SD) of the random center effect in this model was 0.24, indicating that a 1 SD increase in the residual center effect corresponds to an odds ratio for 1 year survival of 1.27, similar in magnitude to the effect of high-volume center or of presence of a dedicated survivorship program.
Table 3:
Univariate analysis of center characteristics and 1-year survival
| Centers, N | Patients, N | 1-year survival, % | P-value* | |
|---|---|---|---|---|
| Affiliation with teaching hospital | 0.724 | |||
| No | 15 | 807 | 60.7 | |
| Yes | 68 | 10730 | 61.0 | |
| Ownership status | 0.580 | |||
| Government | 22 | 2748 | 59.3 | |
| Private | 61 | 8789 | 61.5 | |
| Hospital size (inpatient beds) | 0.624 | |||
| <500 | 30 | 3705 | 63.0 | |
| 500–999 | 42 | 6240 | 60.4 | |
| ≥1000 | 11 | 1592 | 58.4 | |
| NCI Comprehensive Cancer Center | 0.295 | |||
| No | 46 | 3731 | 59.2 | |
| Yes | 37 | 7806 | 61.8 | |
| EHR in inpatient and/or outpatient area | 0.725 | |||
| No | 6 | 477 | 60.2 | |
| Yes | 77 | 11060 | 61.0 | |
| Inpatient beds exclusively dedicated to HCT | 0.327 | |||
| No | 6 | 473 | 56.7 | |
| Yes | 77 | 11064 | 61.2 | |
| Separate outpatient clinic for HCT patients | 0.581 | |||
| No | 29 | 2752 | 60.2 | |
| Yes | 54 | 8785 | 61.2 | |
| Stem cell processing lab on site/campus | 0.852 | |||
| No | 9 | 641 | 61.5 | |
| Yes | 74 | 10896 | 60.9 | |
| FACT accreditation for allogeneic HCT | 0.766 | |||
| No | 5 | 93 | 58.1 | |
| Yes | 78 | 11444 | 61.0 | |
| Participation in cooperative group clinical trials | 0.832 | |||
| No | 14 | 538 | 60.4 | |
| Yes | 69 | 10999 | 61.0 | |
| Patients enrolled on IRB approved protocols | 0.471 | |||
| None | 4 | 120 | 52.5 | |
| <25% | 34 | 2895 | 60.7 | |
| 25–49% | 25 | 5274 | 60.6 | |
| ≥50% | 20 | 3248 | 62.1 | |
| Affiliated with hematology-oncology fellowship program | 0.994 | |||
| No | 17 | 893 | 61.9 | |
| Yes | 66 | 10644 | 60.9 | |
| Long-term follow-up or survivorship program | 0.005 | |||
| No | 55 | 5941 | 58.0 | |
| Yes | 28 | 5596 | 64.1 | |
| Graft-versus-host disease clinic | 0.013 | |||
| No | 68 | 7577 | 59.4 | |
| Yes | 15 | 3960 | 64.0 | |
| Average inpatient nurse-patient ratio | 0.247 | |||
| ≤1:2 | 20 | 1520 | 62.7 | |
| 1:3 | 51 | 7833 | 61.7 | |
| ≥1:4 | 12 | 2184 | 57.1 | |
| FTE transplant clinical coordinators | 0.034 | |||
| ≤1 | 9 | 302 | 64.9 | |
| 2–3 | 36 | 2385 | 56.3 | |
| 4–6 | 29 | 5561 | 62.4 | |
| ≥7 | 9 | 3289 | 61.5 | |
| FTE pharmacists | 0.048 | |||
| ≤1 | 34 | 2123 | 57.2 | |
| 2–3 | 40 | 6440 | 61.1 | |
| ≥4 | 9 | 2974 | 63.4 | |
| FTE psychosocial clinicians | 0.011 | |||
| ≤1 | 31 | 1656 | 56.3 | |
| 2–3 | 44 | 6284 | 60.3 | |
| ≥4 | 8 | 3597 | 64.4 | |
| Clinical effort of majority of HCT physicians | 0.220 | |||
| See HCT patients only | 21 | 4912 | 62.4 | |
| See HCT and hematologic oncology patients | 55 | 6475 | 60.0 | |
| See HCT and general oncology patients | 7 | 150 | 52.7 | |
| Provider responsible for after hour calls | 0.090 | |||
| Attending physician | 50 | 7485 | 62.4 | |
| Fellow | 24 | 2691 | 58.6 | |
| Other providers (e.g., hospitalists, APPs) | 9 | 1361 | 58.1 | |
| Primary team for patients on ventilator | 0.448 | |||
| HCT team | 7 | 970 | 56.0 | |
| Critical care team | 26 | 3042 | 60.5 | |
| Co-managed by HCT and critical care teams | 50 | 7525 | 61.8 | |
| Primary unit for ventilator patients | 0.563 | |||
| HCT unit | 17 | 3228 | 59.4 | |
| Critical care unit | 66 | 8309 | 61.6 | |
| Physician care model in first 100 days | 0.546 | |||
| Same physician inpatient and outpatient | 11 | 344 | 56.0 | |
| >1 physician inpatient and same outpatient | 51 | 7699 | 60.0 | |
| >1 physician inpatient and outpatient | 21 | 3494 | 63.4 | |
| Outpatient care model till day 100 for most patients | 0.236 | |||
| Seen by attending physician | 47 | 5907 | 59.7 | |
| Seen by APPs and staffed with physician | 32 | 4929 | 62.2 | |
| Seen by APPs independently | 4 | 701 | 63.3 | |
| Discharge practice for most patients without complications | 0.383 | |||
| Varies from provider to provider | 50 | 7474 | 61.2 | |
| Co-followed with referring oncologist | 18 | 2873 | 61.9 | |
| Patients are not discharged from transplant center | 15 | 1190 | 57.2 | |
Abbreviations: alloHCT – allogeneic hematopoietic cell transplantation; HCT – hematopoietic cell transplantation; NCI – National Cancer Institute; EHR – electronic health record; FACT: Foundation for the Accreditation of Cellular Therapy; IRB – Institutional Review Board; FTE – full time equivalent
P-value for univariate random effect logistic regression analysis
Table 4.
Center characteristics associated with 100-day and 1-year overall survival on multivariable analysis
| Characteristic | N |
Odds Ratio*† (95% CI) | P-value | |
|---|---|---|---|---|
| Patients | Centers | |||
| 100-day survival | ||||
| Center volume | ||||
| ≤ 40 alloHCT in 2010 | 1900 | 42 | 1 | |
| > 40 alloHCT in 2010 | 9637 | 41 | 1.41 (1.16–1.72) | <0.001 |
| 1-year survival | ||||
| Center volume | ||||
| ≤ 40 alloHCT in 2010 | 1900 | 42 | 1 | |
| > 40 alloHCT in 2010 | 9637 | 41 | 1.32 (1.13–1.55) | <0.001 |
| Long-term followup/survivorship program | ||||
| No | 5941 | 55 | 1 | |
| Yes | 5596 | 28 | 1.23 (1.05–1.43) | 0.009 |
Abbreviations: alloHCT – allogeneic hematopoietic cell transplantation; CI – confidence intervals
Odds ratio of being alive at 100-days or 1-year; odds ratio >1 indicates better odds of survival
Adjusted for the following patient-level variables that were considered for risk-adjustment in the 2012 CIBMTR center-specific survival analysis: recipient age, recipient race/ethnicity, Karnofsky performance score at transplant, prior autologous transplant, recipient cytomegalovirus status, hematopoietic cell transplant comorbidity index score, disease stage, interval from diagnosis to transplant for acute myeloid leukemia and acute lymphoblastic leukemia, chemotherapy sensitivity for non-Hodgkin lymphoma and Hodgkin lymphoma, graft type, donor type HLA match, donor age for unrelated bone marrow or peripheral blood stem cell recipients, donor recipient sex match for bone marrow or peripheral blood stem cell recipients, conditioning regimen intensity for leukemia and year of transplant; transplant year was also included in the model for 100-day survival (additional information on variables included in the analysis is available at http://www.cibmtr.org/Meetings/Materials/CSOAForum/Pages/index.aspx)
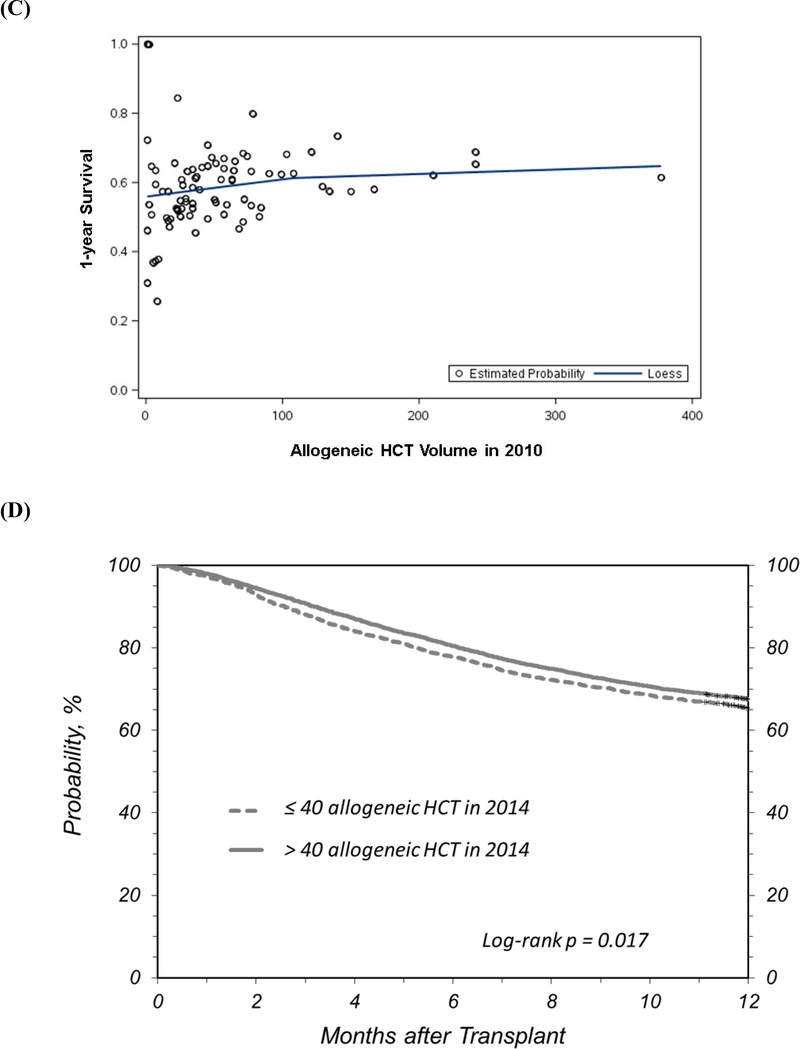
Figure 1A describes the effect of center volume on 1-year survival. The survival probability at 100-days was 86% (95% confidence intervals [CI], 85–87%) for patients transplanted at high volume centers compared to 83% (95% CI, 81–85%) for patients who received HCT at low volume centers (survival difference = 3%; 95% CI, 1–5%; P <0.001). The unadjusted survival probability at 1-year for the two center categories was 62% (95% CI, 61–63%) and 56% (95% CI, 54–58%), respectively (survival difference 6% [95% CI, 3–9%], P<0.001). We performed additional analyses to evaluate center size as deciles of center volume which confirmed the association with 1-year survival and validated our cutpoint of 40 alloHCT for classifying centers as low- and high-volume (Figure 1B). Figure 1C highlights center level variation in 1-year survival based on center volume.
Figure 1:
(a) Unadjusted survival probability by transplant center volume for alloHCT reported by adult transplant centers to the CIBMTR between 2008 and 2010 (categories based on alloHCT performed in 2010), (b) Adjusted probability of 1-year survival by deciles of transplant center alloHCT volume reported to the CIBMTR between 2008 and 2010, (c) Scatter plot of adjusted probability of 1-year survival and transplant center alloHCT volume reported to the CIBMTR between 2008 and 2010 (the line represents the LOESS smoothing function applied to the scatterplot), and (d) Validation analysis showing unadjusted survival probability by center volume for alloHCT reported by adult transplant centers to the CIBMTR between 2012 and 2014 (categories based on alloHCT performed in 2014)
Validation Analysis
A similar association between center volume and 1-year survival was observed in a cohort of alloHCT recipients reported by adult transplant centers from 2012–2014 (Figure 1D). In random effect logistic regression analysis, patients transplanted at centers that performed >40 alloHCT in 2014 had higher odds of 1-year survival (odds ratio 1.27 [95% CI, 1.10–1.46], P<0.001 compared to low-volume centers); 1-year survival was 68% (95% CI, 67–68%) and 65% (95% CI, 64–67%), respectively (P=0.017). Of note, the general better survival in the more recent validation cohort was not surprising since improvement in outcomes of alloHCT over time has been well described.17, 24, 25.
DISCUSSION
Among alloHCT recipients transplanted at adult transplant centers in the US, center volume was associated with 100-day and 1-year survival. In addition, the presence of a survivorship program dedicated to HCT recipients was associated with 1-year survival. We did not identify an association between survival and other physician and healthcare provider characteristics, transplant unit structure and resources, care delivery structure and models, or medical center characteristics included in our survey.
A variety of provider and hospital factors have been investigated as potential modulators of quality of health care, though most research has focused on the volume-outcome relationship.26–29 Studies generally suggest that higher provider and hospital volumes are associated with better outcomes for specific surgical procedures and medical conditions.28, 30–34 However, the mechanisms of the volume-outcome relationship are not fully understood, and volume may serve as a proxy for structural factors and quality measures.35, 36 We designed our study to address this limitation of existing research in the context of alloHCT. Similar to prior studies,12–14, 37 we identified center volume as a significant predictor for survival. However, we were not able to identify specific center structural factors and care delivery models besides the availability of a survivorship program that may explain this association.
The interpretation and implications of our findings have to be considered in the context of the complexity of center volume-survival relationship. For example, should the threshold for alloHCT volume identified in our analysis be the basis for transplant center accreditation (e.g., by FACT/JACIE)? We caution against using our threshold as a benchmark for qualifying individual centers on survival for several reasons. First, the cut point of >40 alloHCT/year is a statistical threshold based on the aggregate dataset that was used for analysis. The magnitude of survival difference between the two center volume categories was relatively small and there was variation in 1-year alloHCT survival among centers. Not all low volume programs had suboptimal survival and vice versa. We queried centers on a comprehensive list of center characteristics, however, we may not have captured nuances such as team interactions, allocation of resources, institutional support, patient care priorities, center expertise and experience, catchment area characteristics and referral and clinical practice patterns that may affect outcomes. Furthermore, we did not have information on patient related variables such as socioeconomic status and distance of residence from the transplant center which have been shown to be associated with alloHCT outcomes.38, 39 There are patient-related issues that need to be considered in any policy discussions about limiting and centralizing transplant care to selected centers (e.g., by volume) given the potential to accentuate healthcare disparities and limit access.39, 40 The other question that can be raised is whether volume reflects the expertise to provide care for more complex transplant patients. For example in one study, 1- and 3-year survival for autologous transplantation was higher in centers that also performed alloHCT.37 Overall, our study emphasizes the need for continued exploration of the volume-survival relationship for alloHCT – to better identify factors that drive this association so that relevant best-practices can be translated to centers that are low-volume or have suboptimal survival and to inform policy decisions that can balance patient access with care at centers with optimal survival.
Our findings should encourage centers to dedicate resources towards setting up programs that focus on health maintenance, preventive care and followup of alloHCT recipients. Several barriers to care after patients are discharged from the transplant center (typically around day 100 after HCT) have been described, and coordinated survivorship care may enhance long-term patient outcomes beyond 1 year after transplantation.11, 41, 42 We acknowledge that presence of survivorship clinic may be a surrogate for other center resources and characteristics that were not captured on our survey. We also recognize the variability in the organization of survivorship clinics that currently exist at transplant centers and optimizing care models for HCT survivors is an area of active research.43, 44 Centers can use guidelines for long-term followup and tools such as treatment summary and survivorship care plans to establish a foundation for coordinated patient-centric survivorship care.44–48
Our study has the limitations of an observational registry based analysis. In addition, we did not take into account autologous HCT experience and volumes, since reporting of autologous HCT activity is voluntary and is not included in the annual center survival analysis. However, we did find good correlation between alloHCT and total transplant volume for included centers. We developed our survey using a systematic process that included qualitative feedback from center medical directors;8 however, we recognize that a survey cannot completely capture the complexity of transplant center structure, resources and experience. We did not include pediatric centers in our analysis since their practice of transplantation and center resources are substantially different from adult centers. We did not find an association between center accreditation and outcomes that has been previously described,14, 16 possibly because nearly all centers included in our analysis were FACT accredited. Since the CSA dataset only included information on overall survival, we are not able to describe causes of death, relapse and complications.
In summary, our study validates the association of alloHCT volume and center survival and highlights the value of a dedicated program for coordinating the care of HCT survivors. Additional research is being planned using qualitative methods to better understand transplant center characteristics that may explain the volume-outcome relationship that we have demonstrated, especially focusing on characteristics that may be generalizable and transferable to centers with the aim of improving alloHCT outcomes across the board.
ACKNOWLEDGEMENTS
We sincerely thank all transplant center Medical Directors who completed the survey. We would like to acknowledge the National Marrow Donor Program’s System Capacity Initiative Program for providing funds for the survey incentive and for providing staff support for implementing and analyzing the survey. We sincerely appreciate review of our manuscript draft and constructive feedback provided by Mary Horowitz, MD, MS, Center for International Blood and Marrow Transplant Research, Milwaukee, and Linda Burns, MD, Center for International Blood and Marrow Transplant Research and National Marrow Donor Program, Minneapolis.
CIBMTR Funding Support: The Center for International Blood and Marrow Transplant Research (CIBMTR) is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10-HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014–15-1–0848 and N00014–16-1–2020 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; AstraZeneca; Be the Match Foundation; *Bluebird Bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cellular Dynamics International, Inc.; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; Mesoblast; MesoScale Diagnostics, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. - Japan; PCORI; Perkin Elmer, Inc.; Pfizer, Inc; *Sanofi US; *Seattle Genetics; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government (*Corporate Members).
Footnotes
Conflict of Interest: None of the authors has any financial conflict of interest to report in relationship to this study.
REFERENCES
- 1.Pasquini MC, Xhu X. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides, 2015. Available at: http://www.cibmtr.org.
- 2.Lee SJ, Astigarraga CC, Eapen M, Artz AS, Davies SM, Champlin R et al. Variation in supportive care practices in hematopoietic cell transplantation. Biol Blood Marrow Transplant 2008; 14(11): 1231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SJ, Joffe S, Artz AS, Champlin RE, Davies SM, Jagasia M et al. Individual physician practice variation in hematopoietic cell transplantation. J Clin Oncol 2008; 26(13): 2162–70. [DOI] [PubMed] [Google Scholar]
- 4.Lee SJ, Vogelsang G, Gilman A, Weisdorf DJ, Pavletic S, Antin JH et al. A survey of diagnosis, management, and grading of chronic GVHD. Biol Blood Marrow Transplant 2002; 8(1): 32–9. [DOI] [PubMed] [Google Scholar]
- 5.Pidala J, Lee SJ, Quinn G, Jim H, Kim J, Anasetti C. Variation in management of immune suppression after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2011; 17(10): 1528–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trifilio S, Verma A, Mehta J. Antimicrobial prophylaxis in hematopoietic stem cell transplant recipients: heterogeneity of current clinical practice. Bone marrow transplantation 2004; 33(7): 735–9. [DOI] [PubMed] [Google Scholar]
- 7.Windrum P, Morris TC, Drake MB, Niederwieser D, Ruutu T. Variation in dimethyl sulfoxide use in stem cell transplantation: a survey of EBMT centres. Bone marrow transplantation 2005; 36(7): 601–3. [DOI] [PubMed] [Google Scholar]
- 8.Majhail NS, Mau LW, Chitphakdithai P, Payton T, Eckrich M, Joffe S et al. National Survey of Hematopoietic Cell Transplantation Center Personnel, Infrastructure, and Models of Care Delivery. Biol Blood Marrow Transplant 2015; 21(7): 1308–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Majhail NS, Murphy EA, Omondi NA, Robinett P, Gajewski JL, LeMaistre CF et al. Allogeneic transplant physician and center capacity in the United States. Biol Blood Marrow Transplant 2011; 17(7): 956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy EA, Ferguson SS, Omondi NA, Getzendaner LC, Gajewski JL, Goldstein GA et al. The National Marrow Donor Program’s symposium on patient advocacy in cellular transplantation therapy: addressing barriers to hematopoietic cell transplantation. Biol Blood Marrow Transplant 2010; 16(2): 147–56. [DOI] [PubMed] [Google Scholar]
- 11.Majhail NS. Optimizing Quality and Efficiency of Healthcare Delivery in Hematopoietic Cell Transplantation. Current hematologic malignancy reports 2015; 10(3): 199–204. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz MM, Przepiorka D, Champlin RE, Gale RP, Gratwohl A, Herzig RH et al. Should HLA-identical sibling bone marrow transplants for leukemia be restricted to large centers? Blood 1992; 79(10): 2771–4. [PubMed] [Google Scholar]
- 13.Loberiza FR Jr., Zhang MJ, Lee SJ, Klein JP, LeMaistre CF, Serna DS et al. Association of transplant center and physician factors on mortality after hematopoietic stem cell transplantation in the United States. Blood 2005; 105(7): 2979–87. [DOI] [PubMed] [Google Scholar]
- 14.Gratwohl A, Sureda A, Baldomero H, Gratwohl M, Dreger P, Kroger N et al. Economics and Outcome After Hematopoietic Stem Cell Transplantation: A Retrospective Cohort Study. EBioMedicine 2015; 2(12): 2101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klingebiel T, Cornish J, Labopin M, Locatelli F, Darbyshire P, Handgretinger R et al. Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplantation in children with very high-risk acute lymphoblastic leukemia: impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood 2010; 115(17): 3437–46. [DOI] [PubMed] [Google Scholar]
- 16.Gratwohl A, Brand R, McGrath E, van Biezen A, Sureda A, Ljungman P et al. Use of the quality management system “JACIE” and outcome after hematopoietic stem cell transplantation. Haematologica 2014; 99(5): 908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn T, McCarthy PL Jr., Hassebroek A, Bredeson C, Gajewski JL, Hale GA et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol 2013; 31(19): 2437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCarthy PL Jr., Hahn T, Hassebroek A, Bredeson C, Gajewski J, Hale G et al. Trends in use of and survival after autologous hematopoietic cell transplantation in North America, 1995–2005: significant improvement in survival for lymphoma and myeloma during a period of increasing recipient age. Biol Blood Marrow Transplant 2013; 19(7): 1116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Health Resources and Services Administration. Blood cell transplant. http://bloodcell.transplant.hrsa.gov/index.html (accessed 01/27/2017).
- 20.Logan BR, Nelson GO, Klein JP. Analyzing center specific outcomes in hematopoietic cell transplantation. Lifetime data analysis 2008; 14(4): 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Center for International Blood and Marrow Transplant Research: U.S. Transplant and Survival Statistics on Related Sites. http://www.cibmtr.org/ReferenceCenter/SlidesReports/USStats (accessed 01/27/2017).
- 22.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106(8): 2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Contal C, O’Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Analysis 1999; 30(3): 253–70. [Google Scholar]
- 24.Majhail NS, Chitphakdithai P, Logan B, King R, Devine S, Rossmann SN et al. Significant improvement in survival after unrelated donor hematopoietic cell transplantation in the recent era. Biol Blood Marrow Transplant 2015; 21(1): 142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horan JT, Logan BR, Agovi-Johnson MA, Lazarus HM, Bacigalupo AA, Ballen KK et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol 2011; 29(7): 805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choudhry NK, Fletcher RH, Soumerai SB. Systematic review: the relationship between clinical experience and quality of health care. Annals of internal medicine 2005; 142(4): 260–73. [DOI] [PubMed] [Google Scholar]
- 27.Reid RO, Friedberg MW, Adams JL, McGlynn EA, Mehrotra A. Associations between physician characteristics and quality of care. Archives of internal medicine 2010; 170(16): 1442–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruen RL, Pitt V, Green S, Parkhill A, Campbell D, Jolley D. The effect of provider case volume on cancer mortality: systematic review and meta-analysis. CA: a cancer journal for clinicians 2009; 59(3): 192–211. [DOI] [PubMed] [Google Scholar]
- 29.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Annals of internal medicine 2002; 137(6): 511–20. [DOI] [PubMed] [Google Scholar]
- 30.Auerbach AD, Maselli J, Carter J, Pekow PS, Lindenauer PK. The relationship between case volume, care quality, and outcomes of complex cancer surgery. Journal of the American College of Surgeons 2010; 211(5): 601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McAteer JP, LaRiviere CA, Drugas GT, Abdullah F, Oldham KT, Goldin AB. Influence of surgeon experience, hospital volume, and specialty designation on outcomes in pediatric surgery: a systematic review. JAMA pediatrics 2013; 167(5): 468–75. [DOI] [PubMed] [Google Scholar]
- 32.Tchouta LN, Park HS, Boffa DJ, Blasberg JD, Detterbeck FC, Kim AW. Hospital Volume and Outcomes of Robot-Assisted Lobectomies. Chest 2017; 151(2): 329–339. [DOI] [PubMed] [Google Scholar]
- 33.Park HS, Detterbeck FC, Boffa DJ, Kim AW. Impact of hospital volume of thoracoscopic lobectomy on primary lung cancer outcomes. The Annals of thoracic surgery 2012; 93(2): 372–9. [DOI] [PubMed] [Google Scholar]
- 34.Lin X, Tao H, Cai M, Liao A, Cheng Z, Lin H. A Systematic Review and Meta-Analysis of the Relationship Between Hospital Volume and the Outcomes of Percutaneous Coronary Intervention. Medicine 2016; 95(5): e2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christian CK, Gustafson ML, Betensky RA, Daley J, Zinner MJ. The volume-outcome relationship: don’t believe everything you see. World journal of surgery 2005; 29(10): 1241–4. [DOI] [PubMed] [Google Scholar]
- 36.Mesman R, Westert GP, Berden BJ, Faber MJ. Why do high-volume hospitals achieve better outcomes? A systematic review about intermediate factors in volume-outcome relationships. Health policy 2015; 119(8): 1055–67. [DOI] [PubMed] [Google Scholar]
- 37.Poirel HA, Vanspauwen M, Macq G, De Geyndt A, Maertens J, Willems E et al. Providing both autologous and allogeneic hematopoietic stem cell transplants (HSCT) may have a stronger impact on the outcome of autologous HSCT in adult patients than activity levels or implementation of JACIE at Belgian transplant centres. Bone marrow transplantation 2019. [DOI] [PubMed] [Google Scholar]
- 38.Baker KS, Davies SM, Majhail NS, Hassebroek A, Klein JP, Ballen KK et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant 2009; 15(12): 1543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Majhail NS, Omondi NA, Denzen E, Murphy EA, Rizzo JD. Access to hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant 2010; 16(8): 1070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Majhail NS, Nayyar S, Santibanez ME, Murphy EA, Denzen EM. Racial disparities in hematopoietic cell transplantation in the United States. Bone marrow transplantation 2012; 47(11): 1385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hashmi S, Carpenter P, Khera N, Tichelli A, Savani BN. Lost in transition: the essential need for long-term follow-up clinic for blood and marrow transplantation survivors. Biol Blood Marrow Transplant 2015; 21(2): 225–32. [DOI] [PubMed] [Google Scholar]
- 42.Khera N, Martin P, Edsall K, Bonagura A, Burns LJ, Juckett M et al. Patient-centered care coordination in hematopoietic cell transplantation. Blood advances 2017; 1(19): 1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Battiwalla M, Hashmi S, Majhail N, Pavletic S, Savani BN, Shelburne N. National Institutes of Health Hematopoietic Cell Transplantation Late Effects Initiative: Developing Recommendations to Improve Survivorship and Long-Term Outcomes. Biol Blood Marrow Transplant 2017; 23(1): 6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashmi SK, Bredeson C, Duarte RF, Farnia S, Ferrey S, Fitzhugh C et al. National Institutes of Health Blood and Marrow Transplant Late Effects Initiative: The Healthcare Delivery Working Group Report. Biol Blood Marrow Transplant 2017; 23(5): 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18(3): 348–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Majhail NS, Murphy E, Laud P, Preussler JM, Denzen EM, Abetti B et al. Randomized controlled trial of individualized treatment summary and survivorship care plans for hematopoietic cell transplantation survivors. Haematologica 2019; 104(5): 1084–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Denzen EM, Preussler JM, Murphy EA, Baker KS, Burns LJ, Foster J et al. Tailoring a Survivorship Care Plan: Patient and Provider Preferences for Recipients of Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2019; 25(3): 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hashmi SK, Lee SJ, Savani BN, Burns L, Wingard JR, Perales MA et al. ASBMT Practice Guidelines Committee Survey on Long-Term Follow-Up Clinics for Hematopoietic Cell Transplant Survivors. Biol Blood Marrow Transplant 2018; 24(6): 1119–1124. [DOI] [PubMed] [Google Scholar]