Abstract
The CYP2D subfamily catalyses the metabolism of about 25% of prescribed drugs, including the majority of antidepressants and antipsychotics. At present, the mechanism of hepatic CYP2D regulation remains largely unknown. This study investigated the role of sex steroid hormones in CYP2D regulation. For this purpose, Cyp2d22 expression was assessed in the distinct phases of the estrous cycle of normocyclic C57BL/6J (WT) female mice. Cyp2d22 was also evaluated in ovariectomised WT and CYP2D6-humanized (hCYP2D6) mice that received hormonal supplementation with either 17β-estradiol (E2) and/or progesterone. Comparisons were also made to male mice. The data revealed that hepatic Cyp2d22 mRNA, protein and activity levels were higher at estrous compared to the other phases of the estrous cycle, and ovariectomy repressed Cyp2d22 expression in WT mice. Tamoxifen, an antiestrogenic compound, also repressed hepatic Cyp2d22 via activation of GH/STAT5b and PI3k/AKT signalling pathways. Both hormones prevented the ovariectomy-mediated Cyp2d22 repression. In case of progesterone, this may be mediated by inhibition of the PI3k/AKT/FOX01 pathway. Notably, Cyp2d22 mRNA levels in WT males were similar to those in ovariectomised mice, and were markedly lower compared to females at estrous, a differentiation potentially regulated by the GH/STAT5b pathway. Sex steroid hormone-related alterations in Cyp2d22 mRNA expression were highly correlated with Hnf1a mRNA. Interestingly, fluctuations in Cyp2d22 in hippocampus and cerebellum followed those in liver. In contrast to WT mice, ovariectomy induced hepatic CYP2D6 expression in hCYP2D6 mice, whereas E2 and/or P prevented this induction. Apparently, sex steroid hormones display a significant gender- and species-specific role in the regulation of CYP2D.
Keywords: Cyp2d22, 17β-estradiol, progesterone, estrous cycle, mice
Introduction
The cytochrome P450 2D (CYP2D) subfamily belongs to the cytochrome P450-dependent mixed function oxidase system that includes several major drug-metabolizing enzymes. CYP2D isoforms are expressed in various tissues, such as the liver, intestine, kidney and brain (Miksys, et al. 2005; Siegle, et al. 2001) and, in humans, catalyze the hepatic metabolism of about 25% of the prescribed drugs including the vast majority of antidepressants and antipsychotics, several analgesics, antiarrythmics and anticancer drugs (Ingelman-Sundberg, et al. 2007; Yu, et al. 2004; Zanger, et al. 2004). CYP2D is also implicated in the synthesis and metabolism of neurotransmitters (dopamine & serotonin) (Hiroi, et al. 1998) and neurosteroids in the human and rat brain (Ning, et al. 2015; Wang, et al. 2014; Yu, et al. 2003), and mainly in brain regions where disturbances in these neurotransmitter systems contribute to the pathophysiology of depression and schizophrenia (Haduch and Daniel 2018). It is also noteworthy that CYP2D6 polymorphisms have been implicated in personality traits and neurological or psychiatric disorders; reduced CYP2D6 activity is associated with increased susceptibility to Parkinson’s disease (Lu, et al. 2014; Smith, et al. 1992). Interestingly, the reduced serotonin metabolism in CYP2D6 poor metabolizers (PMs) has been associated with a cluster of behavioral traits such as anxiety, impulsivity, attention deficits (Bertilsson, et al. 1989; González, et al. 2008). However, the precise role of CYP2D6 in brain physiology remains an enigma (Ning et al. 2015).
To date, the mechanisms involved in CYP2D regulation are largely unidentified. CYP2D isozymes have long been considered resistant to direct hormonal regulation and were known as “un-inducible” CYPs (Daskalopoulos, et al. 2012c). However, previous studies showed that sex steroid hormones affect the expression of CYP2D in the rat brain. In particular, testosterone treatment of ovariectomised female rats dramatically induced the brain CYP2D mRNA, whereas estrogens had a weaker effect (Li, et al. 2015; Wang et al. 2014). Notably, sex-steroid hormones target an array of complex and dynamic regulatory loops, such as those regulating growth hormone (GH) secretion, which in many species, including humans, displays regulatory properties for various hepatic and brain genes, mainly those encoding CYP enzymes that are transcribed in a sex-dependent manner (Waxman and O’Connor 2006; Zhang, et al. 2018). Specifically, GH plays a critical role in the regulation of CYP2C11, CYP2C12, CYP2D9 and CYP3A4, among others (Waxman and O’Connor 2006), but GH does not affect CYP2D6 activity in elderly men (Jürgens, et al. 2002). The limited experimental data though, do not permit a clear association between CYP2D expression and GH/sex steroidal function.
Based on previous studies indicating that the expression of several CYPs is largely dependent on sex hormonal fluctuations within the different phases of the estrus cycle (Bebia, et al. 2004; Chen, et al. 1999; Ingelman-Sundberg 2004; Kim, et al. 1995; Kim, et al. 2007; Konstandi, et al. 2013; Scandlyn, et al. 2008), the present study was developed to investigate the role of estrogens and progesterone in CYP2D regulation. The fluctuation of 17β-estradiol (E2) and progesterone circulating levels during the reproductive cycle is well documented. These female steroid hormones are synthesized and released by the ovaries and play determinant roles in the division of the murine estrous cycle into four stages, namely proestrous, estrous, methestrous, and diestrous. The murine estrous cycle normally lasts 4 to 5 days and E2 levels peak prior to ovulation, as early at estrous. Consequently, progesterone levels start rising late at estrous and remain high at methestrous and diestrous; they start declining from proestrous until the first part of estrous (Fata, et al. 2001; Walmer, et al. 1992).
The considerable differentiation of female and male biological profiles including several drug metabolising systems, and the cross-talk between the steroid receptor-linked signalling pathways and those regulating several CYPs (Scandlyn et al. 2008), necessitate a thorough investigation of the sex-specific differences in CYP2D regulation, emphasizing the role of female sex steroid hormones. Taking mainly into account the wide use of these hormones for therapeutic purposes: a) progesterone is often used for long-term ovarian suppression in dysmenorrhoea, hirtuism, endometriosis and bleeding, in cases where estrogens are contraindicated (Neal 2015), b) the female sex steroid hormones consist the major components of the hormonal replacement therapy followed by menopausal women for the prevention of osteoporosis and cardiovascular disorders and c) the widely used contraceptives contain a combination of estrogen and progestogen (Hel, et al. 2010; Santen, et al. 2010), this study investigated the role of female sex hormones in hepatic CYP2D regulation using ovariectomized wild-type and CYP2D6-humanised (hCYP2D6) mice, supplemented with 17β-estradiol and/or progesterone. In order to further elucidate the role of estrogens in CYP2D regulation, tamoxifen was administered in intact cyclic females. This drug has anti-estrogenic effects in breast tissue and is used as the standard endocrine treatment in women with hormone receptor-positive breast cancer. Under certain circumstances, tamoxifen can exert the properties of an estrogenic agonist depending on target tissue (Moreira, et al. 2007). The pattern of hepatic Cyp2d22 expression was also evaluated in the four distinct phases of the estrous cycle of normo cyclic female mice and compared to that of males. Interestingly, the present data revealed a significant diversity in hepatic Cyp2d22 expression profile in the distinct phases of the estrous cycle and a significant role of female sex steroid hormones in Cyp2d22 regulation.
Materials and Methods
Animals and treatment
Wild-type and CYP2D6-humanized mice, established by insertion of the human CYP2D6 transgene on the C57BL/6J background, were used in this study. These transgenic mice exhibit robust CYP2D6 expression in liver and intestine (Corchero, et al. 2001; Miksys et al. 2005; Scheer, et al. 2012). Mice were housed in groups of 3–5 in plastic cages in a temperature and light-controlled environment. They received the standard rodent chow and tap water ad libitum. One week prior to the experiment all animals were adapted to handling. All procedures were carried out in accordance with Institute of Laboratory Animal Resources guidelines and approved by the National Cancer Institute Animal Care and Use Committee.
Estrous Cycle Monitoring
For a period of two weeks, intact cyclic female 6-week-old wild-type mice were screened for their estrous cycle integrity. In this study, female mice with normal estrous cycle, were divided into four groups according to the estrous cycle phase at the last day of the experiment. The level of CYP2D expression in intact male mice of the same age was also assessed. Estrous cycle phase and normality was monitored by analysis of the cell types in vaginal lavages collected from all cyclic female mice. Vaginal smears were obtained daily between 11:00am-13:00pm for at least 15 consecutive days. The fire-polished and shortened tip of a Pasteur pipette carrying one drop of tap water was placed at the vaginal orifice. Care was taken not to insert it more than 1 mm in order to prevent the cervical stimulation and thus disruption of the estrous cycle cyclicity (Nelson, et al. 1981). Smears were then spread gently on a microscope slide and allowed to dry in the open air. Slides were then fixed with absolute methanol (3 min), drained and stained with Giemsa (Merck) solution 2% for at least 20 min. For the accurate estrous cycle stage identification staining is essential: cytoplasm stained blue and nuclei stained red. Following published methods, the identification of cell types was made microscopically (Zarrow 1964). The four distinct phases of the estrous cycle are: Proesrous (PE), estrous (E), methestrous (ME) and diestrous (DE). All animals with persistent DE or E and cycles lasted longer than 5 days were considered as abnormal and were excluded from the study.
Ovariectomy and hormonal supplementation
Intact cyclic female 5-week-old wild-type mice (C57BL/6J) were subjected to bilateral ovariectomy under gas anaesthesia and implanted with pellets that contained either 17β-estradiol or progesterone (Three-week-release 17β-estradiol or progesterone pellets; Innovative Research of America, Sarasota, FL). The hormone-containing pellets were implanted subcutaneously in the anesthetised animals as follows: In the skin of the mouse’s neck, a 0.5–1.0 cm incision was made, followed by a dissection of a small pocket made caudolaterally, where the pellet was placed using tweezers and the incision then was sealed by sutures. The whole procedure took place under sterile conditions to prevent infection and care was taken to avoid the contact of pellets with solvents and alcohol (Ingberg, et al. 2012). The implanted pellets released either ~25 μg 17β-estradiol or 425 μg progesterone per day. These levels are close to those detected in intact cyclic female mice (Nelson et al. 1981). The whole procedure (anesthesia and operation) lasted approximately 20 min and the mice were returned to their cages after recovery. They remained undisturbed there until the end of the experiment, 21 days later. As controls, mice were sham-operated and implanted with a placebo pellet at estrous.
Anesthesia technique
Spontaneously breathing mice were submitted to anaesthesia using isoflurane (IsoFlo, Abbott Park, Illinois, U.S.A.), which was administered through a vaporizer providing standardized gas concentration to an outlet tube. The anaesthetic gas contained isoflurane that was administered at a constant flow rate of 1.0–2.0 lit/min and oxygen at a concentration of 30–35%. The animals were anesthetised in an anaesthesia induction box of 20 cm diameter and 10 cm height, which was connected to a silicon tube providing the anaesthetic gas mixture. Anaesthesia was induced by inhalation of 4% vaporized isoflurane, which led to a rapid induction of anaesthesia within 15–30 sec, allowing the animals to be placed in a prone position. It also allowed the type of inhalation to be modified to a tube of about 1.5 cm diameter surrounding the head and to reduce the isoflurane vaporization to 3.0–3.5%. This concentration of isoflurane provided deep anaesthesia, allowing the performance of surgical procedures without any clinical sign of pain or changes of macrohaemodynamic parameters (MAP and HR). This consisted of no movements of the animal, no reaction to pain stimuli during surgical procedures (foot pad reaction), and stable blood pressure, HR and respiratory rate. These conditions during anaesthesia were maintained until the end of the operation. After the final incision closure, buprenorphine HCl (0.25 mg/kg s.c., Bedford Labs, USA) was given and the mouse was placed on a heating pad and allowed to recover before returning to its home cage. Evaluation of the body weight progression, locomotor activity, daily food and water consumption showed no abnormalities 24 hours after anaesthesia.
Tamoxifen treatment
Tamoxifen, 2 mg/kg, i.p. was administered to intact cyclic 8-week-old wild-type C57BL/6J female mice for three days and the mice were killed 24 hours after the last dose. Control mice received sodium chloride 0.9% for three days and were killed at estrous. Mice of all groups of treatment used in this study were killed by carbon oxide asphyxiation.
Enzyme activity
Bufuralol 1′-hydroxylation
Bufuralol 1′-hydroxylation reflects the cytochrome CYP2D activity (Matsunaga et al., 1990). In a 200 μl reaction mixture containing potassium phosphate (100 mM, pH 7.4) in the presence of 50 μM bufuralol substrate, liver microsomal protein (40 μg) was pre-incubated at 37°C for 5 min. The reaction, which lasted 7.5 min, was initiated with NADPH (0.5 mM) at 37°C and terminated with 20 μl of perchloric acid 60%. In the supernatant (100 μl) obtained after centrifugation at 14,075 g for 10 min, the concentration of 1’-hydroxy-bufuralol (main metabolite of bufuralol) was determined by HPLC with a fluoroscence detector at 252 and 302 nm (Shimadzu, Japan). A reversed phase Luna C18 column (5 μm, 150 × 3 mm, Phenomenex, USA) was used and the mobile phase contained a mixture of 30% acetonitrile (ACN) and 70% perchlorate buffer 20 mM (pH 2.5). The sample was eluted at a flow rate of 1 ml/min for 15 min.
Quantitative real-time PCR
The Trizol reagent was used (Invitrogen, Carlsbad, CA) for the isolation of total RNA from the liver and brain structures, hippocampus and cerebellum, following the manufacturer’s protocol. The concentration of total RNA was determined spectrophotometrically. Quantitative real-time reverse transcriptase PCR (qPCR) was performed with cDNA generated from 1 μg total RNA with a SuperScript III reverse transcriptase kit (Invitrogen, USA). Gene-specific primers were designed for qPCR using the Primer Express software (Applied Biosystems, Foster City, CA). The sequences for the forward and reverse primers used are shown in the Table 1. For the real-time reactions the SYBR Green PCR master mix (Applied Biosystems, Warrington, UK) was used, and qPCR carried out using the ABIPRISM 7900 HT sequence detection system (Applied Biosystems). The PCR conditions were the following: 95°C for 10 min followed by 40 cycles of 95°C for 15 sec, 60°C for 1 min, and finally, one cycle of 95°C for 15 sec, 60°C for 15 sec and 95°C for 15 sec. Relative mRNA expression levels were normalized to Actb mRNA and values were quantified using the comparative threshold cycle method.
Table 1. Serum estradiol and progesterone levels in wild-type and hCYP2D6 mice.
Assessment of ovariectomy and hormonal replacement effect on serum sex steroid hormone concentrations in wild-type C57BL/6J female mice at estrus or submitted to ovariectomy and hormonal supplementation with 17β-estradiol and/or progestrone. Estradiol and progesterone values are expressed as mean ± SE in pg/ml serum (n=10). All comparisons were made with OV Placebo. The differences between two groups were analyzed using the Student’s t-test,
| WT | Estradiol (pg/ml) | Progesterone (pg/ml) |
|---|---|---|
| Sham at Estrous | 7.2±0.7* | 5154.5±889.0** |
| OV Placebo | 4.1±0.3 | 554.4±79.5 |
| OV Estradiol | 89.5±12.8*** | 3029.4±868.4** |
| OV Progesterone | 7.2±2.8 | 3525.9±362.7*** |
| OV Estradiol plus Progesterone | 56.8±8.9*** | 5618.4±380.9*** |
| hCYP2D6 | ||
| Sham at Estrous | 5.1±0.9** | 569.4±94.9** |
| OV Placebo | 2.6±0.5 | 185.8±32.9 |
| OV Estradiol | 54.1±9.0*** | 603.6±147.0* |
| OV Progesterone | 4.5±0.9 | 1055.7±103.7*** |
| OV Estradiol plus Progesterone | 32.0±4.9*** | 4382.5±900.1*** |
P<0.05,
P<0.01,
P< 0.001.
Western blot analysis
Immunoblot analyses of CYP2D6, CYP2D22, total and phosphorylated-AKT, STAT5b and FOXO1 apoprotein was carried out using microsomes, total cellular proteins or nuclear extracts of liver samples, respectively. RIPA buffer supplemented with protease inhibitors, PMSF (10 μM), BGP (50 μM) and NaF (50 μM) was used for the extraction of liver total cellular proteins. For the preparation of the nuclear extracts and cytosol, the NE-PER nuclear extraction kit (Pierce, Rockford, IL) was used. Protein concentrations were determined in the samples using the BCA protein assay method (Pierce, Rockford, IL, USA). Proteins were subjected to sodium dodecyl sulfate-polyacrylamide (7%) gel electrophoresis and immunoblotting using the following antibodies: human monoclonal CYP2D6 antibody (Cell Signaling Technology), rat monoclonal CYP2D1 IgG (kindly provided by Susumu Imaoka), rabbit monoclonal p-AKT (Ser 473) and total-AKT antibodies (Cell Signalling Technology), mouse monoclonal total STAT5a/b IgG (Santa Cruz Biotechnology) and rabbit monoclonal p-STAT5b IgG (Tyr 694, Cell Signaling Technology), rabbit polyclonal p-FOXO1 (Ser 256) and total FOXO1 IgGs (Santa Cruz Biotechnology). Immunoblotting using mouse monoclonal α-tubulin and GAPDH antibodies (Santa Cruz Biotechnology) was used as loading controls. Secondary anti-rabbit, anti-human, anti-rat or anti-mouse antibodies, conjugated with horseradish peroxidase (Santa Cruz Biotechnology) were used and the proteins were detected using a chemiluminescence detection kit (ECL, Amersham, GE Healthcare).
Hormonal determinations
17β-Estradiol, the major estrogen secreted by the pre-menauposal ovaries, and progesterone concentrations were determined in the serum of intact and tamoxifen-treated female mice at estrous and in those mice submitted to bilateral ovariectomy and supplemented with these hormones. For determinations of estradiol and progesterone serum levels, the EIA corresponding kits (Cayman Chemicals, USA) were used.
Primary hepatocyte cultures
Adult mice were used for the isolation of primary hepatocytes according to a published method (Klaunig 1991) that was later modified (Daskalopoulos, et al. 2012a). In brief, primary hepatocytes were isolated from mice weighing 25–30 g using a two-step collagenase perfusion method. These cells were suspended in William’s Medium E (Gibco) containing 1% L-glutamine (PAA) and 1% penicillin/streptomycin and then, they were counted in a Neubauer cell chamber and plated at a density of 1 × 105 cells per well, in 3.8 square centimeter diameter collagen type I coated dish (BIOCOAT, Cell Environment, Becton Dickinson Labware, UK). The viability of the cells was assessed using a trypan blue dye 0.4% exclusion. In this study, only cells with viability higher than 85% were plated. Primary hepatocytes were cultured at 37°C for 24 hours under an atmosphere of humidified 5% CO2, thus allowing them to adhere to the wells. Optimal conditions related to time and concentration of hormones were determined with time and dose response experiments started 24 hours later. Primary hepatocyte cultures were treated with either 17β-estradiol (1–30 μM) or progesterone (1–30 μM). Primary hepatocytes were also treated with insulin (1 μM) alone (Omiecinski, et al.) or in combination with wortmannin (1 μM), which is an inhibitor of the PI3k/AKT signaling pathway and was added 30 min prior to insulin (Omiecinski et al.). These in vitro experiments employing primary hepatocytes were approved by the Institutional Animal Care and Use Committee of the Faculty of Medicine of the University of Ioannina, Greece.
Statistical analysis
The data are presented as the mean ± SE and were analysed using two-way analysis of variance (ANOVA) followed by multiple comparisons with Bonferroni’s significant difference method. The significance level for all analyses was set at a probability of less than or equal to 0.05. Moreover, correlation statistical analysis was performed using the Pearson’s coefficient correlations to investigate possible correlations between alterations in the relative Cyp2d22 mRNA expression and those observed in several key regulators of this CYP, such as the HNF4α and HNF1α following ovariectomy or hormonal replacement. A probability value of P≤0.05 was considered as significant.
Results
As expected, ovariectomy suppressed serum estradiol (E2) and progesterone levels in both, wild-type (WT) and hCYP2D6 mice (Table 1). Implantation of E2 containing pellets in ovariectomised mice markedly increased serum E2 and progesterone levels in WT and hCYP2D6 mice, while implantation of progesterone containing pellets in ovariectomised mice restored only serum progesterone levels in these two animal models (Table 1). Similarly, the combined treatment with both steroid hormones restored serum E2 and progesterone levels in both, WT and hCYP2D6 mice (Table 1).
Role of sex steroid hormones in the regulation of Cyp2d22 expression profile in wild-type mice.
Analysis of Cyp2d22 mRNA levels by qPCR, of CYP2D protein levels by western blot and the CYP2D-dependent 1΄-hydroxylation of bufuralol by HPLC, revealed that the hepatic expression profile of this isozyme in female WT mice fluctuates within the different phases of the estrous cycle. Hepatic Cyp2d22 mRNA expression was higher at Estrous (E) compared to Proestrous (PE), Methestrous (ME) and Diestrous (DE), (Fig. 1A, P<0.05, P<0.01 and P<0.05, respectively). The levels of 1΄-OH-bufuralol (indicates the activity of CYP2D isozymes) (Fig. 1A, P<0.05) and CYP2D protein levels were also higher at E compared to all other phases of the estrous cycle. Similar fluctuations were detected in hepatic Hnf4a and Hnf1a mRNA expression in the different phases of the estrous cycle (Fig. 1B and 1C) and they were significantly correlated with the hepatic Cyp2d22 mRNA expression (Fig. 1D and 1E, respectively).
Figure 1.

Fluctuations in hepatic Cyp2d22 expression within the distinct phases of the estrous cycle of female mice. (A) Cyp2d22 mRNA expression, CYP2D protein expression and the CYP2D-dependent 1΄-bufuralol hydroxylation. Human monoclonal CYP2D6 IgG (Cell Signalling Technology) was used in the WB capture. (B) and (C) Hnf4α and Hnf1α mRNA expression respectively, within the different phases of the estrous cycle. PE: proestrous, E: estrous, ME: methestrous, DE: diestrous. Mean ±SE, n=22, *P< 0.05, **P< 0.01, ***P< 0.001. (D) and (E) Correlation statistical analysis between relative Cyp2d22 mRNA and Hnf4α or Hnf1α mRNA expression, respectively, was performed using the Pearson’s coefficient correlations (n=5–6 mice per group).
Interestingly, ovariectomy resulted in down-regulation of hepatic Cyp2d22 mRNA (Fig. 2A, P<0.01), CYP2D protein and 1΄-OH bufuralol levels (Fig. 2A). It is also worth noting that Cyp2d22 mRNA expression was lower in the liver of WT males compared to WT females at E (Fig. 2A, P<0.01) and it was equivalent to that detected in ovariectomised females (Fig. 2A). Similar differences were observed in CYP2D protein and 1΄-OH bufuralol levels (Fig. 2A).
Figure 2.
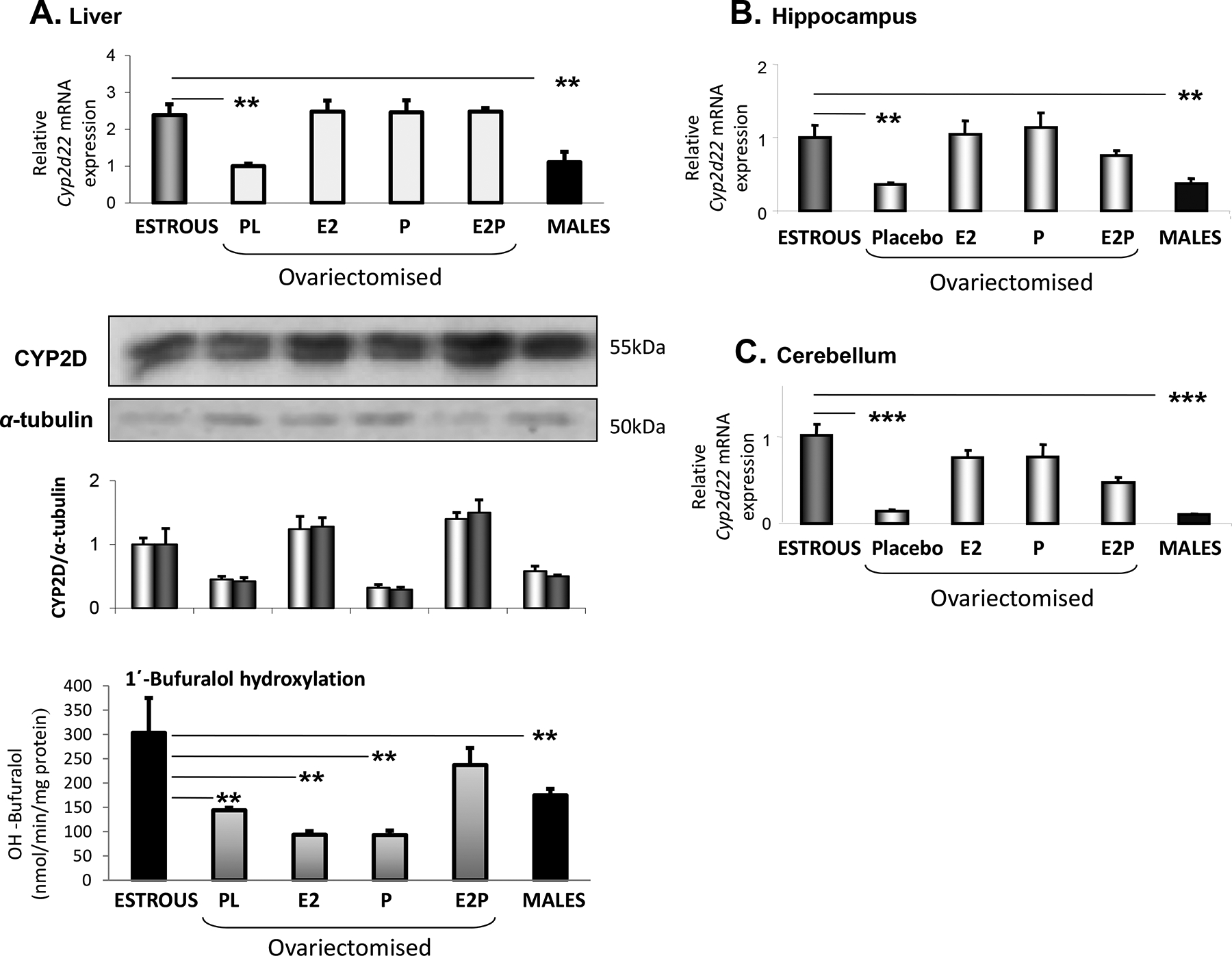
Effect of hormonal supplementation using 17β-estradiol (E2) and/or progesterone (P) or placebo (PL) on Cyp2d22 expression. (A) Fold of change in hepatic Cyp2d22 mRNA, CYP2D protein expression and 1΄-bufuralol hydroxylation between female mice at estrous (E) and ovariectomised mice received hormonal supplementation with 17β-estradiol (E2) and/or progesterone (P). Rat monoclonal CYP2D1 antibody (kindly provided by Susumu Imaoka, JP) was used in the WB capture. (B) and (C) Hippocampal and cerebellum Cyp2d22 mRNA expression pattern, respectively, of female mice at estrous and ovariectomised mice received hormonal supplementation with 17β-estradiol and/or Progesterone. Comparisons were also made between females at E and males. Mean ±SE, (n=5–6 mice per group), **P< 0.01, ***P< 0.001.
In order to investigate the role of female steroid hormones in the differentiation of hepatic Cyp2d22 expression profile during the distinct hormonal states of mice, E2 and/or progesterone were administered to ovariectomised mice. Either E2 or progesterone administered alone or in combination, preserved hepatic Cyp2d22 mRNA expression at levels similar to those detected in intact mice at E (Fig. 2A, P<0.01). Western blot analysis indicated that only the E2-induced Cyp2d22 mRNA expression was correlated with increased CYP2D protein levels (Fig. 2A). Notably, differences in Cyp2d22 activity were not identical to those observed at mRNA and protein expression levels. In particular, in ovariectomised mice, hormonal supplementation using both, E2 and progesterone, preserved the CYP2D-dependent 1΄-bufuralol hydroxylation at levels close to those detected at E (Fig. 2A), whereas E2 or P alone had no effect (Fig. 2A). These data clearly indicate that ovariectomy repressed Cyp2d22 expression at levels detected in males, whereas hormonal supplementation using both, E2 and P, blocked this repression (Fig. 2A).
The hippocampal and cerebellum Cyp2d22 mRNA expression pattern was similar to that observed in the liver of WT mice, females at estrus, males and ovariectomised females with or without hormonal supplementation (Fig. 2B and 2C, respectively).
As previously mentioned, the fluctuations in hepatic Cyp2d22 mRNA expression throughout the distinct phases of the estrous cycle are highly correlated to those in Hnf1a mRNA expression (Fig. 1E, R2=0.7545, P<0.01; Figs. 1A & 1C). This correlation appears to be preserved in the different sex steroid hormonal states of ovariectomised mice received hormonal supplementation with E2 and/or progesterone (Fig. 3D, R2= 0.821, P<0.01; Figs. 2A and 3C), indicating a potential determinant role of sex steroid hormones in the HNF1α-dependent regulation of the Cyp2d22 gene. No significant correlation between Hnf4a and Cyp2d22 mRNA expression patterns was observed in the distinct hormonal states of WT female mice, after ovariectomy and received hormonal supplementation with either E2 and/or progesterone (Fig. 3B. R2= 0,3492; Figs. 2A & 3A). Nonetheless, differences in hepatic Cyp2d22 mRNA expression between females at E and males appear to be associated with those observed in both, Hnf4α and Hnf1α expression (Fig. 3A and 3C, respectively). It is of note that the fluctuations observed in the Cyp2d22 mRNA expression pattern in the liver, mainly correlate to serum progesterone levels and less to serum E2 levels (Fig. 3E and 3F, respectively).
Figure 3.
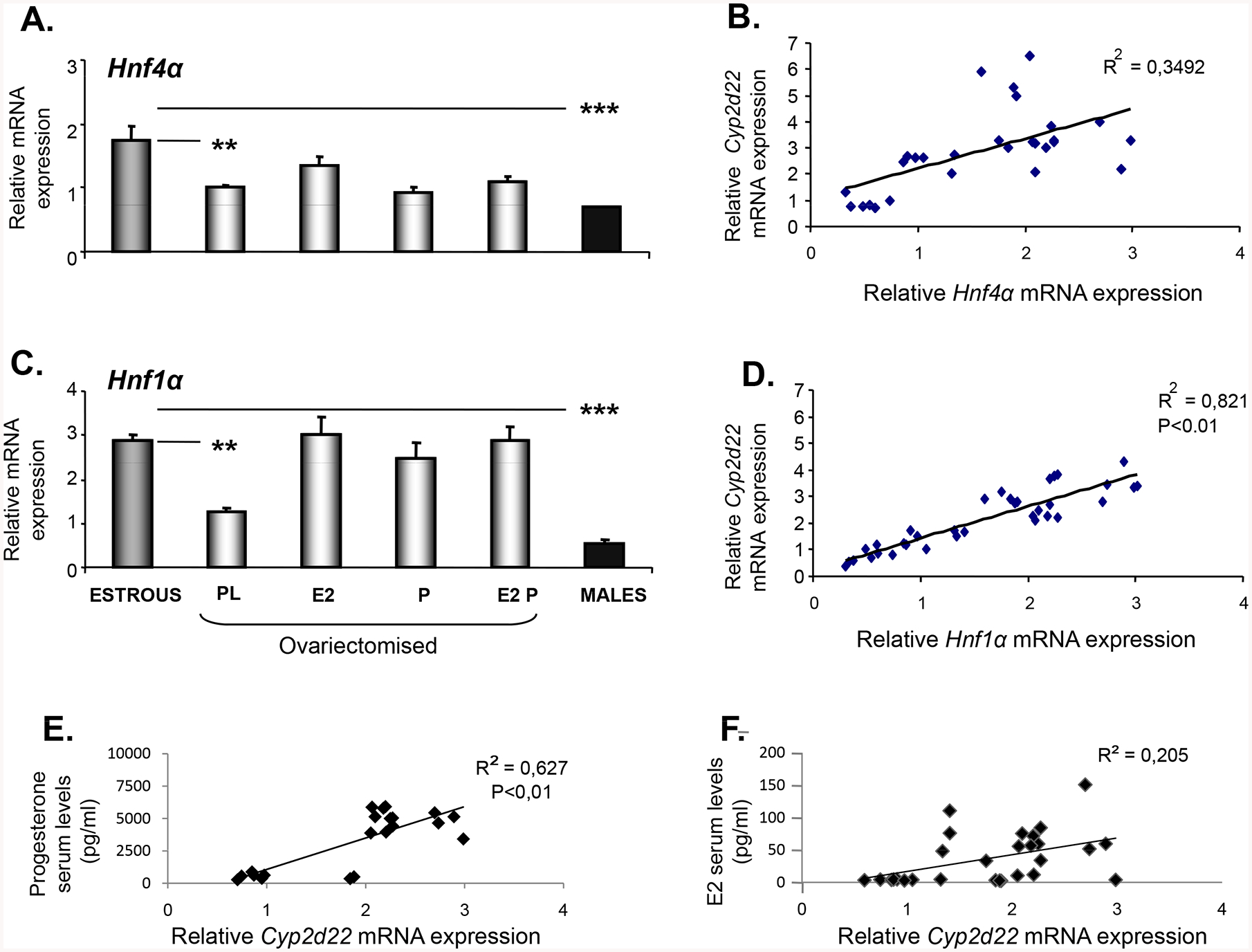
Effect of hormonal supplementation using 17β-estradiol (E2) and/or progesterone (P) or placebo (PL) on Hnf4a and Hnf1a mRNA expression pattern in the liver of male and female mice. (A) Differentiation in hepatic Hnf4α mRNA expression between female mice at estrous (E) and ovariectomised mice received hormonal supplementation with 17β-estradiol (E2) and/or progesterone (P), and males. (B) Correlation between relative Cyp2d22 and Hnf4α mRNA expression in liver. (C) Differentiation in hepatic Hnf1α mRNA expression between female mice at estrous (E) and ovariectomised mice received hormonal supplementation with 17β-estradiol (E2) and/or progesterone (P), and males. (D) Correlation between alterations in hepatic Cyp2d22 and Hnf1α mRNA expression. (E) Correlation between alterations in hepatic Cyp2d22 mRNA expression and serum progesterone levels and (F) Correlation between alterations in hepatic Cyp2d22 mRNA expression and serum estradiol (E2) levels. All correlation statistical analyses were performed using the Pearson’s coefficient correlations. Mean ±SE, (n=5–6 mice per group), **P< 0.01.
The effect of sex steroid hormones on hepatic Cyp2d22 mRNA expression appears to be indirect as in vitro experiments employing murine primary hepatocytes treated with E2 did not significantly change the expression of this cytochrome (Fig. 4A). In addition, treatment of primary hepatocytes with progesterone markedly repressed Cyp2d22 mRNA expression (Fig. 4B, P<0.01), an effect that is opposite to that observed in ovariectomised WT mice following hormonal supplementation with progesterone (Fig. 2A, P< 0.01).
Figure 4.
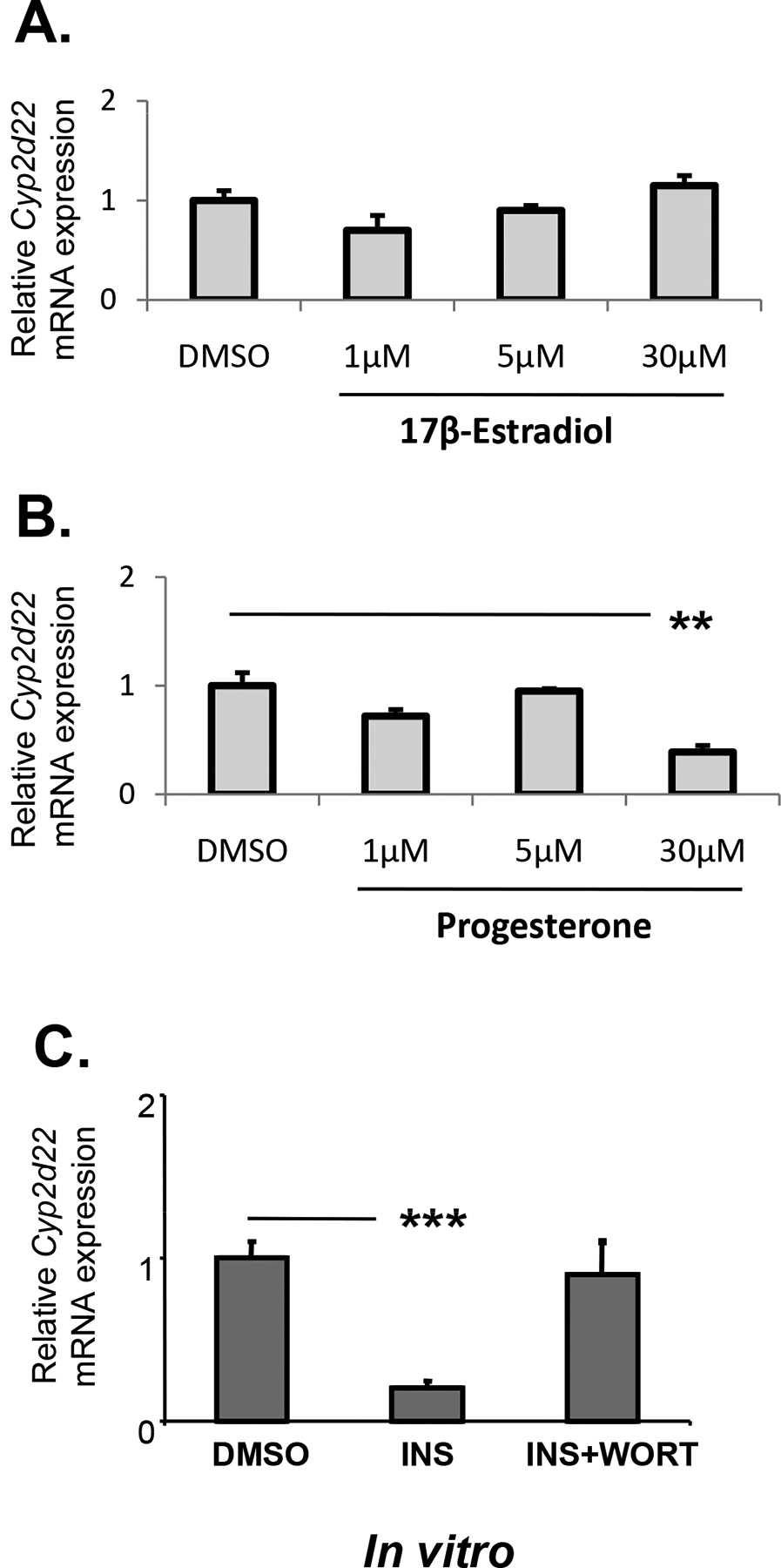
In vitro assessment of the effect of estradiol, progesterone and insulin on Cyp2d22 mRNA expression using primary hepatocytes. INS: insulin, WORT: Wortmannin: a PI3k/AKT pathway inhibitor; **P< 0.01, ***P<0.001
Dissecting signaling pathways mediating the sex steroid hormone-dependent regulation of hepatic Cyp2d22
Hepatic Cyp2d22 mRNA expression was lower in male mice compared to females at estrous and this differentiation could be attributed to the higher activation levels of STAT5b that was detected in male mice (Fig. 5). No significant differentiation in STAT5b phosphorylation was observed in females at distinct hormonal states (Fig. 5). These findings confirm the gender differentiation in the regulation of various CYPs including Cyp2d and the critical role of growth hormone (GH)/STAT5b signaling in this differentiation (Daskalopoulos et al. 2012a; Daskalopoulos et al. 2012c; Waxman and O’Connor 2006).
Figure 5.
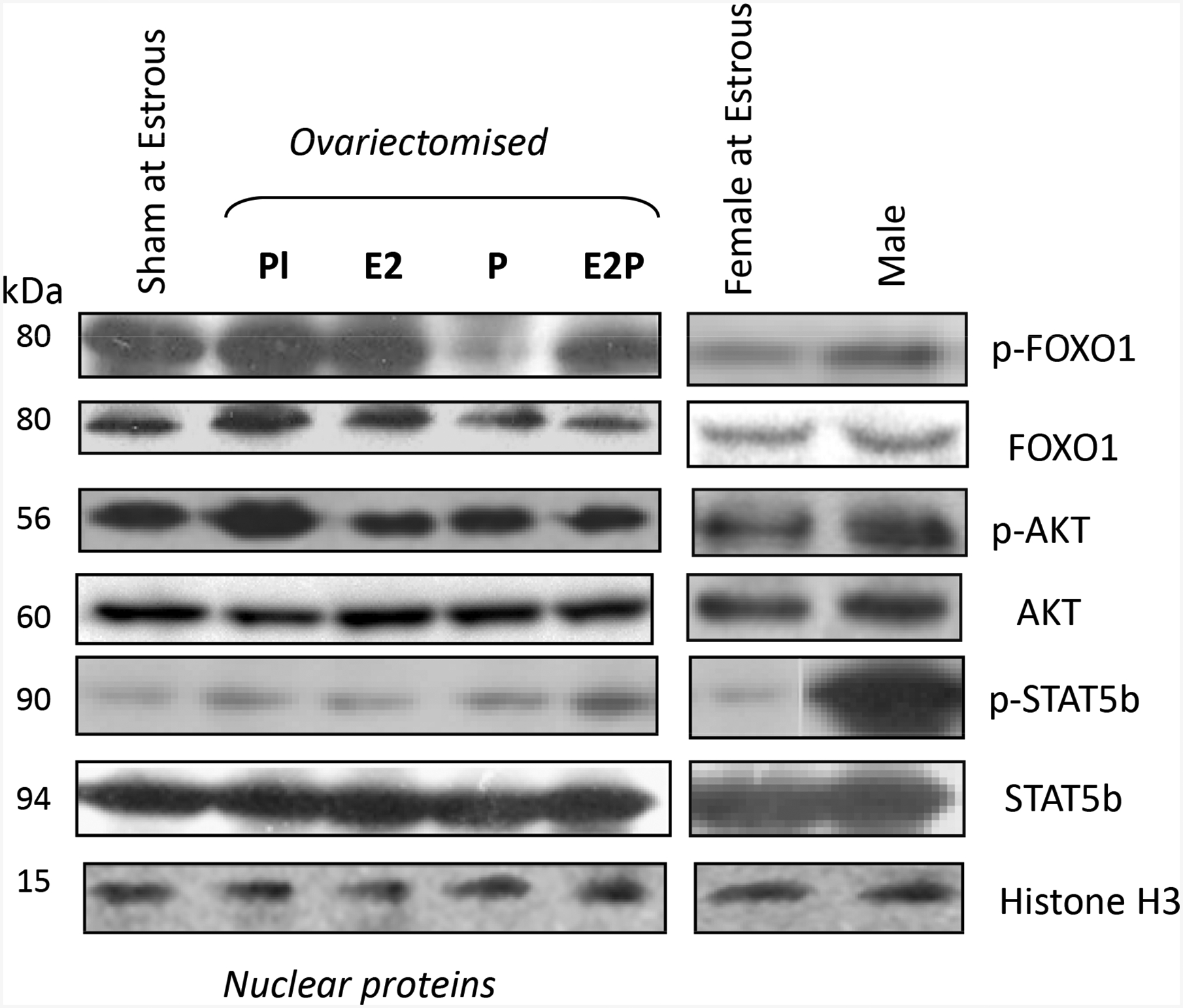
Assessment of the involvement of PI3k/AKT/FOXO1 and GH/STAT5b activation in the female sex steroid hormone impact on Cyp2d22 expression using Western blot analysis. The level of AKT, FOXO1b and STAT5b phosphorylation (p) was assessed in hepatic nuclear proteins of sham operated normo-cyclic females at estrus, OV female mice implanted with pellets containing either placebo (PL), or Estradiol (E2) and/or Progesterone (P), and in normo-cyclic females at estrus and males. Each lane in the western blot capture corresponds to a sample from one mouse, representative of 3 samples per treatment group tested.
The ovariectomy-induced repression of hepatic Cyp2d22 expression may be mediated by the PI3k signaling pathway as phosphorylation of AKT, a downstream element in this pathway, was higher in ovariectomised mice than in those that received hormonal supplementation using either E2, or progesterone or both hormones (Fig. 5). In particular, progesterone supplementation markedly reduced FOXO1 phosphorylation in ovariectomised mice (Fig. 5). In contrast, FOXO1 phosphorylation was higher in males than in females at estrous (Fig. 5). To further elucidate the role of this pathway in Cyp2d22 regulation, primary hepatocytes were treated with insulin, which activated the PI3k/AKT pathway (Kim and Novak 2007; Kodama, et al. 2004), and also repressed Cyp2d22 mRNA expression, an effect that was prevented by wortmannin, an inhibitor of this pathway (Fig. 4C) (Kim and Novak 2007).
Furthermore, tamoxifen, an anti-estrogenic compound, repressed Cyp2d22 expression in the liver of female mice compared to intact mice at E (Fig. 6A, P<0.01), and exerted its suppressive effect potentially via activation of the GH/STAT5b linked pathway (Fig. 6A and 6C) and PI3k/AKT signaling pathway, as depicted by the drug-induced phosphorylation of STAT5b and AKT, respectively (Fig. 6A and 6C). Both, HNF4α and HNF1α, appear to participate in the tamoxifen-mediated down-regulation of Cyp2d22 (Fig. 6B).
Figure 6.
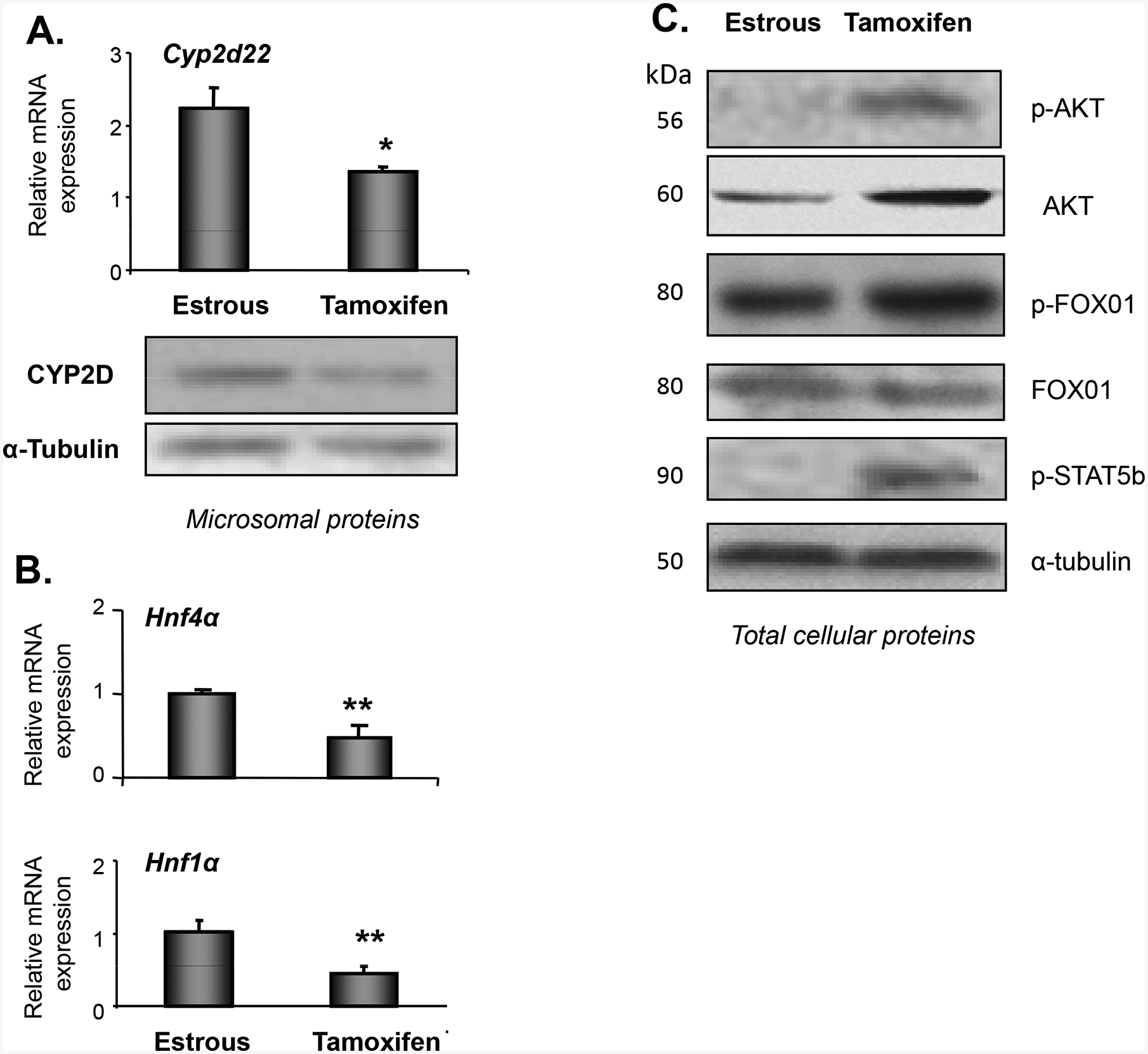
Assessment of tamoxifen effect on (A) Cyp2d22 mRNA and CYP2D protein expression. (B) Effect of tamoxifen on Hnf4a and Hnf1a mRNA expression in liver nuclear proteins *P<0.05, **P<0.01. (C) Effect of tamoxifen on PI3k/AKT/FOXO1 and GH/STAT5b activation (p) in total cellular proteins using western blot analysis. Each lane in the western blot capture corresponds to a sample from one mouse, representative of 3 samples per treatment group tested.
Role of sex steroid hormones in the regulation of hepatic CYP2D6 expression profile in hCYP2D6 mice
Analysis of CYP2D6 mRNA levels revealed a phenotype in the hepatic expression of this cytochrome, as hepatic Cyp2d22 and CYP2D6 expression profiles in WT and hCYP2D6 mice, respectively, were distinct (Figs. 1A and 7A). In particular, in contrast to what observed in WT mice, ovariectomy markedly increased CYP2D6 mRNA and protein expression in the liver of hCYP2D6 mice compared to sham operated controls at E, a fact that was reversed by hormonal replacement therapy using either E2 or P (Fig. 7A, P<0.001). The combined treatment using both steroid hormones was less effective (Fig. 7A, P<0.05). There is also a phenotype in hepatic expression of CYP2D6 in males. It was observed that, although, the hepatic Cyp2d22 mRNA expression was lower in WT males compared to females at E (Fig. 1A, P<0.001), CYP2D6 mRNA expression was markedly higher in the liver of hCYP2D6 males than that of hCYP2D6 females at E and it was close to levels detected in hCYP2D6 ovariectomised females (Fig. 7A).
Figure 7.

Effect of hormonal supplementation using 17β-estradiol (E2) and/or Progesterone (P) on CYP2D6 expression pattern in the liver of male and female hCYP2D6 mice. (A) Fold of change in hepatic CYP2D6 mRNA and protein expression, and alterations in CYP2D6 activity between female mice at estrous (E) and ovariectomised mice received hormonal supplementation with 17β-estradiol (E2) and/or progesterone (P), PL: placebo; Mean ±SE, (n=5–6 mice per group), **P<0.01, ***P< 0.001. Numbers below lanes in the western blot capture indicate the ratio between CYP2D6 expression and the corresponding GAPDH and that of females at estrous is considered as 1. (B) Correlation statistical analysis between alterations in hepatic CYP2D6 mRNA and serum 17β-estradiol levels and (C) Correlation between alterations in hepatic CYP2D6 mRNA expression and serum Progesterone levels was performed using the Pearson’s coefficient correlations (n=5–6 mice per group).
Discussion
Previous studies reported the involvement of several endogenous and exogenous compounds in the regulation of CYP2D (Baum and Strobel 1997; Waxman and O’Connor 2006; Waxman and Holloway 2009). Although CYP2D isozymes have long been considered as non-hormone inducible drug-metabolizing enzymes (Ingelman-Sundberg et al. 2007; Yu et al. 2004; Zanger et al. 2004), recent studies reported possible roles of sex steroid hormones in CYP2D regulation (Ning et al. 2015; Wang et al. 2014). However, the mechanisms involved in this regulation were not elucidated.
This study focused on the role of female sex steroid hormones in the regulation of CYP2D employing wild type and humanized CYP2D6 (hCYP2D6) mouse models. The present data revealed a fluctuation in the hepatic CYP2D expression pattern within the different phases of the estrous cycle of female mice, with higher expression levels at estrous compared to proestrous, methestrous and diestrous. This differentiation was detected at Cyp2d22 mRNA, CYP2D protein and enzyme activity level (1΄-bufuralol hydroxylation). Interestingly, fluctuations in hepatic Cyp2d22 mRNA expression in the distinct phases of the estrous cycle follow those observed in the hepatic nuclear factors, HNF4α and HNF1α, which have significant roles in CYP2D regulation (Cheung, et al. 2003; Congiu, et al. 2009; Daskalopoulos, et al. 2012b; Daskalopoulos et al. 2012c; Dickmann, et al. 2008; Hara and Adachi 2002; Ning et al. 2015). In particular, alterations in Cyp2d22 mRNA expression were highly correlated with Hnf1a mRNA expression.
The role of E2 and progesterone in the distinction of the estrous cycle in four phases is fundamental. Specifically, E2 levels come to a peak early at estrous, prior to ovulation, whereas progesterone levels start rising late at estrous and reach high levels at methestrous remaining high at diestrous. From proestrous they start declining until the first part of estrous (Fata et al. 2001; Walmer et al. 1992). Therefore, to elucidate the role of female sex steroids in Cyp2d22 regulation, female mice were ovariectomised and submitted to hormonal supplementation using either E2 and/or progesterone. Interestingly, ovariectomy resulted in hepatic Cyp2d22 down-regulation and the expression of Cyp2d22 remained at levels similar to those detected in males. Hormonal supplementation using either E2 and/or progesterone restored Cyp2d22 mRNA expression levels close to those observed in intact females at estrous. It is worth noting that the Cyp2d22 mRNA expression pattern in the hippocampus and cerebellum of wild-type male mice and females in different hormonal states was similar to that observed in the liver. These findings clearly indicate a critical role of female steroid hormones in the regulation of Cyp2d22 in the liver and the brain tissues tested. Notably, variations in hepatic Cyp2d22 mRNA expression were highly correlated to those in serum progesterone levels and less to those in E2 levels. The positive effect of progesterone is apparent in the wild-type murine animal model used in the present study. It should be noted though that previous work reported a down-regulating effect of progesterone and an up-regulating effect of testosterone on CYP2D in female rat brain (Baum and Strobel 1997), effects that are profoundly in agreement with the hormone-related hepatic CYP2D6 expression pattern of hCYP2D6 males and ovariectomised female mice that followed progesterone supplementation in the present study. The assumption that the CYP2D6 hormone-related alterations in rat liver follow the same direction with those observed in their brain is based on present findings coming from wild-type mice indicating that the hormone-related alterations in brain Cyp2d22 mRNA expression are similar to those observed in their liver. It should be mentioned here, that Cyp2d22 and not CYP2D6 is expressed in the brain of transgenic hCYP2D6 mice (Miksys et al. 2005) and the hormone-related fluctuations in their brain Cyp2d22 mRNA are similar to those observed in wild-type mice. It is noteworthy that unlike what was observed in wild-type mice, estradiol has a down-regulating effect on hepatic CYP2D6 in hCYP2D6 mice. The discrepancy observed in these two studies regarding the data coming from wild-type mice and rats, is likely due to the differences in the methodological approaches followed in these two studies. Notably, female Sprague Dawley rats were used in the earlier study (Baum and Strobel 1997), while female and male C57BL/6J mice were used in the present study. These animal species display a genetic heterogeneity translated into phenotypic variability in several physiological, molecular, and genomic indices, when deciphering their roles in drug metabolism, among others (Boverhof, et al. 2006; Johnson 2012). Furthermore, CYP2D expression was assessed in the whole brain of female Sprague Dawley rats, while in the present study, Cyp2d22 expression was assessed in two brain regions of mice, the hippocampus and cerebellum, with significant and distinct roles in brain function. Brain is a heterogeneous tissue with a high degree of numerical, morphological and functional cellular diversity and lipid content within the distinct brain regions, conditions that should be taken into account in experimental studies in order to increase the validity of data and facilitate the extrapolation from preclinical species to humans (Gustafsson, et al. 2019). In the present study, comparisons on alterations in Cyp2d22 expression patterns were made between the different phases of the estrous cycle. In the assessment of the role of female sex steroid hormones in the regulation of this isozyme all comparisons were made with respect to estrous, always taking into account the hormonal state of the animals, an approach that was not followed in the previous study (Baum and Strobel 1997).
In the experimental setting of the present study, the fluctuations observed in the hepatic Cyp2d22 mRNA expression pattern, appear to correlate with serum progesterone levels and less with E2 levels. Nonetheless, fluctuations in Cyp2d22 mRNA expression within the different phases of the estrous cycle did not follow those observed in serum progesterone levels, indicating that the effect of other factors is potentially more prominent than that of progesterone in determining Cyp2d22 expression profile in the distinct phases of the menstrual cycle.
In vitro experiments employing murine primary hepatocytes treated with either E2 or progesterone indicated that the effect of female sex steroid hormones on hepatic Cyp2d22 expression is potentially indirect as E2 did not significantly alter the expression of this cytochrome in the hepatocytes and progesterone markedly repressed it, effect opposite to that observed in ovariectomised WT mice receiving progesterone. Based on the fact though, that there is a differentiation at some point in cell biology between in vivo hepatocytes and primary hepatocytes in culture, such as stimulation of several signaling pathways during the isolation of primary hepatocytes including those related to MAPK and NFκB that down-regulates CYP2D; (Shimamoto, et al. 2014), the direct effect of sex steroid hormones should not be excluded, which is apparently overridden by their peripheral effects (Francavilla, et al. 1989), (Clayton and Darnell 1983), (Shimamoto et al. 2014).
In the sex steroid hormone-induced Cyp2d22 regulation, HNF1α appears to play a significant role, because the fluctuations observed in the hepatic Cyp2d22 mRNA expression pattern within the distinct phases of the estrous cycle and in the different sex steroid hormonal states of ovariectomised mice receiving or not hormonal supplementation were highly correlated with Hnf1a mRNA levels. The role of HNF4α appears to be less significant.
It is well established that GH regulates a diverse set of target genes in the liver, including those encoding for receptors, transcription factors and drug metabolizing enzymes, among others. Binding of GH to its receptor on the cell membrane activates several signalling pathways and in particular, that of the transcription factor, signal transducer and activator of transcription 5b (STAT5b) (O’Shea, et al. 2002), with an essential role in liver sexual dimorphism (Clodfelter, et al. 2006; Mode and Gustafsson 2006; Waxman and O’Connor 2006; Waxman and Holloway 2009). However, the GH/STAT5b signaling pathway appears not to play a significant role in the fluctuation of Cyp2d22 expression within the different phases of the estrus cycle, as no significant differentiation in STAT5b phosphorylation was observed in females in the distinct hormonal states of the menstrual cycle. But it is of interest to note that the activation level of the GH/STAT5b pathway was higher in males than in females at estrous, followed by lower hepatic Cyp2d22 expression in males compared to females. Therefore, GH may have a critical role in gender differentiation and regulation of Cyp2d22, which could be mainly attributed in differentiation in the GH/STAT5b signaling activation between male and female mice (Scheme 1) (Daskalopoulos et al. 2012b; Daskalopoulos et al. 2012c; Waxman and O’Connor 2006; Waxman and Holloway 2009; Zhang et al. 2018).
Scheme 1.

Panel A. Down-regulating effect of growth hormone (GH) on Cyp2d22. GH activates its receptor (GHR) on hepatocyte membrane, which then activates Jak2, an effect followed by phosphorylation of the signal transducer and activator of transcription 5b (STAT5b), which dimerizes and then binds to a specific element on the promoter of Cyp2d22 gene, thus inhibiting its transcription. Panel B. Up-regulating effect of progesterone on Cyp2d22. Progesterone inhibits the insulin-induced activation of the PI3k/AKT/FOXO1β signaling pathway (Bruns and Kemnitz 2004), thus preventing the translocation of the forkhead box protein O1β (FOXO1β) into the cytoplasm, an effect that favors the transcription of Cyp2d22. Dotted lines depict inhibition.
Taken together with previous reports, the current study suggests that sex steroid hormones play a significant gender- and species-specific role in the regulation of CYP2D in the liver and brain, with progesterone holding a distinct role in this regulation, highly dependent on HNF1α. The progesterone induced up-regulating effect on Cyp2d22 is profoundly mediated by inhibition of the PI3k/AKT/FOXO1 pathway (Fig. 5; Scheme 1) (Bruns and Kemnitz 2004). It is known that FOXO1 is a transcription factor, downstream to the PI3k/AKT signaling pathway that upon activation trans-locates from nucleus to cytoplasm, thus terminating the transcription of various FOXO1-dependent genes, which encode several proteins including CYPs (Daskalopoulos et al. 2012a). Our hypothesis of the role of PI3k/AKT signaling pathway in the regulation of Cyp2d22 expression pattern in the distinct hormonal states tested is supported by the finding that treatment of primary hepatocytes with insulin activated the PI3k/AKT pathway (Kim and Novak 2007; Kodama et al. 2004), and also repressed Cyp2d22 mRNA expression, effects that were prevented by wortmannin, an inhibitor of this pathway (Kim and Novak 2007).
In the gender-related differentiation in CYP2D regulation the role of GH/STAT5b signaling activation is of fundamental significance (Scheme 1). The present data support the accumulating evidence suggesting that disturbances in CYP2D regulation are associated with the development of neurologic and psychiatric disorders and the impaired outcome of pharmacotherapy, in particular, of women in menopause receiving or not hormonal supplementation or of those under treatment with contraceptives (Haduch and Daniel 2018; Hiroi et al. 1998; Wang et al. 2014; Yu et al. 2003). Although the current experimental data cannot be extrapolated to humans, they clearly indicate that the fluctuations in Cyp2d22 and Cyp2e1 (Konstandi et al. 2013) observed within the distinct phases of the menstrual cycle of female mice along with those dependent on the sex hormonal state of females and males, set the necessity of reconsidering the therapeutic schemes followed in female patients that are in principle based on results coming from studies employing only male patients. These data also indicate that the sex and hormonal status (females with normal or abnormal estrous cycle, being in menopause or following hormonal supplementation, or females receiving contraceptives and males) is a determinant parameter for the successful outcome of pharmacotherapy and should be always taken into account when designing preclinical and clinical studies or prescribing drugs-substrates of the above mentioned CYPs.
Acknowledgements
We would like to thank Tsutomu Matsubara for the training on the isolation of primary hepatocytes, as well as Kristopher W. Krausz and John Buckley for valuable technical assistance. This study was supported in part, by the National Cancer Institute Intramural Program.
Footnotes
Declaration of interest
There are no conflicting interests, financial or otherwise, that should be declared by the authors.
REFERENCES
- Baum LO & Strobel HW 1997. Regulation of expression of cytochrome P-450 2D mRNA in rat brain with steroid hormones. Brain Research 765 67–73. [DOI] [PubMed] [Google Scholar]
- Bebia Z, Buch SC, Wilson JW, Frye RF, Romkes M, Cecchetti A, Chaves-Gnecco D & Branch RA 2004. Bioequivalence revisited: Influence of age and sex on CYP enzymes. Clinical Pharmacology & Therapeutics 76 618–627. [DOI] [PubMed] [Google Scholar]
- Bertilsson L, Alm C, De Las Carreras C, Widen J, Edman G & Schalling D 1989. Debrisoquine hydroxylation polymorphism and personality. Lancet 1 555. [DOI] [PubMed] [Google Scholar]
- Boverhof DR, Burgoon LD, Tashiro C, Sharratt B, Chittim B, Harkema JR, Mendrick DL & Zacharewski TR 2006. Comparative Toxicogenomic Analysis of the Hepatotoxic Effects of TCDD in Sprague Dawley Rats and C57BL/6 Mice. Toxicological Sciences 94 398–416. [DOI] [PubMed] [Google Scholar]
- Bruns CM & Kemnitz JW 2004. Sex hormones, insulin sensitivity, and diabetes mellitus. ILAR J 45 160–169. [DOI] [PubMed] [Google Scholar]
- Chen GF, Ronis MJJ, Ingelman-Sundberg M & Badger TM 1999. Hormonal regulation of microsomal cytochrome P4502E1 and P450 reductase in rat liver and kidney. Xenobiotica 29 437–451. [DOI] [PubMed] [Google Scholar]
- Cheung C, Akiyama TE, Kudo G & Gonzalez FJ 2003. Hepatic expression of cytochrome P450s in hepatocyte nuclear factor 1-alpha (HNF1alpha)-deficient mice. Biochemical pharmacology 66 2011–2020. [DOI] [PubMed] [Google Scholar]
- Clayton DF & Darnell JE Jr. 1983. Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Molecular and cellular biology 3 1552–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clodfelter KH, Holloway MG, Hodor P, Park S-H, Ray WJ & Waxman DJ 2006. Sex-Dependent Liver Gene Expression Is Extensive and Largely Dependent upon Signal Transducer and Activator of Transcription 5b (STAT5b): STAT5b-Dependent Activation of Male Genes and Repression of Female Genes Revealed by Microarray Analysis. Molecular Endocrinology 20 1333–1351. [DOI] [PubMed] [Google Scholar]
- Congiu M, Mashford ML, Slavin JL & Desmond PV 2009. Coordinate regulation of metabolic enzymes and transporters by nuclear transcription factors in human liver disease. J Gastroenterol Hepatol 24 1038–1044. [DOI] [PubMed] [Google Scholar]
- Corchero J, Granvil CP, Akiyama TE, Hayhurst GP, Pimprale S, Feigenbaum L, Idle JR & Gonzalez FJ 2001. The CYP2D6 humanized mouse: effect of the human CYP2D6 transgene and HNF4alpha on the disposition of debrisoquine in the mouse. Mol Pharmacol 60 1260–1267. [DOI] [PubMed] [Google Scholar]
- Daskalopoulos EP, Lang MA, Marselos M, Malliou F & Konstandi M 2012a. D2-Dopaminergic Receptor-Linked Pathways: Critical Regulators of CYP3A, CYP2C, and CYP2D. Molecular Pharmacology 82 668. [DOI] [PubMed] [Google Scholar]
- Daskalopoulos EP, Lang MA, Marselos M, Malliou F & Konstandi M 2012b. D2-Dopaminergic Receptor-Linked Pathways: Critical Regulators of CYP3A, CYP2C, and CYP2D. Molecular Pharmacology 82 668–678. [DOI] [PubMed] [Google Scholar]
- Daskalopoulos EP, Malliou F, Rentesi G, Marselos M, Lang MA & Konstandi M 2012c. Stress is a critical player in CYP3A, CYP2C, and CYP2D regulation: role of adrenergic receptor signaling pathways. American Journal of Physiology-Endocrinology and Metabolism 303 E40–E54. [DOI] [PubMed] [Google Scholar]
- Dickmann LJ, Tay S, Senn TD, Zhang H, Visone A, Unadkat JD, Hebert MF & Isoherranen N 2008. Changes in maternal liver Cyp2c and Cyp2d expression and activity during rat pregnancy. Biochemical pharmacology 75 1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Chaudhary V & Khokha R 2001. Cellular Turnover in the Mammary Gland Is Correlated with Systemic Levels of Progesterone and Not 17β-Estradiol During the Estrous Cycle1. Biology of Reproduction 65 680–688. [DOI] [PubMed] [Google Scholar]
- Francavilla A, Polimeno L, DiLeo A, Barone M, Ove P, Coetzee M, Eagon P, Makowka L, Ambrosino G, Mazzaferro V, et al. 1989. The effect of estrogen and tamoxifen on hepatocyte proliferation in vivo and in vitro. Hepatology 9 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González I, Peñas-Lledó EM, Pérez B, Dorado P, Álvarez M & LLerena A 2008. Relation between CYP2D6 phenotype and genotype and personality in healthy volunteers. Pharmacogenomics 9 833–840. [DOI] [PubMed] [Google Scholar]
- Gustafsson S, Sehlin D, Lampa E, Hammarlund-Udenaes M & Loryan I 2019. Heterogeneous drug tissue binding in brain regions of rats, Alzheimer’s patients and controls: impact on translational drug development. Scientific Reports 9 5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haduch A & Daniel WA 2018. The engagement of brain cytochrome P450 in the metabolism of endogenous neuroactive substrates: a possible role in mental disorders. Drug Metab Rev 50 415–429. [DOI] [PubMed] [Google Scholar]
- Hara H & Adachi T 2002. Contribution of hepatocyte nuclear factor-4 to down-regulation of CYP2D6 gene expression by nitric oxide. Mol Pharmacol 61 194–200. [DOI] [PubMed] [Google Scholar]
- Hel Z, Stringer E & Mestecky J 2010. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocrine reviews 31 79–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi T, Imaoka S & Funae Y 1998. Dopamine formation from tyramine by CYP2D6. Biochem Biophys Res Commun 249 838–843. [DOI] [PubMed] [Google Scholar]
- Ingberg E, Theodorsson A, Theodorsson E & Strom JO 2012. Methods for long-term 17beta-estradiol administration to mice. Gen Comp Endocrinol 175 188–193. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M 2004. Pharmacogenetics of cytochrome P450 and its applications in drug therapy: the past, present and future. Trends in Pharmacological Sciences 25 193–200. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M, Sim SC, Gomez A & Rodriguez-Antona C 2007. Influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol Ther 116 496–526. [DOI] [PubMed] [Google Scholar]
- Jürgens G, Lange KHW, Reuther LØ, Rasmussen BB, Brøsen K & Christensen HR 2002. Effect of growth hormone on hepatic cytochrome P450 activity in healthy elderly men. Clinical Pharmacology & Therapeutics 71 162–168. [DOI] [PubMed] [Google Scholar]
- Johnson M 2012. Laboratory Mice and Rats. MATER METHODS 2. [Google Scholar]
- Kim RB, O’Shea D & Wilkinson GR 1995. Interindividual variability of chlorzoxazone 6-hydroxylation in men and women and its relationship to CYP2E1 genetic polymorphisms. Clinical Pharmacology & Therapeutics 57 645–655. [DOI] [PubMed] [Google Scholar]
- Kim SK & Novak RF 2007. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther 113 88–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Yim HK, Jung YS, Park JH & Kim SY 2007. Hepatic injury induces contrasting response in liver and kidney to chemicals that are metabolically activated: Role of male sex hormone. Toxicology and Applied Pharmacology 223 56–65. [DOI] [PubMed] [Google Scholar]
- Klaunig JE 1991. Alterations in intercellular communication during the stage of promotion. Proc Soc Exp Biol Med 198 688–692. [DOI] [PubMed] [Google Scholar]
- Kodama S, Koike C, Negishi M & Yamamoto Y 2004. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Molecular and cellular biology 24 7931–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstandi M, Cheng J & Gonzalez FJ 2013. Sex steroid hormones regulate constitutive expression of Cyp2e1 in female mouse liver. American journal of physiology. Endocrinology and metabolism 304 E1118–E1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xie M, Wang X, Ouyang X, Wan Y, Dong G, Yang Z, Yang J & Yue J 2015. Sex hormones regulate cerebral drug metabolism via brain miRNAs: down-regulation of brain CYP2D by androgens reduces the analgesic effects of tramadol. British journal of pharmacology 172 4639–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Peng Q, Zeng Z, Wang J, Deng Y, Xie L, Mo C, Zeng J, Qin X & Li S 2014. CYP2D6 phenotypes and Parkinson’s disease risk: A meta-analysis. Journal of the Neurological Sciences 336 161–168. [DOI] [PubMed] [Google Scholar]
- Miksys SL, Cheung C, Gonzalez FJ & Tyndale RF 2005. Human CYP2D6 and mouse CYP2Ds: organ distribution in a humanized mouse model. Drug Metab Dispos 33 1495–1502. [DOI] [PubMed] [Google Scholar]
- Mode A & Gustafsson JA 2006. Sex and the liver - a journey through five decades. Drug Metab Rev 38 197–207. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Custódio JBA, Nunes E, Moreno A, Seiça R, Oliveira CR & Santos MS 2007. Estradiol affects liver mitochondrial function in ovariectomized and tamoxifen-treated ovariectomized female rats. Toxicology and Applied Pharmacology 221 102–110. [DOI] [PubMed] [Google Scholar]
- Neal MJ 2015. Medical Pharmacology at a Glance: Wiley. [Google Scholar]
- Nelson JF, Felicio LS, Osterburg HH & Finch CE 1981. Altered Profiles of Estradiol and Progesterone Associated with Prolonged Estrous Cycles and Persistent Vaginal Cornification in Aging C578L/6J Mice1. Biology of Reproduction 24 784–794. [DOI] [PubMed] [Google Scholar]
- Ning M, Koh KH, Pan X & Jeong H 2015. Hepatocyte nuclear factor (HNF) 4α transactivation of cytochrome P450 (Cyp) 2d40 promoter is enhanced during pregnancy in mice. Biochemical pharmacology 94 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea JJ, Gadina M & Schreiber RD 2002. Cytokine Signaling in 2002: New Surprises in the Jak/Stat Pathway. Cell 109 S121–S131. [DOI] [PubMed] [Google Scholar]
- Omiecinski CJ, Redlich CA & Costa P 1990. Induction and developmental expression of cytochrome P450IA1 messenger RNA in rat and human tissues: detection by the polymerase chain reaction. Cancer Res 50 4315–4321. [PubMed] [Google Scholar]
- Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, Burger HG, Colditz GA, Davis SR, Gambacciani M, et al. 2010. Postmenopausal hormone therapy: an Endocrine Society scientific statement. The Journal of clinical endocrinology and metabolism 95 s1–s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandlyn MJ, Stuart EC, Somers-Edgar TJ, Menzies AR & Rosengren RJ 2008. A new role for tamoxifen in oestrogen receptor-negative breast cancer when it is combined with epigallocatechin gallate. British journal of cancer 99 1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer N, Kapelyukh Y, McEwan J, Beuger V, Stanley LA, Rode A & Wolf CR 2012. Modeling Human Cytochrome P450 2D6 Metabolism and Drug-Drug Interaction by a Novel Panel of Knockout and Humanized Mouse Lines. Molecular Pharmacology 81 63–72. [DOI] [PubMed] [Google Scholar]
- Shimamoto Y, Watanabe K, Ikadai H, Okamura M & Ishizuka M 2014. Repression of hepatic cytochrome P450 2D expression in mice during Babesia microti infection. J Vet Med Sci 76 1147–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle I, Fritz P, Eckhardt K, Zanger UM & Eichelbaum M 2001. Cellular localization and regional distribution of CYP2D6 mRNA and protein expression in human brain. Pharmacogenetics 11 237–245. [DOI] [PubMed] [Google Scholar]
- Smith CAD, Wolf CR, Gough AC, Spurr NK, Leigh PN, Summers BA, Harding AE, Maranganore DM, Sturman SG, Williams AC, et al. 1992. Debrisoquine hydroxylase gene polymorphism and susceptibility to Parkinson’s disease. The Lancet 339 1375–1377. [DOI] [PubMed] [Google Scholar]
- Walmer DK, Wrona MA, Hughes CL & Nelson KG 1992. Lactoferrin expression in the mouse reproductive tract during the natural estrous cycle: correlation with circulating estradiol and progesterone. Endocrinology 131 1458–1466. [DOI] [PubMed] [Google Scholar]
- Wang X, Li J, Dong G & Yue J 2014. The endogenous substrates of brain CYP2D. Eur J Pharmacol 724 211–218. [DOI] [PubMed] [Google Scholar]
- Waxman DJ & O’Connor C 2006. Growth Hormone Regulation of Sex-Dependent Liver Gene Expression. Molecular Endocrinology 20 2613–2629. [DOI] [PubMed] [Google Scholar]
- Waxman DJ & Holloway MG 2009. Sex differences in the expression of hepatic drug metabolizing enzymes. Molecular Pharmacology 76 215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AM, Idle JR & Gonzalez FJ 2004. Polymorphic cytochrome P450 2D6: humanized mouse model and endogenous substrates. Drug Metab Rev 36 243–277. [DOI] [PubMed] [Google Scholar]
- Yu AM, Idle JR, Byrd LG, Krausz KW, Kupfer A & Gonzalez FJ 2003. Regeneration of serotonin from 5-methoxytryptamine by polymorphic human CYP2D6. Pharmacogenetics 13 173–181. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Raimundo S & Eichelbaum M 2004. Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol 369 23–37. [DOI] [PubMed] [Google Scholar]
- Zarrow MX 1964. Experimental endocrinology: a sourcebook of basic techniques: Academic Press. [Google Scholar]
- Zhang F, Li J, Na S, Wu J, Yang Z, Xie X, Wan Y, Li K & Yue J 2018. The Involvement of PPARs in the Selective Regulation of Brain CYP2D by Growth Hormone. Neuroscience 379 115–125. [DOI] [PubMed] [Google Scholar]


