Abstract
T-cell depletion of an HLA-haploidentical (haplo) graft is often used to reduce the risk of graft-versus-host disease (GVHD), but the lack of donor T cells in the infused product may lead to graft failure, slow T-cell reconstitution, infections, and relapse. More selective T-cell depletion targeting CD45RA can effectively deplete naïve T cells but preserve large numbers of memory T cells leading to robust engraftment of diverse T-cell populations and reduction of viremia in the early post-transplant period. Herein, we report the outcome of 143 pediatric and young adult hematologic malignancy patients receiving a first allogeneic hematopoietic cell transplantation (HCT) on 6 consecutive ex vivo T-cell depleted haploHCT protocols over the past 15 years at a single institution - including the first 50 patients on an active CD45RA-depleted haploHCT study in which patients also received NK-cells and pharmacological GvHD prophylaxis post transplant. Our data demonstrated an increase in the 3-year overall survival and event-free survival in non-chemorefractory recipients receiving CD45RA-depleted grafts (78.9% and 77.7%, respectively) compared to historic T-cell depleted haploHCT cohorts (46.7% and 42.7%, respectively, p=0.004, and 0.003). This improvement was primarily due to a reduction in transplant related mortality without significant increase in the rates of GVHD.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) remains the definitive therapeutic option for patients with high risk hematologic malignancies.1, 2 Significant improvement in survival following HCT has been achieved in the last two decades through advances in HLA typing, donor selection, and supportive care.3 Although increased efforts in detecting and treating infections and graft-vs.-host disease (GVHD) have been made, these complications along with disease recurrence remain the primary causes of death.4–8
Matched related and matched unrelated donors continue to be the standard for transplantation in patients with hematologic malignancies, however 25–60% of eligible pediatric recipients lack such donors.9 Ex vivo T-cell depleted haploidentical donor (haplo) grafts can reliably achieve donor engraftment with manageable side effects.10–13 However, extensive T-cell depletion resulted in delayed immune recovery, and higher risk of relapse and infection.14, 15 Hence, more selective T-cell depletion techniques that effectively remove naïve alloreactive T cells but save potentially beneficial donor T-cell subsets have been studied.16, 17 CD45RA-depletion has been shown to preserve diverse donor memory cell populations that may be effective in providing protection against infections and relapse, with little added risk of severe GVHD.18–21 Therefore, we recently incorporated CD45RA-depletion into our institutional haploHCT protocols for treatment of patients with hematologic malignancies.22–24 Our previous analysis showed a robust recovery of effector memory and central memory subsets by Day +30 post-transplant that were a direct recapitulation of the CD45RA-depleted donor graft. This early quantitative T-cell recovery compared favorably to older CD3-depletion techniques and was associated with a reduction in incidence and duration of viremia during the first 6 months after HCT. Herein, we analyze the early clinical outcomes in the first 50 patients on this ongoing study and find significant improvement in overall and event-free survival for non-refractory patients with hematologic malignancies compared to similar patients treated on historic institutional haploHCT trials.
Subjects and Methods
Patients
Analyses include 186 high risk hematologic malignancy patients who received first allogeneic HCT on IRB approved trials at St. Jude Children’s Research Hospital from 2002 to 2017. Informed consent was obtained for all participants. Of these, 143 were treated on T-cell depleted haploHCT trials under various IND or IDE granted by the FDA; 43 were treated on an IRB approved standard-of-care HLA-matched donor protocol.
CD45RA-depleted haploHCT cohort (n = 50)
Initiated in 2013 (ClinicalTrials.gov Identifier: NCT01807611). Eligibility criteria include a hematologic malignancy with an indication for HCT, and lack of matched related or unrelated donor, or unsuitability for conventional matched donor transplant due to refractory malignancy. If more than one suitable haploidentical donor was available, the donor with the greatest level of KIR mismatch was preferred. KIR mismatch was defined by the presence of inhibitory KIR genes for which the recipient lacked the cognate HLA gene.25 Recipients received conditioning consisting of total lymphoid irradiation (8Gy), cyclophosphamide (60mg/kg), fludarabine (150mg/m2), thiotepa (10mg/kg), and melphalan (140mg/m2), followed by two hematopoietic progenitor cell grafts that were T-cell depleted using a CliniMACS device (Miltenyi Biotec, Auburn, CA). The first graft was enriched for CD34+ cells, and the second was CD45RA-depleted with a combined CD34+ cell dose goal of 5 × 106/kg (accepted range: 2 – 50 × 106/kg). Finally, purified donor NK cells were infused on Day +6, and sirolimus (n=9) or MMF (n=41) was initiated for GVHD prophylaxis 7 days after NK cell infusion and continued to Day +60 if no GVHD or mixed chimerism occurred. There was no minimum or maximum T-cell dose in this cohort, only the requirement of >2 log depletion of all CD45RA+ cells.
Historic haploHCT cohorts (n = 93)
Two historic cohorts with similar eligibility requirements were retrospectively analyzed as comparator groups. Goal CD34+ dose for all patients in these two historic haploHCT cohorts was 10 × 106/kg (accepted range: 2 – 100 × 106/kg).
Initial haploHCT cohort (n = 27):
From 2002 through 2004; patients received CD34+ enriched (n=7) or CD3-depleted (n=20) progenitor cell grafts. Patients received total body irradiation (TBI) (12Gy), thiotepa (10mg/kg), cyclophosphamide (120mg/kg), and rabbit ATG (10mg/kg) without post-transplant GVHD prophylaxis.
Recent haploHCT cohort (n = 66):
From 2004 to 2013; patients were treated on 4 consecutive clinical trials utilizing several advances compared to the Initial haploHCT cohort, including: i) use of submyeloablative melphalan, ii) weekly viral monitoring by PCR with preemptive treatment, EBV prophylaxis with rituximab, iii) selection of the donor with the greatest level of KIR mismatch, and iv) frequent chimerism monitoring with donor lymphocyte infusion (DLI) intervention. Patients received CD3-depletion (n=56) or CD34+ enriched (n=10) progenitor cell grafts preceded by a preparative regimen including fludarabine (150–200mg/m2), thiotepa (10mg/kg), and melphalan (120–140mg/m2), and either OKT3 (n=46) or campath (n=20). Recipients of CD3-depleted grafts received MMF for two months post-transplant. In the second of these 4 trials, T-cell addback with a goal of 0.15 × 106/kg T cells was provided. Upon completion of that trial, the T-cell dose of CD3-depleted grafts was capped at <0.1 × 106/kg.
Contemporary HLA-matched donor transplant cohort (n = 43)
Recipients who received standard myeloablative transplantation with an HLA-matched donor for hematologic malignancy parallel (2013–2017) to the CD45RA-depleted haploHCT cohort were also assessed. Donors were required to be matched at a minimum of 7 out of 8 alleles when considering HLA-A, B, C, and DRB1. Matched donor recipients were conditioned with TBI (12Gy) and cyclophosphamide (120mg/kg) or IV Busulfan (0.8mg/kg/dose q6hr initially, then targeted) and cyclophosphamide (200mg/kg). Matched unrelated donor recipients (n=29) received rabbit ATG (7mg/kg), whereas matched sibling donor recipients (n=14) did not. All received MMF to Day +30 and Cyclosporine to Day +100 followed by a 4-week taper. Bone marrow graft was utilized in 40 (93%).
Defining disease status
Pre-transplant disease status was categorized as standard or refractory. Refractory patients included those with active disease, including leukemia >5% blasts in the marrow with count recovery or <5% blasts but without blood count recovery, MDS with >5% blasts, or active lymphoma. Standard patients included anyone in CR (even if detectible minimal residual disease), lymphoma with PR or VGPR, MDS with <5% blasts, or any CML.
Statistics
The first 50 CD45RA-depleted haploHCT recipients, who were at least 100 days post-transplant, were compared to the Initial haploHCT cohort (n=27), the Recent haploHCT cohort (n=66), and the contemporary HLA-matched donor cohort (n=43). Patient characteristics and outcomes data were compared using t-test/Wilcoxon rank-sum test based on normality assumption for continuous variables and chi-square test/fisher exact test for categorical variables. Overall survival (OS) was defined as the time from transplantation until death from any cause, censoring those alive at last follow-up. Survival probabilities were estimated using Kaplan-Meier method and compared using log-rank test. Event-free survival (EFS) was defined as the time from transplantation until relapse or death due to any cause, all other patients are censored. The cumulative incidence (CIN) of relapse, transplant related mortality (TRM), acute GVHD II-IV, acute GVHD III-IV and chronic GVHD were estimated by Kalbfleisch–Prentice method and compared using Gray’s test, deaths were considered as competing events.26, 27 For patients with acute GVHD III-IV events, overall survival from time of diagnosis of acute GVHD was performed as above. The assumption of proportional hazard was confirmed in all analyses. All the reported P values are two-sided and considered statistically significant if < 0.05. Statistical analyses were performed with SAS software version 9.4 Institute (Cary, NC).
Results
HaploHCT recipients
Overall, patient characteristics are similar for the three haploHCT cohorts (Table 1). The majority were of racial or ethnic minority, as only 56 (39%) were non-Hispanic white. Most patients had acute leukemia: 55 acute myeloid leukemia (AML), 55 acute lymphoid leukemia (ALL; 45 B-lineage, 10 T-lineage), and 6 biphenotypic leukemia. Twelve (8%) had MDS (5 with RCC, 3 with RAEB, 4 in CR1 after chemotherapy for presence of blasts). Nearly one-quarter of patients were unable to achieve remission prior to transplant (deemed refractory). Flow cytometry and DNA-based minimal residual disease (MRD) testing was only consistently available in the CD45RA-depleted haploHCT cohort, and of the 36 patients in remission, 22 (61%) were MRD negative, 10 (28%) MRD positive, and 4 had no MRD test available. High progenitor cell doses (CD34+ cells >10×106/kg) were achieved in a majority of recipients in all haploHCT cohorts. The infused CD3+ (T-cell) dose was markedly lower in the historic cohorts in which pan T-cell depletion was used (median infused T-cell dose: 0.05×106/kg (Initial), 0.05×106/kg (Recent) and 75.85×106/kg in the CD45RA-depleted haploHCT cohort). Forty-six (92%) patients in the CD45RA-depleted cohort received no detectible CD45RA+ T cells (<0.001 × 106/kg) from their progenitor cell grafts, the remaining four patients received 0.041 to 0.128 × 106/kg. Five patients did not receive NK cells, and the 45 remaining patients received a median of 11.9 (range 1.26 to 67.97) × 106/kg NK cells and a median of <0.001 (range <0.001 to 0.014) × 106/kg T cells.
Table 1:
HaploHCT Recipient Characteristics
| CD45RA-depleted haploHCT | Initial haploHCT | P value | Recent haploHCT | P value | |||
| Number | 50 | 27 | 66 | ||||
| Age, yr (median, range) | 8.1 (0.6–20.8) | 11.9 (2.7–22.1) | 0.269 | 12.7 (0.5–26.5) | 0.227 | ||
| Gender | 0.81 | 1 | |||||
| Female | 19 (38%) | 9 (33%) | 25 (38%) | ||||
| Male | 31 (62%) | 18 (67%) | 41 (62%) | ||||
| Race | 0.44 | 0.8 | |||||
| Asian | 2 (4%) | 2 (7%) | 2 (3%) | ||||
| Black | 10 (20%) | 7 (26%) | 18 (27%) | ||||
| White | 37 (74%) | 16 (59%) | 44 (67%) | ||||
| Other | 1 (2%) | 2 (7%) | 2 (3%) | ||||
| Ethnicity | 0.45 | 0.43 | |||||
| Hispanic | 18 (36%) | 7 (26%) | 19 (29%) | ||||
| Non-Hispanic | 32 (64%) | 20 (74%) | 47 (71%) | ||||
| Diagnosis | 0.044 | 0.048 | |||||
| acute leukemia | 47 (94%) | 21 (78%) | 48 (73%) | ||||
| lymphoma | 1 (2%) | 1 (4%) | 5 (8%) | ||||
| MDS | 2 (4%) | 2 (7%) | 8 (12%) | ||||
| JMML | 3 (4%) | ||||||
| CML | 3 (11%) | 2 (3%) | |||||
| Disease status | 0.15 | 0.53 | |||||
| Standard | 36 (72%) | 24 (89%) | 51 (77%) | ||||
| CR1 | 17 | 8 | 26 | ||||
| CR2 | 14 | 10 | 9 | ||||
| CR>2 | 3 | 3 | 8 | ||||
| Other | 2 | 3 | 8 | ||||
| Refractory | 14 (28%) | 3 (11%) | 15 (23%) | ||||
| PIF | 8 | ||||||
| Relapse 1 | 4 | 1 | 10 | ||||
| Relapse ≥2 | 2 | 1 | 2 | ||||
| RAEB | 1 | 3 | |||||
| Prior Autologous HCT | 1 (2%) | 1 (3.7%) | 1.000 | 3 (4.5%) | 0.63 | ||
| Donor | 0.76 | 0.580 | |||||
| Father | 17 (34%) | 9 (33%) | 28 (42%) | ||||
| Mother | 29 (58%) | 18 (67%) | 32 (48%) | ||||
| Other related | 1 (2%) | 1 (2%) | |||||
| Sibling | 3 (6%) | 5 (8%) | |||||
| Unrelated | |||||||
| HLA mismatch (#/10) | 1 | 1 | |||||
| 1 (2%) | 1 (4%) | 2 (3%) | |||||
| 49 (98%) | 26 (96%) | 64 (97%) | |||||
| CD34+ cell dose (x106/kg) | 0.029 | 0.023 | |||||
| Median (range) | 16.7(2.95–67.55) | 10.4(1.5–39.0) | 10.9(1.7–49.75) | ||||
| CD3+ cell dose (x106/kg) | <0.001 | <0.001 | |||||
| Median (range) | 75.85(16.1–528.6) | 0.05(0.004–0.15) | 0.05(0.01–0.25) | ||||
Engraftment
In the CD45RA-depleted cohort, 47 (94%) engrafted neutrophils at a median of Day +11 (range 9 to 13). Two (4%) experienced acute rejection, and all other patients uniformly achieved full donor chimerism by Day +30. Both patients with rejection had AML with myelodysplasia-related changes, and both achieved complete donor engraftment following salvage transplantation from the same donor. A third patient died of transplant related causes during engraftment (ANC>500/μL × 2 days). Neutrophil engraftment occurred in 93% in the Initial haploHCT cohort at a median of Day +14 (range 8–35), and 88% in the Recent haploHCT cohort at a median of Day +11 (range 9 to 26).
Platelet engraftment (>20K/μL) occurred in 92% CD45RA-depleted haploHCT recipients by Day +100 (median Day +17, range 10–84), 89% Initial haploHCT (median Day +17, range 14–45), and 86% Recent haploHCT recipients (median Day +15, range 7 to 50).
GVHD
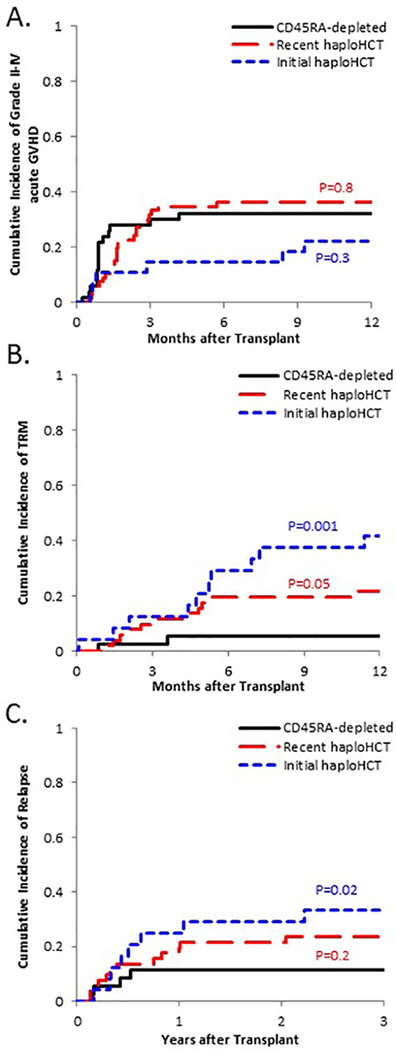
The CIN of acute GVHD II-IV in CD45RA-depleted haploHCT recipients was 32.1±6.7% at one year. This was comparable to 22.2±8.2% (p=0.3) in Initial haploHCT and 36.4±6.0% (p=0.8) in Recent haploHCT recipients (Figure 1A). Overall, 16 CD45RA-depleted haploHCT recipients experienced grade II-IV acute GVHD at a median of Day +27 (range 7 to 125), with a skewing towards higher grades of GVHD (II = 2, III = 7, IV = 7). Six Initial haploHCT and 24 Recent haploHCT recipients experienced grade II-IV acute GVHD (II = 12, III = 10, IV = 8) at a median of Day +49 (range 16 to 278). Notably, grade II-IV acute GVHD at one year was more common in the historic haploHCT recipients who received ≥0.1×106 CD3+-cell/kg (14/28=50%) compared to those receiving <0.1×106 CD3+-cell/kg (16/65=25%) (p=0.006).
Figure 1: Cumulative incidence of key adverse events for successive generations of T-cell depleted haploHCT.
(A) There was no significant difference between the rate of moderate to severe acute GVHD between CD45RA-depleted cohort and the Recent haploHCT cohort (p=0.8) or the Initial haploHCT cohort (p=0.3); GVHD analysis was not stratified by disease status. (B) TRM at one year for non-refractory patients in the CD45RA-depleted cohort trended lower than in the Recent haploHCT cohort (p=0.05) and was significantly lower than in the Initial haploHCT cohort (p=0.001). (C) Rate of relapse at three years for non-refractory patients in the CD45RA-depleted cohort trended lower than in the Recent haploHCT cohort (p=0.2) and was significantly lower than in the Initial haploHCT cohort (p=0.02).
The CIN of chronic GVHD was 25.9±6.6% at two years in CD45RA-depleted, 25.9±8.7% (p=1.0) in Initial and 27.3±5.5% (p=0.7) in Recent haploHCT recipients. The NIH severity score for chronic GVHD was mild in 5, moderate in 5, and severe in 2 patients. Of the 7 Initial and 18 Recent haploHCT recipients who experienced chronic GVHD, the severity of chronic GVHD was limited in 11 and extensive in 14, and an infused CD3+-cell dose of ≥0.1×106/kg was associated with an increased rate of chronic GVHD 14/28=50% vs. 11/65=17% (p=0.00048). Organs typically involved by acute GVHD included skin, gut and liver in all cohorts. Interestingly, there was a trend towards an increased presence of visceral GVHD without skin GVHD in CD45RA-depleted recipients, as skin GVHD was present in only 56% of CD45RA-depleted recipients with any GVHD compared to 79%, 91%, and 100% in the other cohorts. All patients with moderate to severe acute GVHD received standard GVHD therapy consisting of corticosteroids and maintenance agent, usually tacrolimus. In patients with suboptimal initial response, second line therapy was initiated, usually infliximab.
Treatment Related Mortality and Relapse
Because patients with refractory disease generally have higher rates of TRM and relapse, these analyses were stratified by disease status (Table 2).
Table 2:
Clinical Outcomes
| CD45-depleted haploHCT | Initial haploHCT | P value | Recent haploHCT | P value | Contemporary HLA-matched | P value | CD45-depleted haploHCT | Recent haploHCT | P value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 36 | 24 | 51 | 40 | 14 | 15 | ||||
| 1 year OS | 88.1% (±6.1%) | 45.8% (±9.7%) | 68.6% (±6.4%) | 84.2% (±6.2%) | 53.9% (±14.9%) | 33.3% (±11.1%) | ||||
| 3 year OS | 78.9% (±13.7%) | 33.3% (±9.6%) | 0.0005 | 52.9% (±7.0%) | 0.03 | 66.0% (±10.3%) | 0.4 | 32.3% (±18.8%) | 20.0% (±10.3%) | 0.4 |
| 1 year EFS | 82.9% (±7.2%) | 37.5% (±9.4%) | 58.8% (±6.8%) | 65.2% (±8.0%) | 41.7% (±15.9%) | 26.7% (±10.2%) | ||||
| 3 year EFS | 77.7% (±13.9%) | 25.0% (±8.8%) | 0.0001 | 51.0% (±7.0%) | 0.02 | 62.0% (±10.6%) | 0.2 | 27.8% (±16.7%) | 20.0% (±10.3%) | 0.64 |
| 100 day TRM | 2.8% (±2.8%) | 12.5% (±9.6%) | 11.8% (±4.6%) | 2.5% (±2.5%) | 21.4% (±11.4%) | 6.7% (±6.7%) | ||||
| 1 year TRM | 5.6% (±3.9%) | 41.7% (±10.4%) | 0.001 | 21.6% (±5.8%) | 0.05 | 7.9% (±4.4%) | 0.7 | 21.4% (±11.4%) | 33.3% (±13.0%) | 0.67 |
| 1yr relapse rate | 11.5% (±5.5%) | 25.0% (±9.1%) | 19.6% (±5.6%) | 27.0% (±7.5%) | 36.9% (±14.1%) | 53.3% (±13.6%) | ||||
| 3yr relapse rate | 11.5% (±5.5%) | 33.3% (±10.1%) | 0.02 | 23.5% (±6.0%) | 0.2 | 30.3% (±7.9%) | 0.1 | 36.9% (±14.1%) | 53.3% (±13.6%) | 0.3 |
| CD45-depleted haploHCT | Initial haploHCT | P value | Recent haploHCT | P value | Contemporary HLA-matched | ||
|---|---|---|---|---|---|---|---|
| Number | 50 | 27 | 66 | 43 | |||
| II-IV acute GVHD | 32.1% (±6.7%) | 22.2% (±8.2%) | 0.3 | 36.4% (±6.0%) | 0.8 | 32.9 (±7.3%) | 0.9 |
| III-IV acute GVHD | 28.1% (±6.4%) | 7.4% (±5.1%) | 0.04 | 24.2% (±5.3%) | 0.5 | 18.75 (±6.1%) | 0.2 |
| chronic GVHD | 25.9% (±6.6%) | 25.9% (±8.7%) | 1.00 | 27.3% (±5.5%) | 0.7 | 10.3 (±5.0%) | 0.05 |
Four CD45RA-depleted haploHCT recipients experienced TRM by Day +100 (3 were refractory). The CIN of TRM at 1 year in standard risk patients was lower with each generation as CD45RA-depleted haploHCT recipients were 5.6±3.9% compared to 41.7±10.4% (p=0.001) for Initial haploHCT and 21.6±5.8% (p=0.05) for Recent haploHCT (Figure 1B). There was no difference in TRM amongst refractory patients (p=0.67).
Nine CD45RA-depleted haploHCT recipients experienced relapse: 5/14 refractory and 4/36 standard risk patients. The 3-year CIN of relapse in standard risk patients receiving CD45RA-depleted haploHCT (11.5±5.5%) was lower than the Initial haploHCT cohort (33.3±10.1%; p=0.02), and similar to the Recent haploHCT cohort (23.5±6.0%; p=0.2; Figure 1C). The relapse rate in refractory patients was similar, with a 3-year CIN of 36.9±14.1% in CD45RA-depleted haploHCT recipients compared to 53.3±13.6% (p=0.3) in Recent haploHCT recipients. There were only 3 refractory patients in the Initial haploHCT cohort, precluding analysis.
Survival
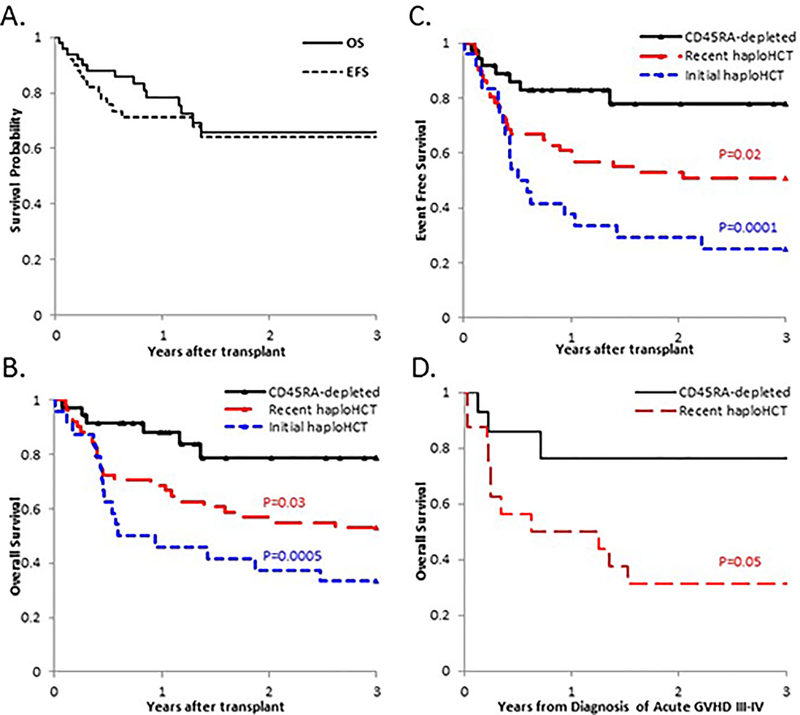
Thirty-six CD45RA-depleted haploHCT recipients remain alive at a median of 532 days post-transplant (range 106 to 1538). Overall survival and event-free survival at 3 years was 65.8±7.8% and 64.1±7.6%, respectively (Figure 2A). The 3-year overall survival was significantly worse in refractory patients 32.3%±18.8% than standard risk patients 78.9±13.7% (p=0.002; Table 2). Therefore, survival analysis was stratified by disease status.
Figure 2: Probabilities for overall and event free survival improved with successive generations of T-cell depleted haploHCT.
(A) The overall survival and event-free survival at 3 years for the first 50 patients receiving CD45RA-depleted haploHCT. (B) 3-year overall survival for non-chemorefractory patients was significantly improved in the CD45RA-depleted cohort compared to the Recent haploHCT cohort (p=0.03) and the Initial haploHCT cohort (p=0.0005). (C) 3-year event-free survival for non-refractory patients was significantly improved in the CD45RA-depleted cohort compared to the Recent haploHCT cohort (p=0.02) and the Initial haploHCT cohort (p=0.0001). (D) 3-year Overall survival from the diagnosis of acute GVHD grade III-IV demonstrated a trend towards improved survival in CD45RA-depleted haploHCT recipients compared to Recent haploHCT recipients (p=0.08).
3-year overall survival in standard risk patients was improved for CD45RA-depleted haploHCT recipients (78.9±13.7%) compared to Initial haploHCT (33.3±9.6%; p=0.0005) and Recent haploHCT (52.9±7.0%; p=0.03) recipients (Figure 2B). Event-free survival (alive without relapse) was also improved at 77.7±13.9% vs. 25.0±8.8% (p=0.0001) and 51.0±7.0% (p=0.02), respectively (Figure 2C).
Due to the observation that TRM was low despite a skewing towards severe GVHD in CD45RA-depleted haploHCT recipients, the overall survival and TRM from the onset of grade III-IV GVHD was analyzed.(Figure 2D) The 2-year OS was 76.2±16.6% in the CD45RA-depleted haploHCT cohort versus 31.2±10.6% in the Recent haploHCT cohort (p=0.05). With relapse considered as a competing event, the 2-year CIN of TRM was 14.3±9.7% in CD45RA-depleted haploHCT recipients versus 56.3±13.1% in Recent haploHCT recipients (p=0.08).
Contemporary matched donor recipients
Patients in the matched donor cohort were more likely to receive bone marrow grafts and less likely to have refractory disease compared to CD45RA-depleted haploHCT recipients (Supplemental Table 1). CD34+ and CD3+ cell doses were lower in matched donor recipients compared to CD45RA-depleted haploHCT recipients.
There were only 3 matched donor recipients with refractory disease at the time of transplantation, and all experienced early relapse (two before Day +30 and the other before 6 months). For standard-risk patients, the TRM at one year was below 10% for both contemporary cohorts, and the relapse rate at 3 years was 11.5%±5.5% vs. 30.3%±7.9% (p=0.1) (Table 2). The 3-year overall survival (66.0±10.3%; p=0.4) and event free survival (62.0±10.6%; p=0.2) in matched donor recipients were also similar (Supplemental Figure 1).
Discussion
HaploHCT has been increasingly used since the implementation of post-transplant cyclophosphamide (PTCY) as a form of in vivo T-cell depletion.11, 28–30 PTCY induces bidirectional tolerance, thereby allowing use of various preparative regimens with consistently low rates of TRM and GVHD yet infections and relapse remain problematic.31–34 Early attempts to limit GVHD in haploHCT recipients utilizing ex vivo T-cell depletion were generally successful, but recipients were plagued by delayed donor T-cell reconstitution and high rates of dangerous viral infections and disease recurrence.35, 36 Subsequent strategies combating delayed reconstitution in T-cell depleted haploHCT included adoptive transfer of conventional and regulatory or genetically modified T cells.37–41 Current efforts are focused on selective forms of T-cell depletion which spare effector T-cell subsets with a low risk of GVHD, out of which the most widely utilized is TCRαβ-depletion.42–45 In a recent series of 80 patients with acute leukemia in remission who received TCRαβ-depleted haploHCT, robust TCRγδ+ T-cell reconstitution with a very low non-relapse mortality rate was reported.45 Furthermore, these patients demonstrated a 5-year leukemia-free survival of 71% although best outcomes were restricted to patients receiving myeloablative TBI preparative regimens.
We report the outcome of 143 patients receiving a first allogeneic HCT for high risk hematologic malignancy on 6 consecutive ex vivo T-cell depleted haploHCT protocols over the past 15 years at a single institution, which includes a cohort of 50 patients on an ongoing haploHCT study utilizing CD45RA-targeted depletion. We previously reported robust recovery of diverse donor memory T-cell subsets, and a reduction in the incidence and severity of viremia in patients who received CD45RA depleted grafts.23, 24 In this expanded report, we show that overall survival and survival without relapse are superior in non-refractory recipients of CD45RA-depleted haploHCT compared to historical haploHCT recipients. Importantly, current outcomes are similar to a contemporaneous HLA-matched donor transplant cohort. While we have not formally compared other donor variables, it is very unlikely that there were significant differences between the haploHCT cohorts, since there were no significant differences regarding recipient age, race, and ethnicity, and the gender of the donor (Table 1). Since the HLA-matched donor transplant cohort was treated on a standard of care protocol and no research immune monitoring was performed, we could not compare the memory T-cell recovery between both groups.
Improved outcome in successive haploHCT cohorts appears to be primarily due to a reduction in TRM, though there is a trend towards lower relapse risk as well. Reduction of TRM may be related in part to advances in supportive care over the 10-year period from the initiation of the historic haploHCT trials to the start of the current trial. However, this narrow timeframe of supportive care advances is disproportional to the magnitude of TRM reduction observed here. Replacing myeloablative TBI with submyeloablative melphalan and ATG or Campath with TLI was well tolerated in CD45RA-depleted haploHCT recipients, and allowed consistent donor engraftment with rapid and robust donor T-cell recovery. The observation that the outcomes of the CD45RA-depleted haploHCT cohort were similar to the matched HLA-matched donor transplant cohort that received TBI should be an impetus to compare TBI and non-TBI based regimen in the HLA-matched donor graft setting.
Our previously reported immune reconstitution data for the first 26 patients showed that CD45RA- depleted haploHCT recipients have robust early engraftment (day 30) of CD4- and CD8-positive T cells in contrast to recipients of CD3-depleted haploHCT grafts, and that this was associated with a reduced incidence of Cytomegalovirus and Adenovirus viremia post HCT.23 Of interest, despite receiving NK-cell infusions, CD45RA-depleted haploHCT recipients had significantly lower number of circulating NK cells in comparison to CD3-deleted haploHCT graft recipients at Day+30, suggesting that the robust early memory T-cell reconstitution in CD45RA-depleted haploHCT recipients is critical for protection against viral-associated diseases.23 Viral infections plague transplantation in an HLA-mismatched setting due to the requirement for extensive ex vivo or in vivo T-cell depletion or suppression. Aggressive viral monitoring and preemptive therapy are required to reduce morbidity and mortality from these infections,15 though prolonged use of these preemptive antiviral agents are associated with potentially severe acute and chronic toxicities such as bone marrow suppression and renal injury.46, 47
Historic haploHCT cohorts demonstrated increased rates of acute and chronic GVHD when the infused T-cell dose was ≥ 0.1 million/kg. In comparison, 1000-fold higher T-cell doses were infused without a significant increase in GVHD when CD45RA-depletion was utilized, although a potential skewing towards more severe acute GVHD was noted in these patients. Interestingly, survival remained high and TRM low even in patients afflicted with grade III-IV acute GVHD. In this regard, it might be instructive not only to calculate the GVHD free, relapse free survival (GFRS) rate but also the current GFRS (CGRFS; accounts for patients who experience GVHD but are successfully weaned off of systemic immune suppression) in the future. The low observed TRM in the CD45RA-depleted haploHCT cohort may be related to an enhanced ability to control viral infections during acute GVHD treatment, and possibly improved response to GVHD therapy.
The early clinical outcomes from this ongoing CD45RA-depleted haploHCT trial were also compared to a contemporary HLA-matched donor cohort receiving identical clinical care. Overall survival and survival without relapse were found to be similar in the two contemporaneous cohorts. However, direct comparison of these cohorts was limited due to differences in the patient characteristics and selection bias, as patients lacking a matched donor or not suitable for a standard transplant due to their disease status were offered the haploHCT trial. This selection bias though likely skews haploHCT recipients towards a higher risk group, and therefore would not influence our report of survival outcome when comparing these two 2 cohorts.
In our report, CD45RA-depleted haploHCT recipients had improved outcomes compared to recent historical haploHCT cohorts which was predominantly due to a reduction in TRM and to a lesser degree a reduction in the incidence of relapse. Thus, further active exploration of memory T-cell infusions is warranted, especially since their anti-leukemia activity can now be improved by expressing tumor-specific chimeric antigen receptors.48
Supplementary Material
Acknowledgements
The authors would like to thank our colleagues for data collection and clinical management. Our thanks also go out to the many patients and families who participated in the transplantation and cellular therapy research program. We would like to recognize additional Principle Investigators of the historic T-cell depleted haploidentical donor therapeutic transplant trials initiated at this institution: Rupert Handgretinger, Eli Benaim, and Greg Hale. This work is supported in part by the National Institutes of Health Cancer Center Support (CORE) grant P30 CA021765, and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Disclosure of Conflicts of Interest
W.L. is currently an employee of Miltenyi Biotech, all efforts contributing to this work except for final manuscript review occurred prior to this employment. B.M.T. received travel support from Miltenyi Biotech to present previously published work at EBMT annual meeting 2018. The other authors have no financial relationships or other conflicts of interest to disclose relevant to this manuscript. No author received an honorarium, grant, or other form of payment to produce the manuscript.
References
- 1.Oliansky DM, Camitta B, Gaynon P, Nieder ML, Parsons SK, Pulsipher MA et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review. ASBMT Position Statement. Biol Blood Marrow Transplant 2012; 18(7): 979–981. e-pub ahead of print 2012/04/12; doi: 10.1016/j.bbmt.2012.03.011 [DOI] [PubMed] [Google Scholar]
- 2.Oliansky DM, Rizzo JD, Aplan PD, Arceci RJ, Leone L, Ravindranath Y et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of acute myeloid leukemia in children: an evidence-based review. Biol Blood Marrow Transplant 2007; 13(1): 1–25. e-pub ahead of print 2007/01/16; doi: 10.1016/j.bbmt.2006.10.024 [DOI] [PubMed] [Google Scholar]
- 3.Brissot E, Rialland F, Cahu X, Strullu M, Corradini N, Thomas C et al. Improvement of overall survival after allogeneic hematopoietic stem cell transplantation for children and adolescents: a three-decade experience of a single institution. Bone Marrow Transplant 2016; 51(2): 267–272. e-pub ahead of print 2015/12/08; doi: 10.1038/bmt.2015.250 [DOI] [PubMed] [Google Scholar]
- 4.Gratwohl A, Brand R, Frassoni F, Rocha V, Niederwieser D, Reusser P et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone Marrow Transplant 2005; 36(9): 757–769. e-pub ahead of print 2005/09/10; doi: 10.1038/sj.bmt.1705140 [DOI] [PubMed] [Google Scholar]
- 5.Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 2015; 125(8): 1333–1338. e-pub ahead of print 2015/01/17; doi: 10.1182/blood-2014-10-609032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solh M, Zhang X, Connor K, Brown S, Solomon SR, Morris LE et al. Factors Predicting Graft-versus-Host Disease-Free, Relapse-Free Survival after Allogeneic Hematopoietic Cell Transplantation: Multivariable Analysis from a Single Center. Biol Blood Marrow Transplant 2016; 22(8): 1403–1409. e-pub ahead of print 2016/04/21; doi: 10.1016/j.bbmt.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 7.Kuhlen M, Willasch AM, Dalle JH, Wachowiak J, Yaniv I, Ifversen M et al. Outcome of relapse after allogeneic HSCT in children with ALL enrolled in the ALL-SCT 2003/2007 trial. Br J Haematol 2018; 180(1): 82–89. e-pub ahead of print 2017/12/02; doi: 10.1111/bjh.14965 [DOI] [PubMed] [Google Scholar]
- 8.Avigan D, Hari P, Battiwalla M, Bishop MR, Giralt SA, Hardy NM et al. Proceedings from the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation: part II. Autologous Transplantation-novel agents and immunomodulatory strategies. Biol Blood Marrow Transplant 2013; 19(12): 1661–1669. e-pub ahead of print 2013/09/11; doi: 10.1016/j.bbmt.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med 2014; 371(4): 339–348. doi: 10.1056/NEJMsa1311707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang P, Handgretinger R. Haploidentical SCT in children: an update and future perspectives. Bone Marrow Transplant 2008; 42 Suppl 2: S54–59. e-pub ahead of print 2008/11/26; doi: 10.1038/bmt.2008.285 [DOI] [PubMed] [Google Scholar]
- 11.Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008; 14(6): 641–650. e-pub ahead of print 2008/05/21; doi: 10.1016/j.bbmt.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung W, Campana D, Yang J, Pei D, Coustan-Smith E, Gan K et al. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood 2011; 118(2): 223–230. e-pub ahead of print 2011/05/27; doi: 10.1182/blood-2011-01-333070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klingebiel T, Cornish J, Labopin M, Locatelli F, Darbyshire P, Handgretinger R et al. Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplantation in children with very high-risk acute lymphoblastic leukemia: impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood 2010; 115(17): 3437–3446. e-pub ahead of print 2009/12/31; doi: 10.1182/blood-2009-03-207001 [DOI] [PubMed] [Google Scholar]
- 14.Yanir AD, Martinez CA, Sasa G, Leung K, Gottschalk S, Omer B et al. Current Allogeneic Hematopoietic Stem Cell Transplantation for Pediatric Acute Lymphocytic Leukemia: Success, Failure and Future Perspectives-A Single-Center Experience, 2008 to 2016. Biol Blood Marrow Transplant 2018; 24(7): 1424–1431. e-pub ahead of print 2018/03/20; doi: 10.1016/j.bbmt.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 15.Federmann B, Bornhauser M, Meisner C, Kordelas L, Beelen DW, Stuhler G et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: a phase II study. Haematologica 2012; 97(10): 1523–1531. e-pub ahead of print 2012/04/12; doi: 10.3324/haematol.2011.059378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vadakekolathu J, Rutella S. T-Cell Manipulation Strategies to Prevent Graft-Versus-Host Disease in Haploidentical Stem Cell Transplantation. Biomedicines 2017; 5(2). e-pub ahead of print 2017/06/22; doi: 10.3390/biomedicines5020033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertaina A, Zecca M, Buldini B, Sacchi N, Algeri M, Saglio F et al. Unrelated donor vs HLA-haploidentical alpha/beta T-cell- and B-cell-depleted HSCT in children with acute leukemia. Blood 2018; 132(24): 2594–2607. e-pub ahead of print 2018/10/24; doi: 10.1182/blood-2018-07-861575 [DOI] [PubMed] [Google Scholar]
- 18.Huang W, Chao NJ. Memory T cells: A helpful guard for allogeneic hematopoietic stem cell transplantation without causing graft-versus-host disease. Hematol Oncol Stem Cell Ther 2017; 10(4): 211–219. e-pub ahead of print 2017/06/22; doi: 10.1016/j.hemonc.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 19.Teschner D, Distler E, Wehler D, Frey M, Marandiuc D, Langeveld K et al. Depletion of naive T cells using clinical grade magnetic CD45RA beads: a new approach for GVHD prophylaxis. Bone Marrow Transplant 2014; 49(1): 138–144. doi: 10.1038/bmt.2013.114 [DOI] [PubMed] [Google Scholar]
- 20.Bleakley M, Heimfeld S, Loeb KR, Jones LA, Chaney C, Seropian S et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest 2015. doi: 10.1172/JCI81229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sisinni L, Gasior M, de Paz R, Querol S, Bueno D, Fernandez L et al. Unexpected High Incidence of Human Herpesvirus-6 Encephalitis after Naive T Cell-Depleted Graft of Haploidentical Stem Cell Transplantation in Pediatric Patients. Biol Blood Marrow Transplant 2018; 24(11): 2316–2323. doi: 10.1016/j.bbmt.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 22.Shook DR, Triplett BM, Eldridge PW, Kang G, Srinivasan A, Leung W. Haploidentical stem cell transplantation augmented by CD45RA negative lymphocytes provides rapid engraftment and excellent tolerability. Pediatr Blood Cancer 2015; 62(4): 666–673. e-pub ahead of print 2015/01/07; doi: 10.1002/pbc.25352 [DOI] [PubMed] [Google Scholar]
- 23.Triplett BM, Muller B, Kang G, Li Y, Cross SJ, Moen J et al. Selective T-cell depletion targeting CD45RA reduces viremia and enhances early T-cell recovery compared with CD3-targeted T-cell depletion. Transpl Infect Dis 2018; 20(1). e-pub ahead of print 2017/11/28; doi: 10.1111/tid.12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Triplett BM, Shook DR, Eldridge P, Li Y, Kang G, Dallas M et al. Rapid memory T-cell reconstitution recapitulating CD45RA-depleted haploidentical transplant graft content in patients with hematologic malignancies. Bone Marrow Transplant 2015; 50(7): 1012 e-pub ahead of print 2015/07/02; doi: 10.1038/bmt.2015.139 [DOI] [PubMed] [Google Scholar]
- 25.Perez-Martinez A, Leung W, Munoz E, Iyengar R, Ramirez M, Vicario JL et al. KIR-HLA receptor-ligand mismatch associated with a graft-versus-tumor effect in haploidentical stem cell transplantation for pediatric metastatic solid tumors. Pediatr Blood Cancer 2009; 53(1): 120–124. e-pub ahead of print 2009/02/14; doi: 10.1002/pbc.21955 [DOI] [PubMed] [Google Scholar]
- 26.Prentice RL, Kalbfleisch JD, Peterson AV Jr., Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics 1978; 34(4): 541–554. [PubMed] [Google Scholar]
- 27.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics 1988; 16(3): 1141–1154. [Google Scholar]
- 28.Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 2015; 126(8): 1033–1040. e-pub ahead of print 2015/07/02; doi: 10.1182/blood-2015-04-639831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu X, Liu L, Xie Z, Dong C, Zhao L, Zhang J et al. Bone marrow versus peripheral blood as a graft source for haploidentical donor transplantation in adults using post-transplant cyclophosphamide-A systematic review and meta-analysis. Crit Rev Oncol Hematol 2019; 133: 120–128. e-pub ahead of print 2019/01/22; doi: 10.1016/j.critrevonc.2018.05.017 [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Chang YJ, Chen L, Xu LP, Bian ZL, Zhang XH et al. Low-dose post-transplant cyclophosphamide can mitigate GVHD and enhance the G-CSF/ATG induced GVHD protective activity and improve haploidentical transplant outcomes. Oncoimmunology 2017; 6(11). ARTN e1356152 doi: 10.1080/2162402X.2017.1356152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein OR, Buddenbaum J, Tucker N, Chen AR, Gamper CJ, Loeb D et al. Nonmyeloablative Haploidentical Bone Marrow Transplantation with Post-Transplantation Cyclophosphamide for Pediatric and Young Adult Patients with High-Risk Hematologic Malignancies. Biol Blood Marrow Transplant 2017; 23(2): 325–332. e-pub ahead of print 2016/11/27; doi: 10.1016/j.bbmt.2016.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Copelan OR, Sanikommu SR, Trivedi JS, Butler C, Ai J, Ragon BK et al. Higher Incidence of Hemorrhagic Cystitis Following Haploidentical Related Donor Transplantation Compared with Matched Related Donor Transplantation. Biol Blood Marrow Transplant 2018. e-pub ahead of print 2018/12/24; doi: 10.1016/j.bbmt.2018.12.142 [DOI] [PubMed] [Google Scholar]
- 33.Mohyuddin GR, Roller J, Shune L, Lin T, Dias A, Ganguly S et al. Epstein-Barr viremia and post-transplant lymphoproliferative disorders in patients undergoing haploidentical stem cell transplantation with post-transplant cyclophosphamide. Hematol Oncol Stem Cell Ther 2018. e-pub ahead of print 2018/12/14; doi: 10.1016/j.hemonc.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 34.Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant 2014; 20(10): 1573–1579. doi: 10.1016/j.bbmt.2014.05.029 [DOI] [PubMed] [Google Scholar]
- 35.Or-Geva N, Reisner Y. The evolution of T-cell depletion in haploidentical stem-cell transplantation. Br J Haematol 2016; 172(5): 667–684. doi: 10.1111/bjh.13868 [DOI] [PubMed] [Google Scholar]
- 36.Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol 2005; 23(15): 3447–3454. e-pub ahead of print 2005/03/09; doi: JCO.2005.09.117 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Roux C, Harbi S, Devillier R, Legrand F, Furst S, Granata A et al. Donor Lymphocyte Infusion after Haploidentical Transplantation with Post-Transplant Cyclophosphamide. Blood 2016; 128(22). [Google Scholar]
- 38.Martelli MF, Ianni MD, Ruggeri L, Falzetti F, Carotti A, Reisner Y et al. Next generation HLA-haploidentical HSCT. Bone Marrow Transpl 2015; 50: S63–S66. doi: 10.1038/bmt.2015.98 [DOI] [PubMed] [Google Scholar]
- 39.Rujkijyanont P, Morris C, Kang G, Gan K, Hartford C, Triplett B et al. Risk-adapted donor lymphocyte infusion based on chimerism and donor source in pediatric leukemia. Blood Cancer J 2013; 3 UNSP e137. doi: 10.1038/bcj.2013.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Locatelli F, Merli P, Li Pira G, Bertaina V, Lucarelli B, Brescia LP et al. Clinical Outcome after Adoptive Infusion of BPX-501 Cells (donor T cells transduced with iC9 suicide gene) in Children Given Alpha/Beta T-Cell Depleted HLA-Haploidentical Hematopoietic Stem Cell Transplantation (haplo-HSCT): Preliminary Results of a Phase I-II Trial. Blood 2015; 126(23). [Google Scholar]
- 41.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood 2011; 117(14): 3921–3928. doi: 10.1182/blood-2010-10-311894 [DOI] [PubMed] [Google Scholar]
- 42.Lang P, Feuchtinger T, Teltschik HM, Schwinger W, Schlegel P, Pfeiffer M et al. Improved immune recovery after transplantation of TCRalphabeta/CD19-depleted allografts from haploidentical donors in pediatric patients. Bone Marrow Transplant 2015; 50 Suppl 2: S6–10. doi: 10.1038/bmt.2015.87 [DOI] [PubMed] [Google Scholar]
- 43.Maschan M, Shelikhova L, Ilushina M, Kurnikova E, Boyakova E, Balashov D et al. TCR-alpha/beta and CD19 depletion and treosulfan-based conditioning regimen in unrelated and haploidentical transplantation in children with acute myeloid leukemia. Bone Marrow Transplant 2016; 51(5): 668–674. e-pub ahead of print 2016/01/26; doi: 10.1038/bmt.2015.343 [DOI] [PubMed] [Google Scholar]
- 44.Zvyagin IV, Mamedov IZ, Tatarinova OV, Komech EA, Kurnikova EE, Boyakova EV et al. Tracking T-cell immune reconstitution after TCRalphabeta/CD19-depleted hematopoietic cells transplantation in children. Leukemia 2017; 31(5): 1145–1153. e-pub ahead of print 2016/11/05; doi: 10.1038/leu.2016.321 [DOI] [PubMed] [Google Scholar]
- 45.Locatelli F, Merli P, Pagliara D, Li Pira G, Falco M, Pende D et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after alphabeta T-cell and B-cell depletion. Blood 2017; 130(5): 677–685. doi: 10.1182/blood-2017-04-779769 [DOI] [PubMed] [Google Scholar]
- 46.Upadhyayula S, Michaels MG. Ganciclovir, Foscarnet, and Cidofovir: Antiviral Drugs Not Just for Cytomegalovirus. J Pediatric Infect Dis Soc 2013; 2(3): 286–290. e-pub ahead of print 2013/09/01; doi: 10.1093/jpids/pit048 [DOI] [PubMed] [Google Scholar]
- 47.Symeonidis N, Jakubowski A, Pierre-Louis S, Jaffe D, Pamer E, Sepkowitz K et al. Invasive adenoviral infections in T-cell-depleted allogeneic hematopoietic stem cell transplantation: high mortality in the era of cidofovir. Transpl Infect Dis 2007; 9(2): 108–113. e-pub ahead of print 2007/04/28; doi: 10.1111/j.1399-3062.2006.00184.x [DOI] [PubMed] [Google Scholar]
- 48.Chan WK, Suwannasaen D, Throm RE, Li Y, Eldridge PW, Houston J et al. Chimeric antigen receptor-redirected CD45RA-negative T cells have potent antileukemia and pathogen memory response without graft-versus-host activity. Leukemia 2015; 29(2): 387–395. e-pub ahead of print 2014/06/04; doi: 10.1038/leu.2014.174 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.