Abstract
Electronic nicotine delivery systems (ENDS) have the potential to help smokers living with HIV/AIDS (PLWHA) to reduce harms from tobacco use. However, little is known about ENDS use among people living with HIV/AIDS. This study’s aim was to evaluate the acceptability of two types of ENDS among PLWHA not planning to quit smoking. The study utilized a cross-over design where participants used two ENDS in a random order, a cigalike and a button-operated device, as smoking substitutes during two use periods separated by 7 days. Exhaled carbon monoxide (CO) was analyzed and participants reported daily cigarette and ENDS use and completed subjective ratings on ENDS acceptability. Paired t-tests were used to evaluate within-subject differences in CO, cigarettes per day (CPD), and subjective ratings from baseline to follow-up for each use period. Independent t-tests evaluated differences between ENDS devices. Participants (n=17) were a mean age of 49.1 years (SD=8.8), were 53% white, and 59% male. All participants had undetectable HIV RNA viral loads and their mean CD4 count was 765.1 cells/mm3 (SD=281.6). Participants smoked a mean of 16.9 (SD=7.9) CPD at baseline. Overall, CPD significantly decreased during both ENDS use periods (p<.01) but there were no differences in reduction between the different devices (48% cigalike v. 55% button-operated reduction, p=.44). CO decreased from baseline to follow-up only during the button-activated ENDS use period (p=.03), but there were no differences between ENDS devices. There were no significant differences in subjective ratings of acceptability between ENDS devices. These results suggest that ENDS could be a harm reduction tool for smokers with HIV.
Keywords: Electronic cigarettes, HIV, tobacco use
Introduction
Among people living with HIV or AIDS (PLWHA), tobacco use is highly prevalent, with smoking rates greater than the general population (Frazier, Sutton, Brooks, Shouse, & Weiser, 2018). High rates of tobacco use are particularly concerning because PLWHA have greater susceptibility to illnesses associated with smoking, including cardiovascular disease, non-AIDS defining cancers, and HIV-related illnesses, like tuberculosis (TB) (Mdege, Shah, Ayo-Yusuf, Hakim, & Siddiqi, 2017; O’Cleirigh et al., 2014). Although tobacco use is not directly related to HIV disease progression, PLWHA who smoke cigarettes are more likely to experience treatment failure (Hile, Feldman, Alexy, & Irvine, 2016; Pollack, Duong, Pham, Do, & Colby, 2017).
While most of the general smoking population reports wanting to quit smoking (Centers for Disease Control and Prevention, 2017), readiness to quit among PLWHA is lower (Burkhalter, Springer, Chhabra, Ostroff, & Rapkin, 2005; Niaura et al., 1999; Niaura et al., 2000). Although multiple smoking cessation methods (including Food and Drug Administration (FDA) approved aids) are available to PLWHA smokers, successful smoking reduction or cessation is often not sustained over time (Calvo-Sanchez & Martinez, 2014; Cioe, 2013). Therefore, novel methods are needed to help PLWHA who smoke to reduce the burden of their tobacco use.
Switching to an electronic nicotine delivery system (ENDS) may be a new way to help PLWHA to reduce the harms of tobacco use. ENDS are a diverse group of battery-powered tobacco products that heat liquid containing nicotine into an aerosol (Breland et al., 2017). ENDS are capable of delivering nicotine similarly to a cigarette (Hajek, Przulj, Phillips, Anderson, & McRobbie, 2017; St Helen, Havel, Dempsey, Jacob, & Benowitz, 2016; Yingst et al., 2019), but with fewer harmful toxicants compared with combustible cigarettes (Caponnetto et al., 2013; National Academies of Science, 2018; Polosa et al., 2014). Additionally, unlike many traditional smoking cessation medications, ENDS use replaces the behavioral aspects of smoking (Baweja et al., 2016; McQueen, Tower, & Sumner, 2011). ENDS have been perceived as more satisfying and rewarding than traditional nicotine replacement and may increase the desire to quit smoking (Steinberg et al., 2014). More recently, a large randomized trial reported that ENDS were a more effective smoking cessation aid compared with nicotine replacement (18% v 9.9% at one year) (Hajek et al., 2019).
The extent to which PLWHA find ENDS to be an acceptable alternative for cigarette smoking is uncertain. Additionally, multiple ENDS products are available on the market and variations in device features are known to affect nicotine delivery and product likeability (Schroeder & Hoffman, 2014; Yan & D’Ruiz, 2014; Yingst et al., 2015). There are no data to suggest which of the many ENDS products are appropriate for PLWHA smokers. The aim of this pilot study was to evaluate the acceptability of ENDS among PLWHA by examining cigarette consumption, ENDS use, and subjective ratings of acceptability during periods in which smokers were asked to use ENDS as smoking substitutes. In addition, this study examined differences in acceptability between two common ENDS device types, those that are puff activated and look like a cigarette (“cigalike”) and those with a larger battery that produce aerosol only after the user has pressed a button (button-operated).
Methods
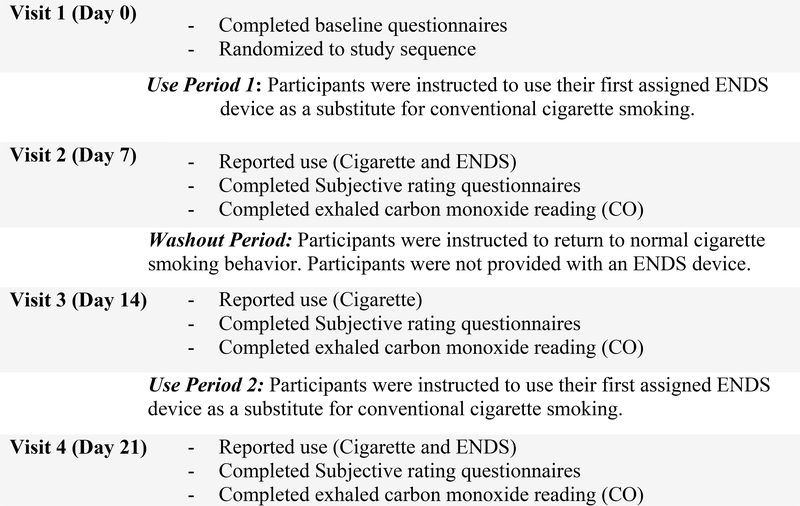
The study was a 3-week, cross-over study to test the acceptability of ENDS among smokers living with HIV/AIDS. Participants were assigned to use two different ENDS devices in a random order, a cigalike and a button-activated device (Table 1), for separate 7-day periods, with a washout period of 7 days between each ENDS use period (Figure 1). Participants were current adult (age ≥18) smokers (≥10 cigarettes daily) not planning to quit smoking with a documented history of a positive HIV status. Participants were recruited from PLWHA seeking care at the Penn State Health HIV Comprehensive Care Program.
Table 1:
ENDS devices provided to participants based on randomized group (Cigalike then Button-operated or Button-operated then Cigalike)
| Devices Used | Flavor | Nicotine Concentration | Propylene glycol/Vegetable glycerin Ratio | Nicotine Delivery |
|---|---|---|---|---|
| Cigalike Device (Blu) | Tobacco | 24 mg/ml | 70/30 | 4.56 ng/ml after 20 puffs in 10 minutes (D’Ruiz, O’Connell, Graff, & Yan, 2017). |
| Button-operated Device (eGO) | Tobacco | 36 mg/ml | 70/30 | 6.9 ng/ml after 10 puffs in 5 minutes (Hiler et al., 2017) |
Figure 1:
Study Flow Chart
Participants were provided with a paper daily diary and were asked to record the number of cigarettes smoked per day and the ENDS puffs taken per day throughout the study. These data were collected at each study visit via a 5-day timeline follow-back procedure. Mean CPD and puffs per day were calculated by averaging the data from the past 5 days at each study visit. Participants also completed a series of subjective ratings on ENDS acceptability (Table 2). In addition, exhaled carbon monoxide (CO) concentration was measured at each visit. Participants who reported no cigarette smoking in the past 5 days and had a CO level of less than 10ppm were considered abstinent.
Table 2:
Subjective Rating Questionnaires
| Questionnaire | Measures | Score Range | Completed at: |
|---|---|---|---|
| Modified Product Evaluation Scale for ENDS (HATSUKAMI, Zhang, O’Connor, & Severson, 2013) | Satisfaction, psychological reward, aversion, and relief | 0 (not at all) to 4 (a lot) | Visit 2 and Visit 4 after e-cig use periods |
| ENDS Experiences Questionnaire | Experiences using ENDS device | 1 (not at all) to 10 (extremely) | Visit 2 and Visit 4 after e-cig use periods |
| ENDS Comparison Questionnaire | Perceptions of satisfaction, ease of use, taste, appearance, and acceptability | 1 (not at all) to 7 (extremely) | Visit 4 |
Data were analyzed using SAS Version 9.4 (Cary, NC). Paired T-tests were used detect within-subject differences in CPD and CO from baseline to follow-up for both use periods. Baseline for these analyses was considered the time period before the respective use period. Independent t-tests were also used to test for differences between the ENDS device use periods for the following variables: CPD, CO, puffs per day, evaluation scale questions, experience questions, and ENDS comparison questions.
Results
Participants (n=17) were a mean age of 49.1 years (SD=8.8), were 52.9% white, and 58.8% male. All participants had controlled HIV disease with an undetectable HIV RNA viral load and a mean CD4 count of 765.1 cells/mm3 (SD=281.6). Participants reported smoking cigarettes for a mean of 30.4 years (SD=8.9) and smoked a mean of 16.9 (SD=7.9) CPD at baseline. Mean baseline exhaled CO was 22.4ppm (SD=13.1).
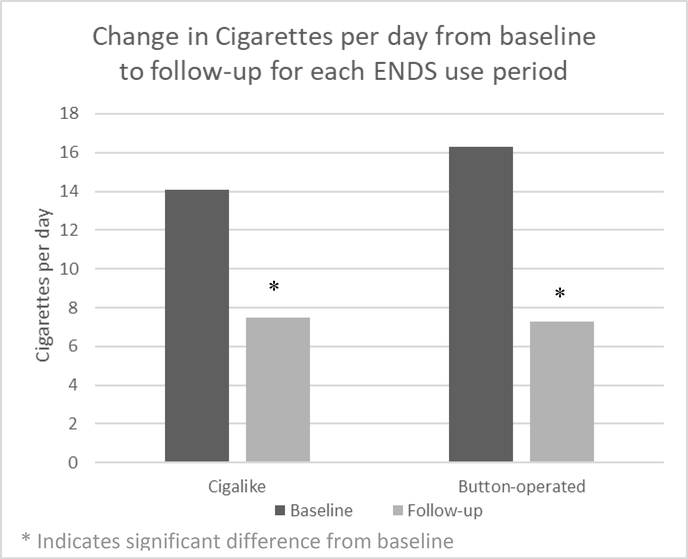
Overall, participants’ self-reported daily cigarette consumptions significantly decreased from baseline during both ENDS use periods (Figure 2). For the cigalike use period, baseline CPD was 14.41 (SD=7.4) and follow-up CPD was 7.5 (SD=5.4) (48% reduction, p<.01). For the button-operated use period, baseline CPD was 16.3 (SD=6.9) and follow-up CPD was 7.3 (SD=6.4) (55% reduction, p<.01). There were no differences in cigarette reduction between use of the cigalike and button-operated devices (p=.44). CO significantly decreased from baseline during the button-operated ENDS use period from 24.1ppm (SD=12.2) to 18.4ppm (SD=13.8) (p=.03), but did not significantly decrease during the cigalike use period (22.4ppm (SD=12.8) vs. 18.1ppm (SD=13.8) (p=.07). Total CO reduction was not different between ENDS devices (p=.75). Only one participant reported ceasing cigarette smoking (no use in the past 5 days), during the button-operated use period, however self-reported quitting was not verified by a CO level less than 10ppm.
Figure 2:
Change in Cigarette smoking from baseline to follow-up for each ENDS use period
Participants reported slightly greater puffs/day while using the button-operated device versus the cigalike device (p=.81). During the cigalike use period, participants took a mean of 45.6 (SD=42.6) (range 1.4–161.2) puffs/day. During the button-operated use period, participants took a mean of 61.6 (SD=65.1) (range 3.6–232.7) puffs/day. Overall, 1 participant reported stopping ENDS use during the cigalike use period, 1 stopped ENDS use during the button-operated use period, and 1 participant reported stopping ENDS use during both use periods because they did not like the products.
There were no significant differences between use of the cigalike or button-operated ENDS on any of the acceptability scales (Table 3).
Table 3.
ENDS Evaluation Scale, Experiences Scale, and Comparison Scale Mean Scores (SD)
| Cigalike (n=17) Mean score (SD) | Button-Operated (n=17) Mean score (SD) | P-value | |
|---|---|---|---|
| ENDS Evaluation Scale (Score range: 0–4) | |||
| Satisfaction | 1.6 (1.1) | 2.1 (2.0) | .19 |
| Taste | 1.5 (1.3) | 1.6 (1.7) | .90 |
| Sensation in throat and chest | 1.2 (1.2) | 1.3 (1.5) | .90 |
| Calm down | 1.6 (1.5) | 1.6 (1.3) | .77 |
| Awake | 0.6 (0.9) | 1.2 (1.3) | .14 |
| Less irritable | 1.3 (1.3) | 1.5 (1.3) | .55 |
| Help concentrate | 1.1 (1.4) | 1.4 (1.2) | .49 |
| Hunger | 0.9 (0.9) | 1.1 (1.4) | .56 |
| Dizziness | 0.7 (1.0) | 0.9 (1.4) | .68 |
| Nauseous | 0.5 (1.1) | 0.5 (1.1) | 1.0 |
| Relief | 1.5 (1.5) | 1.7 (1.4) | .50 |
| ENDS Experiences Questionnaire (Score range: 1–10) | |||
| Helpful in keeping from smoking | 5.5 (2.4) | 5.8 (2.8) | .75 |
| Embarrassing to use | 1.4 (0.7) | 1.3 (0.5) | .58 |
| Similar to a cigarette | 3.9 (2.4) | 4.1 (2.8) | .89 |
| Easy to use | 8.8 (2.2) | 8.9 (1.5) | .86 |
| Acceptable to smokers | 7.2 (2.4) | 7.8 (1.8) | .45 |
| Favorable or “cool” | 6.5 (2.4) | 7.2 (2.7) | .46 |
| Effective in helping to stop smoking | 5.4 (2.8) | 7.4 (2.4) | .08 |
| ENDS Comparison Questionnaire (Score range: 1–7) | |||
| Overall satisfaction | 4.0 (2.2) | 4.7 (2.1) | .38 |
| Easy to use | 5.9 (2.0) | 5.4 (2.1) | .37 |
| Good taste | 3.8 (2.2) | 3.8 (2.4) | .93 |
| Nice appearance | 5.5 (2.0) | 5.0 (2.1) | .43 |
| Acceptable | 4.6 (2.4) | 5.1 (2.2) | .54 |
| Helpful in reducing tobacco smoking | 4.2 (2.5) | 4.6 (2.3) | .64 |
| Likelihood of continuing to use | 4.0 (2.5) | 5.1 (2.3) | .14 |
Discussion
Overall, use of an ENDS device was associated with a significant decrease in self-reported CPD among PLWHA not interested in quitting smoking, with no differences in reduction between ENDS devices. In addition, during the button-operated ENDS use period, exhaled CO was significantly reduced compared with baseline. Participants generally found ENDS to be acceptable. Of importance, participants reported little to no adverse effects (dizziness, nausea) related to use. In regards to replacing cigarettes, participants felt that ENDS were somewhat helpful and effective in helping them keep from smoking and reported that ENDS were very easy to use and were “cool”. While differences were found overall and within ENDS device groups from baseline to follow-up, we did not find any significant differences in CPD reduction, CO, ENDS puffs/day, or subjective ratings between the use of two ENDS device types.
Participants in the present study had no immediate plans to quit smoking, though they reported a willingness to try to replace their cigarette smoking with ENDS use. However, none were able to do so completely. It is clear that attempts to use ENDS for a short period of time by smokers with no serious plans to quit could facilitate smoking reduction but do not often result in immediate smoking cessation.
This study is limited by the small sample size. A prospective study with larger sample size may be needed to confirm these differences in outcomes between the two ENDS device types in the current study. Additionally, there were differences between the two devices that could have impacted results, including the difference in nicotine concentration. Also, the button-activated device included a puff-counter, which could have aided participants in providing more accurate puff per day data during that use period compared with the cigalike use period.
In conclusion, our pilot study found that the use of ENDS was associated with significant decreases in self-reported conventional cigarette smoking among PLWHA. Additionally, during use of the button-activated device, participants experienced a significant lessening in their toxicant exposure to smoke, measured via CO. Participants rated ENDS as moderately satisfying and reported few adverse effects related to use. These findings suggest that the use of ENDS among smokers living with HIV may help reduce the burden of harm from tobacco use. However, PLWHA interested in quitting should be advised to use FDA smoking cessation medications before initiating ENDS use.
Acknowledgments
Funding
This study was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036107. JY is also funded by the Penn State Cancer Institute (PSCI) and TE is also supported by U54DA036105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration.
Footnotes
Declaration of interests
JF has done paid consulting for pharmaceutical companies involved in producing smoking cessation medications, including GSK, Pfizer, Novartis, J&J, and Cypress Bioscience. TE is a paid consultant in litigation against the tobacco industry and is named on a patent application for a device that measures the puffing behavior of electronic cigarette users. There are no other competing interests to report for other authors.
References
- Baweja R, Curci K, Yingst J, Veldheer S, Wilson S, Nichols T,… Foulds J. (2016). Views of Experienced Electronic Cigarette Users. Addition Research and Theory, 21(1), 80–88. doi: 10.3109/16066359.2015.1077947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland A, Soule E, Lopez A, Ramôa C, El‐Hellani A, & Eissenberg T (2017). Electronic cigarettes: what are they and what do they do? Annals of the New York Academy of Sciences, 1394(1), 5–30. doi:doi: 10.1111/nyas.12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhalter JE, Springer CM, Chhabra R, Ostroff JS, & Rapkin BD (2005). Tobacco use and readiness to quit smoking in low-income HIV-infected persons. Nicotine Tob Res, 7(4), 511–522. doi: 10.1080/14622200500186064 [DOI] [PubMed] [Google Scholar]
- Calvo-Sanchez M, & Martinez E (2014). How to address smoking cessation in HIV patients. HIV Med. doi: 10.1111/hiv.12193 [DOI] [PubMed] [Google Scholar]
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, & Polosa R (2013). EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLOS ONE, 8(6), e66317. doi: 10.1371/journal.pone.0066317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2017). Smoking & Tobacco Use Fast Facts. Retrieved from https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm
- Cioe PA (2013). Smoking Cessation Interventions in HIV-Infected Adults in North America: A Literature Review. J Addict Behav Ther Rehabil, 2(3), 1000112. doi: 10.4172/2324-9005.1000112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ruiz CD, O’Connell G, Graff DW, & Yan XS (2017). Measurement of cardiovascular and pulmonary function endpoints and other physiological effects following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Regulatory Toxicology and Pharmacology, 87, 36–53. doi: 10.1016/j.yrtph.2017.05.002 [DOI] [PubMed] [Google Scholar]
- Frazier EL, Sutton MY, Brooks JT, Shouse RL, & Weiser J (2018). Trends in cigarette smoking among adults with HIV compared with the general adult population, United States - 2009–2014. Prev Med, 111, 231–234. doi: 10.1016/j.ypmed.2018.03.007 [DOI] [PubMed] [Google Scholar]
- Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N,… McRobbie HJ (2019). A Randomized Trial of E-Cigarettes versus Nicotine-Replacement Therapy. N Engl J Med. doi: 10.1056/NEJMoa1808779 [DOI] [PubMed] [Google Scholar]
- Hajek P, Przulj D, Phillips A, Anderson R, & McRobbie H (2017). Nicotine delivery to users from cigarettes and from different types of e-cigarettes. Psychopharmacology (Berl), 234(5), 773–779. doi: 10.1007/s00213-016-4512-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Zhang Y, O’Connor RJ, & Severson HH (2013). Subjective responses to oral tobacco products: scale validation. Nicotine Tob Res, 15(7), 1259–1264. doi: 10.1093/ntr/nts265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hile SJ, Feldman MB, Alexy ER, & Irvine MK (2016). Recent Tobacco Smoking is Associated with Poor HIV Medical Outcomes Among HIV-Infected Individuals in New York. AIDS Behav, 20(8), 1722–1729. doi: 10.1007/s10461-015-1273-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiler M, Breland A, Spindle T, Maloney S, Lipato T, Karaoghlanian N,… Eissenberg T (2017). Electronic cigarette user plasma nicotine concentration, puff topography, heart rate, and subjective effects: Influence of liquid nicotine concentration and user experience. Exp Clin Psychopharmacol, 25(5), 380–392. doi: 10.1037/pha0000140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen A, Tower S, & Sumner W (2011). Interviews With “Vapers”: Implications for Future Research With Electronic Cigarettes. Nicotine Tob Res, 13(9). doi: 10.1093/ntr/ntr088 [DOI] [PubMed] [Google Scholar]
- Mdege ND, Shah S, Ayo-Yusuf OA, Hakim J, & Siddiqi K (2017). Tobacco use among people living with HIV: analysis of data from Demographic and Health Surveys from 28 low-income and middle-income countries. Lancet Glob Health, 5(6), e578–e592. doi: 10.1016/S2214-109X(17)30170-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Science. (2018). Public Health Consequences of e-cigarettes. Retrieved from Washington DC: [PubMed] [Google Scholar]
- Niaura R, Shadel W, Morrow K, Tashima K, Flanigan T, & Abrams D (1999). Smoking among HIV-positive persons. Annals of Behavioral Medicine, 21. [Google Scholar]
- Niaura R, Shadel W, Morrow K, Tashima K, Flanigan T, & Abrams D (2000). Human Immunodeficiency Virus Infection, AIDS, and Smoking Cessation: The Time is Now. Clinical Infectious Diseases, 31, 808–812. [DOI] [PubMed] [Google Scholar]
- O’Cleirigh C, Valentine SE, Pinkston M, Herman D, Bedoya CA, Gordon JR, & Safren SA (2014). The Unique Challenges Facing HIV-Positive Patients Who Smoke Cigarettes: HIV Viremia, Art Adherence, Engagement in HIV care, and Concurrent Substance Use. AIDS Behav. doi: 10.1007/s10461-014-0762-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack TM, Duong HT, Pham TT, Do CD, & Colby D (2017). Cigarette smoking is associated with high HIV viral load among adults presenting for antiretroviral therapy in Vietnam. PLOS ONE, 12(3), e0173534. doi: 10.1371/journal.pone.0173534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Morjaria JB, Caponnetto P, Campagna D, Russo C, Alamo A,… Fisichella A (2014). Effectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational study. Intern Emerg Med, 9(5), 537–546. doi: 10.1007/s11739-013-0977-z [DOI] [PubMed] [Google Scholar]
- Schroeder MJ, & Hoffman AC (2014). Electronic cigarettes and nicotine clinical pharmacology. Tob Control, 23 Suppl 2, ii30–35. doi: 10.1136/tobaccocontrol-2013-051469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Helen G, Havel C, Dempsey DA, Jacob P 3rd, & Benowitz NL (2016). Nicotine delivery, retention and pharmacokinetics from various electronic cigarettes. Addiction, 111(3), 535–544. doi: 10.1111/add.13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MB, Zimmermann MH, Delnevo CD, Lewis MJ, Shukla P, Coups EJ, & Foulds J (2014). E-cigarette versus nicotine inhaler: comparing the perceptions and experiences of inhaled nicotine devices. J Gen Intern Med, 29(11), 1444–1450. doi: 10.1007/s11606-014-2889-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan XS, & D’Ruiz C (2014). Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regul Toxicol Pharmacol. doi: 10.1016/j.yrtph.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Yingst, Foulds J, Veldheer S, Hrabovsky S, Trushin N, Eissenberg TT,… Hobkirk AL (2019). Nicotine absorption during electronic cigarette use among regular users. PLOS ONE, 14(7), e0220300. doi: 10.1371/journal.pone.0220300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingst, Veldheer S, Hrabovsky S, Nichols TT, Wilson SJ, & Foulds J (2015). Factors Associated With Electronic Cigarette Users’ Device Preferences and Transition From First Generation to Advanced Generation Devices. Nicotine Tob Res, 17(10), 1242–1246. doi: 10.1093/ntr/ntv052 [DOI] [PMC free article] [PubMed] [Google Scholar]