Abstract
Streptococcus mutans is a major etiologic agent of dental caries, which is the most common chronic infectious disease worldwide. S. mutans is particularly adept at causing caries due to its exceptional capacity to form biofilms and its ability to survive acidic conditions that arrest acid production and growth in many more benign members of the oral microbiota. Two mechanisms utilized by S. mutans to tolerate acid are: modulation of the membrane fatty acid content and utilization of the F1F0-ATPase to pump protons out of the cytosol. In this study, the role of the spxA2 transcriptional regulator in these two pathways, and overall cell envelope homeostasis, was examined. Loss of spxA2 resulted in an increase in the proportion of saturated fatty acids in the S. mutans membrane and altered transcription of several genes involved in the production of these membrane fatty acids, including fabT and fabM. Furthermore, activity of the F1F0-ATPase was increased in the ΔspxA2 strain. Transcription of spxA2 was elevated in the presence of a variety of membrane stressors, and highly dependent on the liaR component of the LiaFSR system, which is known to sense cell envelope stress in many Gram-positive bacteria. Finally, deletion of ΔspxA2 led to altered susceptibility of S. mutans to membrane stressors. Overall, the results of this study indicate that spxA2 serves a crucial role in transmitting the signal of cell wall/membrane damage from the LiaFSR sensor to downstream effectors in the SpxA2 regulon which restore and maintain membrane and cell wall homeostasis.
Keywords: Streptococcus mutans, Dental caries, Spx, Fatty acids, Environmental regulation
Introduction
Dental caries is an oral disease affecting greater than 50% of the population worldwide, and is caused by biofilms of acid-producing bacteria on the tooth surface which demineralize the enamel (Pitts et al., 2017). Dental caries is often associated with elevated numbers of Streptococcus mutans (Gross et al., 2012), which is considered a major etiologic agent of the disease. S. mutans contributes substantially to caries pathogenesis through its exceptional capacity to form biofilms and its ability to produce acid from a wide variety of carbohydrate sources (Bowen et al., 2018; Lemos et al., 2019). Furthermore, S. mutans employs an acid-tolerance response (ATR) that utilizes multiple mechanisms to survive better in acidic conditions than many of its neighbors in the oral microbiota (Baker, Faustoferri, & Quivey, 2017; Lemos & Burne, 2008). This allows S. mutans to contribute to the destruction of the underlying tooth even at low pH values that would arrest growth and glycolysis in many more benign species.
An important component of the S. mutans ATR is a shift from a cell membrane consisting of mainly saturated fatty acids (SFAs) to one comprised mainly of unsaturated fatty acids (UFAs) that is accompanied by an increase in fatty acid chain length by ~2 carbons (Fozo & Quivey, Jr., 2004b). These longer-chained UFAs are synthesized de novo by S. mutans using FabM, a trans-2/cis-3-decenoyl-ACP isomerase enzyme (Fozo & Quivey, Jr., 2004a). Blockage of this pathway through inhibition of the FabF enzyme (the next enzyme downstream of FabM in the UFA synthesis pathway) via the drug cerulenin impaired the ability of S. mutans to generate UFAs, which rendered it more sensitive to acid (Fozo & Quivey, Jr., 2004b). Alternatively, deletion of the fabM gene resulted in a strain unable to produce UFAs (0% UFA content), which was severely impaired in its ability to survive an acid challenge and, crucially, cause disease in a rodent model of dental caries (Fozo & Quivey, Jr., 2004a; Fozo et al., 2007). The acid-sensitive phenotype of the ΔfabM strain could be partially rescued by addition of exogenous fatty acids to the growth media, which were incorporated into the membrane and protective against acid stress (Fozo & Quivey, Jr., 2004a).
The transcriptional regulator denoted Spx, for suppressor of ClpX, has been described to play a major role in the general stress response in Gram-positive bacteria (Nakano et al., 2001). Canonical Spx regulators serve as sensors that monitor redox state and regulate transcription accordingly (Zuber, 2004). S. mutans UA159 encodes two Spx homologs, spxA1 (SMU.1142) and spxA2 (SMU.2084c) (Kajfasz et al., 2010). SpxA1 plays a major role in the oxidative stress response and survival in the presence of oxidative stressors, serving as a positive transcriptional regulator of a number of genes that ameliorate oxidative stress including superoxide dismutase, NADH oxidase, and glutathione oxidoreductase (Baker et al., 2014; Kajfasz et al., 2010; Kajfasz et al., 2015). Interestingly, microarray analysis revealed that the regulons of SpxA1 and SpxA2 had little overlap, suggesting distinct regulatory duties for each Spx protein (Kajfasz et al., 2010). While the regulon of SpxA1 heavily featured genes involved in oxidative stress, the regulon of SpxA2 included many genes involved in cell division, the cell envelope, and fatty acid biosynthesis (FAB) (Kajfasz et al., 2010). Indeed, additional research illustrated that the SpxA2 deletion mutant (ΔspxA2) could not form the chains of cells typical of Streptococci, suggesting a defect in the cell division process (Kajfasz et al., 2010; Kajfasz et al., 2015). Furthermore, ΔspxA2 exhibited enhanced biofilm formation due to increased expression of the glycosyltransferase enzymes that form the extracellular glucan matrix (Galvao et al., 2017). Finally, expression of ΔspxA2 was also dependent on the LiaFSR three-component system (Shankar et al., 2015; Suntharalingam et al., 2009), which governs the response to cell envelope stress across a diverse number of Gram-positive bacteria (Mascher, Helmann, & Unden, 2006).
The hypothesis of this study was that S. mutans SpxA2 modulates membrane composition, which is likely to have significant downstream physiological effects on the ability of the organism to deal with cell envelope stress. This theory was well supported by the fact that loss of spxA2 affects transcription of several genes in the fatty acid biosynthesis operon (fab) (Kajfasz et al., 2010), and that deletion of spxA2 decreased colonization and virulence in a rodent model of dental caries (Galvao et al., 2017). The data presented below illustrates that SpxA2 is indeed required for S. mutans to have the full complement of UFAs in its membrane, and that SpxA2 influences UFA production at the transcriptional level. Furthermore, SpxA2 transcription is increased in the presence of membrane stressors, and the deletion of spxA2 rendered S. mutans more sensitive to several peptidoglycan-targeting antibiotics, yet more resistant to osmotic stress and the fatty acid biosynthesis inhibitor, cerulenin.
Materials and Methods
Bacterial strains and growth conditions
Strains in this study are listed in Table 1. Bacterial strains were grown in brain heart infusion (BHI) medium (BD/Difco, Franklin Lakes, NJ) at 37°C in a 5% (vol/vol) CO2/95% air atmosphere. Overnight cultures of S. mutans strains UA159 and ΔspxA2 were grown in BHI medium, sub-cultured 1:20 into fresh BHI medium, and incubated at 37°C in a 5% (vol/vol) CO2/95% air atmosphere until cultures reached an optical density at 600 nm (OD600) ~ 0.3. Where indicated, cerulenin (10 μg mL−1) was added to BHI medium and S. mutans strains were seeded to a final OD600 of 0.05 in a 96 well plate (Corning, Corning, NY). Growth was assessed through measurement of OD600 in a Bioscreen C (Growth Curves USA, Haverhill, MA).
Table 1:
Strain and plasmids used in this study.
| Strains | Description | Source or reference |
|---|---|---|
| Streptococcus mutans | ||
| UA159 | genomic type strain | Murchison et al. (1986), Ajdic et al. (2002) |
| JL13 | spxA2 mutant strain | Kajfasz et al. (2009) |
| UR222 | UA159 carrying fabT-cat construct | Faustoferri et al. (2015) |
| UR309 | JL13 carrying fabT-cat construct | This study |
| UR211 | UA159 carrying fabM-cat construct | Faustoferri et al. (2015) |
| UR312 | JL13 carrying fabM-cat construct | This study |
| ΔliaR | liaR deletion strain, ΔSMU.487 | Quivey et al., 2015 |
| UA159 (pJLspxA2) | UA159 carrying spxA2-cat construct | Rivera-Ramos (2015) and gift from Lemos Lab |
| UR378 | liaR carrying spxA2-cat construct | This study |
| Plasmids | Description | Source or reference |
| pJLmarR | fabT (SMU.1745c) promoter-cat construct | Faustoferri et al. (2015) |
| pJLfabM | fabM (SMU.1746c) promoter-cat construct | Faustoferri et al. (2015) |
| pJLspxA2 | spxA2 (SMU.2084c) promoter-cat construct | Rivera-Ramos (2015) and gift from Lemos Lab |
Chemostat culture conditions
S. mutans strains UA159 and ΔspxA2 were grown under continuous culture conditions using a BioFlo 2000 fermenter (New Brunswick Scientific, Edison, NJ) in TY medium (3% tryptone, 0.1% yeast extract, 0.5% KOH, and 1 mM H3PO4) containing 1% glucose, as described previously (Faustoferri et al., 2015). Cultures were grown at a constant dilution rate of 0.24 h−1 under glucose-limiting (2.3 mM) conditions. Culture pH was continually maintained throughout the experiment via the addition of 2 N KOH and verified by a pH probe (Mettler Toledo, Billerica, MA). Cultures were harvested after 10 generations at steady-state pH 7, followed by addition of excess glucose (20 mM), thereby lowering culture pH. Cultures were similarly harvested at pH 5 after 10 generations at steady-state and stored at −80°C.
ATPase activity assay
ATPase activity from 3 independent cultures of S. mutans strains UA159 and ΔspxA2 was measured as a function of release of inorganic phosphate from ATP, as previously described (Cross et al., 2016). Briefly, 25 mL cultures were grown in BHI medium overnight at 37°C in a 5% (vol/vol) CO2/95% air atmosphere, harvested, and washed in membrane buffer (75 mM Tris pH 7, 10 mM MgSO4). Cells were permeabilized by addition of toluene, followed by two freeze–thaw cycles in dry ice/ethanol, and pelleted in a microcentrifuge for 10 min at 16,100 × g. The supernatant was decanted and cells were resuspended in 1 mL ATPase buffer (50 mM Tris maleate pH 6, 10 mM MgSO4) and stored in 500 μL aliquots at −80°C.
An aliquot (300 μL) of the toluenized cell extract was added to 9 mL ATPase buffer. ATP (pH 6, 5 mM final concentration) was added and 500 μL aliquots were removed at time points 0, 5, 10, 20, 30 and 45 min, in triplicate. Reactions were stopped with 2 mL 20% trichloroacetic acid. Samples were centrifuged at 4°C for 15 min at 2,272 × g. The supernatant (1 mL) was transferred to tubes containing 1.5 mL H2O, followed by addition of 0.5 mL acid molybdate solution (1.25 g dL−1 ammonium molybdate tetrahydrate in 2.5 N sulfuric acid). Fiske and Subbarow Reducing solution (125 μL) (Fiske & Subbarow, 1925) was added to each tube, samples were incubated at room temperature for 10 min, and absorbance was measured at 660 nm. Protein concentration was estimated using the bicinchoninic acid (BCA) assay (Smith et al., 1985) (Sigma, St. Louis, MO). ATPase activity was assessed relative to a phosphate standard curve, normalized to OD660 at time zero, and expressed as mM PO4 released per min per μg protein.
Real-time quantitative RT-PCR
Real-time quantitative reverse transcription-PCR (qRT-PCR) was performed as described previously (Kovacs, Faustoferri, & Quivey, 2017) using a StepOnePlus real-time PCR system (Applied Biosystems, Carlsbad, CA) with gene-specific primers described previously (Faustoferri et al., 2015). RNA was isolated from 3 independent cultures of UA159 or ΔspxA2 grown to steady-state in the chemostat to pH values of 7 and 5 (described above). The mRNA copy number was quantified based on a standard curve of PCR product, as previously described (Kovacs, Faustoferri, & Quivey, 2017).
Membrane fatty acid determination
Membrane fatty acid content was determined from aliquots of steady-state cultures of S. mutans UA159 and ΔspxA2 grown to pH 7 and 5 (described above). Samples (20 mL) were stored at −80°C until sent to Microbial ID (Newark, DE) for analysis of membrane fatty acid composition by gas chromatography of fatty acid methyl esters (GC-FAME), as described elsewhere (Fozo & Quivey, 2004).
Chloramphenicol acetyltransferase (CAT) activity assay
S. mutans strains carrying a promoter-cat fusion were grown overnight and sub-cultured in BHI medium, BHI medium buffered to pH 7 (+ 50mM KPO4 buffer), or BHI titrated to pH 5 (with HCl), where indicated, in conditions described above. Cultures were harvested at OD600 ~ 0.5 and pellets were stored at −80°C. For experiments measuring spxA2 transcription in cultures exposed to cell wall stress agents, overnight cultures were sub-cultured in BHI medium and grown to an OD600 ~ 0.4. Test agents were added to a final concentration of 0.5X MIC [i.e., 4 μg mL−1 tunicamycin; 1 μg mL−1 vancomycin; 2.5% NaCl; or 10 μg mL−1 cerulenin] and cultures were incubated at 37°C for 30 minutes. Cells were harvested and pellets stored at −80°C.
CAT assays for the measurement of transcriptional activity were performed according to the method of Shaw et al., as modified by Kuhnert et al. (Kuhnert et al., 2004; Shaw, 1975). In brief, pellets from 3 independent 50 mL cultures per test strain were resuspended in 1mL 10 mM Tris-Cl, pH 7.8 and lysed for two 1-min intervals using 0.1-mm glass beads in a Mini-Bead Beater (BioSpec Products, Bartlesville, OK). Cell debris was removed by centrifugation at 4°C, 16,100 × g for 10 min. The lysates were removed and used for CAT assays and total protein quantification using the Bradford method and assayed in triplicate. Each CAT assay reaction mixture consisted of 50 μL whole-cell lysate, 100 mM Tris-Cl, pH 7.8, 0.1 mM acetyl-coenzyme A (acetyl-CoA), and 0.4 mg mL−1 5,5-dithio-bis (2-nitrobenzoic acid) (DTNB) in a total volume of 1 mL. Reactions were initiated by addition of 0.1 mM chloramphenicol. The reaction rate was determined at an optical density of 412 nm over a period of 3 min and is presented as μmol of chloramphenicol acetylated per min per mg total protein.
MIC determination
Compounds used in MIC testing were as follows: bacitracin, chloramphenicol, daptomycin, penicillin, polymixin B, tunicamycin, and vancomycin (Sigma-Aldrich, St. Louis, MO). To determine the MICs of test compounds against S. mutans UA159 and ΔspxA2, overnight cultures were grown in BHI medium, diluted 1:50 in fresh TYG medium, and grown to exponential phase (OD600 ~ 0.3). A 96-well plate (Corning, Inc., Corning, NY) containing fresh TYG medium was inoculated with 105 CFU. A dilution series of the test compound (concentrations ranged from 0 to 64 μg mL−1) was added to the plate. The plates were incubated at 37°C in a 5% (vol/vol) CO2/95% air atmosphere for 24 h. The MIC was considered the lowest compound concentration that inhibited bacterial growth, as measured by the OD600. MICs are presented as concentration in μg mL−1 from 3 independent cultures assayed in triplicate.
Statistical analysis
Statistical significance (p < 0.05) was determined by pairwise comparison using either a paired t-test, or a two-way ANOVA with a Tukey’s Multiple Comparisons Post Test, where indicated.
Results
Deletion of spxA2 results in aberrant membrane composition.
Previous transcriptional analysis indicated that the fabF and fabG genes within the fab operon were down-regulated in the ΔspxA2 strain, compared to the UA159 parent strain, suggesting that SpxA2 may be an enhancer of their transcription (Kajfasz et al., 2010). To examine whether this dysregulation of FAB in the ΔspxA2 strain affected the membrane composition of S. mutans, GC-FAME was performed on cultures of UA159 or ΔspxA2 in steady-state growth at pH 7 and pH 5, as described in the Materials and Methods. ΔspxA2 exhibited a significant reduction in the UFA:SFA ratio, compared to UA159, at both pH 7 and 5 (Figure 1A). Similarly, the length of the fatty acid chains was reduced in the ΔspxA2 strain compared to UA159 at both pH 7 and pH 5. Specifically, increased proportions of 12-, 14-, and 16-carbon chains and decreased proportions of 18- and 20-carbon chains were observed in ΔspxA2 compared to UA159 (Figure 1B). This increased chain length may be reflective of the increase in transcription of fabF and fabG observed in ΔspxA2 (Kajfasz et al., 2010). The full list of fatty acid types detected by the GC-FAME analysis is available in Supplemental Table 1. Although both fatty acid saturation and chain length were affected by the loss of spxA2, the ΔspxA2 strain was still able to significantly increase the percentage of UFAs, and 18 and 20 carbon chains, in its membrane through the transition from pH 7 to pH 5. The fold-change in UFA:SFA ratio at pH 5, compared to pH 7, in ΔspxA2 (3.31-fold) was comparable to that observed in UA159 (3.02-fold), as was the fold-change in the (C14+C16)/(C18+C20) ratio from pH 5 to pH 7 (0.18-fold in UA159 and 0.16-fold in ΔspxA2). Overall, these data indicate that in ΔspxA2, the ATR is still able to increase the proportion of UFAs upon a reduction in environmental pH; however, the proportion of UFAs at both neutral and acidic pH is significantly lower. The reduction of the UFA:SFA ratio in the ΔspxA2 strain, compared to UA159, concurs with a previous study that examined membrane composition in batch culture growth conditions (Rivera-Ramos, 2015).
Figure 1: Membrane fatty acid composition is altered in ΔspxA2.
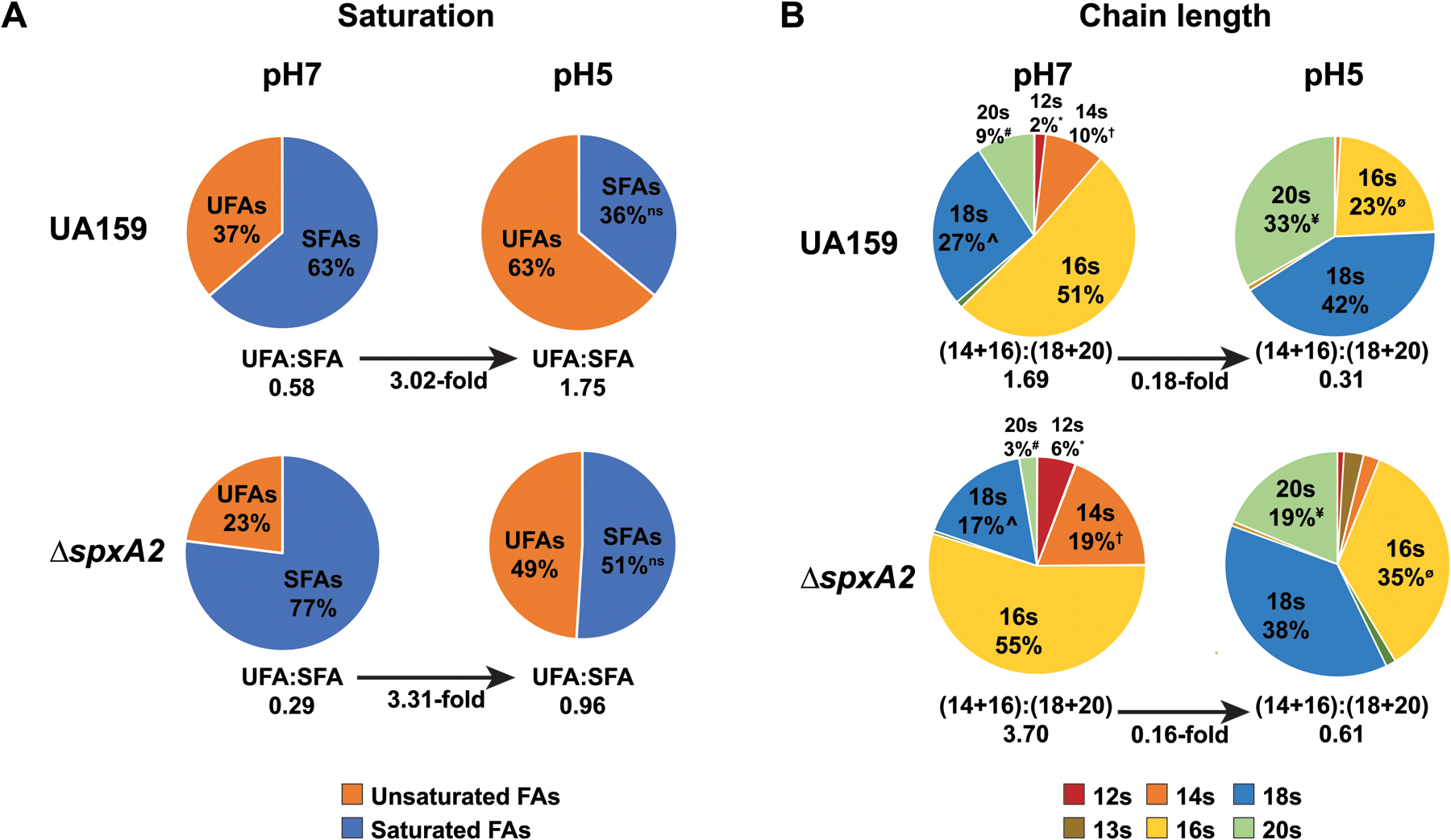
(A) GC-FAME analysis was used to determine membrane fatty acid composition from cultures of S. mutans UA159 and ΔspxA2 grown to steady-state pH values of 7 and 5 as described in Materials and Methods. Percentage of membrane composition is denoted in each sector where saturated fatty acids are shown in blue and unsaturated fatty acids in orange. All values are statistically significant in pairwise comparisons, except for ns, as determined by Student’s t-test, p < 0.05. Below each pie graph is the UFA:SFA ratio, and values are statistically significant in pairwise comparisons, the fold-change between pH 7 and pH 5 is also indicated. (B) Fatty acid chain lengths are displayed for cultures of S. mutans UA159 and ΔspxA2 grown to steady-state pH values of 7 and 5. Percentage of membrane composition representing fatty acids composed of indicated carbon chain-lengths is denoted in each sector. Only chain lengths representing at least 1% of the total are shown. Statistical significance is designated for major chain length groups, between pairs of like symbols, determined by Student’s t-test, p < 0.05. Below each pie graph is the ratio of C14 + C16 carbon chains to C18 + C20 carbon chains. The fold-change in this ratio between pH 7 and pH 5 is also indicated. Ratios were statistically significant in pairwise comparisons.
SpxA2 influences transcription of fabT and fabM.
Since previous microarray transcriptomics indicated that SpxA2 may be a positive regulator of transcription of the fabF and fabG components of the fatty acid biosynthesis elongation module (Kajfasz et al., 2010), and loss of spxA2 led to an increase in SFAs, we examined the role of SpxA2 on transcription of fabT and fabM, two genes that are involved specifically with UFA biosynthesis. qRT-PCR and a chloramphenicol acetyltransferase (CAT) assay were utilized, which are more sensitive than microarray analysis. qRT-PCR of steady-state cultures of UA159 and ΔspxA2 at pH 7 and pH 5 indicated a modest reduction in transcription of fabT in the ΔspxA2 strain (Figure 2A). Transcription of fabM was also slightly reduced in the ΔspxA2 background, however the decrease was not statistically significant (data not shown). fabT encodes a transcriptional regulator of the fab operon (Faustoferri et al., 2015), while fabM encodes the isomerase that allows for UFA production (Fozo & Quivey, Jr., 2004a). To provide further evidence for this regulation by SpxA2, and in a batch culture setting, transcriptional activity from the fabT and fabM promoters was measured using a chloramphenicol acetyltransferase (CAT) reporter system. In this assay, transcription of fabT was decreased in ΔspxA2 compared to UA159 in media buffered either to pH 7 or pH 5 (Figure 2B). fabT transcription at pH 5 was elevated compared to pH 7 in both genetic backgrounds (Figure 2B), in agreement with the qRT-PCR data (Figure 2A). Transcription of fabM was increased in ΔspxA2, compared to UA159, at pH 7, but was similar to UA159 at pH 5. The discrepancy in results between the CAT assay and qRT-PCR in fabM transcription may either result from the physiological differences between steady-state and batch growth or subtle differences between qRT-PCR and the CAT assay.
Figure 2: spxA2 affects transcription of fabT and fabM.

(A) qRT-PCR enumerating fabT transcripts in RNA extracted from the indicated strains during steady-state growth at pH 7 or pH 5. (B) CAT assay quantifying transcription of fabT in the indicated strain from batch cultures buffered to either pH 7 or pH 5. (C) CAT assay quantifying transcription of fabM in the indicated strain from batch cultures buffered to either pH 7 or pH 5. All data is derived from 3 independent cultures assayed in triplicate. Asterisks indicate expression levels which are statistically significant between indicated conditions based on a Tukey’s Multiple Comparisons Test following a two-way ANOVA (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****P < 0.0001; n = 3)
Loss of spxA2 results in elevated ATPase activity.
In addition to an increase in the UFA:SFA ratio, another major component of the S. mutans ATR is increased expression and activity of the F1F0-ATPase (Baker et al., 2017; Kuhnert et al., 2004). This complex hydrolyzes ATP to extrude protons from the cell interior in order to maintain a relatively alkaline cytoplasm during growth of the organism in acidic conditions (Kuhnert et al., 2004). The transcriptional profile of ΔspxA2 revealed that expression of the genes encoding 3 ATPase subunits (atpC, atpD, and atpG) was elevated, relative to UA159, during growth in standard, non-stressed conditions (Kajfasz et al., 2010). To examine whether the increased gene expression of ATPase subunits in ΔspxA2 was correlated with changes in enzyme activity, cultures of UA159 and ΔspxA2 were grown and ATPase activity was measured from permeabilized cells by assaying for release of inorganic phosphate relative to a standard curve. The ATPase activity measured in the spxA2 mutant strain (1.67 ± 0.14 mmol L−1 μg protein−1) was elevated, compared to UA159 (1.23 ± 0.02 mmol L−1 μg protein−1) (Figure 3). These results suggest that the higher transcript levels of ATPase subunits observed in ΔspxA2 are indeed directly correlated with an overall increase in ATPase activity.
Figure 3: ATPase activity is increased in the ΔspxA2 strain.

Graph illustrating ATPase activity, measured as described in Materials and Methods, in the indicated strains over time. Data is derived from 3 independent cultures assayed in triplicate. The ATPase activities of UA159 and ΔspxA2 were significantly different based on a paired Student’s t-test (p = 0.0162; n = 3)
Expression of spxA2 is dependent on LiaR, and is elevated in the presence of membrane and cell wall stressors.
Previous work illustrated that transcription of spxA2 is highly dependent on the LiaFSR three-component system (Suntharalingam et al., 2009). This phenomenon was confirmed here using the CAT reporter system. In batch cultures buffered to either to pH 7 or pH 5, expression of spxA2 was reduced to background levels in a ΔliaR strain (Figure 4A). Since the LiaFSR system plays a major role in the cell envelope stress response, and induces transcription in the presence of membrane stressors (Suntharalingam et al., 2009), it was likely that spxA2 responded to membrane stressors as well. To examine this hypothesis, a CAT assay was used to examine the expression of spxA2 in the presence of membrane-targeting antibiotics tunicamycin and vancomycin, the osmotic stressor NaCl, and the fatty acid synthesis inhibitor, cerulenin. Transcription of spxA2 was significantly increased in the presence of all four of these membrane perturbing agents (Figure 4B). When this experiment was repeated in a ΔliaR background, transcription of spxA2 was reduced to background levels in the presence of all stressors (Figure 4B), further indicating that the expression of spxA2 is dependent on liaR.
Figure 4: spxA2 transcription is dependent on LiaR, and responds to membrane stressors.
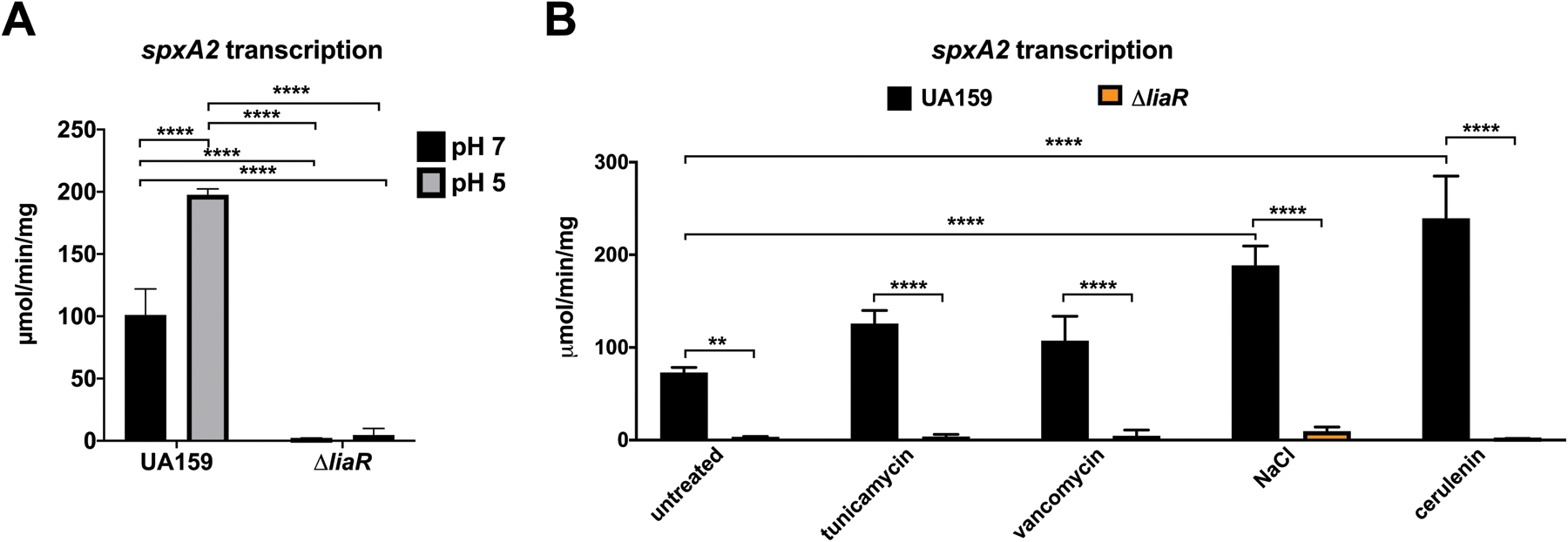
(A) CAT assay quantifying transcription of spxA2 in the indicated strain from batch cultures buffered to either pH 7 or pH 5. (B) CAT assay quantifying transcription of spxA2 in the indicated strain from batch cultures containing the indicated membrane stressor, as described in Materials and Methods. Data is derived from 3 independent cultures assayed in triplicate. Asterisks indicate expression levels which are statistically significant between indicated conditions based on a Tukey’s Multiple Comparisons Test following a two-way ANOVA (**, P < 0.01; ****P < 0.0001; n = 3).
Loss of spxA2 results in increased sensitivity to certain peptidoglycan-targeting antibiotics, but increased resistance to osmotic stress and the FAB inhibitor, cerulenin.
Since spxA2 expression was increased in the presence of the membrane stressors tested above, the susceptibility of the ΔspxA2 strain to these agents, and several others, was examined. The MIC of UA159 and ΔspxA2 was determined for a number of antibiotics that target the cell membrane or peptidoglycan through various modes of action, and chloramphenicol, a protein synthesis inhibitor (Table 2). ΔspxA2 had the same MIC as UA159 for chloramphenicol, bacitracin, daptomycin, and penicillin. ΔspxA2 did have an increased susceptibility to polymyxin B, tunicamycin, and vancomycin, which may indicate a defect in peptidoglycan homeostasis. The fact that bacitracin and penicillin, which also target the peptidoglycan layer, and daptomycin, which targets the membrane, did not have lowered MIC values in the ΔspxA2 strain may seem contradictory; however, likely explanations are described below in the Discussion section.
Table 2:
Minimum inhibitory concentrations (MIC) of S. mutans strains, presented in μg mL−1 (n=3)
| UA159 | ΔspxA2 | |
|---|---|---|
| bacitracin | 256 | 256 |
| chloramphenicol | 8 | 8 |
| daptomycin | 64 | 64 |
| penicillin | 0.25 | 0.25 |
| polymixin B | 64 | 32 |
| tunicamycin | 8 | 0.125 |
| vancomycin | 2 | 1 |
The susceptibility of ΔspxA2 to osmotic stress in the form of 2.5% NaCl was examined. The hyperosmotic stress induced by the addition of NaCl (2.5%) to log phase cultures resulted in severe growth inhibition of UA159, but only moderate inhibition of ΔspxA2, suggesting that ΔspxA2 is resistant to osmotic stress (Figure 5A). Finally, the susceptibility of ΔspxA2 was investigated in the presence of the fatty acid biosynthesis inhibitor, cerulenin. While cultures of both UA159 and ΔspxA2 were inhibited by cerulenin, the ΔspxA2 strain exhibited a faster growth rate and achieved a higher cell density than UA159 (Figure 5B).
Figure 5: The ΔspxA2 strain is resistant to osmotic stress (NaCl) and the fatty acid biosynthesis inhibitor, cerulenin.

Growth of the indicated strain with or without 2.5% NaCl (A) or 10 μg/mL cerulenin (B). n = 10 for each condition.
Discussion
Spx proteins are transcriptional regulators that are widespread across Gram-positive bacteria (Zuber, 2004). Spx is most well-understood in B. subtilis, where the protein utilizes an N-terminal CXXC motif, a conserved glycine residue at position 52, and a C-terminal RPI motif to sense redox state and subsequently interact with a specific DNA sequence and the RNA polymerase to influence transcription of its regulon (Nakano et al., 2005). S. mutans encodes two Spx homologs, encoded by spxA1 and spxA2 (Nakano et al., 2005). SpxA1 plays a major role in sensing oxidative stress and positively regulating the transcription of genes that alleviate oxidative stress, such as superoxide dismutase, NADH oxidase, and glutathione oxidoreductase (Kajfasz et al., 2010; Kajfasz et al., 2015). Deletion of spxA1 results in increased sensitivity to acid and oxidative stress, as well as attenuated virulence, and a reduced ability to produce mutacins and compete with neighboring health-associated bacteria (Galvao et al., 2017). Deletion of spxA2 resulted in a more subtle phenotype, and its role remains poorly understood. Microarray analysis indicated that the regulons of spxA1 and spxA2 have little overlap, suggesting a distinct role for each regulator (Kajfasz et al., 2010). The regulon of SpxA2 primarily consisted of genes involved in cell division, the cell membrane, and cell wall homeostasis (Kajfasz et al., 2010). Further evidence of a role of spxA2 in these pathways has accumulated. The spxA2 deletion mutant was unable to form cell chains, and also displayed increased biofilm formation due to elevated expression of glucosyltransferase enzymes (Galvao et al., 2017; Kajfasz et al., 2015; Kajfasz et al., 2010). Lastly, transcription of spxA2 was significantly decreased upon disruption of the LiaFSR three-component system, which is known to sense, and respond to, cell envelope stress (Shankar et al., 2015; Suntharalingam et al., 2009).
To cause disease, S. mutans must be able to survive and continue to produce acid below the critical pH of 5.5, the point where the adjacent enamel of the tooth begins to demineralize (Bowen et al., 2018; Pitts et al., 2017). To this end, S. mutans employs a complex ATR that allows it to survive in acidic conditions that many of the more health-associated dental plaque residents cannot tolerate (Lemos & Burne, 2008; Lemos et al., 2019). A crucial component of the ATR is a coordinated increase in the proportion of UFAs in the cell membrane, concurrent with an increase in the length of these fatty acid chains (Fozo & Quivey, Jr., 2004b). Blockage of this response via deletion of FabM, the enzyme responsible for unsaturated fatty acid production, resulted in greatly increased acid sensitivity and significantly reduced virulence in a rodent model of dental caries (Fozo & Quivey, Jr., 2004a; Fozo et al., 2007). Although the transcription of fabM is regulated in part by the FabT regulator (Faustoferri et al., 2015), the precise induction mechanism of this shift has not been elucidated. Another well-established component of the S. mutans ATR is increased expression of the F1F0 ATPase, which is used to actively pump protons out of the cytosol to maintain a more neutral pH (Kuhnert et al., 2004; Sheng & Marquis, 2006). Previous examination of the SpxA2 regulon using microarrays suggested that SpxA2 may serve as a positive regulator of genes responsible for both of these components of the ATR (Kajfasz et al., 2010). The goal of this study was to further explore the role of SpxA2 in these ATR pathways, and overall membrane homeostasis.
Deletion of spxA2 resulted in a membrane fatty acid composition that was distinct from the parent strain, UA159 (Figure 1). Although the ΔspxA2 strain was able to increase the proportion of UFAs in its membrane upon environmental acidification, the level of UFAs was significantly reduced compared to the parent strain in both neutral and acidic conditions. The reduced level of UFAs in ΔspxA2 agreed with a preliminary experiment examining the UFA:SFA ratio of ΔspxA2 in batch cultures (Rivera-Ramos, 2015). This result suggests that although the ATR is largely intact in the absence of SpxA2, normal FAB is disrupted. Typically, the UFAs present in the S. mutans membrane are mainly 18 and 20 carbon chains, while the SFAs are 14 and 16 carbon chains. This was observed here, indicating that loss of spxA2 did not decouple unsaturation from the increase in chain length. Although it is not known why the increase in UFAs in S. mutans is protective against acid, a hypothesis is that the fluidity of the membrane is altered by this shift. A change in fluidity could alter the activity of transmembrane proteins, such as ATPases, which are known to be required for maintenance of a neutral cytosol in an acidic extracellular environment (Sheng & Marquis, 2006). Indeed, the ΔfabM strain displayed aberrant ATPase activity in the complete absence of UFAs (Fozo & Quivey, Jr., 2004a). As opposed to the ΔfabM strain, which was extremely sensitive to acid, the ΔspxA2 strain was only slightly more acid sensitive than the parent strain UA159 (Kajfasz et al., 2010). This makes sense, as the ΔfabM strain has no UFAs in its membrane, while the ΔspxA2 strain does have UFAs, but fewer than UA159. It is also worth noting that an ΔspxA1/A2 double mutant was significantly more acid-sensitive than either the ΔspxA1 or the ΔspxA2 single mutant strains (Kajfasz et al., 2010).
Because the fatty acid composition was disrupted in the ΔspxA2 strain, and microarray analysis had suggested that SpxA2 may regulate genes in the fab operon (Kajfasz et al., 2010), transcription of fabT and fabM, specific genes involved in UFA production, was examined in the genetic background of the ΔspxA2 strain. Similar to the microarray data that showed a decrease in fabF and fabG expression in the ΔspxA2 strain (Kajfasz et al., 2010), qRT-PCR of the ΔspxA2 strain grown in steady-state cultures showed a decrease in fabT transcription (Figure 2A). Data from a CAT assay performed using batch cultures confirmed the decrease in fabT expression in the absence of spxA2 (Figure 2B). It appears that SpxA2 positively regulates fabT, therefore the elevated fabM expression in the ΔspxA2 background observed in the CAT assay could be explained by the fact that FabT represses fabM transcription (Faustoferri et al., 2015). Although it is clear that SpxA2 regulates FAB, the collective transcriptional data examined here does not yet provide a coherent picture of why UFA production is reduced in the ΔspxA2 strain. This is not terribly surprising given that the transcriptome of UA159 has been examined through the acid-induced shift of membrane saturation, and an overt mechanistic induction of UFA biosynthesis was not seen at the transcriptional level (Baker et al., 2015). Expression of fab genes appears to be dependent on a complex network of regulators including FabT, CcpA, and SpxA2 (Faustoferri et al., 2015). Furthermore, it is likely that production of UFAs is also governed at least in part at the proteomic and metabolomic levels; therefore, more in-depth transcriptional, as well as biochemical, studies are needed to completely elucidate how the shift to a UFA-dominant membrane occurs, how UFA production is disrupted in ΔspxA2, and whether spxA2 regulates the FAB machinery expression in a direct or indirect manner.
Microarray analysis also indicated that SpxA2 may be a repressor of the operon encoding the F1F0 ATPase (Kajfasz et al., 2010), which is another crucial component of the S. mutans ATR (Baker et al., 2017). We confirmed that ATPase activity was indeed significantly increased in the ΔspxA2 strain concurrent with the increased transcriptional expression (Figure 3). This may suggest a number of different hypotheses. It is possible that the increased expression of the ATPase may mitigate the acid-sensitive phenotype that might be expected due to the decreased UFAs in the ΔspxA2 strain, leading to the mild acid-sensitivity observed. Alternatively, increased ATPase activity was also seen in the ΔfabM strain, despite that strain being severely acid-sensitive (Fozo & Quivey, Jr., 2004a). As the ATPase assay utilized in both studies is not performed on intact cells, it is possible that in vivo, the altered membrane composition observed in both ΔfabM and ΔspxA2 renders the ATPase less effective at providing protection from acid, despite its increased expression. Despite these uncertainties, the research presented here does provide concrete evidence that SpxA2 regulates multiple arms of the ATR and overall membrane homeostasis.
This study confirmed that transcription of spxA2 is dependent on LiaR. Like Spx, the Lia system is widespread among Gram-positive bacteria, and has been shown to sense cell envelope stress in the form of disruption in Lipid II cycling and subsequently regulate transcription of genes that alleviate membrane and cell wall stress (Mascher et al., 2006). In S. mutans, liaR transcription is elevated in the presence of several antibiotics, therefore the expression of spxA2 in the presence of membrane stressors was examined in both UA159 and a liaR deletion mutant (Suntharalingam et al., 2009). Indeed, expression of spxA2 was significantly increased in the presence of all of the membrane stressors tested (Figure 4B). In the absence of liaR, this response was not observed and expression of spxA2 was nearly ablated, indicating that spxA2 requires LiaR to sense membrane stress.
Because deletion of spxA2 would be likely to disrupt signaling from LiaFSR to the cell envelope repair machinery in the SpxA2 regulon, the sensitivity of the ΔspxA2 strain to a number of envelope stressors was examined. The ΔspxA2 strain had increased sensitivity to polymyxin B, tunicamycin, and vancomycin, and no change in sensitivity to bacitracin, daptomycin, penicillin, and chloramphenicol (Table 2). Chloramphenicol was included as essentially a negative control, since it targets protein synthesis, and not the cell envelope or peptidoglycan synthesis. Therefore, it was not surprising that ΔspxA2 did not have an altered susceptibility against this antibiotic. The increased susceptibility of ΔspxA2 to polymyxin B, tunicamycin, and vancomycin was expected, given that these antibiotics target cell wall synthesis, and that increased susceptibility to vancomycin was also observed in a ΔliaR strain (Suntharalingam et al., 2009). Since bacitracin, daptomycin, and penicillin also target the cell wall and/or membrane, it was initially surprising that the ΔspxA2 strain was not more sensitive to these drugs. Although bacitracin disrupts the Lipid II step, which is sensed by LiaFSR, S. mutans is generally recalcitrant to bacitracin, which is included in a media formulation commonly utilized to isolate S. mutans (Gold, Jordan, & Van Houte, 1973). Although the ΔliaR strain was more susceptible to bacitracin than UA159 (Suntharalingam et al., 2009), it is possible that there is a compensatory mechanism at play in ΔspxA2, such as increased expression of the bacitracin resistance genes. Penicillin targets the transpeptidation step of peptidoglycan synthesis, which is downstream of the Lipid II step, the step sensed by LiaFSR. Furthermore, the ΔliaR strain showed a similar lack of increased susceptibility to penicillin (Suntharalingam et al., 2009). Thus, the lack of increased penicillin susceptibility in ΔspxA2 is perhaps not unanticipated. Daptomycin creates pores in the cell envelope, thus it was unexpected that ΔspxA2 was not more sensitive to daptomycin than UA159. It is unclear why this is the case, however it is worth noting that the ΔliaR strain was not more susceptible to nisin (Suntharalingam et al., 2009), which also creates pores in the membrane. Furthermore, in Enterococcus faecalis, exogenous fatty acids protect against daptomycin-induced membrane stress independent of LiaR (Harp et al., 2016). The ΔspxA2 strain was more resistant to osmotic stress in the form of 2.5% NaCl (Figure 5A). This was surprising given that the ΔliaR strain was more sensitive to NaCl than UA159 (Suntharalingam et al., 2009). This result illustrates that although the ΔspxA2 and ΔliaR strains have many overlapping phenotypes, there are subtle differences, highlighting the need for further work exploring the effect of the Lia system on membrane composition and other traits. The ΔspxA2 strain was also more resistant to the FabF inhibitor, cerulenin. It is presently unclear why this would be the case, however the reduced abundance of longer-chained UFAs in ΔspxA2 could indicate reduced flux through the FabF elongation enzyme, which is the target of cerulenin, causing a reduction in sensitivity.
Overall, the results of this study illustrate that SpxA2 responds to a variety of membrane stresses in a LiaR-dependent manner. Furthermore, SpxA2 plays a crucial role in two elements of the S. mutans ATR that are intertwined with cell membrane/wall homeostasis: modulation of the fatty acid composition of the cell membrane, and ATPase expression and activity. The growth phenotypes observed in the ΔspxA2 strain in the presence of membrane stressors indicate that SpxA2 is an integral part of the S. mutans cell envelope stress response. The conserved Spx RPI motif is encoded as SPI in S. mutans SpxA2, thus it has been postulated that SpxA2 may not be able to sense redox state in the same manner as SpxA1 and other canonical Spx proteins (Kajfasz et al., 2010). Perhaps this is why SpxA2 has evolved a distinct regulatory role (Kajfasz et al., 2010). The number of genes predicted to be directly regulated by SpxA2 is much larger than the predicted direct regulon of LiaR, further supporting the idea that SpxA2 transmits the signal of membrane stress detected by the Lia system to downstream effectors. Additional investigations to further examine and refine this regulatory network and its relationship to virulence in this important oral pathogen are currently in progress.
Supplementary Material
Acknowledgements:
This study was supported by NIH/NIDCR DE013683 (R.G.Q.), DE017425 (R.G.Q.), DE021985 (J.L.B. and S.S.), and DE026947 (J.L.B.).
Footnotes
Conflict of Interest: The authors do not declare any conflicts of interest.
References
- Baker JL, Abranches J, Faustoferri RC, Hubbard CJ, Lemos JA, Courtney MA, & Quivey R Jr. (2015). Transcriptional profile of glucose-shocked and acid-adapted strains of Streptococcus mutans. Mol Oral Microbiol, 30(6), 496–517. doi: 10.1111/omi.12110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JL, Derr AM, Karuppaiah K, MacGilvray ME, Kajfasz JK, Faustoferri RC, … Quivey RG Jr. (2014). Streptococcus mutans NADH oxidase lies at the intersection of overlapping regulons controlled by oxygen and NAD+ levels. J Bacteriol, 196(12), 2166–2177. doi: 10.1128/JB.01542-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JL, Faustoferri RC, & Quivey RG Jr. (2017). Acid-adaptive mechanisms of Streptococcus mutans - the more we know, the more we don’t. Mol Oral Microbiol, 32(2), 107–117. doi: 10.1111/omi.12162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen WH, Burne RA, Wu H, & Koo H (2018). Oral biofilms: pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol, 26(3), 229–242. doi: 10.1016/j.tim.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross B, Garcia A, Faustoferri R, & Quivey RG (2016). PlsX deletion impacts fatty acid synthesis and acid adaptation in Streptococcus mutans. Microbiology, 162(4), 662–671. doi: 10.1099/mic.0.000252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustoferri RC, Hubbard CJ, Santiago B, Buckley AA, Seifert TB, & Quivey RG Jr. (2015). Regulation of fatty acid biosynthesis by the global regulator CcpA and the local regulator FabT in Streptococcus mutans. Mol Oral Microbiol, 30(2), 128–146. doi: 10.1111/omi.12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske CH, & Subbarow Y (1925). The colorimetric determination of phosphorus. J Biol Chem, 66, 375–400. [Google Scholar]
- Fozo EM, & Quivey RG Jr. (2004a). The fabM gene product of Streptococcus mutans is responsible for the synthesis of monounsaturated fatty acids and is necessary for survival at low pH. J Bacteriol, 186(13), 4152–4158. doi: 10.1128/JB.186.13.4152-4158.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozo EM, & Quivey RG Jr. (2004b). Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic environments. Appl Environ Microbiol, 70(2), 929–936. doi: 10.1128/aem.70.2.929-936.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozo EM, Scott-Anne K, Koo H, & Quivey RG Jr. (2007). Role of unsaturated fatty acid biosynthesis in virulence of Streptococcus mutans. Infect Immun, 75(3), 1537–1539. doi: 10.1128/IAI.01938-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvao LC, Rosalen PL, Rivera-Ramos I, Franco GC, Kajfasz JK, Abranches J, … Lemos JA (2017). Inactivation of the spxA1 or spxA2 gene of Streptococcus mutans decreases virulence in the rat caries model. Mol Oral Microbiol, 32(2), 142–153. doi: 10.1111/omi.12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold OG, Jordan HV, & Van Houte J (1973). A selective medium for Streptococcus mutans. Arch Oral Biol, 18(11), 1357–1364. doi: 10.1016/0003-9969(73)90109-x [DOI] [PubMed] [Google Scholar]
- Gross EL, Beall CJ, Kutsch SR, Firestone ND, Leys EJ, & Griffen AL (2012). Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS One, 7(10), e47722. doi: 10.1371/journal.pone.0047722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harp JR, Saito HE, Bourdon AK, Reyes J, Arias CA, Campagna SR, & Fozo EM (2016). Exogenous fatty acids protect Enterococcus faecalis from daptomycin-induced membrane stress independently of the response regulator LiaR. Appl Environ Microbiol, 82(14), 4410–4420. doi: 10.1128/AEM.00933-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajfasz JK, Rivera-Ramos I, Abranches J, Martinez AR, Rosalen PL, Derr AM, … Lemos JA (2010). Two Spx proteins modulate stress tolerance, survival, and virulence in Streptococcus mutans. J Bacteriol, 192(10), 2546–2556. doi: 10.1128/JB.00028-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajfasz JK, Rivera-Ramos I, Scott-Anne K, Gregoire S, Abranches J, & Lemos JA (2015). Transcription of oxidative stress genes is directly activated by SpxA1 and, to a lesser extent, by SpxA2 in Streptococcus mutans. J Bacteriol, 197(13), 2160–2170. doi: 10.1128/JB.00118-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs CJ, Faustoferri RC, & Quivey RG Jr. (2017). RgpF is required for maintenance of stress tolerance and virulence in Streptococcus mutans. J Bacteriol, 199(24). doi: 10.1128/JB.00497-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhnert WL, Zheng G, Faustoferri RC, & Quivey RG Jr. (2004). The F-ATPase operon promoter of Streptococcus mutans is transcriptionally regulated in response to external pH. J Bacteriol, 186(24), 8524–8528. doi: 10.1128/JB.186.24.8524-8528.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JA, & Burne RA (2008). A model of efficiency: stress tolerance by Streptococcus mutans. Microbiology, 154(Pt 11), 3247–3255. doi: 10.1099/mic.0.2008/023770-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JA, Palmer SR, Zeng L, Wen ZT, Kajfasz JK, Freires IA, … Brady LJ (2019). The biology of Streptococcus mutans. Microbiol Spectr, 7(1). doi: 10.1128/microbiolspec.GPP3-0051-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher T, Helmann JD, & Unden G (2006). Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol Mol Biol Rev, 70(4), 910–938. doi: 10.1128/MMBR.00020-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MM, Hajarizadeh F, Zhu Y, & Zuber P (2001). Loss-of-function mutations in yjbD result in ClpX- and ClpP-independent competence development of Bacillus subtilis. Mol Microbiol, 42(2), 383–394. 10.1046/j.1365-2958.2001.02639.x [DOI] [PubMed] [Google Scholar]
- Nakano S, Erwin KN, Ralle M, & Zuber P (2005). Redox-sensitive transcriptional control by a thiol/disulphide switch in the global regulator, Spx. Mol Microbiol, 55(2), 498–510. doi: 10.1111/j.1365-2958.2004.04395.x [DOI] [PubMed] [Google Scholar]
- Pitts NB, Zero DT, Marsh PD, Ekstrand K, Weintraub JA, Ramos-Gomez F, … Ismail A (2017). Dental caries. Nat Rev Dis Primers, 3, 17030. doi: 10.1038/nrdp.2017.30 [DOI] [PubMed] [Google Scholar]
- Quivey RG Jr., Grayhack EJ, Faustoferri RC, Hubbard CJ, Baldeck JD, Wolf AS, … Marquis RE (2015). Functional profiling in Streptococcus mutans: construction and examination of a genomic collection of gene deletion mutants. Mol Oral Microbiol, 30(6), 474–495. doi: 10.1111/omi.12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Ramos I (2015). Characterization of the Spx global regulator in Streptococcus mutans. Retrieved from http://hdl.handle.net/1802/29365
- Shankar M, Mohapatra SS, Biswas S, & Biswas I (2015). Gene regulation by the LiaSR two-component system in Streptococcus mutans. PLoS One, 10(5), e0128083. doi: 10.1371/journal.pone.0128083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw WV (1975). Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol, 43, 737–755. 10.1016/0076-6879(75)43141-X [DOI] [PubMed] [Google Scholar]
- Sheng J, & Marquis RE (2006). Enhanced acid resistance of oral streptococci at lethal pH values associated with acid-tolerant catabolism and with ATP synthase activity. FEMS Microbiol Lett, 262(1), 93–98. doi: 10.1111/j.1574-6968.2006.00374.x [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, … Klenk DC (1985). Measurement of protein using bicinchoninic acid. Anal Biochem, 150(1), 76–85. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3843705 [DOI] [PubMed] [Google Scholar]
- Suntharalingam P, Senadheera MD, Mair RW, Levesque CM, & Cvitkovitch DG (2009). The LiaFSR system regulates the cell envelope stress response in Streptococcus mutans. J Bacteriol, 191(9), 2973–2984. doi: 10.1128/JB.01563-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P (2004). Spx-RNA polymerase interaction and global transcriptional control during oxidative stress. J Bacteriol, 186(7), 1911–1918. doi: 10.1128/jb.186.7.1911-1918.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


