Abstract
Reptiles are reservoirs of a wide range of pathogens, including many protozoa, helminths, pentastomids, and arthropod parasitic species, some of which may be of public health concern. In this review we discuss the zoonotic risks associated with human–reptile interactions. Increased urbanization and introduction of exotic species of reptile may act as drivers for the transmission of zoonotic parasites through the environment. In addition, being a part of human diet, reptiles can be a source of life-threatening parasitoses, such as pentastomiasis or sparganosis. Finally, reptiles kept as pets may represent a risk to owners given the possibility of parasites transmitted by direct contact or fecal contamination. Awareness of reptile-borne zoonotic parasitoses is important to advocate control, prevention, and surveillance of these neglected diseases.
Keywords: zoonotic parasites, food-borne, vector-borne, reptile pet trade, pentastomiasis, sparganosis
Highlights
Species of protozoa, helminths, pentastomids, and arthropod vectors exploit reptiles as definitive or paratenic hosts, which may represent a public health concern.
The zoonotic risk is associated with human–reptile interactions and includes environmental contamination, reptile consumption, or keeping reptiles as pets.
Exotic reptile species may introduce new zoonotic parasites in a previously nonendemic region.
Pentastomiasis and sparganosis are life-threatening food-borne parasitoses.
In our households, if precautions are not taken, reptiles may transmit zoonotic parasites by direct contact or fecal contamination.
Trained veterinarians, physicians, and public health officials are important to advocate for proper diagnostics, parasite identification and treatment, as well as for surveillance strategies and food inspection in areas where reptiles are consumed.
Humans, Reptiles, and…Their Parasites
Crawling creatures, identified as reptiles (from the Latin repere 'to crawl'), include a polyphyletic (see Glossary) group of animals belonging to different orders and a large number of species (Box 1 ) [1]. In many aspects, reptiles represent enigmatic creatures and arouse an incredible range of feelings in humans as they are perceived, according to different cultures, as fascinating and even worshipped in some African and Asian societies, such as the ball pythons (Python regius) and black cobra (Naja melanoleuca) in Africa, or scary and disgusting wild creatures crawling on earth, mainly in the western world [2]. Accordingly, the symbolic usage of reptiles varied through history in many cultures. The range of attitudes of humans toward reptiles commonly affects their conservation, being often persecuted as dangerous creatures (P. Luís Ceríaco, Master’s Thesis, Évora University, 2010). Nowadays, reptiles have become popular exotic pets and account for an estimated 21% of the value of the live animal trade [3]. In addition, in some parts of the world, the reptiles are used as important source of food, medicines, and materials (e.g., the leather industry) (Box 2 ).
Box 1. Evolution, Biodiversity, and Ecological Relevance.
Reptiles are among the most diverse group of animals that inhabit almost all continents and environments and, as a matter of fact, one of the most ancient groups of animals alive, having their ancestors in dinosaurs (i.e., reptiliomorph tetrapods) that colonized the earth during the Carboniferous period, around 300 million years ago [91]. However, a relatively low number of species survived the mass extinction events during the Cretaceous–Paleogene period (i.e., around 65 million years ago), spreading through almost all the biotopes on earth and becoming increasingly adapted to life on dry land [92]. This class of animals includes about 1200 genera, and more than 10 000 species have been described, mainly within the group Squamata (i.e., 10 417 species of lizards, snakes, and worm-like amphisbaenians), Testudines (i.e., 351 species of turtles and tortoises), Crocodylia (i.e., 24 species), and Rhynchocephalia (i.e., 1 species of tuataras) [93]. Some reptiles are of great ecological significance as they represent in nature either the first (e.g., lizards, geckos) or the higher level (e.g., crocodiles) of the food chain. In the latter case, given their aquatic habits and longevity (generally >50 years), crocodilians have also been suggested as potential indicators for environmental pollution as they reflect changes in an area over longer periods.
Alt-text: Box 1
Box 2. Reptiles as Food and Medicaments.
Reptiles have represented an important source of protein in the diet of humans in many parts of the world and are still important in tropical countries and as gourmet specialties in Europe and North America (i.e., crocodilian meat) [94]. Infection by the food-borne pathogens closely depends on the way in which the reptile meat is prepared and eaten. In many areas of the world reptiles are eaten as any other protein source; however, it is also broadly believed that reptile meat and organs have medicinal properties [95]. While the usage of dry or alcohol-based remedies is rather safe, most of the clinical reports of reptile-associated helminthiases of humans are connected with the consumption of raw meat, organs, blood, or bile of snakes or lizards. In some part of Asia, applying snake meat as a poultice to a wound represents an alternative route of human infection by Spirometra [52]. Usually, when reptiles are cooked as a dish, the risk of parasite transmission relates mainly to the way in which the animal was kept at home prior its slaughtering, evisceration, and food preparation, rather than to consumption of the resulting meal.
To illustrate, here is the traditional finger-licking recipe from Gabon made with African rock python: 'Take medium to large size African rock python. Skin the snake, chop to small pieces and place into a large pot. Sprinkle with salt, add cold water and lemongrass (to reduce snake smell), bring to boil and simmer shortly. Remove snake pieces from water, put into larger pan with hot vegetable oil and gently fry the meat with curry and black pepper. Add little water, bring to the boil, cover with lid and boil for another 20 minutes. Serve with tomato sauce and steamed rice.'
Interestingly, similar recipes are popping up in the USA as a reaction to the growing presence of invasive Burmese pythons, for example, in the Everglades.
Alt-text: Box 2
As other animals, reptiles are hosts of viral, bacterial, and parasitic pathogens, some of which are of zoonotic concern [4]. In particular, Salmonella bacteria are the most common causative agents of zoonotic disease due to their biological characteristics (e.g., fast logarithmic growth rate, tolerance to a wide variety of environmental temperatures and humidity, and potential to colonize artificial environments created by humans), as well as their natural affinity with this group of hosts [5]. Most of the available studies on parasites of reptiles have dealt with ecological investigations of hosts and have focused on the conservational aspects rather than on the zoonotic potential [6]. As a result, zoonotic parasites have been less studied, and information on the biology, ecology, and zoonotic potential of most of them is scarce, being historically neglected even in endemic areas. The transmission of reptile-borne parasitic zoonoses is mainly related to organisms for which these animals are intermediate or paratenic, rather than definitive hosts [4]. In the first case, humans are infected usually through consumption of reptiles, whereas in the latter, the parasite stages are shed into the environment, where humans are exposed to them (Figure 1 ). Moreover, reptiles are a source of blood meal for arthropods, acting as reservoirs of several zoonotic vector-borne diseases (VBDs) (Table 1 ). Overall, zoonotic parasites may be transmitted through different modalities (e.g., environmental contamination, food, or by direct contact with captive animals), and also according to the geographical area and human level of interaction with reptiles (Table 1). In this article we discuss the risks of transmission of reptile-associated parasitic zoonotic diseases in different contexts, answering practical questions from a veterinary and medical perspective.
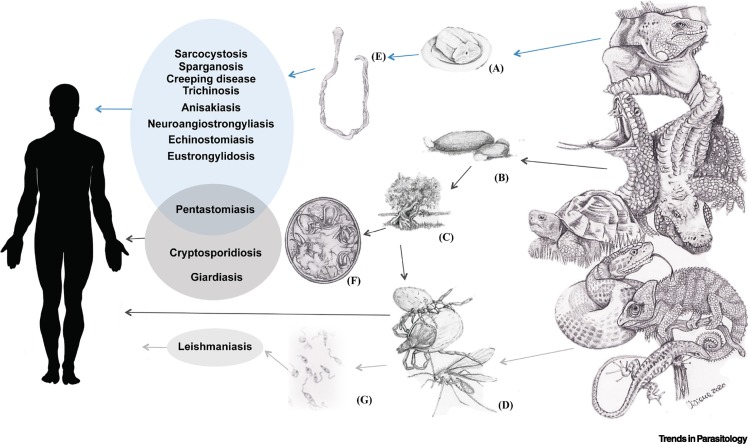
Figure 1.
Transmission Pathways of Main Reptile-Borne Zoonotic Parasitic Diseases.
Representation of the main transmission modalities of reptile-borne zoonotic parasitic diseases that are (A) food-borne, (B) transmitted by direct contact with feces, (C) spread through environmental contamination, and (D) vector-borne. The most representative parasites for each pathway are also illustrated: (E) Spirometra spp., (F) egg of Armillifer spp., (G), Leishmania spp.
Table 1.
Zoonotic Parasites of Reptiles Grouped According to the Modality of Transmission
| Pathogens (zoonosis) | Geographic origin of reports | Reptile hosts | Categories of zoonotic importancea | Main clinical signs in humans | Refs |
|---|---|---|---|---|---|
| Protozoa | |||||
|
Giardia duodenalis (giardiasis) |
Spain | Lizards | UI | Diarrhea | [10] |
|
Cryptosporidium parvum subtype: IIaA15G2R1 (cryptosporidiosis) |
Italy | Snakes, lizards and turtles | UI | Acute to persistent diarrhea | [75,76,78] |
|
Sarcocystis nesbitti (sarcocystosis) |
Mainly Southeast Asia | Snakes | LI | Muscular sarcocystosis | [17] |
|
Leishmania tropica, Leishmania. donovani, Leishmania turanica (cutaneous and visceral leishmaniasis) |
Asia | Lizards, snakes | UI | Unknown | [40] |
|
Trypanosoma brucei (sleeping sickness) |
Africa | Monitor lizards | UI | Unknown | see Box S1b |
| Cestoda | |||||
|
Spirometra erinaceieuropaei, Spirometra mansonoides (sparganosis) |
Americas, Europe, Asia, Australia | Snakes | HI | Blindness, paralysis, death | [51] |
| Pentastomida | |||||
|
Armillifer armillatus, Armillifer moniliformis, Armillifer grandis, Armillifer agkistrodontis (pentastomiasis) |
Asia, Africa | Snakes | HI | Organ damage by larvae | [83] |
|
Raillietiella hemidactyli (creeping disease) |
SE Asia | Lizards | LI | Subcutaneous pentastomiasis | [59] |
| Nematoda | |||||
|
Trichinella zimbabwensis, Trichinella papuae (trichinosis) |
Worldwide | Crocodiles, snakes, Monitor lizards, turtles |
LI | Fever, myalgia, gastrointestinal symptoms | [47] |
|
Contracaecum spp., Anisakis spp., Pseudoterranova spp. (anisakiasis) |
Worldwide | Crocodiles | UI | Eosinophilic granulomas | [47] |
|
Eustrongylides spp. (eustrongylidosis) |
Worldwide | Crocodiles | UI | No specific symptoms recorded so far | [84] |
|
Gnathostoma binucleatum, Gnathostoma doloresi, Gnathostoma hispidum, Gnathostoma nipponicum, Gnathostoma spingerum (gnathostomosis) |
Africa, Asia, Central America | Snakes (but mainly fish) | LI | Cutaneous or visceral larvae migrans symptoms | [85] |
|
Angiostrongylus cantonensis (neuroangiostrongyliasis) |
Subtropics | Monitor lizards | HI | Eosinophilic meningitis | [86] |
| Trematoda | |||||
|
Alaria and Echinostoma (echinostomiasis) |
Asia | Turtles and crocodiles | LI | Catarrhal inflammation Peripheral eosinophilia |
[87] |
| Ixodida | |||||
| Amblyomma spp. | Africa, Asia | Monitor lizards, tortoises, snakes | LI | Dermatitis and VBDs | [44,88] |
| Bothriocroton hydrosauri | Australia | Snakes, lizards | HI | Vector of Flinders Island spotted fever | [33] |
| Haemaphysalis spp. | Europe, Africa, Asia | Lizards, viperid snakes | LI | Dermatitis and VBDs | [88] |
| Hyalomma aegyptium | Africa | Tortoises | LI | Dermatitis and VBDs | [88] |
| Ixodes spp. | Europe, North America | Snakes, lizards | HI | Dermatitis and VBDs | [35] |
| Ornithodoros spp. | Africa, North America | Tortoises and viperid snakes | LI | Dermatitis and VBDs | [88] |
| Mesostigmata | |||||
| Ophionyssus natricis | Worldwide | Snakes, lizards | LI | Dermatitis | [89,90] |
| Prostigmata | |||||
| Eutrombicula spp. | Americas | Snakes, lizards, turtles | LI | Dermatitis | [35,90] |
| Neotrombicula autumnalis | Europe | Lizards, snakes | HI | Dermatitis | [35,90] |
Zoonotic importance categorized as follows: high importance (HI): causing severe or lethal cases in humans clearly associated with reptiles; low importance (LI): causing human cases that are likely linked to reptiles, even though rare or accidental; unknown importance (UI): possibly zoonotic parasites that were isolated from reptiles (including spurious parasites) but causative link of reptiles to a human disease was not proved.
For a full reference list see Box S1 in the supplemental information online.
Parasitic Zoonotic Infections in Our Backyard: Synanthropic and Invasive Species
Reptiles live in our houses, backyards, or any place where they can find shelter, food, and heat. Thus, it is not surprising that increasing urbanization and habitat loss have facilitated the encounters with humans and enabled numerous reptile species to adapt to peridomestic environments [7]. As a matter of fact, parasite transmission from reptiles to humans depends not only on their biology and abundance in a host population but also on environmental conditions. Thus, the transmission of some parasites could be trigged, but also limited, by urbanization [8]. Undoubtedly, in peridomestic areas, the synanthropic species (i.e., geckos and lizards) may transmit zoonotic protozoa and pentastomids through contamination of the environment (Table 1) [9,10]. Meanwhile, invasive/exotic reptile species may be a source of spill-over of parasites to native species, eventually representing a further threat for public health [11,12]. It was an invasive Burmese python (Python bivittatus), introduced into Florida and bringing Asiatic pentastomids (i.e., Raillietiella orientalis), which eventually infected native species of snake [12]. Although the majority of human infections by pentastomids occur through the ingestion of snake meat (which is typical in Asian and African countries), clinicians should be aware that alternative routes of infection exist, for example, through exposure to snake nasal secretions, saliva, and feces [13], suggesting the potential risk for humans. Such transmission routes may explain some of the human cases recorded in the USA, where this infection is unexpected or considered to be of travel-medicine concern. In addition, when the same species of python was transported back to Asia it carried American pentastomids (i.e., Raillietiella bicaudata) into local populations [14]. In tropical Asia, muscular sarcocystosis of humans has been associated with ingestion of sporocysts of Sarcocystis nesbitti (Figure 2A), probably originating from snake feces [15., 16., 17.]. Overall, veterinarians play a pivotal role in avoiding zoonotic infections through coprological screening of captive snakes.
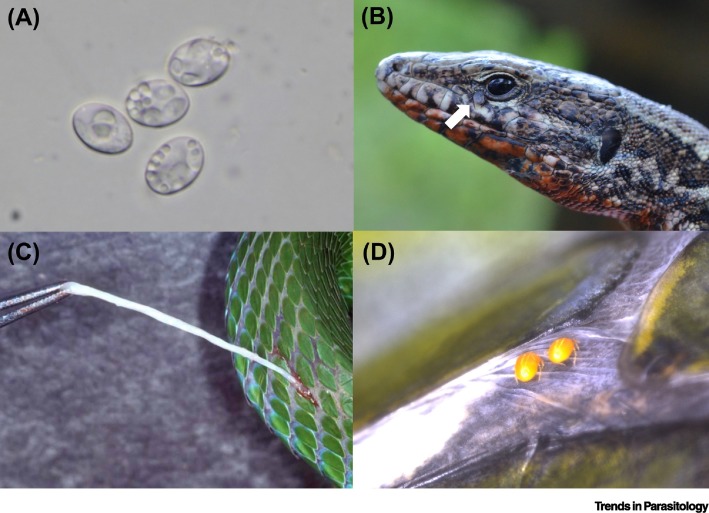
Figure 2.
Representative Examples of Reptile-Borne Zoonotic Parasites.
Endoparasites and ectoparasites of reptiles causing zoonotic diseases through (A) direct contact with feces (Sarcocystis singaporensis), (B) infestation by ticks from the environment (white arrow is a nymph of Ixodes ricinus), (C) ingestion of contaminated food (Spirometra spp. in subcutaneous tissue), or (D) chigger mites (Eutrombicula alfreddugesi) from the environment.
Reptiles serve also as a blood source for many species of hematophagous arthropods (e.g., mites, ticks, sand flies, mosquitoes) and then as possible reservoirs for bacterial, viral, and protozoan pathogens (Table 1) [18,19]. For example, some species of tick (Ixodidae and Argasidae) and mites (Macronyssidae, Trombiculidae) often feed on reptiles as well as on humans (Table 1). The macronyssid mite Ophionyssus natricis, one of the most widely distributed mite species of snakes, is a mechanical vector of Aeromonas hydrophila, the causative agent of hemorrhagic disease in reptiles as well as of gastroenteritis, and in rare cases, of necrotizing fasciitis in humans [20,21]. In the past decade, the epidemiological role of argasid and ixodid ticks as vectors of zoonotic pathogens has received great attention. For example, Ornithodoros turicata, an argasid tick parasitizing mainly tortoises, is the vector of Borrelia turicatae, of the relapsing fever clade [22], which has been associated with tick-borne relapsing fever in five cases among employees who worked in caves in the USA [23]. Exotic tick species, along with the pathogens they carry, may be imported in nonendemic areas together with reptiles (Box 3 ). This is the case of tortoises infested by Hyalomma aegyptium illegally imported into Italy [24]. This tick species has been found to be molecularly positive for pathogens of zoonotic concern (Box 3) [25., 26., 27.]. In addition, some of the tick species parasitizing reptiles were vectors of Coxiella burnetii, the agent of Q fever (e.g., H. aegyptium ticks in the Mediterranean basin) [28]. Reptiles also participate directly in the epidemiology of some pathogens of the Rickettsiaceae family [29]. This is so in the case of African fever, a human disease caused by Rickettsia africae and transmitted by Amblyomma variegatum [30], detected in ticks imported, with reptiles, into North America from Africa [31]. In Australia, Rickettsia honei, the causative agent of the Flinders Island spotted fever in humans, has been detected in ticks (e.g., Bothriocroton hydrosauri) infesting a range of reptile species, which act as the main reservoirs for this pathogen [32,33]. Also, Rickettsia anan was detected in ticks from the species Amblyomma exornatum in varanid lizards imported into the USA [34]. Finally, lacertid lizards are primary hosts of immature stages of Ixodes ricinus ticks (Figure 2B), playing a role in the epidemiology of some tick-borne pathogens, such as Borrelia burgdorferi sensu lato group, causing Lyme borreliosis [35].
Box 3. The Reptile Trade, and the Risk of Importing New Tick Species.
The worldwide trade in live reptiles has increased considerably during the past decades. The 96.8% of the reptiles imported globally (e.g., over 18.8 million, between 1996 and 2012) for commercial purposes are captive-bred, or from farming/wild caught, with Green iguana (Iguana iguana) being at the top of the list followed by ball python (Python regius) [96]. However, a portion of the whole global trade is still represented by illegal trading [96] and this poses concerns about the potential risk for the dissemination of infectious agents, as well as exotic tick species and the pathogens they may transmit. For example, Testudo graeca (also known as the Eastern Spur-thighed Tortoise) is an endangered species present in southeastern Europe, western Asia and North Africa and it is the most frequently reported host of the tortoise tick Hyalomma aegyptium [97]. These tortoises are often illegally treated in southern Mediterranean and Middle East regions. For example, of the 585 tortoises illegally imported into Italy from North Africa, 37.8% (n = 221) were infested with H. aegyptium [24]. Although H. aegyptium adults are typically associated with tortoises of the genus Testudo, immature stages parasitize a wide range of reptiles, birds, and mammals, including humans [98]. This tick species has also been collected from tortoises in other countries where they have been associated with Anaplasma phagocytophylum (in Romania; [25]), with the Crimean Congo Hemorrhagic fever virus (in the Middle East; [26]), and with spotted fever group Rickettsia (in Qatar; [27]). Similarly, four species of Amblyomma ticks, parasitizing lizards and tortoises, were introduced into Florida, USA, and were found to be infected with Ehrlichia ruminantium or 'Heartwater' disease as well as C. burnetii, the agent of Q fever [31].
Alt-text: Box 3
Phlebotomine flies, mainly of the genus Sergentomyia, are herpetophilic hematophagous invertebrates that were, until recently, associated with the transmission of the nonpathogenic species Leishmania tarentolae [36]. However, recent studies have demonstrated that Sergentomyia spp. can also harbor pathogenic Leishmania, such as Leishmania infantum and Leishmania major [37., 38., 39.]. Moreover, other pathogenic species of Leishmania have been detected in lizards (e.g., Leishmania turanica, Leishmania tropica, and Leishmania donovani) [40,41] (Table 1), raising the hypothesis that lizards and associated sand flies could play a role in the epidemiological cycle of leishmaniasis. This issue should be better and fully addressed. Indeed, studies on vector-borne pathogens represent a unique opportunity to integrate veterinarians, physicians, and public health officials toward elucidating the impact of reptile vector-borne pathogens under the context of One Health.
Parasitic Zoonotic Infection of Reptiles in Our Dish
Reptiles represent an essential part of the food web in many ecosystems [42]. While large carnivorous reptile species (e.g., crocodilians, monitor lizards, a range of snakes, marine and aquatic turtles) act as top predators, the smaller reptile taxa (mainly in Squamata) represent a common prey for many carnivores. Therefore, it is not surprising that a wide range of heteroxenous protozoan and metazoan parasites cycle through reptiles, using them as intermediate or final hosts [43]. Also, humans, as large omnivorous primates, play their role in the food chains that involve reptiles (Box 2). The largest carnivorous reptiles, such as crocodiles, can occasionally hunt and consume even adult humans, however, these are rather unusual extreme cases [44]. Conversely, various reptiles represent an important part of human diet throughout the world and, in many areas, this habit persists until today. Besides consumption of wild-caught reptiles, reptile farming was developed in past decades in order to cover growing demand and to replace the animals from diminished wild populations [45]. Among the farmed reptiles, crocodiles probably form the largest proportion; however, snake and turtle farming are fast developing in South Eastern (SE) Asia, and iguanas are locally farmed in South America [46]. This farming system is in urgent need of defined food-inspection protocols as well as trained professionals, through updating animal science curricula. In addition, reptiles are used as part of traditional medicine, mainly in Asia (Box 2).
Cases of zoonotic helminthic infections are reported throughout the world, being associated with reptile consumption or with reptile meat used in medicinal practices [47]. In all food-borne parasitoses associated with reptile consumption (e.g., Anisakis spp., Gnathostoma spp., Spirometra spp., Alaria, Angiostrongylus cantonensis, Trichinella spp. and several pentastomids, Table 1) humans represent aberrant or dead-end hosts. Pseudophyllidean tapeworms of the genus Spirometra (Diphyllobothriidae) are the most frequent reptile-borne zoonotic helminths – Spirometra erinaceieuropaei, Spirometra mansonoides, and Spirometra proliferum, being among the most commonly reported species. The life cycle of Spirometra spp. typically involves carnivorous mammals as definitive hosts and freshwater crustaceans (i.e., cyclops) and poikilothermic vertebrates as first and second intermediate hosts, respectively. The infective larval stages (i.e., the plerocercoids, commonly referred to as sparganum, or spargana in plural) are typically found in subcutaneous tissue (Figure 2C) and/or muscles of reptiles and may become infective to humans by eating uncooked meat. After the infection, plerocercoids migrate to various organs and tissues in the human body, including subcutaneous tissue, muscles, lungs, the pleural cavity, urogenital and abdominal viscera, and, importantly, to the central nervous system [48,49]. Therefore, depending on which organs are invaded, disease is usually classified as subcutaneous, ocular, cerebral, or visceral sparganosis [50]. It may occur that plerocercoids asexually multiply within the human host, causing a severe clinical condition known as proliferative sparganosis [51]. Most of the cases of human sparganosis are confined to SE Asia, apparently due to locally common consumption of raw or inadequately cooked snakes, frogs, and tadpoles infected with the plerocercoid. Cases recently reported from Europe were foreigners who had probably acquired infection in other continents [49]. Drinking untreated water with infected copepods (i.e., first intermediate hosts) or applying the meat of infected snake or frog as a poultice to a wound, are alternative routes of human infection [52]. As the disease in humans is difficult to identify at a preoperative stage, the anamnesis is pivotal to expedite diagnosis and treatment.
A. cantonensis is a metastrongyloid nematode of emerging concern causing eosinophilic meningitis in humans [53]. This parasite exploits various species of rat as a definitive host and a broad range of molluscs as intermediate hosts [54]. Amphibians and reptiles represent paratenic hosts that are able to accumulate infective L3 larvae in liver and other tissues. Although the major source of infections by A. cantonensis in SE Asia is through the consumption of molluscs, prawns, and shrimps, eating raw meat of varanids was documented as an alternative infection route [55,56]. Nonetheless, the importance of poikilothermic hosts in the circulation of A. cantonensis in the food chain is not fully understood. Trichinella spp. are nematodes with a high potential for zoonotic infections. While the majority of species (the encapsulated ones) are infective only for mammals, two nonencapsulated species – Trichinella zimbabwensis and Trichinella papuae – are found also in large carnivorous reptiles, namely in crocodiles and monitor lizards [57]. Completion of the Trichinella spp. life cycle in these hosts was confirmed also experimentally [58]. Trichinellosis cases associated with the consumption of meat from monitor lizards and turtles have been documented in Thailand, even though the Trichinella species involved was not identified.
Pentastomids are currently considered as a unique lineage of crustaceans, adapted to endoparasitic life in vertebrates. Without doubt, pentastomids are parasites typically associated with Reptilia, as the vast majority of existing species are described from these hosts. They are known as serious pathogens in captive reptiles, and several species may cause zoonotic infections in Africa (i.e., Armillifer grandis, Armillifer armillatus) and Asia (i.e., Armillifer moniliformis, Armillifer agkistrodontis). Other published zoonotic records (such as 'Porocephalus taiwana' in China and Porocephalus crotali in South America) need careful scrutiny [59,60]. The life cycle of Armillifer spp. is typically heteroxenous in that vermiform adults reside in the lungs of large snakes (i.e., pythons, large vipers, cobras) and shed infective eggs with typical larvae in feces. A range of mammals serve as intermediate hosts, in which the larvae develop in connective tissues and parenchymatous organs. Even though they occur frequently in some areas of West and Central Africa, human infections are usually asymptomatic, diagnosed incidentally as liver calcifications by X-ray or ultrasonography, or, at autopsy [61., 62., 63.]. Human cases of pentastomiasis are associated with consumption of snake meat, though infection by eggs in the contaminated environment represents a risk in endemic areas [64], as well as from captive reptiles (see later). Massive infections leading to rare lethal cases are tentatively associated with incidental ingestion of the entire gravid female of Armillifer spp. [65]. Historically, postmortem prevalence in humans has been recorded up to 23% in Central (Congo) and southern Africa (Zimbabwe) and 40% in SE Asia (i.e., Malaysia, [66]).
Parasitic Zoonotic Infection of Reptiles in Our Bedroom: The Pet Scenario
Reptiles are common animals kept as pets. From calm ball pythons, charming green iguanas, to long-living tortoises, nowadays keeping reptiles in our houses is becoming a regular habit, rather than an exclusive hobby [67]. For example, in the USA 2.4% of the pet population is represented by reptiles and amphibians [68., 69., 70.]. The information regarding husbandry and basic care for many kept species is scant, and these animals are still among the most inhumanely treated in the pet market [71]. Therefore, veterinarians need to be aware of the increasing need for medical care given that keeping reptiles in captive conditions usually comes with unnatural events (e.g., metabolic imbalances, low immunity, scarce hygiene of enclosure), which facilitates transmission of parasites. Therefore, in poor husbandry conditions, and without regular veterinary follow-up, zoonotic parasites could infect the pet owners, especially those with immunosuppression, or children [4,71., 72., 73.]. The risk of zoonotic agents (mostly bacteria but also parasites) being transmitted to the above-mentioned groups of people has raised concerns by public health agencies, such as the Center for Disease Control and Prevention (CDC), which discourage the tenure of reptiles as pets [71]. Moreover, the reptile pet trade has been pointed out as a possible source of introduction and emergence of exotic parasites in nonendemic countries [71,74].
Zoonotic parasites associated with pet reptiles (Table 1) include protozoa (e.g., Cryptosporidium, Sarcocystis), pentastomids, and ectoparasites (e.g., Trombiculidae and Macronyssidae) (Figure 2D) [71,75,76]. In recent years Cryptosporidium species with zoonotic potential have been isolated from pet reptiles by the molecular detection of Cryptosporidium muris, Cryptosporidium tyzzeri and, most importantly, subtypes of Cryptosporidium parvum previously detected in humans [76., 77., 78.]. The detection of zoonotic Cryptosporidium spp. in snakes is associated with the ingestion of infected prey (e.g., rodents) since they do not develop in the ophidian host. However, also a number of lizards, snakes, and tortoises have been found excreting Cryptosporidium oocysts potentially pathogenic to humans [75., 76., 77., 78.]. Larger species of pet snake, such as ball pythons or large African vipers, are typical hosts of pentastomids [79], which may represent a serious zoonotic risk through fecal egg contamination of the environment [67,68].
In addition, the parasitic fauna of captive reptiles, besides some species having zoonotic potential, can help to distinguish animals that were caught from their natural environment from those animals that were bred in captivity [80]. This is particularly true for various hemoprotozoa that are found only in reptiles that had contact with the infected vector in the natural environment to which these animals are native [81]. Therefore, the proper identification of the parasitic fauna of captive reptiles has turned into a useful tool to control and prevent the illegal reptile trade, as well as a surveillance strategy for potential public health risks associated with this diverse group of animals.
Finally, in order to minimize the risk of zoonotic pathogen transmission in the household setting, good and proper sanitary precautions should be taken along with personal hygiene when handling these animals – as well as good husbandry conditions and separating reptiles from areas where food is prepared. Also, when keeping reptiles as pets, animals need to be periodically checked and examined for parasites and other pathogens, and proper quarantine measures should be carried out to avoid introducing diseases to a healthy population of reptiles. It is also important for public health officials to have updated population statistics of reptiles kept as pets to advocate policies of good care and hygiene.
Concluding Remarks
Reptile-borne zoonotic pathogens have been generally associated with bacterial infections (e.g., Salmonella). On the other hand, parasitic zoonotic diseases have received little attention in the past decade. Although pentastomiasis and sparganosis are among the main parasitic zoonoses, the role of reptiles as reservoirs and hosts of zoonotic parasites, mainly for VBDs, has not been fully elucidated (see Outstanding Questions). Therefore, the risk of parasites of reptiles with zoonotic potential that could be introduced by invasive species is difficult to estimate given the scarce data available. Furthermore, the recent COVID-19 pandemic has highlighted the risk that anthropic pressures may be exerted on wild-animal populations, resulting in the emergence of new zoonotic diseases [82]. Thus, health authorities, veterinarians, and the general public should be aware of the risks that reptile parasites represent, so that preventative and control measures can be undertaken in all of the levels discussed herein (i.e., environmental, food, captive, or pets). Undoubtedly, multicentric studies monitoring reptile-borne parasitic pathogens are warranted, in order to elucidate the origin of introduced parasites and the role of reptiles as definitive, intermediate, and paratenic hosts in nonendemic areas. This could contribute to reducing the risk of zoonotic transmission and, at the same time, in improving welfare and conservation efforts for these creatures. Finally, as the consumption of reptile meat has increased in recent years, veterinarians should advocate for proper food inspection and the control of parasitic diseases and animal welfare of ectothermic tetrapods. Hence, given all of the above, there is still much crawling to do.
Outstanding Questions.
Do we really know the role of reptiles as reservoirs or sentinels of VBDs?
What is the risk of introducing new exotic parasites with reptiles entering trade directly from the wild?
What is the risk of spill-over of parasites, with zoonotic potential, from invasive reptile species to native fauna?
Which are the most useful parasitic species for the surveillance of illegal trafficking of reptiles?
Should we advocate avoiding consumption of reptile meat given the high zoonotic risk?
Alt-text: Outstanding Questions
Acknowledgments
The authors thank Viviana Domenica Tarallo (Dipartimento di Medicina Veterinaria, Università degli Studi di Bari, Italy) for drawing Figure 1. J.A.M.-R. dedicates this article to his parents (Luz Mery Roldan and Hugo Alfonso Mendoza) and his brothers (Miguel Angel Mendoza-Roldan, Juan Salvador Mendoza-Roldan) for inspiring him in working in this field and for their support and patience in allowing him to have a household full of crawling creatures since his infancy in Barranquilla.
Glossary
- Ophidian
a reptile of the group Ophidia (snakes).
- Plerocercoid
an elongate flat vermiform larval stage of pseudophyllidean tapeworm.
- Poikilothermic
refers to organisms that cannot regulate their body temperature except by using behavioral options such as basking or burrowing. Therefore, their body temperature depends on that of the environment where they live.
- Polyphyletic
refers to a group of organisms derived from more than one common ancestor.
- Reptiliomorpha
a clade of late Paleozoic tetrapods (i.e., four-legged animals) more closely related to amniotes than to amphibians.
- Varanids
any lizards of the saurian family Varanidae (monitor lizards).
Footnotes
Supplemental information associated with this article can be found online at https://doi.org/10.1016/j.pt.2020.04.014.
Supplemental Information
Relevant reference list
References
- 1.Pincheira-Donoso D. Global taxonomic diversity of living reptiles. PLoS One. 2013;8:3. doi: 10.1371/journal.pone.0059741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alves R., Albuquerque U., editors. Ethnozoology: Animals in Our Lives. Academic Press; 2018. [Google Scholar]
- 3.Engler M., Parry-Jones R., editors. Opportunity or Threat: The Role of the European Union in Global Wildlife Trade. TRAFFIC Europe; 2007. [Google Scholar]
- 4.Mitchell M. Zoonotic diseases associated with reptiles and amphibians: an update. Vet. Clin. North. Am. Exot. Anim. Pract. 2011;14:439–456. doi: 10.1016/j.cvex.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Corrente M. Risk for zoonotic Salmonella transmission from pet reptiles: a survey on knowledge, attitudes and practices of reptile-owners related to reptile husbandry. Prev. Vet. Med. 2017;146:73–78. doi: 10.1016/j.prevetmed.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Bower D. A review of the role of parasites in the ecology of reptiles and amphibians. Austral. Ecol. 2019;44:433–448. [Google Scholar]
- 7.Wolfe K. Does urbanization influence the diet of a large snake? Curr. Zool. 2017;64:311–318. doi: 10.1093/cz/zox039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.French S. Town and country reptiles: a review of reptilian responses to urbanization. Integr. Comp. Biol. 2018;58:948–966. doi: 10.1093/icb/icy052. [DOI] [PubMed] [Google Scholar]
- 9.Kelehear C. Invasive parasites in multiple invasive hosts: the arrival of a new host revives a stalled prior parasite invasion. Oikos. 2013;122:1317–1324. [Google Scholar]
- 10.Reboredo-Fernández A. Detection of zoonotic and livestock-specific assemblages of Giardia duodenalis in free-living wild lizards. Rev. Bras. Parasitol. Vet. 2017;26:395–399. doi: 10.1590/S1984-29612017034. [DOI] [PubMed] [Google Scholar]
- 11.Hoyer J. Mammal decline, linked to invasive Burmese python, shifts host use of vector mosquito towards reservoir hosts of a zoonotic disease. Biology. 2017;13:10. doi: 10.1098/rsbl.2017.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller A. Parasite spillover: indirect effects of invasive Burmese pythons. Ecol. Evol. 2018;8:830–840. doi: 10.1002/ece3.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dakubo J. Totemism and the transmission of human pentastomiasis. Ghana Med. J. 2008;42:165. [PMC free article] [PubMed] [Google Scholar]
- 14.Westfall A. Host-specific phenotypic variation of a parasite co-introduced with invasive Burmese pythons. PLoS One. 2019;14:1. doi: 10.1371/journal.pone.0209252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Italiano C. Sarcocystis nesbitti causes acute, relapsing febrile myositis with a high attack rate: description of a large outbreak of muscular sarcocystosis in Pangkor Island, Malaysia, 2012. PLoS Negl. Trop. Dis. 2014;8:5. doi: 10.1371/journal.pntd.0002876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau Y. Sarcocystis nesbitti infection in human skeletal muscle: possible transmission from snakes. Am. J. Trop. Med. Hyg. 2014;90:361–364. doi: 10.4269/ajtmh.12-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fayer R. Human infections with Sarcocystis species. Clin. Microbiol. Rev. 2015;28:295–311. doi: 10.1128/CMR.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yared S. A molecular analysis of sand fly blood meals in a visceral leishmaniasis endemic region of northwestern Ethiopia reveals a complex host–vector system. Heliyon. 2019;5:7. doi: 10.1016/j.heliyon.2019.e02132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosco-Lauth A. Reptiles and amphibians as potential reservoir hosts of Chikungunya virus. Am. J. Trop. Med. Hyg. 2018;98:841–844. doi: 10.4269/ajtmh.17-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsujimoto Y. Necrotizing fasciitis and sepsis caused by Aeromonas hydrophila. Infez. Med. 2019;4:21. [PubMed] [Google Scholar]
- 21.Kwon J. A case of mortality caused by Aeromonas hydrophila in wild-caught red-eyed crocodile skinks (Tribolonotus gracilis) Vet. Sci. 2020;7:4. doi: 10.3390/vetsci7010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnavajhala A. Vector competence of geographical populations of Ornithodoros turicata for the tick-borne relapsing fever spirochete Borrelia turicatae. Appl. Environ. Microbiol. 2018;84:5–18. doi: 10.1128/AEM.01505-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell S. Evaluating the risk of tick-borne relapsing fever among occupational cavers-Austin, TX, 2017. Zoonoses Public Health. 2019;66:579–586. doi: 10.1111/zph.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brianti E. Risk for the introduction of exotic ticks and pathogens into Italy through the illegal importation of tortoises, Testudo graeca. Med. Vet. Entomol. 2010;24:336–339. doi: 10.1111/j.1365-2915.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- 25.Paștiu A. Zoonotic pathogens associated with Hyalomma aegyptium in endangered tortoises: evidence for host-switching behaviour in ticks? Parasit. Vectors. 2012;5:301. doi: 10.1186/1756-3305-5-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Široký P. Hidden threat of tortoise ticks: high prevalence of Crimean-Congo haemorrhagic fever virus in ticks Hyalomma aegyptium in the Middle East. Parasit. Vectors. 2014;7:101. doi: 10.1186/1756-3305-7-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barradas P. Pathogenic Rickettsia in ticks of spur – thighed tortoise (Testudo graeca) sold in a Qatar live animal market. Transbound. Emerg. Dis. 2019;67:461–465. doi: 10.1111/tbed.13375. [DOI] [PubMed] [Google Scholar]
- 28.Široký P. Tortoise tick Hyalomma aegyptium as long term carrier of Q fever agent Coxiella burnetii – evidence from experimental infection. Parasitol. Res. 2010;107:1515–1520. doi: 10.1007/s00436-010-2037-1. [DOI] [PubMed] [Google Scholar]
- 29.Novakova M. Rickettsial infections in ticks from reptiles, birds and humans in Honduras. Ticks Tick Borne Dis. 2015;6:737–742. doi: 10.1016/j.ttbdis.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Mediannikov O. Rickettsia africae, western Africa. Emerg. Infect. Dis. 2010;16:571. doi: 10.3201/eid1603.090346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burridge M., Simmons L. Exotic ticks introduced into the United States on imported reptiles from 1962 to 2001 and their potential roles in international dissemination of diseases. Vet. Parasitol. 2003;113:289–320. doi: 10.1016/s0304-4017(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 32.Unsworth N. Flinders Island spotted fever rickettsioses caused by 'marmionii' strain of Rickettsia honei, Eastern Australia. Emerg. Infect. Dis. 2007;13:566. doi: 10.3201/eid1304.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiley H. Rickettsia detected in the reptile tick Bothriocroton hydrosauri from the lizard Tiliqua rugosa in South Australia. Pathogens. 2016;5:41. doi: 10.3390/pathogens5020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reeves W. A spotted fever group Rickettsia from an exotic tick species, Amblyomma exornatum (Acari: Ixodidae), in a reptile breeding facility in the United States. J. Med. Entomol. 2006;43:1099–1101. doi: 10.1603/0022-2585(2006)43[1099:asfgrf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 35.Mendoza-Roldan J. Borrelia burgdorferi (sensu lato) in ectoparasites and reptiles in southern Italy. Parasit. Vectors. 2019;12:35. doi: 10.1186/s13071-019-3286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bravo-Barriga D. First molecular detection of Leishmania tarentolae-like DNA in Sergentomyia minuta in Spain. Parasitol. Res. 2016;115:1339–1344. doi: 10.1007/s00436-015-4887-z. [DOI] [PubMed] [Google Scholar]
- 37.Campino L. The first detection of Leishmania major in naturally infected Sergentomyia minuta in Portugal. Mem. Inst. Oswaldo Cruz. 2013;108:516–518. doi: 10.1590/0074-0276108042013020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pereira S. First molecular detection of Leishmania infantum in Sergentomyia minuta (Diptera, Psychodidae) in Alentejo, southern Portugal. Acta Trop. 2017;174:45–48. doi: 10.1016/j.actatropica.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Latrofa M. Detection of Leishmania infantum DNA in phlebotomine sand flies from an area where canine leishmaniosis is endemic in southern Italy. Vet. Parasitol. 2018;253:39–42. doi: 10.1016/j.vetpar.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J. Molecular detection, identification and phylogenetic inference of Leishmania spp. in some desert lizards from Northwest China by using internal transcribed spacer 1 (ITS1) sequences. Acta Trop. 2016;162:83–94. doi: 10.1016/j.actatropica.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J. Pathogenic Leishmania spp. detected in lizards from Northwest China using molecular methods. BMC Vet. Res. 2019;15:446. doi: 10.1186/s12917-019-2174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valencia-Aguilar A. Ecosystem services provided by amphibians and reptiles in Neotropical ecosystems. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2013;9:257–272. [Google Scholar]
- 43.Greiner E. Coccidiosis in reptiles. Semin. Avian Exot. Pet Med. 2003;12:49–56. [Google Scholar]
- 44.Patro S., Padhi S. Saltwater crocodile and human conflict around Bhitarkanika National Park, India: a raising concern for determining conservation limits. Ocean Coast. Manag. 2019;182:104923. [Google Scholar]
- 45.Hossain M. Food consumption of saltwater crocodile (Crocodylus porosus) in a reptile farm of Bangladesh. Bangladesh J. Zool. 2013;41:173–179. [Google Scholar]
- 46.Bulte E. Resource intensity, institutions, and development. World Dev. 2005;33:1029–1044. [Google Scholar]
- 47.Magnino S. Biological risks associated with consumption of reptile products. Int. J. Food Microbiol. 2009;134:163–175. doi: 10.1016/j.ijfoodmicro.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Kwon J., Kim J. Sparganosis presenting as a conus medullaris lesion: case report and literature review of the spinal sparganosis. Arch. Neurol. 2004;61:1126–1128. doi: 10.1001/archneur.61.7.1126. [DOI] [PubMed] [Google Scholar]
- 49.Presti A. Cerebral sparganosis: case report and review of the European cases. Acta Neurochir. 2015;157:1339–1343. doi: 10.1007/s00701-015-2466-9. [DOI] [PubMed] [Google Scholar]
- 50.Liu L. Serodiagnosis of sparganosis by ELISA using recombinant cysteine protease of Spirometra erinaceieuropaei spargana. Parasitol. Res. 2015;114:753–757. doi: 10.1007/s00436-014-4270-5. [DOI] [PubMed] [Google Scholar]
- 51.Anantaphruti M. Human sparganosis in Thailand: an overview. Acta Trop. 2011;118:171–176. doi: 10.1016/j.actatropica.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 52.Liu Q. Human sparganosis, a neglected food borne zoonosis. Lancet Infect. Dis. 2015;15:1226–1235. doi: 10.1016/S1473-3099(15)00133-4. [DOI] [PubMed] [Google Scholar]
- 53.Barratt J. Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology. 2016;143:1087–1118. doi: 10.1017/S0031182016000652. [DOI] [PubMed] [Google Scholar]
- 54.Prociv P. Neuro-angiostrongyliasis: unresolved issues. Int. J. Parasitol. 2000;30:1295–1303. doi: 10.1016/s0020-7519(00)00133-8. [DOI] [PubMed] [Google Scholar]
- 55.Parameswaran K. Case series of eosinophilic meningoencephalitis from South India. Ann. Indian Acad. Neurol. 2006;9:217. [Google Scholar]
- 56.Johny J. Eosinophilic meningitis caused by consumption of meat of monitor lizard (Varanus bengalensis) Neurol. India. 2018;66:1166. doi: 10.4103/0028-3886.237031. [DOI] [PubMed] [Google Scholar]
- 57.Pozio E. Trichinella papuae and Trichinella zimbabwensis induce infection in experimentally infected varans, caimans, pythons and turtles. Parasitology. 2004;128:333. doi: 10.1017/s0031182003004542. [DOI] [PubMed] [Google Scholar]
- 58.Pozio E. Trichinella papuae in saltwater crocodiles (Crocodylus porosus) of Papua New Guinea. Emerg. Infect. Dis. 2004;10:1507–1509. doi: 10.3201/eid1008.040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paré J. An overview of pentastomiasis in reptiles and other vertebrates. J. Exot. Pet Med. 2008;17:285–294. [Google Scholar]
- 60.Chen S.-H. Multi-host model-based identification of Armillifer agkistrodontis (Pentastomida), a new zoonotic parasite from China. PLoS Negl. Trop. Dis. 2010;4:647. doi: 10.1371/journal.pntd.0000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.du Plessis V. Pentastomiasis (Armillifer armillatus infestation) S. Afr. Med. J. 2007;97:928–930. [PubMed] [Google Scholar]
- 62.Tappe D., Büttner D. Diagnosis of human visceral pentastomiasis. PLoS Negl. Trop. Dis. 2009;3 doi: 10.1371/journal.pntd.0000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tappe D. Imported Armillifer pentastomiasis: Report of a symptomatic infection in The Netherlands and mini-review. Travel Med. Infect. Dis. 2014;12:129–133. doi: 10.1016/j.tmaid.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 64.Tappe D. Transmission of Armillifer armillatus ova at snake farm, The Gambia, West Africa. Emerg. Infect. Dis. 2011;17:251. doi: 10.3201/eid1702.101118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yapo H. Human pentastomiasis discovered post-mortem. Forensic Sci. Int. 2003;137:52–54. doi: 10.1016/s0379-0738(03)00281-0. [DOI] [PubMed] [Google Scholar]
- 66.Burns-Cox C. Porocephaliasis in Western Malaysia. Trans. R. Soc. Trop. Med. Hyg. 1969;63:409–411. doi: 10.1016/0035-9203(69)90021-2. [DOI] [PubMed] [Google Scholar]
- 67.Pantchev N., Tappe D. Pentastomiasis and other parasitic zoonoses from reptiles and amphibians. Berl. Munch. Tierarztl. 2011;124:528–535. [PubMed] [Google Scholar]
- 68.Rataj A. Parasites in pet reptiles. Acta Vet. Scand. 2011;53:33. doi: 10.1186/1751-0147-53-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schuppli C. Welfare of non-traditional pets. Rev. Sci. Tech. 2014;33:221–231. doi: 10.20506/rst.33.1.2287. [DOI] [PubMed] [Google Scholar]
- 70.Pasmans F. Future of keeping pet reptiles and amphibians: towards integrating animal welfare, human health and environmental sustainability. Vet. Rec. 2017;181:7. doi: 10.1136/vr.104296. [DOI] [PubMed] [Google Scholar]
- 71.Cervone M. Internal and external parasitic infections of pet reptiles in Italy. J. Herpetol. Med. Surg. 2016;26:122–130. [Google Scholar]
- 72.Chomel B. Wildlife, exotic pets, and emerging zoonoses. Emerg. Infect. Dis. 2007;13:6. doi: 10.3201/eid1301.060480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mader D., Divers S., editors. Current Therapy in Reptile Medicine and Surgery. Elsevier Health Sciences; 2013. [Google Scholar]
- 74.Nowak M. The international trade in reptiles (Reptilia) – the cause of the transfer of exotic ticks (Acari: Ixodida) to Poland. Vet. Parasitol. 2010;169:373–381. doi: 10.1016/j.vetpar.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 75.Traversa D. Cryptosporidium from tortoises: genetic characterisation, phylogeny and zoonotic implications. Mol. Cell. Probe. 2008;22:122–128. doi: 10.1016/j.mcp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 76.Rinaldi L. Prevalence and molecular identification of Cryptosporidium isolates from pet lizards and snakes in Italy. Parasite. 2012;19:437. doi: 10.1051/parasite/2012194437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pedraza-Díaz S. Molecular characterisation of Cryptosporidium isolates from pet reptiles. Vet. Parasitol. 2009;160:204–210. doi: 10.1016/j.vetpar.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 78.Díaz P. Cryptosporidium in pet snakes from Italy: molecular characterization and zoonotic implications. Vet. Parasitol. 2013;197:68–73. doi: 10.1016/j.vetpar.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 79.Galecki R. Tongue worm (Pentastomida) infection in ball pythons (Python regius) – a case report. Ann. Parasitol. 2016;62:363–365. doi: 10.17420/ap6204.76. [DOI] [PubMed] [Google Scholar]
- 80.Lyons J., Natusch D. AC28 Inf. Vol. 9. IUCN-SSC Boa and Python Specialist Group; 2015. Methodologies for differentiating between wild and captive-bred CITES-listed snakes; p. 35. [Google Scholar]
- 81.Wolf D. Diagnosis of gastrointestinal parasites in reptiles: comparison of two coprological methods. Acta Vet. Scand. 2014;56:44. doi: 10.1186/s13028-014-0044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Decaro N. COVID-19 from veterinary medicine and One Health perspectives: What animal coronaviruses have taught us. Res. Vet. Sci. 2020;131:21–23. doi: 10.1016/j.rvsc.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tappe D., Warrell D. Pentastomiasis. In: Ryan E.T., editor. Hunter's Tropical Medicine and Emerging Infectious Diseases. Elsevier; 2019. pp. 1030–1032. [Google Scholar]
- 84.Boomker J. Eustrongylides sp. (Nematoda: Dioctophymatoidea) from the stomach of a Nile crocodile, Crocodylus niloticus Laurenti, 1768, in Botswana: research communication. Onderstepoort J. Vet. Res. 2006;73:315–317. [PubMed] [Google Scholar]
- 85.Nawa Y. Ocular gnathostomiasis – update of earlier survey. Am. J. Trop. Med. Hyg. 2017;97:1232–1234. doi: 10.4269/ajtmh.17-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eamsobhana P. Review paper eosinophilic meningitis caused by Angiostrongylus cantonensis – a neglected disease with escalating importance. Trop. Biomed. 2014;31:569–578. [PubMed] [Google Scholar]
- 87.Pauwels O., Pantchev N. Risks for human health related to invasive alien reptiles and amphibians. In: Mazza G., Tricarico E., editors. Invasive Species and Human Health. CABI International; 2018. pp. 108–119. [Google Scholar]
- 88.Sánchez-Montes S. Rickettsia species in ticks that parasitize amphibians and reptiles: novel report from Mexico and review of the worldwide record. Ticks Tick Borne Dis. 2019;10:987–994. doi: 10.1016/j.ttbdis.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 89.Amanatfard E. Human dermatitis caused by Ophionyssus natricis, a snake mite. Iran. J. Parasitol. 2014;9:594. [PMC free article] [PubMed] [Google Scholar]
- 90.Mendoza-Roldan J. Mites and ticks of reptiles and amphibians in Brazil. Acta Trop. 2020 doi: 10.1016/j.actatropica.2020.105515. Published online May 11, 2020. [DOI] [PubMed] [Google Scholar]
- 91.Shaw G. End of Cretaceous extinction: the end of the dinosaurs. In: Shaw G., editor. Great Moments in the History of Life. Springer; 2018. pp. 65–68. [Google Scholar]
- 92.Mohabey D., Samant B. Cretaceous–paleogene transition of reptilian tetrapods across deccan volcanism in India. Open J. Geol. 2019;9:639. [Google Scholar]
- 93.Uetz P. A global catalogue of primary reptile type specimens. Zootaxa. 2019;4695:438–450. doi: 10.11646/zootaxa.4695.5.2. [DOI] [PubMed] [Google Scholar]
- 94.European Food Safety Authority (EFSA) Public health risks involved in the human consumption of reptile meat – Scientific Opinion of the Panel on Biological Hazards. EFSA J. 2007;5:578. [Google Scholar]
- 95.da Nóbrega A. Reptiles used in traditional folk medicine: conservation implications. Biodivers. Conserv. 2008;17:2037–2049. [Google Scholar]
- 96.Robinson J. Dynamics of the global trade in live reptiles: Shifting trends in production and consequences for sustainability. Biol. Conserv. 2015;184:42–50. [Google Scholar]
- 97.Tavasoli E. Hyalomma aegyptium on spur-thighed tortoise (Testudo graeca) in Urmia Region West Azerbaijan, Iran. Iran. J. Parasitol. 2007;2:40–47. [Google Scholar]
- 98.Vatansever Z. Ticks biting humans in the urban area of Istanbul. Parasitol. Res. 2008;102:551–553. doi: 10.1007/s00436-007-0809-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relevant reference list