To the Editor
A novel coronavirus (SARS CoV-2) spread in China in December 2019, becoming soon a relevant problem of international public health concern [1]. In Italy the SARS-CoV-2 officially spread around the 20th of February 2020 and the country became the first in Europe to register a high number of infections and deaths. The beta-coronavirus mainly creates a severe acute respiratory syndrome (COVID-19), with fever, cough, fatigue, pneumonia and acute respiratory distress syndrome, eventually. The patient management mainly focuses on supportive care: oxygenation, fluid management, and treatments with multiple drugs as antiviral therapies, chloroquine or hydroxyichloroquine, antibiotics, steroids, nonsteroidal anti-inflammatory drugs, bronchodilators and immunosuppressive drugs. Many patients require invasive ventilation, whereas others are treated with non-invasive ventilation (NIV) support or C-PAP (Continuous Positive Airway Pressure). In the available studies, COVID-19 patients showed alterations of coagulation test, with significant increase of D-Dimer levels associated with severity of illness and adverse outcomes [2]. Besides, a high risk for venous thromboembolism has been recently highlighted with high prevalence of symptomatic acute pulmonary embolism and deep vein thrombosis in patients [3]. Therefore, currently low molecular weight heparin (LMWH) has become part of the clinical management of the hospitalized COVID-19 patients, even if evidences about the right prophylactic dose are still lacking. In this scenario, we describe two cases of spontaneous abdominal internal bleeding in hospitalized patients with bilateral interstitial pneumonia and SARS-CoV-2 throat swab positive, supported with C-PAP ventilation, as the invasive ventilation was not recommended for both.
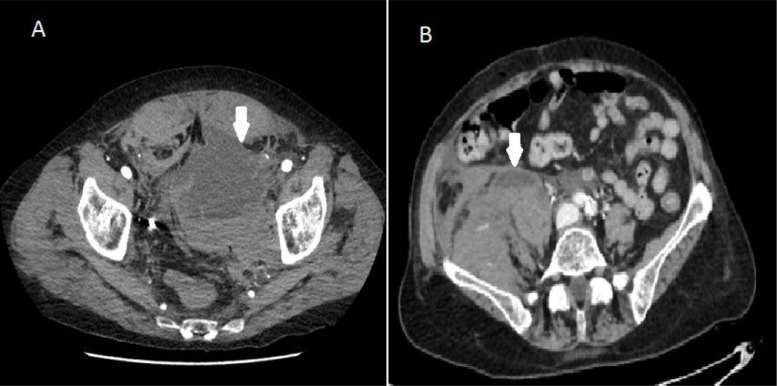
The first patient, a 76-year-old man, was supported with C-PAP helmet, with PEEP 12.5 and FiO2 50%. The comorbidity were arterial hypertension and chronic ischemic heart disease. He was treated with antiviral drugs, azythromicine, steroids and LMWH 6000 UI daily. Suddenly, after 7 days from the admission to the hospital, he started complaining of severe abdominal pain, the blood pressure decreased to 80/60 mmHg and the blood test showed Hb 8.6 g/dl (from 12 g/dl), fibrinogen of 324 mg/dl; normal protrombine time (PT) and platelet count (PTL). The abdominal CT scan demonstrated a large pelvic blood collection anterior to the left ileo-psoas muscle (size. 9×13 cm) (Fig. 1 A). The lesion showed enhanced contrast tardive spot of 10 mm, above the ischiopubic branch, as sign of active arterial bleeding. The second case refers to a 72-year-old woman with severe respiratory insufficiency, treated with C-PAP helmet, PEEP 12 cm H2O, FiO2 60%. The comorbidities were arterial hypertension and anxious syndrome. During a sonography performed to place a CVC, a venous femoral thrombosis was detected; LMWH at therapeutic dose 100UI/kg/BID was promptly started. Suddenly, after 10 days from the admission, similarly to the first case we described, the patient complained of severe abdominal pain, with clinical signs of hemorrhagic shock. CT scan showed large pelvic blood collection (size:16×10 cm) not dissociable from the right ileo-psoas muscle (Fig. 1B), with two late enhanced spots, both of 5 mm, as sign of active bleeding. Hemoglobin was 8.1 g/dl (from 11 g/dl). PTL, PT and PTT were normal. Heparin concentration in the blood was not available.
Fig. 1.
Computed Tomography image of 9×13 cm (A) and 16×10 cm (B) abdominal hematoma.
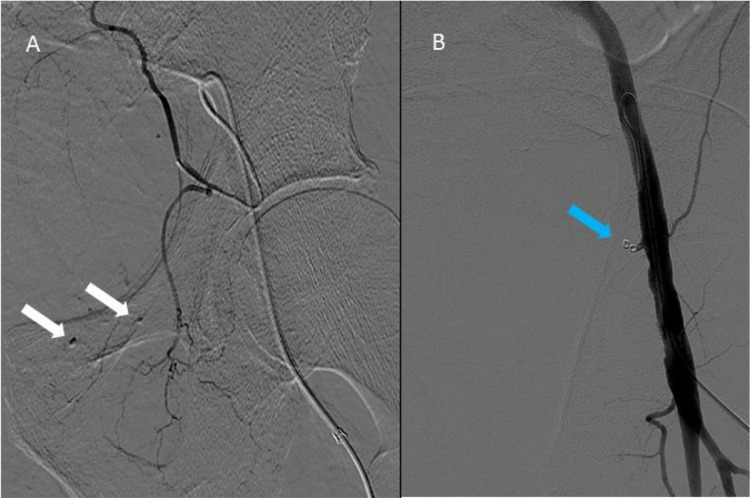
The two patients were both treated with interventional radiology. The male received radiological embolization of the left epigastric inferior artery using the insertion of coil (Contour 150-250 micron) (Fig. 2 A). However, in the following days the bleeding continued, confirmed by the imaging, with a precarious hemodynamical balance. Therefore, a second embolization, occluding completely the epigastric inferior artery by mechanical spirals (Fig. 2B), was mandatory. The woman received radiological embolization of the right epigastric inferior artery, with the insertion of coil as well. In the following days, similarly, the bleeding apparently continued, as the hemoglobin levels did not increase, despite 3 Units red blood cell transfusion. However, the lack of enlargement of the lesion at imaging, the hemodynamic stability and the need to transfer the patient to a different hospital for a possible second embolization session, led to the successfully choice of a conservative treatment, with local abdominal compression and cryotherapy.
Fig. 2.
Angiographic image of left inferior epigastric artery bleeding (A) and after treatment with mechanical spirals (B).
The genesis of the bleeding of inferior epigastric artery appears unclear. However, many different factors need to be considered. Firstly, the presence of the cough, which is a common symptom of the COVID-19, could have led to a relevant increase in the abdominal pressure and, therefore, to the arterial rupture with consequent bleeding. In literature there are some description of intercostal arterial rupture [4] and a rare case of a gastroduodenal artery rupture after severe cough [5]. Secondly, all the patients were supported with C-PAP. During the SARS COV-1 epidemic in 2003, spontaneous pneumomediastinum and pneumothorax were reported in patients treated with NIV or C-PAP. The incidence of NIV-associated barotrauma in a study on SARS COV-1 patients ranged from 6.6% to 15% [6]. However, up to now, no data are available about the possible association of NIV or C-PAP and abdominal internal bleeding. Importantly, the two patients had been treated with LMWH at different dosages, both prophylactic and therapeutic. Regarding the available data about spontaneous pelvic hematomas, the ileo-psoas hematoma is a potentially lethal condition that can arise during hospital stay. In an ICU cohort of 40 patients the mortality rate was of 50% following this complication (versus a general mortality rate of 22% for the patients without psoas hematoma, over the same period of time) [7]. However, a large number of patients are treated worldwide with LMWH, above all hospitalized and post-operative patients. Nevertheless, only few studies reported cases of ileo-psoas hematomas, usually occurring as a result of trauma, iatrogenic etiology, rupture of the aortic aneurysm, and hematologic diseases, and no study reported cases of inferior epigastric artery rupture with blood pelvic collection, that therefore can be considered very rare [8]. Moreover, the diffuse microvascular damage was described as an important cause of death in critically ill patients with COVID-19, and it has been related to the “cytokine storm” syndrome caused by immune disorder [2,9]. Again, a study [2] on the coagulation pattern of the COVID-19 in China found that, next to an increased risk of thrombosis, patients seem to have an increased risk of bleeding as well, due to imbalances in platelet production and disruption, and disorders of the coagulation system which need to be further better clarified. In addition, in a study on the autopsy results, pulmonary thrombosis and hemorrhagic lesions were found to be mixed in patients with COVID-19 [9].
Hence, COVID-19 is a very new disease, with systemic involvement. Abnormal coagulation findings, including thrombocytopenia, elevated D-dimer, prolonged PT and disseminated intravascular coagulation, have been described [10]. If on one hand the protrombotic features have been underlined and few reports about minor or obscure/occult GI bleeding appeared in literature, up to now no major internal bleeding have been observed in COVID-19 patients. To our knowledge, our notification is the first one reporting two cases of a major hemorrhagic complication in severe COVID-19, apparently without other predisposing causes. Further studies are therefore needed to investigate these aspects.
Declaration of Competing Interest
None
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. Feb 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Li D., Wang X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang T., Chen R., Liu C., Liang W., Guan W., Tang R. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol. 2020 doi: 10.1016/S2352-3026(20)30109-5. Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang J.Y., Lim Y.S., Woo J.H. Spontaneous rupture of intercostal artery after severe cough. Am J Emerg Med. 2015;33(1):131. doi: 10.1016/j.ajem.2014.06.033. Jane1-3. [DOI] [PubMed] [Google Scholar]
- 5.Lee C.H., Lan C.C., Wang C.C. Spontaneous rupture of gastroduodenal artery pseudoaneurysm following vigorous cough. Am J Gastroenterol. 2009;104(2):529–530. doi: 10.1038/ajg.2008.52. Feb. [DOI] [PubMed] [Google Scholar]
- 6.Yam L.Y., Chen R.C., Zhong N.S. SARS: ventilatory and intensive care. Respirology. 2003;Nov; 8 Suppl(Supp l):S31–S35. doi: 10.1046/j.1440-1843.2003.00521.x. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Artzner T., Clere-Jehl R., Schenck M. Spontaneous ilio-psoas hematomas complicating intensive care unit hospitalizations. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0211680. Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo J.G., Yang J.C., Kim T.V., Park K.H. Intramuscular hematoma on the psoas muscle. Korean J Neurotrauma. 2019;15(2):234–238. doi: 10.13004/kjnt.2019.15.e29. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang H., Liu L., Guo T. Improving the safety of CAR-T cell therapy by controlling CRS-related coagulopathy. Ann Hematol. 2019;98(7):1721–1732. doi: 10.1007/s00277-019-03685-z. [DOI] [PubMed] [Google Scholar]
- 10.Giannis D., Ziogas I.A., Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104362. Apr 9. [DOI] [PMC free article] [PubMed] [Google Scholar]