Abstract
Recent studies have shown that statins and Metformin may have beneficial effects on seizure through different mechanisms. In the current study, we investigated whether Metformin, Atorvastatin, and concomitant uses of them have beneficial effects on pentylenetetrazole (PTZ)-induced kindling. Adult male C57BL/6 mice were randomly divided into four experimental groups with seven mice in each group. Group 1, control group; group 2, received Metformin (200 mg/kg, i.p); group 3, received Atorvastatin (10 mg/kg, i.p.); group 4, received Atorvastatin (10 mg/kg, i.p.) plus Metformin (200 mg/kg, i.p.). Twenty minutes after injection of the mentioned drugs, the experimented mice received 37/5 mg/kg of PTZ intraperitoneally on alternating days. Then the convulsive behavior signs were evaluated for 20 min after each PTZ injection. There were significant differences in the stage 2 latency parameter among group 2 (p = 0.033, F = 8.46)/group 3 (p = 0.032, F = 10.42)/group 4 (p = 0.008, F = 24.57) as compared to the control group, while no significant differences were found comparing only group 2,3, and 4 with eachother excluding the control group. Pretreatment with Atorvastatin (p = 0.002, F = 33), Atorvastatin + Metformin (p = 0.006, F = 20.77), and Metformin alone increased stage 5 latency as compared to the PTZ group, significantly. Also, our results have shown that pretreatment with Atorvastatin (p = 0.013, F = 14.48), Metformin (p = 0.015, F = 16.67), and concomitant usage of them significantly decreased stage 5 duration as compared to the control group. Our findings clearly demonstrate that concomitant use of Metformin and Atorvastatin has no more protective effect against the development of kindling as compare to these drugs alone. Thus, we concluded that, these drugs may inhibit kindling via a similar mechanism and we suggested that it is probably through regulation of autophagy.
Keywords: Biochemistry, Toxicology, Pharmacology, Clinical toxicology, Medical ethics, Atorvastatin, Metformin, Pentylenetetrazole kindling, Seizure
Biochemistry; Toxicology; Pharmacology; Clinical Toxicology; Medical Ethics; Atorvastatin; Metformin; Pentylenetetrazole kindling; seizure
1. Introduction
Epilepsy is a common chronic neurological disorder that annually, about 50,000 to 100,000 new cases of it are reported and there are an estimated at least sixty-five million worldwide people living with this disease [1]. Approximately 30–40% of epileptic patients have shown refractoriness to medications [2].
Large body of evidence shows that statins are very effective in decreasing neuroinflammation, oxidative stress, neurotoxicity, and declining nitric oxide (NO). Neuroprotective effect of statins is really efficient in improving various life threatening conditions including brain injury, stroke, and cerebral ischemia [3]. Recent studies have shown that Atorvastatin has a protective role in seizure and this effect is independent from cholesterol-lowering properties [4, 5, 6]. Lee et al. have indicated that Atorvastatin inhibits kainic acid-induced seizure, and hippocampal cell death [7]. Several mechanisms and pathways have been proposed for anticonvulsant effects of statins, such as; regulating the glycogen synthase kinase-3β (GSK-3β) pathway [8], inhibition of neuroinflammation [9], modulating hippocampal levels of dopamine, glutamate, and gamma-aminobutyric acid (GABA) [10].
Metformin, an oral antidiabetic drug, is another medicine that has a protective effect against seizures, memory, learning injuries, and oxidative damage [11]. It has been reported that reactive oxygen species (ROS) may have a vital function in the development and progression of epilepsy [12], hence, antioxidant agents have been recommended as new therapeutic medications for the treatment of epilepsy. The previous study revealed that Metformin could decrease ROS [13], and inflammation [14]. In addition, Metformin has a beneficial effect on the antioxidant defense system by inducing Nrf2 [nuclear factor (erythroid-derived 2)-like 2; NFE2L2] target gene activation [15], upregulating the uncoupled proteins 2 (UCP2) [16], and improving blood-brain barrier (BBB) function by activating AMP-activated protein kinase (AMPK) [14, 17]. The previous study indicated that BBB leakage occurs during epileptogenesis and lead to the progression of epilepsy [18], and an increase in the BBB permeability was observed in mice with generalized convulsive seizures induced by acute pentylenetetrazole (PTZ) injection [19].
Despite of the potential benefits of Metformin and Atorvastatin, only a few studies have been conducted on the anticonvulsant effect of these drugs. Therefore, to further investigate the impact of Metformin and Atorvastatin on seizure activity, in the current study, we investigated whether Metformin and Atorvastatin treatment has any favourable impression on seizure induced by PTZ in the most widely-accepted animal model. This way we tried to understand the process of epileptogenesis and discover novel compounds with anticonvulsant activity.
2. Materials and methods
2.1. Animals, drugs, and chemicals
Adult male C57BL/6 mice, weighing 20 ± 2 g (6–8 weeks old) were used in this study. They were kept under controlled light and condition (12:12 h light/dark cycle, 25±1C, 55% relative humidity) with free access to water and food. All experimental procedures were approved by the Ethics Committee of the Golestan University of Medical Sciences (No. IR.GOUMS.REC.1394.89).
Atorvastatin, Metformin, and PTZ were purchased from Sigma-Aldrich, Germany. Metformin and PTZ were dissolved in physiological saline and Atorvastatin was dissolved in 40% dimethyl sulfoxide (DMSO), and 60 % physiological saline. All drugs were prepared freshly prior to the injections.
2.2. Induction of kindling and design of the experiment
For induction of kindling a subconvulsive dose of PTZ (40 mg/kg, i.p.) was injected intraperitoneally (i.p.) every 48 h.The injections were repeated until all of the mice showed the full kindling state [20]. After PTZ injection, the convulsive behavior was observed for a period of 20 minuts [20, 21]. Seizure stage was evaluated using the following scale: stage 0, no response; stage 1, ear and facial twitching; stage 2, convulsive waves axially through the body; stage 3, myoclonic jerks and rearing; stage4, clonic convulsions with the animal falling on its sides; and stage 5, repeated severe tonic-clonic or lethal convulsions [11, 22, 23]. Stage 2 and 5 latencies (S2L, S5L), stage 5 duration (S5D), and seizure stage (SS) were evaluated during the experiment.
The animals were randomly divided into four experimental groups with seven mice in each group. Group 1, control group, received PTZ (40 mg/kg, i.p.) [20]; group 2, received Metformin (200 mg/kg, i.p) [11]; group 3, received Atorvastatin (10 mg/kg, i.p.) [6]; group 4, received Atorvastatin (10 mg/kg, i.p.) + Metformin (200 mg/kg, i.p.). Mice in the last three groups received PTZ twenty minutes after pretreatment.
2.3. Statistical analysis
Statistical significance was carried out using repeated-measures ANOVA and one-way ANOVA for multi-group comparisons. Tukey test was used for the post hoc comparison. Differences were considered significant where p < 0.05.
3. Results
According to the present study, Metformin, Atorvastatin, and concomitant use of them showed conspicious anticonvulsant and antiepileptogenic effects, while there are no significant differences between the experimented groups.
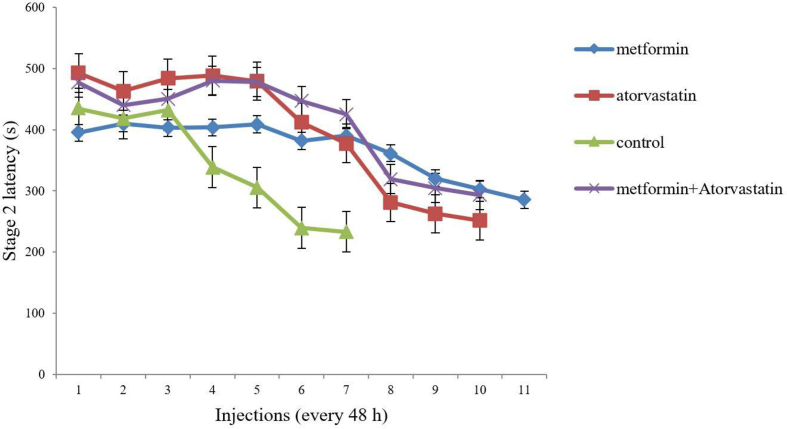
As shown in Figure 1, there were significant differences in S2L between group 2 (p = 0.033, F = 8.46)/group 3 (p = 0.032, F = 10.42)/group 4 (p = 0.008, F = 24.57) as compared to the control group, however, no considerable differences were seen among groups 2, 3, and 4 excluding the control group.
Figure 1.
Comparison of the effect of ‘Metformin’, ‘Atorvastatin’ and ‘Metformin + Atorvastatin’ treatments on stage 2 latency in PTZ kindling mice. Values are expressed as mean ± SD (n = 7 in each group), and are shown for each injection (⁄p < 0.05, ⁄⁄p < 0.01).
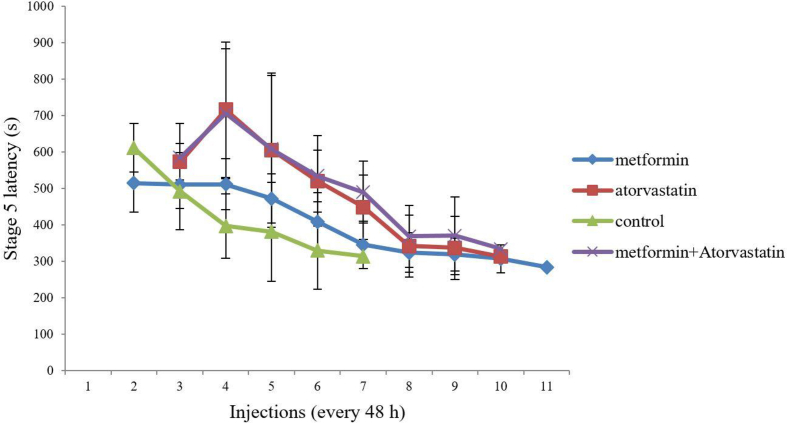
As shown in Figure 2, pretreatment with Atorvastatin (p = 0.002, F = 33), Metformin, or combination of them (p = 0.006, F = 20.77) increased S5L as compared to the control group, significantly.
Figure 2.
Comparison of the effect of ‘Metformin’, ‘Atorvastatin’ and ‘Metformin + Atorvastatin’ treatments on stage 5 latency in PTZ kindling mice. Values are expressed as mean ± SD (n = 7 in each group), and are shown for each injection (⁄p < 0.05, ⁄⁄p < 0.01).
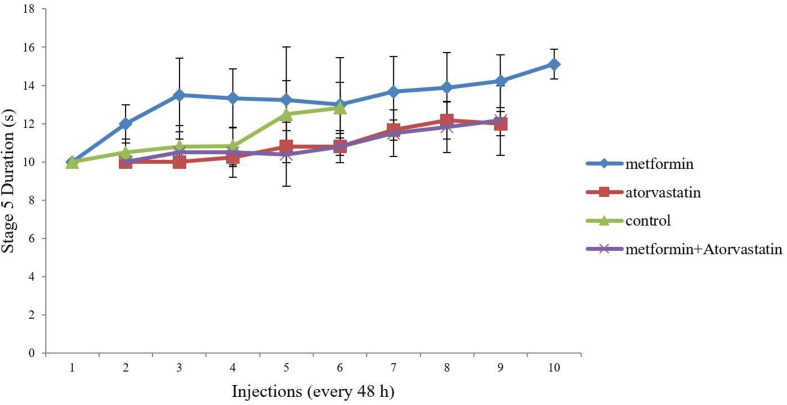
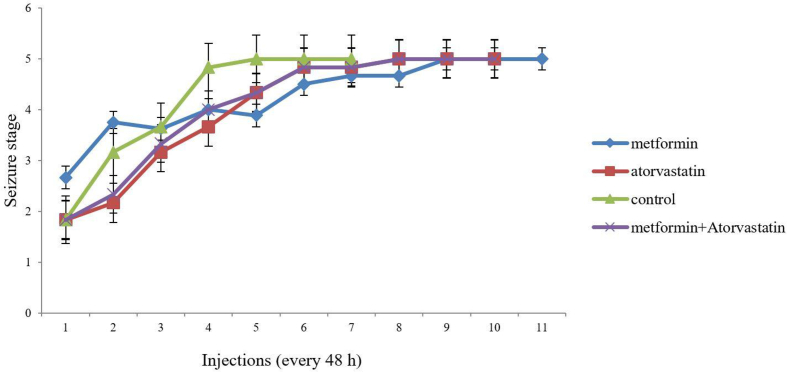
Similarly, pretreatment with Atorvastatin (p = 0.013, F = 14.48), Metformin (p = 0.015, F = 16.67) and concomitant use of them significantly decreased S5D as compared to the control group (Figure 3). Despite of the previous mentioned results, our studies showed pretreatment with these drugs has no significant effect on SS (Figure 4).We need to mention that our pretreated groups received more injections to become full-kindle as compared to the control group (11 vs 7).
Figure 3.
Comparison of the effect of ‘Metformin’, ‘Atorvastatin’ and ‘Metformin + Atorvastatin’ treatments on stage 5 duration in PTZ kindling mice. Values are expressed as mean ± SD (n = 7 in each group), and are shown for each injection (⁄p < 0.05, ⁄⁄p < 0.01).
Figure 4.
Comparison of the effect of ‘Metformin’, ‘Atorvastatin’ and ‘Metformin + Atorvastatin’ treatments on seizure stage in different injections in PTZ kindling mice. Values are expressed as mean ± SD (n = 7 in each group), and are shown for each injection (⁄p < 0.05, ⁄⁄p < 0.01).
4. Discussion
Earlier studies demonstrated that Metformin can cross the BBB and have anti-inflammatory, antioxidant [24], and neuroprotective [25] properties. Besides, it is reported that Metformin has beneficial effects on neurological disorders [24, 26, 27]. Metformin could directly and indirectly modulate some signaling pathways like Sonic hedgehog (Shh), bone morphogenetic protein (BMP), and atypical protein kinase C (aPKC) [28, 29, 30]. In a research done by Wing and his colleagues, it was shown that aPKCs ζ and ι play a critical role in the development of embryonic precursors. They also showed that PKCs-CBP is essential for human forebrain neurogenesis. Their invivo study demonstrated that Metformin enhances neurogenesis in a CBP-dependent fashion in adult mice [24]. In another study, Dadwal et al. developed a cell replacement strategy. They found that, administration of Metformin in hypoxia-ischemia (H/I) injury model activates endogenous neural progenitor cells (NPCs) [31]. It is proven that, Shh and BMP signaling pathways have a very important role in neurogenesis [32, 33]. Gajera and his team have stated that, LRP2 receptor in BMP signaling pathway is so vital to coordinate the specification of proliferative and non-proliferative cells fates and Loss of LRP2 activity suppresses progenitor cell proliferation, hence, neurogenic output is decreased in subependymal zone (SEZ) of the brain which is considered as the stem cell niche [34]. In another paper, Ding et al. have concluded that Shh pathway contributes to brain plasticity and following functional recovery after treating the stroke by bone marrow stromal cells in mice [35]. Consequently, it could be elucidated that Metformin which is used as an efficient drug in modulating signaling pathways such as BMP, Shh, and aPKC can induce neurogenesis.
Since free radicals and reactive oxygen species are supposed to mediate the seizure development, researches on antiepileptic compounds modulating signaling pathways such as BMP, Shh, and aPKC with antioxidant and neuroprotective effects have been considered [11].
In the present study, we found that Metformin significantly increased S2L and S5L parameters as compared to the control group. Moreover, the pretreatment with Metformin. elevated the number of injections to reach full kindling.Therefore, anticonvulsant activity of Metformin in epilepsy progression by these results was proven. Previous studies demonstrated that BBB dysfunction occurs during epileptogenesis and contributes to the development of epilepsy [18], and it is known that BBB permeability was increased in mice with generalized convulsive seizures induced by acute PTZ [19]; meanwhile, it has been reported that Metformin can have considerable therapeutic benefits to prevent the development and formation of BBB disruption [17]. Furthermore, studies have proved that Metformin could activate AMPK, an important sensor of energy balance [36]. Metformin could promote neurogenesis and improve spatial memory function, by activation of AMPK pathway [28]. Therefore, the beneficial effect of Metformin on epilepsy could be modulated by AMPK pathway and it could prevent the increment of BBB disruption.
Atorvastatin is categorized as a class of Hypolipidemic statins. several studies have shown neurogenesis effect and protective role of atorvastatin in the seizure. Statins function as neuroprotective agent in various life compromising conditions such as stroke and traumatic brain injury [3]. Several reports are available on the anticonvulsant effect of statins and a few of them contain disputable report of atorvastatin impact on experimental seizure models [37, 38].
In some studies, statins have demonstrated a neuroprotective effect on an animal model for Alzheimer's disease and ischemic brain injury through NO-dependent pathways. It reveals the role of NO as a second messenger system of statins. It functions via various mechanisms like upregulation of endothelial NO synthase, antiapoptotic effects, reduction in oxidative stress and inducible nitric oxide synthase [3, 37, 39, 40]. In a paper, Mahmood et al. investigated the impact of a combination therapy of marrow stromal cells (MSCs) and statins (atorvastatin) on brain injury caused by trauma in rats. When they administered statins orally, after the traumatic brain injury, functional outcome and neuroplasticity were improved [41].
In the present study, we also demonstrated that Atorvastatin, and Atorvastatin plus Metformin treatments increased the latency to PTZ-induced seizure. Studies have revealed that statins, particularly atorvastatin, have beneficial effects on several excitotoxic disorders, including stroke [42], traumatic brain injury [39], glutamate exposure [43], and cerebral ischemia [44]. In agreement with our study results, Lee and colleagues showed that atorvastatin treatment (10 mg/kg/day for 7 days) significantly decreases the severity of kainate-induced seizures [7]. In addition, Piermartiri et al. reported that atorvastatin treatment prevents the incidence of tonic and/or clonic seizures induced by quinolinic acid in around 33% of tested mice [5]. However, Lee et al. claimed that the seizure score does not change with a single injection of atorvastatin (30 min after kainate administration) [7]. It has also been reported that atorvastatin withdrawal facilitated the occurrence of PTZ-induced seizures, as evidenced by a decline in the latency to clonic and generalized seizures [6].
Increasing evidence indicates that BBB disruption is an important etiologic factor in seizure disorders [45, 46]. Interestingly, several studies have revealed that atorvastatin inhibits BBB breakdown [47, 48]. On the other hand, Van Vliet et al. have demonstrated that atorvastatin treatment did not affect BBB leakage [49]. Moreover, the anti-epileptic effects of atorvastatin might be due to reduced cholesterol levels. Therefore, it seems that the molecular mechanisms of atorvastatin are not necessarily limited to its neuroprotective properties, and further studies are needed. Furthermore, several studies have shown that inhibition of mTOR pathway can prevent seizure [50, 51]. Indeed, Atorvastatin, and Metformin could effectively promote autophagy through mTOR inhibition [52, 53], and studies displayed that, suitable activation of autophagy has antiepileptic effects [54]. Our findings clearly indicate that Metformin, Atorvastatin, and the combination of these two drugs have anticonvulsant effects which could be through regulation of autophagy.
Declarations
Author contribution statement
Mohammad Ali Zeyghami, Ebrahim Hesam: Conceived and designed the experiments; Performed the experiments.
Parand Khadivar, Halimeh Khaton Hesam: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Ali Ahmadnia: Analyzed and interpreted the data; Wrote the paper.
Abolfazl Amini: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by Golestan University of Medical Sciences, Gorgan, IRAN (No. 940409078).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Contributor Information
Ebrahim Hesam, Email: hesam_ebrahim@yahoo.com.
Abolfazl Amini, Email: amini_ab@msn.com.
References
- 1.Ngugi A.K., Bottomley C., Kleinschmidt I., Sander J.W., Newton C.R. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51(5):883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sander J.W. The epidemiology of epilepsy revisited. Curr. Opin. Neurol. 2003;16(2):165–170. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- 3.Shafaroodi H., Moezi L., Fakhrzad A., Hassanipour M., Rezayat M., Dehpour A.R. The involvement of nitric oxide in the anti-seizure effect of acute atorvastatin treatment in mice. Neurol. Res. 2012;34(9):847–853. doi: 10.1179/1743132812Y.0000000080. [DOI] [PubMed] [Google Scholar]
- 4.Uzüm G., Akgün-Dar K., Aksu U. The effects of atorvastatin on memory deficit and seizure susceptibility in pentylentetrazole-kindled rats. Epilepsy Behav. 2010;19(3):284–289. doi: 10.1016/j.yebeh.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Piermartiri T.C., Vandresen-Filho S., de Araújo Herculano B., Martins W.C., Dal’Agnolo D., Stroeh E. Atorvastatin prevents hippocampal cell death due to quinolinic acid-induced seizures in mice by increasing Akt phosphorylation and glutamate uptake. Neurotox. Res. 2009;16(2):106–115. doi: 10.1007/s12640-009-9057-6. [DOI] [PubMed] [Google Scholar]
- 6.Funck V.R., de Oliveira C.V., Pereira L.M., Rambo L.M., Ribeiro L.R., Royes L.F.F. Differential effects of atorvastatin treatment and withdrawal on pentylenetetrazol-induced seizures. Epilepsia. 2011;52(11):2094–2104. doi: 10.1111/j.1528-1167.2011.03261.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee J.-K., Won J.-S., Singh A.K., Singh I. Statin inhibits kainic acid-induced seizure and associated inflammation and hippocampal cell death. Neurosci. Lett. 2008;440(3):260–264. doi: 10.1016/j.neulet.2008.05.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C.-Y., Jaw T., Tseng H.-C., Chen I.-C., Liou H.-H. Lovastatin modulates glycogen synthase kinase-3β pathway and inhibits mossy fiber sprouting after pilocarpine-induced status epilepticus. PLoS One. 2012;7(6):e38789. doi: 10.1371/journal.pone.0038789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gouveia T.L.F., Scorza F.A., Silva M.J.V., de Aquino Bandeira T., Perosa S.R., Argañaraz G.A. Lovastatin decreases the synthesis of inflammatory mediators in the hippocampus and blocks the hyperthermia of rats submitted to long-lasting status epilepticus. Epilepsy Behav. 2011;20(1):1–5. doi: 10.1016/j.yebeh.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Sehar N., Agarwal N.B., Vohora D., Raisuddin S. Atorvastatin prevents development of kindling by modulating hippocampal levels of dopamine, glutamate, and GABA in mice. Epilepsy Behav. 2015;42:48–53. doi: 10.1016/j.yebeh.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Zhao R-r, Xu X-c, Xu F., Zhang W-l, Zhang W-l, Liu L-m. Metformin protects against seizures, learning and memory impairments and oxidative damage induced by pentylenetetrazole-induced kindling in mice. Biochem. Biophys. Res. Commun. 2014;448(4):414–417. doi: 10.1016/j.bbrc.2014.04.130. [DOI] [PubMed] [Google Scholar]
- 12.Sudha K., Rao A.V., Rao A. Oxidative stress and antioxidants in epilepsy. Clin. Chim. Acta. 2001;303(1):19–24. doi: 10.1016/s0009-8981(00)00337-5. [DOI] [PubMed] [Google Scholar]
- 13.Algire C., Moiseeva O., Deschênes-Simard X., Amrein L., Petruccelli L., Birman E. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Canc. Prev. Res. 2012;5(4):536–543. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Montalvo A., Mercken E.M., Mitchell S.J., Palacios H.H., Mote P.L., Scheibye-Knudsen M. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Onken B., Driscoll M. Metformin induces a dietary restriction–like state and the oxidative stress response to extend C. elegans healthspan via AMPK, LKB1, and SKN-1. PLoS One. 2010;5(1):e8758. doi: 10.1371/journal.pone.0008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anedda A., Rial E., González-Barroso M.M. Metformin induces oxidative stress in white adipocytes and raises uncoupling protein 2 levels. J. Endocrinol. 2008;199(1):33–40. doi: 10.1677/JOE-08-0278. [DOI] [PubMed] [Google Scholar]
- 17.Takata F., Dohgu S., Matsumoto J., Machida T., Kaneshima S., Matsuo M. Metformin induces up-regulation of blood–brain barrier functions by activating AMP-activated protein kinase in rat brain microvascular endothelial cells. Biochem. Biophys. Res. Commun. 2013;433(4):586–590. doi: 10.1016/j.bbrc.2013.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Van Vliet E., da Costa Araujo S., Redeker S., Van Schaik R., Aronica E., Gorter J. Blood–brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130(2):521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- 19.Danjo S., Ishihara Y., Watanabe M., Nakamura Y., Itoh K. Pentylentetrazole-induced loss of blood–brain barrier integrity involves excess nitric oxide generation by neuronal nitric oxide synthase. Brain Res. 2013;1530:44–53. doi: 10.1016/j.brainres.2013.06.043. [DOI] [PubMed] [Google Scholar]
- 20.Ammon-Treiber S., Grecksch G., Angelidis C., Vezyraki P., Höllt V., Becker A. Pentylenetetrazol-kindling in mice overexpressing heat shock protein 70. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2007;375(2):115–121. doi: 10.1007/s00210-007-0143-0. [DOI] [PubMed] [Google Scholar]
- 21.Claycomb R.J., Hewett S.J., Hewett J.A. Characterization of the effect of oral rofecoxib treatment on PTZ-induced acute seizures and kindling. Epilepsia. 2011;52(2):273–283. doi: 10.1111/j.1528-1167.2010.02889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palizvan M., Esmaeili A., Rajabian H., Jand Y., Mirzazadeh E. Effect of progesterone administration in newborns rats on Pentylenetetrazol kindling susceptibility after maturity. J. Adv. Med. Biomed. Res. 2006;14(56):24–31. [Google Scholar]
- 23.Naderi M., Jand A., Jand Y., Rahjoo T., Palizvan M. Effect of ursodeoxycholic acid on pentylenetetrazole kindling and kindling induced memory impairment in rat. J. Babol Univ. Med. Sci. 2018;20(1):50–56. [Google Scholar]
- 24.Łabuzek K., Suchy D., Gabryel B., Bielecka A., Liber S., Okopień B. Quantification of metformin by the HPLC method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol. Rep. 2010;62(5):956–965. doi: 10.1016/s1734-1140(10)70357-1. [DOI] [PubMed] [Google Scholar]
- 25.El-Mir M.-Y., Detaille D., Gloria R., Delgado-Esteban M., Guigas B., Attia S. Neuroprotective role of antidiabetic drug metformin against apoptotic cell death in primary cortical neurons. J. Mol. Neurosci. 2008;34(1):77–87. doi: 10.1007/s12031-007-9002-1. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Benashski S.E., Venna V.R., McCullough L.D. Effects of metformin in experimental stroke. Stroke. 2010;41(11):2645–2652. doi: 10.1161/STROKEAHA.110.589697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Deng J., Sheng W., Zuo Z. Metformin attenuates Alzheimer's disease-like neuropathology in obese, leptin-resistant mice. Pharmacol. Biochem. Behav. 2012;101(4):564–574. doi: 10.1016/j.pbb.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J., Gallagher D., DeVito L.M., Cancino G.I., Tsui D., He L. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11(1):23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Iwata N., Hasegawa T., Fujita S., Nagao S., Nakano Y., Nada T. Effect of the interaction of metformin and bone morphogenetic proteins on ovarian steroidogenesis by human granulosa cells. Biochem. Biophys. Res. Commun. 2018;503(3):1422–1427. doi: 10.1016/j.bbrc.2018.07.058. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura M., Ogo A., Yamura M., Yamaguchi Y., Nakashima H. Metformin suppresses sonic hedgehog expression in pancreatic cancer cells. Anticancer Res. 2014;34(4):1765–1769. [PubMed] [Google Scholar]
- 31.Dadwal P., Mahmud N., Sinai L., Azimi A., Fatt M., Wondisford F.E. Activating endogenous neural precursor cells using metformin leads to neural repair and functional recovery in a model of childhood brain injury. Stem Cell Reports. 2015;5(2):166–173. doi: 10.1016/j.stemcr.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L., Chopp M., Meier D.H., Winter S., Wang L., Szalad A. Sonic hedgehog signaling pathway mediates cerebrolysin-improved neurological function after stroke. Stroke. 2013;44(7):1965–1972. doi: 10.1161/STROKEAHA.111.000831. [DOI] [PubMed] [Google Scholar]
- 33.Lim D.A., Tramontin A.D., Trevejo J.M., Herrera D.G., García-Verdugo J.M., Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28(3):713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 34.Gajera C.R., Emich H., Lioubinski O., Christ A., Beckervordersandforth-Bonk R., Yoshikawa K. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J. Cell Sci. 2010;123(11):1922–1930. doi: 10.1242/jcs.065912. [DOI] [PubMed] [Google Scholar]
- 35.Ding X., Li Y., Liu Z., Zhang J., Cui Y., Chen X. The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice. J. Cerebr. Blood Flow Metabol. 2013;33(7):1015–1024. doi: 10.1038/jcbfm.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramirez C., Tercero I., Pineda A., Burgos J.S. Simvastatin is the statin that most efficiently protects against kainate-induced excitotoxicity and memory impairment. J. Alzheimers Dis. 2011;24(1):161–174. doi: 10.3233/JAD-2010-101653. [DOI] [PubMed] [Google Scholar]
- 38.Moezi L., Shafaroodi H., Hassanipour M., Fakhrzad A., Hassanpour S., Dehpour A.R. Chronic administration of atorvastatin induced anti-convulsant effects in mice: the role of nitric oxide. Epilepsy Behav. 2012;23(4):399–404. doi: 10.1016/j.yebeh.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 39.Lu D., Goussev A., Chen J., Pannu P., Li Y., Mahmood A. Atorvastatin reduces neurological deficit and increases synaptogenesis, angiogenesis, and neuronal survival in rats subjected to traumatic brain injury. J. Neurotrauma. 2004;21(1):21–32. doi: 10.1089/089771504772695913. [DOI] [PubMed] [Google Scholar]
- 40.Lu D., Li Y., Wang L., Chen J., Mahmood A., Chopp M. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J. Neurotrauma. 2001;18(8):813–819. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- 41.Mahmood A., Lu D., Qu C., Goussev A., Chopp M. Treatment of traumatic brain injury with a combination therapy of marrow stromal cells and atorvastatin in rats. Neurosurgery. 2007;60(3):546–553. doi: 10.1227/01.NEU.0000255346.25959.99. [DOI] [PubMed] [Google Scholar]
- 42.Nagotani S., Hayashi T., Sato K., Zhang W., Deguchi K., Nagano I. Reduction of cerebral infarction in stroke-prone spontaneously hypertensive rats by statins associated with amelioration of oxidative stress. Stroke. 2005;36(3):670–672. doi: 10.1161/01.STR.0000155732.27333.3c. [DOI] [PubMed] [Google Scholar]
- 43.Zacco A., Togo J., Spence K., Ellis A., Lloyd D., Furlong S. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors protect cortical neurons from excitotoxicity. J. Neurosci. 2003;23(35):11104–11111. doi: 10.1523/JNEUROSCI.23-35-11104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laufs U., Gertz K., Huang P., Nickenig G., Böhm M., Dirnagl U. Atorvastatin upregulates type III nitric oxide synthase in thrombocytes, decreases platelet activation, and protects from cerebral ischemia in normocholesterolemic mice. Stroke. 2000;31(10):2442–2449. doi: 10.1161/01.str.31.10.2442. [DOI] [PubMed] [Google Scholar]
- 45.Marchi N., Tierney W., Alexopoulos A.V., Puvenna V., Granata T., Janigro D. The etiological role of blood-brain barrier dysfunction in seizure disorders. Cardiovasc. Psychiatry Neurol. 2011;2011:482415. doi: 10.1155/2011/482415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seiffert E., Dreier J.P., Ivens S., Bechmann I., Tomkins O., Heinemann U. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J. Neurosci. 2004;24(36):7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kahles T., Luedike P., Endres M., Galla H.-J., Steinmetz H., Busse R. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38(11):3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 48.Taoufiq Z., Pino P., N'dilimabaka N., Arrouss I., Assi S., Soubrier F. Atorvastatin prevents Plasmodium falciparum cytoadherence and endothelial damage. Malar. J. 2011;10(1):52. doi: 10.1186/1475-2875-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Vliet E.A., Holtman L., Aronica E., Schmitz L.J., Wadman W.J., Gorter J.A. Atorvastatin treatment during epileptogenesis in a rat model for temporal lobe epilepsy. Epilepsia. 2011;52(7):1319–1330. doi: 10.1111/j.1528-1167.2011.03073.x. [DOI] [PubMed] [Google Scholar]
- 50.Ryther R.C., Wong M. Mammalian target of rapamycin (mTOR) inhibition: potential for antiseizure, antiepileptogenic, and epileptostatic therapy. Curr. Neurol. Neurosci. Rep. 2012;12(4):410–418. doi: 10.1007/s11910-012-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang X., McMahon J., Huang Y. Rapamycin attenuates aggressive behavior in a rat model of pilocarpine-induced epilepsy. Neuroscience. 2012;215:90–97. doi: 10.1016/j.neuroscience.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi W., Xiao D., Wang L., Dong L., Yan Z., Shen Z. Therapeutic metformin/AMPK activation blocked lymphoma cell growth via inhibition of mTOR pathway and induction of autophagy. Cell Death Dis. 2012;3(3):e275. doi: 10.1038/cddis.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Q., Yang Y.-J., Wang H., Dong Q.-T., Wang T.-J., Qian H.-Y. Autophagy activation: a novel mechanism of atorvastatin to protect mesenchymal stem cells from hypoxia and serum deprivation via AMP-activated protein kinase/mammalian target of rapamycin pathway. Stem Cell. Dev. 2012;21(8):1321–1332. doi: 10.1089/scd.2011.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McMahon J., Huang X., Yang J., Komatsu M., Yue Z., Qian J. Impaired autophagy in neurons after disinhibition of mammalian target of rapamycin and its contribution to epileptogenesis. J. Neurosci. 2012;32(45):15704–15714. doi: 10.1523/JNEUROSCI.2392-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]