Abstract
Herbicide contamination of nearshore tropical marine ecosystems is widespread and persistent; however, risks posed by most ‘alternative’ herbicides to tropical marine microalgae remain poorly understood. Experimental exposures of the important but understudied microalgae Rhodomonas salina to seven individual Photosystem II (PSII) inhibitor herbicides (diuron, metribuzin, hexazinone, tebuthiuron, bromacil, simazine, propazine) led to inhibition of effective quantum yield (ΔF/Fm′) and subsequent reductions in specific growth rates (SGR). The concentrations which reduced ΔF/Fm′ by 50% (EC50) ranged from 1.71-59.2 µg L−1, while the EC50s for SGR were 4-times higher, ranging from 6.27-188 µg L−1. Inhibition of ΔF/Fm′ indicated reduced photosynthetic capacity, and this correlated linearly with reduced SGR (R2 = 0.89), supporting the application of ∆F/Fm’ inhibition as a robust and sensitive indicator of sub-lethal toxicity of PSII inhibitors for this microalga. The three non-PSII inhibitor herbicides (imazapic, haloxyfop and 2,4-Dichlorophenoxyacetic acid (2,4-D)) caused low or no toxic responses to the function of the PSII or growth at the highest concentrations tested suggesting these herbicides pose little risk to R. salina. This study highlights the suitability of including R. salina in future species sensitivity distributions (SSDs) to support water quality guideline development for the management of herbicide contamination in tropical marine ecosystems.
Subject terms: Environmental impact, Marine biology, Microbial ecology
Introduction
Herbicides in tropical marine ecosystems
Poor water quality, including pesticide contamination, has long been recognized as a threat to the health and resilience of tropical and subtropical marine ecosystems (Asia Pacific1–5, Central America6, Mexico7, Caribbean8). In north Queensland, Australia, herbicide transport from agriculture runoff into coastal waters peaks with summer rainfall9; however, the persistence of many herbicides10 contributes to herbicide detection year-round in the Great Barrier Reef (GBR) catchment area11–13. The GBR represents the most studied tropical marine location for herbicide contamination14, with annual monitoring conducted by the Marine Monitoring Program (MMP) under the Reef 2050 Long-Term Sustainability Plan assessing long-term trends in water quality in coastal waters of Queensland and the GBR marine park13,15.
Five Photosystem II (PSII) inhibitor herbicides ametryn, atrazine, diuron, hexazinone, and tebuthiuron are the most frequently detected herbicides in GBR waters. For example, maximum sustained concentrations of 778 ng L−1 diuron, 405 ng L−1 atrazine and 134 ng L−1 hexazinone have been reported in recent monitoring programs using passive sampling techniques. However, peak concentrations of up to 22 µg L−1 diuron have previously been identified in grab samples during flood events16. These five herbicides have therefore been identified as ‘priority’ herbicides for reductions by management12–14,17,18. PSII inhibitor herbicides specifically target the PSII of the photosynthetic complex within chloroplasts by competing with plastoquinone for the QB binding site on D1 proteins within the PSII19. This leads to the blocking of the light-induced electron transport chain, reducing photosynthetic efficiency and causing oxidative damage to PSII19. Since PSII is common across photosynthetic organisms, these herbicides can impact non-target marine species, including corals20,21, microalgae22,23, crustose coralline algae24, foraminifera25, and seagrass26–29. Reduced photosynthetic efficiency and damage to PSII caused by PSII inhibitor herbicides results in ecologically-relevant effects, such as inhibition of growth in microalgae23 and seagrass30, and bleaching, partial colony mortality, and reduced fecundity in coral31.
Potential risks of ‘alternative’ herbicides
The risks posed by priority PSII inhibitor herbicides in runoff have led Australian regulators to tighten registrations and regulations for the application of some of these herbicides in agriculture32. As a result, there is a transition towards the application of ‘alternative’ PSII and non-PSII inhibitor herbicides in coastal agriculture33. Compared to PSII inhibitor herbicides, non-PSII inhibitor herbicides exhibit a range of different modes of action, such as inhibiting acetohydroxyacid synthase (AHAS)34,35 or acetyl-CoA carboxylase (ACCase)36 that result in reduced cell growth in plants. In recent years, 55 pesticides, including the five priority PSII inhibitor herbicides, as well as eight alternative PSII inhibitor herbicides (bromacil, fluometuron, metribuzin, terbuthylazine, propazine, simazine, terbutryn, prometryn) and eight alternative non-PSII inhibitor herbicides (2,4-D, fluroxypyr, monochlorophenoxyacetic acid (MCPA), imazapic, metsulfuron-methyl, metolachlor, haloxyfop, fluazifop) (Table 1) have been detected in the GBR and its catchments17,37. While most alternative herbicides detected in coastal waters of the GBR are registered for use in agriculture, these herbicides have only recently been added to pesticide analytical suites conducted as part of GBR MMP38. Many of the alternative herbicides occur at frequencies and concentrations similar to the regulated priority PSII inhibitor herbicides they are replacing (but usually <1 µg L−1)13,17,37 and often exhibit similar chemical properties and toxicities (Table S1). However, knowledge of the likely ecological effects of alternative herbicides in GBR waters is limited and toxicity data that underpins their registrations are generally scarce39.
Table 1.
Summary of detected herbicides in the GBR and its catchment. Comparison of water quality guideline values (WQGVs)39 (all of low reliability) based on freshwater species and proposed water quality guideline values (PGVs)43,44,93 for 99%, 95%, 90% and 80% species protection (based on marine and freshwater species) against toxicity thresholds [no effect concentration (NEC); effect concentration inhibiting the specific growth rate by 10% (EC10))] values derived for Rhodomonas salina in this study (from Table 3). All concentrations in µg L−1. NA signifies no available guideline values. Bold indicates herbicides tested in this study.
| Herbicide | Mode of action | WQGVs | PGVs | Guideline reliability | NEC This study | EC10 This study | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC99 | PC95 | PC90 | PC80 | PC99 | PC95 | PC90 | PC80 | |||||
| ‘Priority’ PSII inhibitor herbicides | ||||||||||||
| Diuron | Inhibition of photosynthesis at PSII | 0.2* | 0.43 | 0.67 | 0.86 | 1.2 | Very high | 1.7 | 1.9 | |||
| Hexazinone | 75* | 1.8 | 2.5 | 3.1 | 4 | Low | 4.6 | 4.0 | ||||
| Tebuthiuron | 0.02 | 2.2 | 20 | 160 | 4.7 | 11 | 17 | 26 | Moderate | 23 | 28 | |
| Ametryn | 0.5 | 1 | 1.6 | 0.10 | 0.61 | 1.3 | 2.8 | Low | ||||
| Atrazine | 0.7 | 13 | 45 | 150 | NA | NA | ||||||
| ‘Alternative’ PSII inhibitor herbicides | ||||||||||||
| Bromacil | Inhibition of photosynthesis at PSII | 180* | 0.23 | 1.1 | 2.2 | 4.8 | Moderate | 5.5 | 4.9 | |||
| Fluometuron | NA | 20 | 40 | 55 | 77 | Low | ||||||
| Metribuzin | NA | 2 | 2.7 | 3.1 | 3.9 | Moderate | 2.2 | 2.7 | ||||
| Terbuthylazine | NA | 0.40 | 0.97 | 1.6 | 2.8 | Moderate | ||||||
| Propazine | NA | 2.2 | 4.6 | 6.4 | 9.2 | Low | 28 | 42 | ||||
| Simazine | 0.2 | 3.2 | 11 | 35 | 28 | 63 | 84 | 130 | Low | 48 | 38 | |
| Terbutryn | NA | 0.079 | 0.26 | 0.51 | 1.2 | Moderate | ||||||
| Prometryn | NA | 0.11 | 0.52 | 1.1 | 2.2 | Low | ||||||
| ‘Alternative’ non-PSII inhibitor herbicides | ||||||||||||
| 2,4-D | Auxin mimic, promotes uncontrolled growth | 140 | 280 | 450 | 830 | 1,000 | 2,500 | 3,800 | 5,800 | Low | >279,000 | >279,000 |
| Fluroxypyr | NA | 87 | 200 | 290 | 440 | Low | ||||||
| MCPA | 1.4* | 1 | 17 | 60 | 240 | Low | ||||||
| Imazapic | Inhibition of AHAS | NA | 0.049 | 0.44 | 1.2 | 3.6 | Very low | 363,000 | 410,000 | |||
| Metsulfuron-methyl | NA | NA | NA | |||||||||
| Metolachlor | Inhibition of cell division | NA | NA | NA | ||||||||
| Haloxyfop | Inhibition of ACCase | NA | 590 | 2,000 | 3,400 | 590 | 2,000 | 3,400 | 6,100 | Low | >3,700 | >3,700 |
| Fluazifop | NA | NA | NA | |||||||||
*Level of protection unknown.
Water quality guideline values (WQGVs) exist for only a handful of alternative herbicides in Australia39 or globally (e.g. Canada40, EU41). According to the Australian and New Zealand Guidelines for Fresh and Marine Water Quality (ANZG)39, there are currently only four WQGVs for alternative PSII and non-PSII inhibitor herbicides (bromacil, simazine, 2,4-D, MCPA) and five guidelines for the priority herbicides (diuron, atrazine, ametryn, tebuthiuron, hexazinone) detected in GBR waters; however, all were derived from freshwater toxicity thresholds and are of low reliability (Table 1). Given that complex mixtures of herbicides are commonly detected in coastal waters, the multisubstance-potentially affected fraction (ms-PAF) method42 has been recently applied as a more comprehensive approach to predict the cumulative risk of herbicide mixtures37. Although exceedances of WQGVs by individual herbicides are rare, when the combined concentrations of multiple co-occurring herbicides are considered using ms-PAF, exceedances are more frequent37. However, the high-reliability WQGVs that are necessary to predict ms-PAFs are not available for most alternative herbicides. Recently, revisions of the WQGVs for 27 GBR-relevant pesticides (including some alternative herbicides) based on all available marine and freshwater toxicity data have been proposed43,44; nevertheless, many data gaps remain, especially for marine species. More targeted toxicity testing is therefore warranted to improve current WQGVs for marine species and to develop WQGVs for alternative herbicides where they do not exist.
Microalgal toxicity tests for derivation of water quality guidelines
National WQGVs (referred to by ANZG39 as default GVs) are derived in Australia to protect 99%, 95%, 90% and 80% (PC99, 95, 90, 80, respectively) of marine and freshwater communities by estimating community sensitivity from species sensitivity distributions (SSDs)45. The minimum data required for SSDs to meet WQGV criteria are toxicity thresholds for at least five species from at least four phyla that are characteristic of the receiving environment45. For a recent and detailed description of the methods and criteria in the Australian context see Warne et al.45. With rapid growth rates that allow for chronic exposure testing in a short period, marine microalgae represent a suitable taxon to contribute to future SSDs. Currently, SSDs are developed using toxicity data from chronic exposures that are ecologically relevant, and for microalgal toxicity testing inhibition of growth is the most common ecologically relevant endpoint39,45. However, strong correlations between effects on microalgae growth and reduced photosynthetic efficiency in estuarine microalgae as measured by Pulse Amplitude Modulation (PAM) fluorometry has been demonstrated for several PSII inhibitor herbicides23. The inhibition of effective quantum yield (ΔF/Fm′) by PSII inhibitor herbicides is proportional to the inhibition of photosynthetic efficiency at a given irradiance46 and could be considered as a rapid, sensitive and non-invasive alternative for growth measurements in microalgae toxicity tests involving PSII inhibitor herbicides23. In previous studies, inhibition of ΔF/Fm′ has been extensively applied for assessing the toxicity of PSII inhibitor herbicides in microalgae23,46–49 and has also revealed herbicide-induced community tolerance in microalgae to PSII inhibitor herbicides over chronic exposures50,51. However, this sensitive photophysiological response may not be suitable as an ecologically relevant measure of whole organism stress for microalgae to non-PSII inhibitor herbicides where the mode of action does not involve PSII34,52. Further comparisons between the inhibition of growth and ΔF/Fm′ as endpoints for herbicide toxicity in marine microalgae are therefore warranted to demonstrate the relevance of using ΔF/Fm′ as an ecological relevant endpoint in future SSDs.
In order to improve WQGVs for herbicides and expand toxicity threshold data for tropical marine species to alternative herbicides, this study tested the effects of several herbicides on growth and ΔF/Fm′ to the marine microalgae Rhodomonas salina. This species was selected as a tropical representative of an understudied phylum, Cryptophyta, generally underrepresented in SSDs. In addition, this study aimed to derive no effect concentrations (NECs), which are the preferred toxicity estimates for inclusion in SSDs to derive WQGVs. Nine herbicides detected in the GBR and catchments17,37 that indicated current toxicity data gaps (based on consultation with the Queensland Department of Environment and Science (DES)) were selected for testing, along with the reference herbicide diuron. The tested herbicides included the PSII inhibitor herbicides tebuthiuron, hexazinone, metribuzin, simazine, propazine, bromacil, and the non-PSII inhibitor herbicides, haloxyfop, 2,4-dichlorophenoxyacetic acid (2,4-D) and imazapic. The toxicity thresholds identified provide valuable toxicity data for alternative herbicides detected in GBR waters and will contribute to new and improved WQGVs for application in risk assessments.
Results
Assay performance
Rhodomonas salina displayed exponential growth in control treatments across all bioassays with SGR ranging between 1.07 ± 0.07 d−1 and 1.29 ± 0.02 d−1 (mean ± SD) (Table 2). ΔF/Fm′ measurements of control treatments varied between 0.45 ± 0.02 and 0.53 ± 0.01 (mean ± SD). The carrier solvents (<0.01% v/v) had no significant influence on SGR compared with filtered seawater after 72 h (ANOVA, Fethanol (1,3) = 1.12; p = 0.37; FDMSO (1,3) = 0.15; p = 0.73). The reference toxicant diuron used in each growth test and fluorescence well plate assay inhibited SGR and ΔF/Fm′ between 30.1 ± 2.2% and 57.2 ± 2.8% and between 78.4 ± 2.0% and 97.7 ± 2.2% (mean ± SD), respectively (Table 2). This level of variability was expected between independent experiments conducted across 10 occasions and may have been due to minor differences in nutrients or the physiology of cells at the start of each test.
Table 2.
Assay performance. Specific growth rate (SGR, d−1) and photosynthetic efficiency (ΔF/Fm′) measurements of control and reference (diuron, 4 µg L−1) treatments and diuron reference percent inhibition effect (Ref. inh (%)) (mean ± SD; n = 5 per treatment).
| Herbicide | SGR d−1 | ΔF/Fm′ | ||||
|---|---|---|---|---|---|---|
| Control | Reference | Ref. inh. (%) | Control | Reference | Ref. Inh. (%) | |
| Diuron | 1.20 ± 0.01 | 0.82 ± 0.03 | 31.4 ± 2.14 | 0.53 ± 0.01 | 0.10 ± 0.01 | 80.2 ± 1.4 |
| Metribuzin | 1.29 ± 0.02 | 0.55 ± 0.04 | 57.2 ± 2.8 | 0.47 ± 0.01 | 0.03 ± 0.01 | 93.9 ± 1.4 |
| Hexazinone | 1.24 ± 0.04 | 0.77 ± 0.06 | 37.7 ± 4.8 | 0.51 ± 0.01 | 0.08 ± 0.01 | 84.8 ± 1.0 |
| Bromacil | 1.07 ± 0.06 | 0.47 ± 0.06 | 55.9 ± 5.8 | 0.45 ± 0.02 | 0.05 ± 0.01 | 89.0 ± 1.8 |
| Tebuthiuron | 1.27 ± 0.02 | 0.59 ± 0.06 | 46.4 ± 4.3 | 0.47 ± 0.01 | 0.01 ± 0.01 | 97.7 ± 2.2 |
| Simazine | 1.18 ± 0.03 | *0.92 ± 0.06 | *22.0 ± 5.1 | 0.49 ± 0.01 | 0.08 ± 0.01 | 83.2 ± 0.8 |
| Propazine | 1.19 ± 0.02 | 0.61 ± 0.05 | 48.3 ± 3.8 | 0.52 ± 0.01 | 0.10 ± 0.01 | 81.4 ± 1.9 |
| Imazapic | 1.22 ± 0.03 | 0.85 ± 0.02 | 30.1 ± 2.2 | 0.52 ± 0.01 | 0.11 ± 0.01 | 78. 7 ± 1.2 |
| Haloxyfop | 1.18 ± 0.05 | 0.80 ± 0.01 | 31.8 ± 1.4 | 0.47 ± 0.01 | 0.10 ± 0.01 | 78.4 ± 2.0 |
| 2,4 D | 1.13 ± 0.07 | 0.71 ± 0.04 | 37.0 ± 3.8 | 0.47 ± 0.01 | 0.07 ± 0.01 | 84.5 ± 1.9 |
*Note for the simazine toxicity bioassay, a reference treatment of diuron, 2 µg L−1 was used instead of 4 µg L−1 and therefore not included in calculations of the total mean.
Physicochemical measurements indicated little variation within each treatment and across all tests over 72 h: pH 8.5 ± 0.4; salinity 34.2 ± 0.6 PSU, dissolved oxygen 8.0 ± 0.4 mg L−1 (± SD, n = 169 for each parameter), temperature 26.0 ± 0.6 °C ( ± SD, 10-min logging intervals). Herbicide concentrations were measured at 0 h and 72 h of each toxicity test to estimate the potential losses of herbicides due to degradation, volatilization or adsorption over the 72-h test duration. Chemical analyses showed that the time-averaged measured concentrations (between 0 h and 72 h samples) were within 20% of nominal concentrations for diuron, metribuzin, hexazinone, bromacil, tebuthiuron, and 2,4-D and between 30-50% of nominal concentrations for propazine, simazine and imazapic. No contaminant was detected in the control treatments and a summary of the nominal and measured concentrations can be found in Table S1.
Effects of PSII inhibitor herbicides on growth
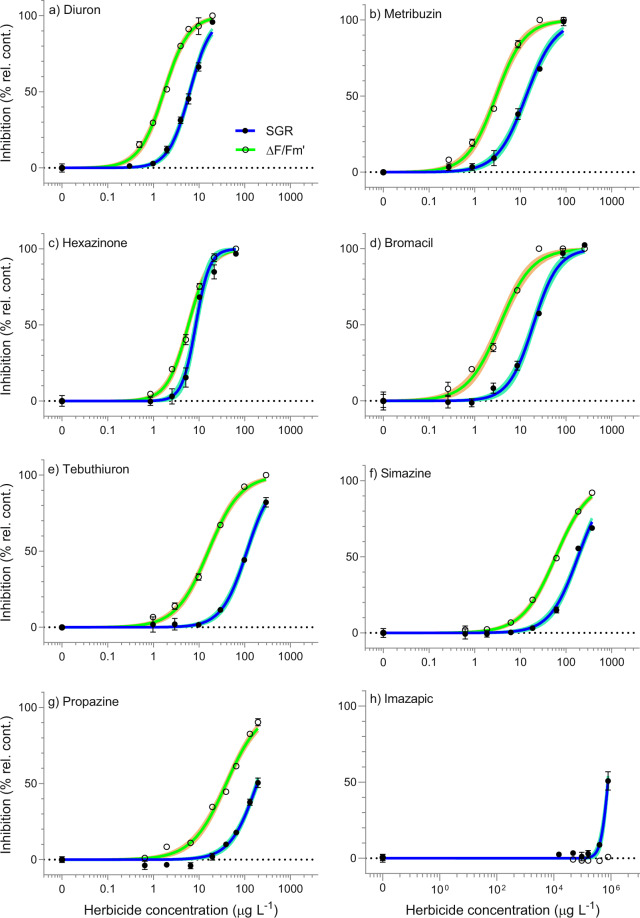
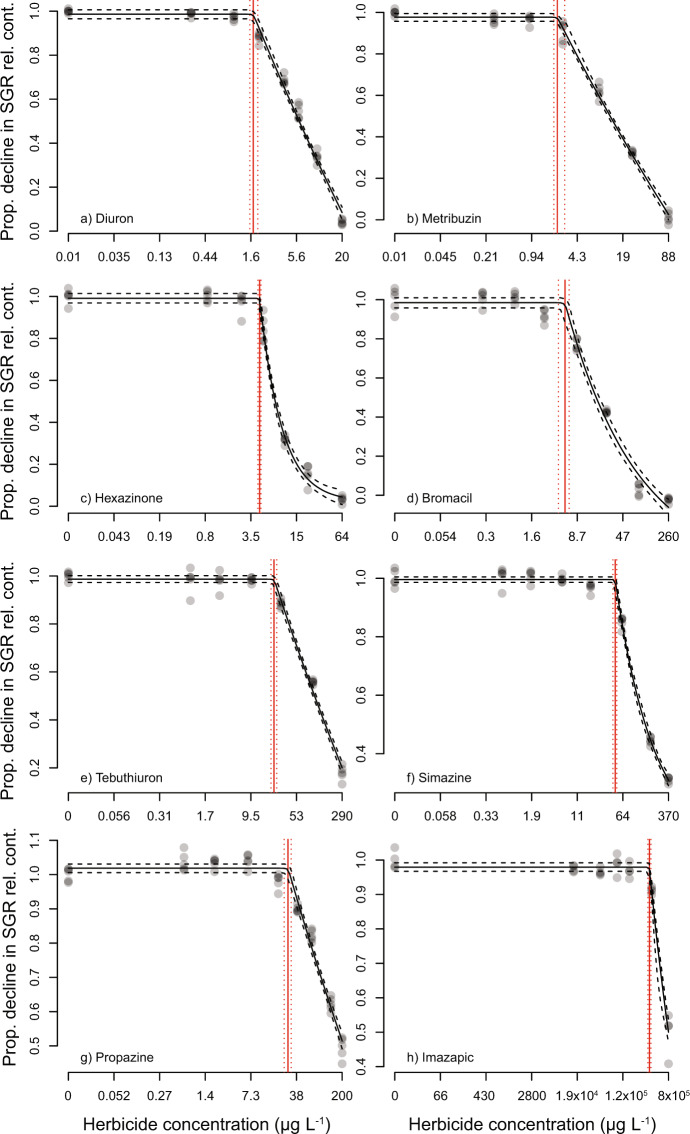
Toxicity tests using R. salina were performed on seven PSII inhibitor herbicides, including the reference herbicide diuron (Table 3). The growth of R. salina was inhibited by all PSII inhibitor herbicides, and diuron was the most toxic of all PSII inhibitor herbicides with an EC50 value of 6.27 µg L−1 (Table 3). A summary of the slope and goodness of fit of each concentration-response curve (Sigmoidal, 4 parameter model) for SGR (Fig. 1) is shown in Table S2. The comparison between relative potencies (ReP) based on EC50 values to the reference herbicide diuron indicated the order of toxicity: diuron > hexazinone > metribuzin > bromacil > tebuthiuron > simazine > propazine (Table 2). The EC10 and modelled no effect concentrations (NECs) were also reported in Table 2 and showed similar orders of toxicity (Fig. 2).
Table 3.
Toxicity threshold summary. Derived effect concentrations (EC10 and EC50 from Fig. 1) and no effect concentrations (NECs from Fig. 2) with 95% confidence intervals for each herbicide, and relative equivalent potencies (ReP). NA indicates values could not be calculated. Concentrations are reported in µg L−1.
| Herbicide | Endpoint | SGR | ΔF/Fm′ | SGR (EC50): ΔF/Fm′ (EC50) |
|---|---|---|---|---|
| Diuron | EC50 | 6.27 (6.02–6.54) | 1.71 (1.63–1.80) | 3.7 |
| EC10 | 1.94 (1.75–2.14) | 0.43 (0.38–0.48) | ||
| NEC | 1.68 (1.53–1.90) | |||
| ReP | 1 | 1 | ||
| Metribuzin | EC50 | 13.4 (12.3–14.5) | 2.95 (2.72–3.18) | 4.6 |
| EC10 | 2.66 (2.21–3.18) | 0.60 (0.50–0.71) | ||
| NEC | 2.21 (1.97–2.82) | |||
| ReP | 0.47 | 0.59 | ||
| Hexazinone | EC50 | 8.50 (7.99–9.06) | 5.85 (5.61–6.09) | 1.5 |
| EC10 | 3.96 (3.40–4.57) | 1.81 (1.63 – 1.99) | ||
| NEC | 4.58 (4.34 – 4.78) | |||
| ReP | 0.71 | 0.29 | ||
| Bromacil | EC50 | 19.3 (17.7–21.0) | 3.56 (3.19 – 3.98) | 5.4 |
| EC10 | 4.89 (4.01–5.91) | 0.59 (0.45–0.75) | ||
| NEC | 5.53 (4.33 – 6.44) | |||
| ReP | 0.33 | 0.47 | ||
| Tebuthiuron | EC50 | 112 (106–119) | 16.0 (15.1–17.0) | 7.0 |
| EC10 | 27.5 (24.2–31.2) | 2.66 (2.31–3.06) | ||
| NEC | 22.7 (20.3–25.2) | |||
| ReP | 0.056 | 0.11 | ||
| Simazine | EC50 | 184 (173–195) | 59.2 (56.7–61.8) | 3.1 |
| EC10 | 38.4 (33.0–44.2) | 9.28 (8.41–10.2) | ||
| NEC | 48.0 (44.0–51.0) | |||
| ReP | 0.034 | 0.029 | ||
| Propazine | EC50 | 188 (177–201) | 39.5 (37.1–42.1) | 4.8 |
| EC10 | 42.0 (37.1–47.3) | 5.85 (4.90–6.91) | ||
| NEC | 27.8 (24.2 – 31.1) | |||
| ReP | 0.033 | 0.043 | ||
| Imazapic | EC50 | 790,000 (760,000–825,000) | >790,000 | NA |
| EC10 | 410,000 (362,000–462,000) | >790,000 | ||
| NEC | 363,000 (341,000–386,000) | |||
| ReP | NA | NA | ||
| Haloxyfop | EC50 | >3,700 | >3,700 | NA |
| EC10 | >3,700 | >3,700 | ||
| NEC | >3,700 | |||
| ReP | NA | NA | ||
| 2,4-D | EC50 | >279,000 | >279,000 | NA |
| EC10 | >279,000 | >279,000 | ||
| NEC | >279,000 | |||
| ReP | NA | NA |
Figure 1.
Concentration-response curves for ECx derivation. Sigmoidal, 4-parameter curve fit (solid line) and 95% confidence intervals (shaded area) on the relative percent inhibition of 3-day specific growth rate (SGR; full ring, mean ± SE) and 24 h effective quantum yield (ΔF/Fm′; open ring, mean ± SE) following herbicide exposure to (a) diuron; (b) metribuzin; (c) hexazinone; (d) bromacil; (e) tebuthiuron; (f) simazine; (g) propazine; and (h) imazapic at increasing concentrations. All concentrations in µg L−1 (n = 5 for each treatment, bars not visible are smaller than symbol).
Figure 2.
Concentration-response curves for NEC derivation. Bayesian non-linear gaussian model fit on the proportional decline in 3-day specific growth rate (SGR) relative to the control treatment (solid black line) and 95% confidence interval (black dashed line) to derive the no effect concentration (NEC) (red line) and 95% confidence interval (red dashed line) of (a) diuron; (b) metribuzin; (c) hexazinone; (d) bromacil; (e) tebuthiuron; (f) simazine; (g) propazine; and h) imazapic. All concentrations in µg L−1.
Effects of PSII inhibitor herbicides on effective quantum yield
Diuron, metribuzin, hexazinone, tebuthiuron, and bromacil, caused 100% steady-state inhibition of ΔF/Fm′ in R. salina after 24 h exposures (Fig. 1). Propazine and simazine did not reach 100% steady-state inhibition, peaking at a maximum of 90% inhibition of ΔF/Fm′ at the highest concentration tested (Fig. 1). A summary of the slope and goodness of fit of each concentration-response curve (Sigmoidal, 4 parameter model) for ΔF/Fm′ (Fig. 1) is shown in Table S2. The comparison of herbicide concentrations inhibiting ΔF/Fm′ by 50% (EC50) revealed the order of toxicity: diuron > metribuzin > bromacil > hexazinone > tebuthiuron > propazine > simazine (Table 3). Comparable patterns were observed for the order of potencies with respect to ΔF/Fm′ EC10 values (Table 3).
Toxicity of non-PSII inhibitor herbicides
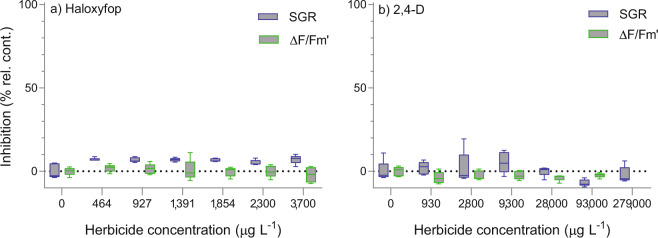
Imazapic inhibited R. salina SGR by 50% at a high concentration of 790,000 µg L−1, while the same concentration had no effect on ΔF/Fm′ (F (5,24) = 2.5, p = 0.06) (Fig. 1h, Table 3). Higher concentrations of imazapic caused a decrease in pH to <7.4, therefore effects of imazapic above this concentration were not considered in data analyses. SGR of R. salina showed significant differences between control and haloxyfop treatments (F (6,28) = 6.9, p < 0.001); however, inhibition effects across all haloxyfop treatments were consistent (5-7% inhibition) and no relationship between SGR and herbicide concentration between treatments was observed (F (5,24) = 1.1, p = 0.37) (Fig. 3a). ΔF/Fm′ of R. salina was not responsive to haloxyfop (F (6,28) = 0.58, p = 0.74) (Fig. 3a) at the maximum concentration of 3,700 µg L−1 (Fig. 3a), which was the highest concentration tested due to its low water solubility. SGR and ΔF/Fm′ of R. salina were nonresponsive to the synthetic auxin-inhibitor 2,4-D at the maximum concentration of 279,000 µg L−1 and 93,000 µg L−1 (Fig. 3b), respectively tested, and no significant differences between treatments by ANOVA (F (6,28) = 2.2, p = 0.07; F (5,28) = 1.5, p = 0.24, respectively) were detected.
Figure 3.
Response of Rhodomonas salina to (a) haloxyfop and (b) 2,4-D. Boxplots showing percent inhibition relative to control treatments in 3-day specific growth rate (SGR d−1) and 24 h effective quantum yield (ΔF/Fm′) (n = 5 for each treatment).
Relationship between inhibition of effective quantum yield and growth
The relationship between EC50 values for SGR and ΔF/Fm′ obtained for each PSII herbicide was compared in two ways, with both demonstrating that inhibition of ΔF/Fm′ was more sensitive than inhibition of growth. Firstly, we compared the EC50 ratios for SGR: ΔF/Fm′ which ranged from 1.5 – 7.0 and averaged 4.3 (Table 3). Secondly, we plotted the linear relationship (R2 = 0.87) of EC50 values for each herbicide for SGR and ΔF/Fm′ (Fig. 4) which yielded a slope of 3.48.
Figure 4.
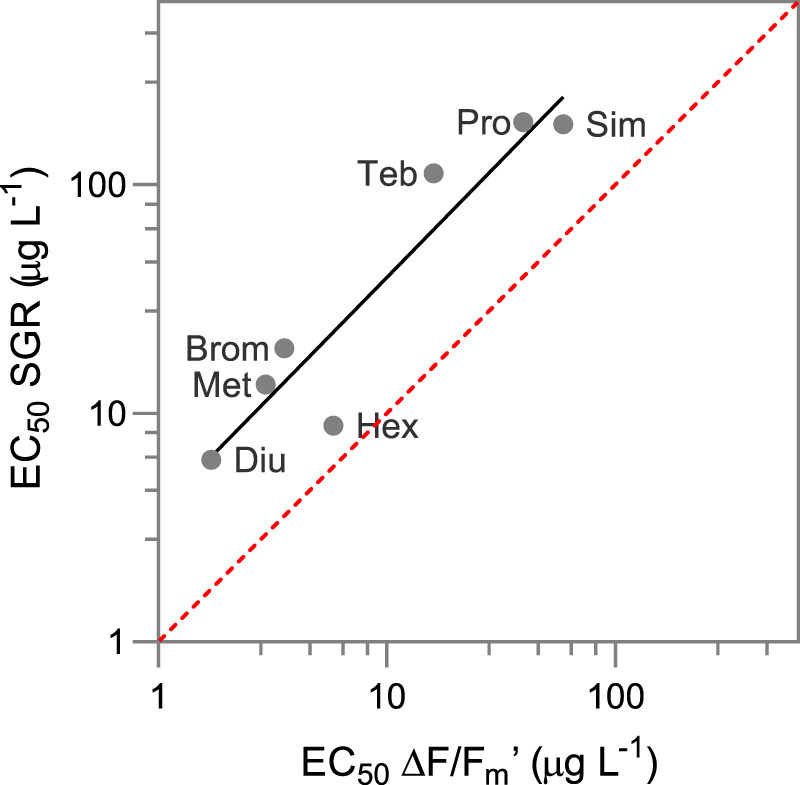
Linear relationship between effective quantum yield (ΔF/Fm′) and specific growth rate (SGR). Comparison of EC50 values (Slope = 3.48; R2 = 0.87) of seven PSII inhibitor herbicides (Diu – diuron, Met - metribuzin, Brom - bromacil, Hex - hexazinone, Teb - tebuthiuron, Pro - propazine, Sim - simazine). Dashed red line indicates 1:1 relationship.
Discussion
Toxicity effects of PSII inhibitor herbicides
Substantial reductions in both ΔF/Fm′ and SGR of R. salina were observed following exposure to all seven PSII inhibitor herbicides from four different chemical classes. Since Photosystem II is conserved across phototrophs53, the response of R. salina to the four classes of herbicides including phenylureas (diuron and tebuthiuron), triazines (simazine and propazine), triazinones (metribuzin and hexazinone) and uracils (bromacil) was expected. The toxicities of PSII inhibitor herbicides varied by over 20-fold with respect to inhibition of SGR and ΔF/Fm′, but a relationship between toxicity and chemical classes was not observed. For example, intra-class variations were wide within the phenylureas with diuron up to 18-times more toxic than tebuthiuron and similar disparities were evident within the triazinones (Table 3). Although all of these herbicides have the same mode of action, differences in PSII activity have been observed in a number of marine phototrophs21,24,28,47. Chesworth et al.54 suggested that herbicides with a greater affinity and faster rate of binding to the QB site accumulate more effectively leading to higher potencies of these herbicides. Furthermore, the binding of some herbicides to the QB site lowers the redox potential of the plastoquinone QA/QA- redox couple within PSII, resulting in increased photooxidative stress and subsequently a higher toxicant PSII activity55,56, potentially explaining some of the differences observed.
Comparative species sensitivity
Several studies investigating toxicity of herbicides to tropical marine algae have applied standard test species, such as the diatom Phaeodactylum tricornutum47,49,57 or symbionts of the family Symbiodiniaceae isolated from corals21,58. Toxicity values (EC10s and EC50s) from these studies and the present study are summarized in Table 4. While some ECx values are similar between species, others differ by up to an order of magnitude (Table 4). Some of these differences will be due to inherent difference is in cell structure, biochemistry and physiology between different species. For example, Millie et al.59 have shown that algae sensitivity to PSII inhibitor herbicides were related to differences in light-harvesting pigments under different light conditions. Guasch and Sabater60 reported that the toxicity of PSII inhibitors was lower for diatom species that were already adapted to low light conditions. In addition, Tang et al.61 observed higher herbicide sensitivity in chlorophytes compared to diatoms, suggesting some diatoms may apply an extra carbon fixation pathway, such as β-carboxylation that could compensate for the shutdown of PSII-based photosynthesis, and allow algal metabolism to continue62. The sensitivity of algal species to PSII inhibitor herbicides has also been shown to be affected by cell size63. Although most growth tests are relatively standardized, care should be taken before directly comparing toxicity values between studies, as even subtle differences in experimental exposure and conditions are likely to affect responses.
Table 4.
Toxicity values for Rhodomonas salina and other marine microalgae. Herbicide toxicity to microalgae including data from the United States Environmental Protection Agency (USEPA) ECOTOX Database94 and other publications using similar methods as those used in the present study (i.e. experimental conditions, ecological endpoint).
| Herbicide | Phyla | Species | Duration | Endpoint growth (µg L−1) | Endpoint ΔF/Fm′ (µg L−1) | Reference | ||
|---|---|---|---|---|---|---|---|---|
| EC10 | EC50 | EC10 | EC50 | |||||
| Metribuzin | Crypto-phyta | Rhodomonas salina | 3 d; 24 h | 2.7 | 13 | 0.60 | 3.0 | Present study |
| Ochro-phyta | Skeletonema costatum | 5 d | 88 | USEPA94 | ||||
| Bromacil | Crypto-phyta | Rhodomonas salina | 3 d; 24 h | 4.9 | 19 | 0.59 | 3.6 | Present study |
| Ochro-phyta | Skeletonema costatum | 5 d | 25 | USEPA94 | ||||
| Hexazinone | Crypto-phyta | Rhodomonas salina | 3 d; 24 h | 4.0 | 8.5 | 1.8 | 5.9 | Present study |
| Ochro-phyta | Navicula sp. | 3 d | 6.5 | 27 | 3.3 | 16 | Magnusson et al.23 | |
| Navicula sp. | 4 h | 1.4 | 5.7 | Magnusson et al.47 | ||||
| Phaeodactylum tricornutum | 4 h | 1.7 | 6.6 | Magnusson et al.47 | ||||
| Phaeodactylum tricornutum | 2 h | 2.9 | 22 | Muller et al.49 | ||||
| Cylindrotheca closteriuma | 4 h | 1.7 | 6.9 | Magnusson et al.47 | ||||
| Chloro-phyta | Nephroselmis pyriformis | 3 d | 4.8 | 10 | 2.1 | 6.2 | Magnusson et al.23 | |
| Nephroselmis pyriformis | 4 h | 0.47 | 2.4 | Magnusson et al.47 | ||||
| Dunaliella sp. | 24 h | 38 | Mercurio et al.58 | |||||
| Dino-flagellata | Symbiodinium sp. | 24 h | 46 | Mercurio et al.58 | ||||
| Symbiodinium sp. | 10 h | 8.8 | Jones and Kerswell21 | |||||
| Tebuthiuron | Crypto-phyta | Rhodomonas salina | 3 d; 24 h | 28 | 112 | 2.7 | 16 | Present study |
| Ochro-phyta | Skeletonema costatum | 5 d | 60 | USEPA94 | ||||
| Navicula sp. | 4 h | 17 | 94 | Magnusson et al.47 | ||||
| Phaeodactylum tricornutum | 4 h | 7.6 | 51 | Magnusson et al.47 | ||||
| Cylindrotheca closteriuma | 4 h | 10 | 77 | Magnusson et al.47 | ||||
| Chloro-phyta | Nephroselmis pyriformis | 4 h | 2.3 | 12 | Magnusson et al.47 | |||
| Dino-flagellata | Symbiodinium sp. | 10 h | 175 | Jones and Kerswell21 | ||||
| Simazine | Crypto-phyta | Rhodomonas salina | 3 d; 24 h | 38 | 184 | 9.3 | 59 | Present study |
| Ochro-phyta | Skeletonema costatum | 5 d | 60 | USEPA94 | ||||
| Ceratoneis closterium | 96 h | 310 | Hook et al.95 | |||||
| Phaeodactylum tricornutum | 3 d | 100 | 580 | Osborn and Hook57 | ||||
| Phaeodactylum tricornutuma | 4 h | 11 | 101 | Magnusson et al.47 | ||||
| Phaeodactylum tricornutum | 2 h | 18 | 400 | Muller et al.49 | ||||
| Navicula sp. | 4 h | 24 | 157 | Magnusson et al.47 | ||||
| Cylindrotheca closteriuma | 4 h | 35 | 242 | Magnusson et al.47 | ||||
| Chloro-phyta | Nephroselmis pyriformis | 4 h | 3.7 | 24 | Magnusson et al.47 | |||
| Dunaliella sp. | 24 h | 87 | Mercurio et al.58 | |||||
| Dino-flagellata | Symbiodinium sp. | 24 h | 84 | Mercurio et al.58 | ||||
| Symbiodinium sp. | 10 h | 150 | Jones and Kerswell21 | |||||
| Propazine | Crypto-phyta | Rhodomonas salina | 3 d; 24 h | 42 | 188 | 5.9 | 40 | Present study |
| Ochro-phyta | Skeletonema costatum | 5 d | 25 | USEPA94 | ||||
| Imazapic | Crypto-phyta | Rhodomonas salina | 3 d; 24 h | 410,000 | 790,000 | Present study | ||
| Ochro-phyta | Skeletonema costatum | 5 d | <45 | USEPA94 | ||||
| Nephroselmis pyriformis | 3,5,10 d | <1,455 | Magnusson et al.69 | |||||
| Navicula sp. | 3,5,10 d | <1,455 | Magnusson et al.69 | |||||
| Haloxyfop | Crypto-phyta | Rhodomonas salina | 3 d; 24 h | >3,700 | >3,700 | >3,700 | >3,700 | Present study |
| 2,4-D | Crypto-phyta | Rhodomonas salina | 3 d; 24 h | >279,000 | >279,000 | >93,000 | >93,000 | Present study |
| Ochro-phyta | Skeletonema costatum | 5 d | >2,000 | USEPA94 | ||||
| Chaetoceros calcitrans | 21 d | 9,200 | His and Seaman96 | |||||
| Chloro-phyta | Dunaliella tertiolecta | 1 h | 160,220 | Mcfeters97 | ||||
| Dunaliella tertiolecta | 24 h | 246,500 | Mcfeters97 | |||||
Relationship between inhibition of effective quantum yield and growth
The inhibition of R. salina growth was on average 4-times less sensitive to PSII herbicide exposures than the photoinhibition endpoint (ratios of EC50s SGR: ΔF/Fm′ for each herbicide can be found in Table 2). The correlation plot of EC50 values for both endpoints had a slope of 3.5, also showing a greater sensitivity of ΔF/Fm′ to PSII inhibitor herbicides. Magnusson et al.23 had demonstrated a relationship between SGR and ΔF/Fm′ inhibition by PSII inhibitor herbicides that was closer to 1:1 for two tropical benthic microalgae; Navicula sp. and Nephroselmis pyriformis. Both studies clearly demonstrated a link between inhibition in ΔF/Fm′ and decreasing growth rates; however, the direct link between the binding of PSII inhibitor herbicides to the D1 protein (reducing electron transport and causing damage to PSII) with growth is not necessarily expected to be 1:1 for all taxa and experimental conditions. Light intensity and light acclimation history have large influences on the relationships between photophysiology, primary production and growth64. Furthermore, it has been shown that the pigment structures in some microalgae, such as red algae (rhodophytes) can shift between PSI and PSII, potentially affecting the path of electron transport65,66 and direct quantitative links between ΔF/Fm′, primary production, and SGR may be less certain in rhodophytes than for some other phototrophs. Cryptophytes also contain phycobiliproteins, the characteristic antennae pigments of the prokaryotic cyanobacteria and the eukaryotic rhodophytes67. The presence of phycobiliproteins in cryptophytes may allow shifting between PSI and PSII in R. salina although, phycobiliproteins are only present in the thylakoid lumen and as phycoerythrin68. While Magnusson et al.23 measured effects on both growth and ΔF/Fm′ over 3 d, our comparison was between a chronic 3-d growth test and an acute 24-h ΔF/Fm′ test (as effects of PSII inhibitor herbicides on microalgae typically peak before 6 h and remain consistent over longer periods69). While these differences in exposure durations make direct comparisons between the techniques (and against prior studies) more difficult, the exposure durations are optimal for each test type and the linear relationship between effects of multiple PSII inhibitor herbicides on photosynthetic efficiency and growth remains strong. The consistency of these results for a variety of marine microalgae and reported in this study for the marine cryptophyte, in combination with the direct mechanistic link between ΔF/Fm′ and SGR for PSII inhibitor herbicides, suggests that ΔF/Fm′ provides a robust and sensitive endpoint for determining sub-lethal effect thresholds for these herbicides.
Toxicity effects of non-PSII inhibitor herbicides
R. salina was far more sensitive to the PSII inhibitor herbicides than the non-PSII inhibitor herbicides tested here. R. salina was insensitive to non-PSII inhibitor herbicides within the phenoxy family, haloxyfop and 2,4-D at the highest concentrations tested. Growth regulator herbicides, such as 2,4-D, inhibit the plant hormone auxin and are primarily used as selective herbicides for controlling broadleaves (dicots)43. This pathway is not present in cryptophytes, explaining the lack of toxicity of 2,4-D to R. salina. Previous studies reported similar observations of the low toxicity of 2,4-D on the growth rate of marine microalgae (Table 4), with the most sensitive species the diatom Chaetoceros calcitrans (21 d, EC50 = 9.2 mg L−1). R. salina was also less responsive to haloxyfop, which targets the acetyl-CoA carboxylase enzyme involved in the synthesis of lipids and fatty acids in plants44. ACCase inhibitors target the homomeric (eukaryotic) form of the enzyme rather than the heteromeric (prokaryotic) form44 and microalgae, such as rhodophytes and chlorophytes contain the heteromeric ACCase enzyme in their plastids70, likely explaining the insensitivity towards haloxyfop in cryptophytes. Imazapic was only toxic to R. salina at high concentrations. Imazapic inhibits the activity of the enzyme acetohydroxy acid synthase (AHAS or ALS), which is responsible for catalyzing the production of several branched-chain aliphatic amino acids across many aquatic phototrophs71. Other marine microalgae are similarly insensitive to imazapic, and the sensitivity to imazapic of enzyme variants in marine microalgae are unknown. No effect on the growth rates of the marine microalgae Navicula sp. and Nephroselmis pyriformis were observed after 10 d exposure at concentrations of up to 1.5 mg L−1 69. Conversely, imazapic is far more toxic to freshwater phototrophs. For example, imazapic has an EC50 of 6.1 µg L−1 for growth in the freshwater macrophyte Lemna gibba (duckweed)72. In macrophytes, imazapic is absorbed through the roots and shoots of plants, possibly explaining the lower toxicity of imazapic to microalgae71. Another factor to consider with respect to the sensitivity of marine species is whether the structure of imazapic may affect its exposure and bioavailability in seawater. Imazapic contains a carboxylic acid (COOH) which may result in complexation with Mg2+ and Ca2+ ions in the seawater73, or stabilize the herbicide at the seawater:air interface74. Both mechanisms could reduce the exposure and bioavailability of imazapic to marine species accounting for the low toxicities reported.
Toxicity thresholds for guideline development
Water quality guidelines are usually developed using SSDs75 and ideally from NEC or EC10 values for multiple diverse taxa45. However, most current herbicide WQGVs for marine communities are of low reliability (e.g. developed from toxicity data for as few as five species), or have not yet been developed due to the lack of data39. Currently, WQGVs exist only for the five priority herbicides (diuron, atrazine, ametryn, tebuthiuron, hexazinone) and four alternative herbicides (bromacil, MCPA, simazine, and 2,4-D)39 (Table 1). There are no WQGVs for metribuzin, propazine, haloxyfop, and imazapic. A comparison of the existing ANZG WQGVs39 and proposed guideline values (PGVs)43,44 against herbicide toxicity thresholds (SGR: NEC and EC10 values) for R. salina is presented in Table 1. The NEC and EC10 values for hexazinone and bromacil were far lower than current PC99 WQGVs; however, the PC99 PGVs would all be protective of R. salina (Table 1). Nevertheless, most of the PGVs are of very low to moderate reliabiltiy43,44 (Table 1) and could be improved by the incorporation of toxicity data from additional species, such as R. salina. Apart from diuron (the most toxic herbicide tested in this study), the NEC and EC10 values were all greater than 2 µg L−1 and above concentrations that have been detected in tropical coastal waters13,16,17,37. However, the risks posed by these herbicides should not be assessed individually as they are usually detected in complex mixtures of multiple herbicides. Instead, their contribution to the total risk can be assessed using ms-PAF42, which accounts for all herbicides that have reliable SSDs (and WQGVs). The ms-PAF method has also been extended to include the additional influence of heatwave conditions on WQGVs for pesticides76. The exceedance of PC99 values for herbicide mixtures has recently been reported in water quality monitoring programs using ms-PAF, where individual herbicide did not exceed their own PC99 values37. The development of SSDs for alternative herbicides detected in the GBR using relevant toxicity data (such as the R. salina data presented here) will allow their contribution in predicting the cumulative risks of herbicide mixtures using ms-PAF.
Conclusion
Alternative herbicides may be practical substitutes for controlling weeds; however, their toxicity to non-target species such as R. salina could contribute to the combined risks posed by herbicide mixtures regularly detected in coastal waters in the tropics. In the present study, exposures of R. salina to increasing herbicide concentrations resulted in inhibition of ΔF/Fm′ within 24 h, indicating reduced photosynthetic efficiency which led to reduced growth rates over 72 h chronic exposures. Photoinhibition was a more sensitive endpoint over 24 h than inhibition of growth over 72 h; however, the relationship between inhibition of ΔF/Fm′ and SGR was linear and consistent. Importantly, the non-PSII inhibitor herbicides (imazapic, 2,4-D, haloxyfop) were substantially less toxic than the most toxic PSII inhibitor herbicides, indicating these herbicides pose little risk to this microalga in the marine environment. The toxicity thresholds (NECs and EC10s) derived here were higher than concentrations detected in tropical marine waters. However, the risk posed by these herbicides to marine species is better assessed by comparing measured values in the field against high-reliability WQGVs that are derived from SSDs. The current study contributes targeted data towards developing SSDs for alternative herbicides that are essential to improve predictions of the cumulative ecological risks posed by herbicide mixtures (using ms-PAF) detected in marine monitoring programs. While this study targeted some of the most frequently detected alternative herbicides in GBR waters, there remains a number of pesticides, including insecticides and fungicides with no current WQGVs and further testing is needed to address this.
Methods
Test species and culture conditions
The cryptophyte Rhodomonas salina (Wislouch)77 (CS 24/01) was purchased from the Australian National Algae Supply Service, Hobart (CSIRO). Cryptophytes are an important component of the primary producers in both freshwater and marine habitats, and changes in their abundance, composition and nutritional value may initiate an indirect bottom-up effect on higher trophic levels78. Many species are widespread and abundant in the sea within wide temperature ranges (5–29 °C), which make this phylum highly suitable for acute and chronic toxicity tests in a short period of time under both temperate and tropical conditions79–81. Cultures of R. salina were established three weeks prior to experimentation in Guillard’s f2 marine medium (0.5 mL of AlgaBoost F/2, AusAqua in 1 L sterile 0.5 µm-filtered seawater (FSW; pH 8.0, salinity 35.0 psu))82. Cultures were maintained in sterile 500 mL Erlenmeyer flasks as batch cultures in exponential growth phase with twice-weekly transfers of 70 mL of a 3- to 4-day-old R. salina suspension to 300 mL f2 medium under sterile conditions. Clean culture solutions were aerated and maintained at 26 ± 1 °C and under a 12:12 h light:dark cycle (90-100 μmol photons m–2 s–1, Osram Lumilux Cool White 36 W).
Herbicide stock preparation
Herbicide stock solutions were prepared using PESTANAL (Sigma-Aldrich) analytical grade products (HPLC ≥ 98%): diuron (CAS 330-54-1), metribuzin (CAS 21087-64-9), hexazinone (CAS 51235-04-2), tebuthiuron (CAS 34014-18-1), bromacil (CAS 314-40-9), propazine (CAS 139-40-2), simazine (CAS 122-34-9), imazapic (CAS 104098-48-8), haloxyfop (CAS 72619-32-0), 2,4-D (CAS 94-75-7). The selection of herbicides was based on consultation with the Queensland DES and detection frequency in coastal waters of the GBR17,37. Stock solutions were prepared in sterile 1 L Schott glass bottles using ultra-pure water (milli-Q, Millipore) or sterile 0.5 µm-FSW. Diuron and simazine were dissolved using HPLC-grade ethanol (<0.001% (v/v) in exposures). Haloxyfop was dissolved in dimethyl sulfoxide (DMSO) (≤ 0.006% (v/v) in exposure). No solvent carrier was used for the preparation of the remaining herbicide stock solutions. A summary of herbicide stocks, solvent carriers, nominal and measured concentrations, as well as chemical properties of the tested herbicides can be found in Table S1.
Toxicity test protocol
Cultures of R. salina were exposed to a range of herbicide concentrations over a period of 72 h. Inoculum was taken from cultures in the exponential growth phase (4-day-old with cell density of approximately 1 × 106 cell mL−1). Prior to the inoculation of the test solutions, 15 mL of algae suspension (of the 4-day-old algal culture) was washed in 30 mL sterile 0.5 µm-FSW by centrifuge in 50 mL Falcon tubes at 1500 g for 5 minutes (Eppendorf Centrifuge 5810 R, Bio-strategy). The supernatant was decanted, and the cell pellet re-suspended in 30 mL of sterile 0.5 µm-FSW and homogenized by vortexing. This process was repeated three times to remove the nutrient-enriched f2 culture medium, which might affect herbicide toxicity83–86. The cell pellet was finally re-suspended in about 15 mL of sterile 0.5 µm-FSW. The cell density of the concentrated algae suspension was measured from two 500 µL sub-samples by flow cytometry. The desired inoculum was calculated to a given starting cell density of 3 × 103 cells mL−1 in the following toxicity test. Individual R. salina working suspensions for each herbicide treatment were prepared in individual 100 mL Schott glass bottles by adding the required algae inoculum and sterile 0.5 µm-FSW. Each Schott glass bottle was finally dosed with a range of herbicide concentrations (Table S1). Bioassays for each herbicide were performed on different days with fresh algae, FSW and herbicide stocks. In each bioassay, a control (no herbicide) and reference (diuron, 4 µg L−1) treatment was added to ensure the response is reproducible. Diuron was chosen as a reference toxicant as it is a widespread contaminant and its toxic mode of action (PSII inhibition) and toxicity to a wide variety of microalgae are well understood (see Magnusson et al.23).
Five replicated aliquots of 10 mL were transferred from the individual 100 mL Schott glass bottles into sterile 20 mL glass scintillation vials and incubated at 26.0 ± 0.6 °C under a 12:12 h light:dark cycle at 90-100 μmol photons m–2 s–1 (Osram Lumilux Cool White 36 W). Vials were randomized and swirled daily. Sub-samples of 500 µL were taken from each replicate to measure cell densities of algal populations at 0 h and 72 h using a flow cytometer (BD Accuri C6, BD Biosciences, CA, USA) equipped with red and blue lasers (14.7 mW 640 nm Diode Red Laser 20 mW 488 nm Solid State Blue Laser) and standard filter setup. The flow rate was set to 35 µL min−1, 16-µm core size with a sample volume of 50 µL. Cell densities were obtained by plotting a two-dimensional cytogram. A fixed gating was used around the viable (chlorophyll fluorescing) cells, which allowed for differentiation of non-algal particles (debris) and dead cells from viable R. salina cells. Viable cells typically represented 75 - 95% of particles counted (control treatment at 72 h). Each 500 µL sub-sample was analyzed by the flow cytometer two times and an average taken of the number of events that occur within the gated region. This process was then repeated for each replicate per treatment. Specific growth rates (SGR) were expressed as the logarithmic increase in cell density from day i (ti) to day j (tj) as per Eq. (1), where SGRi-j is the specific growth rate from time i to j; Xj is the cell density at day j and Xi is the cell density at day i87.
| 1 |
SGR relative to the control treatment was used to derive chronic effect values for growth inhibition. A test was considered valid if the SGR of control replicates was ≥0.92 day−1 87.
Chlorophyll fluorescence measurements
Acute effects of herbicides on the photophysiology of R. salina, measured by chlorophyll fluorescence as the effective quantum yield (ΔF/Fm′), were investigated in non-pyrogenic polystyrene 48 well-plates with lid (Nunclon Delta, Thermo Scientific) using imaging PAM fluorometry (I-PAM, Walz, Germany)46,88, following an exposure period of 24 h at an irradiance of 90-100 μmol photons m–2 s–1. Inoculum was taken from mother cultures in the exponential growth phase (4-day-old with cell density of approximately 1 × 106 cell mL−1). Initial testing of varying cell densities indicated that consistent ΔF/Fm′ measurement signals >0.4546 were obtained at a starting cell density of 3.5 × 105 cells mL−1 (equivalent to cell density after ~3 d in the SGR inhibition test). Individual R. salina working suspensions for each herbicide treatment were prepared in individual 50 mL Schott glass bottles by adding algae inoculum and sterile f2 (0.5 µm-FSW) marine medium. Each 50 mL Schott glass bottle was finally dosed with a range of herbicide concentrations (Table S1). Five replicated aliquots of 1 mL were transferred from the individual 50 mL Schott glass bottles across two 48-well plates (randomly) and incubated at 26.0 ± 0.6 °C under a 12:12 h light:dark cycle at 90-100 μmol photons m–2 s–1 (Osram Lumilux Cool White 36 W). Replicated seawater controls (SWC) (n = 5) or solvent controls (SC) and diuron references (4 µg L−1) were included randomly across each 48-well plate to ensure consistency in inhibition response between replicated algae cultures. Light adapted minimum fluorescence (F) and maximum fluorescence (Fm′) were determined and effective quantum yield was calculated for each treatment as per Eq. (2) 88. The timing of plate preparation and measurements were staggered to ensure a consistent exposure duration of 24 h. Imaging PAM settings were set to actinic light = 1 (corresponding to photosynthetically active radiation (PAR) of 90-100 μmol photons m−2 s−1), measuring intensity = 11, gain = 3; damp = 2.
| 2 |
A screening process of plates containing algae suspension only was performed immediately prior to exposure with herbicides to ensure that ΔF/Fm′ > 0.45.
Physicochemical analyses
Physico-chemical water quality parameters including pH and salinity (LAQUAact-PC110 Meter, HORIBA Scientific) and dissolved oxygen (HQ30D Portable Meter, HACH) were measured from individual 100 mL Schott glass bottles at 0 h and replicated 20 mL glass scintillation vials pooled for each concentration at 72 h. Temperature was logged in 10-min intervals over the total test duration (HOBO, Onset). Analytical samples were also taken from individual 100 mL Schott glass bottles at 0 h and replicated 20 mL glass scintillation vials pooled for each concentration at 72 h. Aliquots (1 mL) were transferred into 1.5 mL Liquid Chromatography amber glass vials and spiked with surrogate standards (i.e. diuron-D6, hexazinone-D6, metribuzin-D3, simazine-D10, propazine-D6, bromacil-D3, haloxyfop-D4, 2,4-D-13C6, and imazapic-D7) at a final concentration of 10 ng mL−1. Prior to analysis samples were stored at −20 °C, defrosted and centrifuged. Herbicide concentrations were determined by HPLC-MS/MS using a SCIEX Triple Quad 6500 QTRAP mass spectrometer (SCIEX, Concord, Ontario, Canada) equipped with a TurboIonSpray probe10,89,90. The mass spectrometer was coupled to a Shimadzu Nexera X2 uHPLC system (Shimadzu Corp., Kyoto, Japan) using a Phenomenex Kinetex Biphenyl column (2.6 μm 50 ×2.1 mm 100 Å) for analyte separation. Five μL of sample was injected on to the column followed by a linear gradient starting at 10% B for 0.5 min, ramped to 100% B in 4.7 min then held at 100% for 4.0 min followed by equilibration at 10% B for 3.0 min (A = 1% methanol in milli-Q water, B = 95% methanol in milli-Q water, both containing 0.1% acetic acid). The mass spectrometer was operated in both positive and negative ion mode using a scheduled multiple reaction-monitoring method (sMRM). Positive samples were confirmed by retention time and by comparing transition intensity ratios between the sample and an appropriate calibration standard from the same run. To provide estimates of ‘measured’ concentrations used for concentration-response modelling the geometric mean from measured start and end concentrations (time-weighted average) was assigned as the ‘actual’ concentration in that sample. The average loss from these measured concentrations was then applied to all nominal concentrations.
Statistical analyses
All statistical analyses were based on measured herbicide concentrations. Mean percent inhibition in SGR and ΔF/Fm′ of each treatment relative to the control treatment was calculated as per Eq. (3) 87, where Xcontrol is the average SGR or ΔF/Fm′ of control and Xtreatment is the average SGR or ΔF/Fm′ of single treatments.
| 3 |
Nonlinear regression (Sigmoidal, 4-parameter) was used to produce concentration-response curves for each herbicide test (GraphPad Prism V 8.0.). Effective concentrations inhibiting ΔF/Fm′ and SGR by 10% and 50% with 95% confidence intervals (EC10/EC50) relative to the control were interpolated from the equations of the curve fit. One-factor analysis of variance (ANOVA with replicates) was used to determine if there were significant differences (p < 0.05) in algal SGR rates and ΔF/Fm′ samples between various herbicide treatments. The relative potencies of the herbicides were determined using the relative equivalent potencies (ReP) compared to the reference herbicide diuron (EC50 diuron/EC50 herbicide)23. ReP values > 1 indicate potencies proportionally greater than diuron and ReP values < 1 indicate potencies less than diuron.
The estimation of no effect concentrations (NEC) was calculated in R (Version 3.6.1). The proportional decline in SGR (1-inhibition) was modelled as a function of log concentration of each herbicide using a Bayesian non-linear gaussian model using the R package jagsNEC91. This model has been specifically developed to derive no effect concentrations (NECs) but also allows the estimation of EC10 and EC50 values and is adapted from Fox92, and more generally defined by Eq. (4) 92:
| 4 |
E[Yi|xi] is the mathematical expectation of Yi (the response, e.g. in this case, the proportional decline in SGR) conditional on a given concentration xi. The model parameters for the generalized case are α (the response at zero or low concentrations, also called ‘top’), −β (the rate of decay in the response after the NEC) and γ (the NEC value)92. For a gaussian Y, as used here, the model has the additional parameters Δ (an offset or intercept) and σ (the random error variance in Y). We used un-informative priors for the model parameters, including: α ~ dnorm(0, 0.1), β ~ dgamma(0.0001,0.0001), γ ~ dnorm(0, 0.01), Δ ~ dnorm(0, 0.1), and σ ~dunif(0, 29). Note that in jags dnorm is parameterized as a mean and precision (rather than mean and SD, as in R). Models were run with 10,000 Markov chain Monte Carlo (MCMC) iterations after an initial ‘burn-in’ period of 20000 iterations and for five separate chains. Trace plots were used to evaluate model fits and were found to have relatively good mixing in all cases.
Supplementary information
Acknowledgements
We thank Carlos Alvarez-Roa from the Symbiont Culture Facility at the Australian Institute of Marine Science and Tom Barker for providing expertise for algae cultivation. Further, we would like to thank Gerard Ricardo for his advice regarding statistical analysis. This research was supported by the Australian Government’s National Environmental Science Program (NESP) Tropical Water Quality Hub Project 3.1.5 Ecotoxicology of pesticides on the Great Barrier Reef for guideline development and risk assessments. M.C.T. was supported by AIMS@JCU: Australian Institute of Marine Science, College of Marine and Environmental Sciences, James Cook University.
Author contributions
M.C.T., F.F. and A.P.N. designed the experiment. M.C.T. and F.F. performed experiments. M.C.T. analyzed the data with input from F.F., R.F. and A.P.N. S.K. preformed physicochemical analyses of samples. M.C.T. wrote the manuscript with input from A.P.N. as well as F.F., R.F. and S.K.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-64116-y.
References
- 1.Basheer C, Obbard JP, Lee HK. Persistent organic pollutants in Singapore’s coastal marine environment: part I, seawater. Water, Air, Soil Pollut. 2003;149:295–313. doi: 10.1023/A:1025689600993. [DOI] [Google Scholar]
- 2.Ali HR, et al. Contamination of diuron in coastal waters around Malaysian Peninsular. Mar. Pollut. Bull. 2014;85:287–291. doi: 10.1016/j.marpolbul.2014.05.049. [DOI] [PubMed] [Google Scholar]
- 3.Okamura H, Aoyama I, Ono Y, Nishida T. Antifouling herbicides in the coastal waters of western Japan. Mar. Pollut. Bull. 2003;47:59–67. doi: 10.1016/S0025-326X(02)00418-6. [DOI] [PubMed] [Google Scholar]
- 4.Roche, H., Salvat, B. & Ramade, F. Assessment of the pesticides pollution of coral reefs communities from French Polynesia. Rev. Ecol. (2011).
- 5.Sarkar SK, et al. Occurrence, distribution and possible sources of organochlorine pesticide residues in tropical coastal environment of India: an overview. Environ. Int. 2008;34:1062–1071. doi: 10.1016/j.envint.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Castillo LE, de la Cruz E, Ruepert C. Ecotoxicology and pesticides in tropical aquatic ecosystems of Central America. Environ. Toxicol. Chem. 1997;16:41–51. doi: 10.1002/etc.5620160104. [DOI] [Google Scholar]
- 7.Hernández-Romero AH, Tovilla-Hernández C, Malo EA, Bello-Mendoza R. Water quality and presence of pesticides in a tropical coastal wetland in southern Mexico. Mar. Pollut. Bull. 2004;48:1130–1141. doi: 10.1016/j.marpolbul.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Carbery K, Owen R, Frickers T, Otero E, Readman J. Contamination of Caribbean coastal waters by the antifouling herbicide Irgarol 1051. Mar. Pollut. Bull. 2006;52:635–644. doi: 10.1016/j.marpolbul.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy K, et al. The influence of a season of extreme wet weather events on exposure of the World Heritage Area Great Barrier Reef to pesticides. Mar. Pollut. Bull. 2012;64:1495–1507. doi: 10.1016/j.marpolbul.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Mercurio P, et al. Degradation of herbicides in the tropical marine environment: Influence of light and sediment. Plos One. 2016;11:e0165890. doi: 10.1371/journal.pone.0165890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lewis, S. E. et al. Using monitoring data to model herbicides exported to the Great Barrier Reef, Australia. In: The 19th International Congress on Modelling and Simulation. Modelling and Simulation Society of Australia and New Zealand. MODSIM2011, 2051–2056 (2011).
- 12.Shaw M, et al. Monitoring pesticides in the Great Barrier Reef. Mar. Pollut. Bull. 2010;60:113–122. doi: 10.1016/j.marpolbul.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 13.Grant, S. et al. Marine Monitoring Program: Annual Report for inshore pesticide monitoring 2015–2016. Report for the Great Barrier Reef Marine Park Authority, Great Barrier Reef Marine Park Authority, Townsville, Australia. (2017).
- 14.Devlin, M. M. et al. Advancing our understanding of the source, management, transport and impacts of pesticides on the Great Barrier Reef 2011–2015. Report for the Queensland Department of Environment and Heritage Protection. Tropical Water & Aquatic Ecosystem Research (TropWATER) Publication, James Cook University, Cairns, Australia. (2015).
- 15.GBRMPA. Reef 2050 integrated monitoring and reporting program: strategy update 2018. Great Barrier Reef Marine Park Authority, Townsville, Australia, http://www.environment.gov.au/marine/gbr/publications/reef-2050-long-term-sustainability-plan-2018 (2018).
- 16.Lewis SE, et al. Herbicides: a new threat to the Great Barrier Reef. Environ. Pollut. 2009;157:2470–2484. doi: 10.1016/j.envpol.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien D, et al. Spatial and temporal variability in pesticide exposure downstream of a heavily irrigated cropping area: application of different monitoring techniques. J. Agric. Food Chem. 2016;64:3975–3989. doi: 10.1021/acs.jafc.5b04710. [DOI] [PubMed] [Google Scholar]
- 18.Radcliffe, J. Pesticide use in Australia. A review undertaken by the Australian Academy of Technological Sciences, Victoria, Australia, https://www.atse.org.au/ (2002).
- 19.Oettmeier, W. Herbicides of photosystems II. In: Barber, J., 1992. Structure, Function and Molecular Biology. Elsevier, 349–408, 10.1016/B978-0-444-89440-3.50018-7 (1992).
- 20.Negri A, et al. Effects of the herbicide diuron on the early life history stages of coral. Mar. Pollut. Bull. 2005;51:370–383. doi: 10.1016/j.marpolbul.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 21.Jones RJ, Kerswell AP. Phytotoxicity of photosystem II (PSII) herbicides to coral. Mar. Ecol. Prog. Ser. 2003;261:149–159. doi: 10.3354/meps261149. [DOI] [Google Scholar]
- 22.Bengston-Nash SM, Quayle PA, Schreiber U, Muller JF. The selection of a model microalgal species as biomaterial for a novel aquatic phytotoxicity assay. Aquat. Toxicol. 2005;72:315–326. doi: 10.1016/j.aquatox.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Magnusson M, Heimann K, Negri AP. Comparative effects of herbicides on photosynthesis and growth of tropical estuarine microalgae. Mar. Pollut. Bull. 2008;56:1545–1552. doi: 10.1016/j.marpolbul.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Negri AP, Flores F, Röthig T, Uthicke S. Herbicides increase the vulnerability of corals to rising sea surface temperature. Limnol. Oceanogr. 2011;56:471–485. doi: 10.4319/lo.2011.56.2.0471. [DOI] [Google Scholar]
- 25.van Dam JW, Negri AP, Mueller JF, Uthicke S. Symbiont-specific responses in foraminifera to the herbicide diuron. Mar. Pollut. Bull. 2012;65:373–383. doi: 10.1016/j.marpolbul.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Haynes D, Ralph P, Prange J, Dennison B. The impact of the herbicide diuron on photosynthesis in three species of tropical seagrass. Mar. Pollut. Bull. 2000;41:288–293. doi: 10.1016/s0025-326x(00)00127-2. [DOI] [Google Scholar]
- 27.Flores, F., Collier, C. J., Mercurio, P. & Negri, A. P. Phytotoxicity of four photosystem II herbicides to tropical seagrasses. Plos One8, 10.1371/journal.pone.0075798 (2013). [DOI] [PMC free article] [PubMed]
- 28.Wilkinson AD, Collier CJ, Flores F, Negri AP. Acute and additive toxicity of ten photosystem-II herbicides to seagrass. Sci. Rep. 2015;5:17443. doi: 10.1038/srep17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ralph PJ. Herbicide toxicity of Halophila ovalis assessed by chlorophyll a fluorescence. Aquat. Bot. 2000;66:141–152. doi: 10.1016/S0304-3770(99)00024-8. [DOI] [Google Scholar]
- 30.Negri AP, Flores F, Mercurio P, Mueller JF, Collier CJ. Lethal and sub-lethal chronic effects of the herbicide diuron on seagrass. Aquat. Toxicol. 2015;165:73–83. doi: 10.1016/j.aquatox.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Cantin NE, Negri AP, Willis BL. Photoinhibition from chronic herbicide exposure reduces reproductive output of reef-building corals. Mar. Ecol. Prog. Ser. 2007;344:81–93. doi: 10.3354/meps07059. [DOI] [Google Scholar]
- 32.King J, Alexander F, Brodie J. Regulation of pesticides in Australia: the Great Barrier Reef as a case study for evaluating effectiveness. Agric., Ecosyst. Environ. 2013;180:54–67. doi: 10.1016/j.agee.2012.07.001. [DOI] [Google Scholar]
- 33.Davis A, Lewis S, Brodie J, Benson A. The potential benefits of herbicide regulation: a cautionary note for the Great Barrier Reef catchment area. Sci. Total Environ. 2014;490:81–92. doi: 10.1016/j.scitotenv.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Duggleby RG, McCourt JA, Guddat LW. Structure and mechanism of inhibition of plant acetohydroxyacid synthase. Plant Physiol. Biochem. 2008;46:309–324. doi: 10.1016/j.plaphy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Shaner DL, Anderson PC, Stidham MA. Imidazolinones: potent inhibitors of acetohydroxyacid synthase. Plant Physiol. 1984;76:545–546. doi: 10.1104/pp.76.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Secor J, Cséke C. Inhibition of acetyl-CoA carboxylase activity by haloxyfop and tralkoxydim. Plant Physiol. 1988;86:10–12. doi: 10.1104/pp.86.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gallen, C. et al. Marine Monitoring Program: Annual Report for inshore pesticide monitoring 2017–18. Report for the Great Barrier Reef Marine Park Authority, Great Barrier Reef Marine Park Authority, Townsville, 118 pp, http://elibrary.gbrmpa.gov.au/jspui/handle/11017/3489 (2019).
- 38.Brodie J, et al. Terrestrial pollutant runoff to the Great Barrier Reef: an update of issues, priorities and management responses. Mar. Pollut. Bull. 2012;65:81–100. doi: 10.1016/j.marpolbul.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 39.ANZG. Revised Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Environment and Conservation Council and Agriculture and Resource Management Council of Australia and New Zealand, Canberra, Australia, https://www.waterquality.gov.au/anz-guidelines/guideline-values/default/water-quality-toxicants/toxicants (accessed 10 March 2020) (2018).
- 40.CCME. Canadian water quality guidelines for the protection of aquatic life. Canadian Council of Ministers of the Environment, Winnipeg, Canada, https://www.ccme.ca/en/resources/canadian_environmental_quality_guidelines/index.html (accessed 26 May 2019) (2014).
- 41.EU. Directive 2013/39/EU of the european parliament and of the council of 12 August 2013 amending directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Official J. Eur. Union 226, http://data.europa.eu/eli/dir/2013/2039/oj (2013).
- 42.Traas, T. P. et al. The potentially affected fraction as a measure of ecological risk in Posthuma, L., Suter, G. W. II, Traas, T. P., eds, Species Sensitivity Distributions in Ecotoxicology. Lewis, Boca Raton, FL, USA, pp 315–344. (2002).
- 43.King, O., Smith, R., Mann, R. & Warne, M. St. J. Proposed aquatic ecosystem protection guideline values for pesticides commonly used in the Great Barrier Reef catchment area: Part 1 (amended) - 2,4-D, Ametryn, Diuron, Glyphosate, Hexazinone, Imazapic, Imidacloprid, Isoxaflutole, Metolachlor, Metribuzin, Metsulfuron-methyl, Simazine, Tebuthiuron. Department of Environment and Science, Brisbane, Australia. 296 pp, https://www.publications.qld.gov.au/dataset/proposed-guideline-values-27-pesticides-used-in-the-gbr-catchment (2017).
- 44.King, O., Smith, R., Warne, M. St. J. & Mann, R. Proposed aquatic ecosystem protection guideline values for pesticides commonly used in the Great Barrier Reef catchment area: Part 2 - Bromacil, Chlorothalonil, Fipronil, Fluometuron, Fluroxypyr, Haloxyfop, MCPA, Pendimethalin, Prometryn, Propazine, Propiconazole, Terbutryn, Triclopyr and Terbuthylazine. Department of Science, Information Technology and Innovation, Brisbane, Australia. 211 pp, https://www.publications.qld.gov.au/dataset/proposed-guideline-values-27-pesticides-used-in-the-gbr-catchment (2017).
- 45.Warne, M. St. J. et al. Revised Method for Deriving Australian and New Zealand Water Quality Guideline Values for Toxicants – update of 2015 version. Prepared for the revision of the Australian and New Zealand Guidelines for Fresh and Marine Water Quality. Australian and New Zealand Governments and Australian state and territory governments, Canberra, Australia. 48 pp, https://www.waterquality.gov.au/anz-guidelines/guideline-values/derive/warne-method-derive, 10.13140/RG.2.2.36577.35686 (2018).
- 46.Schreiber U, Quayle P, Schmidt S, Escher BI, Mueller JF. Methodology and evaluation of a highly sensitive algae toxicity test based on multiwell chlorophyll fluorescence imaging. Biosens. Bioelectron. 2007;22:2554–2563. doi: 10.1016/j.bios.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 47.Magnusson M, Heimann K, Quayle P, Negri AP. Additive toxicity of herbicide mixtures and comparative sensitivity of tropical benthic microalgae. Mar. Pollut. Bull. 2010;60:1978–1987. doi: 10.1016/j.marpolbul.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 48.Bengston-Nash SM, Schreiber U, Ralph PJ, Muller JF. The combined SPE: ToxY-PAM phytotoxicity assay; application and appraisal of a novel biomonitoring tool for the aquatic environment. Biosens. Bioelectron. 2005;20:1443–1451. doi: 10.1016/j.bios.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 49.Muller R, et al. Rapid exposure assessment of PSII herbicides in surface water using a novel chlorophyll a fluorescence imaging assay. Sci. Total Environ. 2008;401:51–59. doi: 10.1016/j.scitotenv.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 50.Magnusson M, Heimann K, Ridd M, Negri AP. Chronic herbicide exposures affect the sensitivity and community structure of tropical benthic microalgae. Mar. Pollut. Bull. 2012;65:363–372. doi: 10.1016/j.marpolbul.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 51.Pesce S, et al. Evaluation of single and joint toxic effects of diuron and its main metabolites on natural phototrophic biofilms using a pollution-induced community tolerance (PICT) approach. Aquat. Toxicol. 2010;99:492–499. doi: 10.1016/j.aquatox.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 52.Grossmann K. Auxin herbicides: current status of mechanism and mode of action. Pest Manage. Sci. 2010;66:113–120. doi: 10.1002/ps.1860. [DOI] [PubMed] [Google Scholar]
- 53.Vinyard DJ, Ananyev GM, Charles Dismukes G, Photosystem II. The reaction center of oxygenic photosynthesis. Annu. Rev. Biochem. 2013;82:577–606. doi: 10.1146/annurev-biochem-070511-100425. [DOI] [PubMed] [Google Scholar]
- 54.Chesworth J, Donkin M, Brown M. The interactive effects of the antifouling herbicides Irgarol 1051 and Diuron on the seagrass Zostera marina (L.) Aquat. Toxicol. 2004;66:293–305. doi: 10.1016/j.aquatox.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Krieger-Liszkay A, Rutherford AW. Influence of herbicide binding on the redox potential of the quinone acceptor in photosystem II: relevance to photodamage and phytotoxicity. Biochemistry. 1998;37:17339–17344. doi: 10.1021/bi9822628. [DOI] [PubMed] [Google Scholar]
- 56.Krieger-Liszkay A. Singlet oxygen production in photosynthesis. J. Exp. Bot. 2005;56:337–346. doi: 10.1093/jxb/erh237. [DOI] [PubMed] [Google Scholar]
- 57.Osborn HL, Hook SE. Using transcriptomic profiles in the diatom Phaeodactylum tricornutum to identify and prioritize stressors. Aquat. Toxicol. 2013;138:12–25. doi: 10.1016/j.aquatox.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 58.Mercurio P, et al. Contribution of transformation products towards the total herbicide toxicity to tropical marine organisms. Sci. Rep. 2018;8:4808. doi: 10.1038/s41598-018-23153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Millie DF, Hersh CM, Dionigi CP. Simazine-induced inhibition in photoacclimated populations of Anabaena circinalis (Cyanophyta) J. Phycol. 1992;28:19–26. doi: 10.1111/j.0022-3646.1992.00019.x. [DOI] [Google Scholar]
- 60.Guasch H, Sabater S. Light history influences the sensitivity to atrazine in periphytic algae. J. Phycol. 1998;34:233–241. doi: 10.1046/j.1529-8817.1998.340233.x. [DOI] [Google Scholar]
- 61.Tang J, Hoagland KD, Siegfried BD. Uptake and bioconcentration of atrazine by selected freshwater algae. Environ. Toxicol. Chem. 1998;17:1085–1090. doi: 10.1002/etc.5620170614. [DOI] [Google Scholar]
- 62.Descolas-Gros C, Oriol L. Variations in carboxylase activity in marine phytoplankton cultures. ß-carboxylation in carbon flux studies. Mar. Ecol. Prog. Ser. 1992;85:163–169. doi: 10.3354/meps085163. [DOI] [Google Scholar]
- 63.Ramakrishnan B, Megharaj M, Venkateswarlu K, Naidu R, Sethunathan N. The impacts of environmental pollutants on microalgae and cyanobacteria. Crit. Rev. Environ. Sci. Technol. 2010;40:699–821. doi: 10.1080/10643380802471068. [DOI] [Google Scholar]
- 64.Ralph P, Smith R, Macinnis-Ng C, Seery C. Use of fluorescence-based ecotoxicological bioassays in monitoring toxicants and pollution in aquatic systems. Toxicol. Environ. Chem. 2007;89:589–607. doi: 10.1080/02772240701561593. [DOI] [Google Scholar]
- 65.Büchel C, Wilhelm C. In vivo analysis of slow chlorophyll fluorescence induction kinetics in algae: progress, problems and perspectives. Photochem. Photobiol. 1993;58:137–148. doi: 10.1111/j.1751-1097.1993.tb04915.x. [DOI] [Google Scholar]
- 66.Schubert, N. Phototprotective mechanisms in red algae, Ph. D. thesis. Universidad Autónoma de Baja California, Mexico, (2008).
- 67.MacColl, R. Phycobilisomes: a structure-function model. In: MacColl, R., 2018. Phycobiliproteins. 9–23 (2018).
- 68.Apt KE, Collier JL, Grossman AR. Evolution of the phycobiliproteins. J. Mol. Biol. 1995;248:79–96. doi: 10.1006/jmbi.1995.0203. [DOI] [PubMed] [Google Scholar]
- 69.Magnusson, M. Effects of priority herbicides and their breakdown products on tropical, estuarine microalgae of the Great Barrier Reef Lagoon. Ph. D. thesis, James Cook University, Australia, (2009).
- 70.Huerlimann R, Heimann K. Comprehensive guide to acetyl-carboxylases in algae. Crit. Rev. Biotechnol. 2013;33:49–65. doi: 10.3109/07388551.2012.668671. [DOI] [PubMed] [Google Scholar]
- 71.Tu, M., Hurd, C. & Randall, J. M. Weed Control Methods Handbook, The Nature Conservancy, https://www.invasive.org/gist/products/handbook/methods-handbook.pdf (accessed 12 September 2019) (2001).
- 72.Durkin, P. & Follansbee, M. Imazapic-human health and ecological risk assessment-final report. Prepared for USDA Forest Service, Forest Health Protection. (2004).
- 73.Tang CY, Huang Z, Allen HC. Interfacial water structure and effects of Mg2+ and Ca2+ binding to the COOH headgroup of a palmitic acid monolayer studied by sum frequency spectroscopy. J. Phys. Chem. B. 2010;115:34–40. doi: 10.1021/jp1062447. [DOI] [PubMed] [Google Scholar]
- 74.Brzozowska A, Duits MH, Mugele F. Stability of stearic acid monolayers on Artificial Sea Water. Colloids Surf. Physicochem. Eng. Aspects. 2012;407:38–48. doi: 10.1016/j.colsurfa.2012.04.055. [DOI] [Google Scholar]
- 75.Posthuma, L., Suter, G. W. II & Traas, T. P. Species sensitivity distributions in ecotoxicology. CRC press (2001).
- 76.Negri AP, et al. Adjusting tropical marine water quality guideline values for elevated ocean temperatures. Environ. Sci. Technol. 2019;54:1102–1110. doi: 10.1021/acs.est.9b05961. [DOI] [PubMed] [Google Scholar]
- 77.Hill DR, Wetherbee R. A reappraisal of the genus Rhodomonas (Cryptophyceae) Phycologia. 1989;28:143–158. doi: 10.2216/i0031-8884-28-2-143.1. [DOI] [Google Scholar]
- 78.Fleeger JW, Carman KR, Nisbet RM. Indirect effects of contaminants in aquatic ecosystems. Sci. Total Environ. 2003;317:207–233. doi: 10.1016/S0048-9697(03)00141-4. [DOI] [PubMed] [Google Scholar]
- 79.Hammer A, Schumann R, Schubert H. Light and temperature acclimation of Rhodomonas salina (Cryptophyceae): photosynthetic performance. Aquat. Microb. Ecol. 2002;29:287–296. doi: 10.3354/ame029287. [DOI] [Google Scholar]
- 80.Guevara M, Arredondo-Vega BO, Palacios Y, Saéz K, Gómez PI. Comparison of growth and biochemical parameters of two strains of Rhodomonas salina (Cryptophyceae) cultivated under different combinations of irradiance, temperature, and nutrients. J. Appl. Phycol. 2016;28:2651–2660. doi: 10.1007/s10811-016-0835-2. [DOI] [Google Scholar]
- 81.Barlow SB, Kugrens P. Cryptomonads from the Salton Sea, California. Hydrobiologia. 2002;473:129–137. doi: 10.1023/A:1016585818665. [DOI] [Google Scholar]
- 82.Guillard RR, Ryther JH. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- 83.Stone S, Adams M, Stauber J, Jolley DF, Warne MSJ. Development and application of a multispecies toxicity test with tropical freshwater microalgae. Environ. Pollut. 2019;250:97–106. doi: 10.1016/j.envpol.2019.03.058. [DOI] [PubMed] [Google Scholar]
- 84.Trenfield MA, et al. Aluminium, gallium, and molybdenum toxicity to the tropical marine microalga Isochrysis galbana. Environ. Toxicol. Chem. 2015;34:1833–1840. doi: 10.1002/etc.2996. [DOI] [PubMed] [Google Scholar]
- 85.Pease, C., Mooney, T., Trenfield, M., Costello, C. & Harford, A. Updated procedure for the 72 hour algal growth inhibition toxicity test using Chlorella sp. Internal Report 645, Department of the Environment and Energy, https://www.environment.gov.au/science/supervising-scientist/publications/internal-reports/updated-procedure-algal-growth-inhibition-toxicity-test (2016).
- 86.Franklin, N., Stauber, J., Markich, S. & Lim, R. A new tropical algal test to assess the toxicity of metals in freshwaters. Supervising Scientists Report 133. (1998).
- 87.OECD. Organisation for Economic Cooperation and Development (OECD) guidelines for the testing of chemicals: freshwater alga and cyanobacteria, growth inhibition test. Test No. 201, https://search.oecd.org/env/test-no-201-alga-growth-inhibition-test-9789264069923-en.htm (2011).
- 88.Schreiber U, Müller JF, Haugg A, Gademann R. New type of dual-channel PAM chlorophyll fluorometer for highly sensitive water toxicity biotests. Photosynth. Res. 2002;74:317–330. doi: 10.1023/A:1021276003145. [DOI] [PubMed] [Google Scholar]
- 89.Mercurio P, Mueller JF, Eaglesham G, Flores F, Negri AP. Herbicide persistence in seawater simulation experiments. PLoS One. 2015;10:e0136391. doi: 10.1371/journal.pone.0136391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mercurio, P. III Herbicide persistence and toxicity in the tropical marine environment, University of Queensland, School of Medicine. 148 pp, 10.14264/uql.2016.722 (2016).
- 91.Fisher, R., Ricardo, G. & Fox, D. jags NEC: A Bayesian No Effect Concentration (NEC) package, https://github.com/AIMS/NEC-estimation (accessed 03 december 2019) (2019).
- 92.Fox DR. A Bayesian approach for determining the no effect concentration and hazardous concentration in ecotoxicology. Ecotoxicol. Environ. Saf. 2010;73:123–131. doi: 10.1016/j.ecoenv.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 93.Warne MSJ, King O, Smith RA. Ecotoxicity thresholds for ametryn, diuron, hexazinone and simazine in fresh and marine waters. Environ. Sci. Pollut. Res. 2018;25:3151–3169. doi: 10.1007/s11356-017-1097-5. [DOI] [PubMed] [Google Scholar]
- 94.USEPA. ECOTOX User Guide: ECOTOXicology Database System. Version 5.0. United States Environmental Protection Agency, http://cfpub.epa.gov/ecotox/, (accessed 05 September 2019) (2019).
- 95.Hook SE, et al. RNA-Seq analysis of the toxicant-induced transcriptome of the marine diatom, Ceratoneis closterium. Marine genomics. 2014;16:45–53. doi: 10.1016/j.margen.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 96.His, E. & Seaman, M. Effect of twelve pesticides on larvae of oysters (Crassostrea gigas) and on two species of unicellular marine algae (Isochrysis galbana and Chaetoceros calcitrans). ICES, Copenhagen, Denmark (1993).
- 97.McFeters GA, Bond PJ, Olson SB, Tchan Y. A comparison of microbial bioassays for the detection of aquatic toxicants. Water Res. 1983;17:1757–1762. doi: 10.1016/0043-1354(83)90197-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.