Abstract
Soil salinity is one of the major plant growth and yield-limiting constraints in arid and semi-arid regions of the world. In addition to the oxidative damage, increasing salt stress is associated with elevated cellular ethylene levels due to the synthesis of 1-aminocyclopropane-1-carboxylic acid (ACC) in large amounts. The objective of the current study was to elucidate the inoculation effect of an ACC deaminase (ACCD)–producing phytobeneficial strain Achromobacter sp. FB-14 on rice plants to alleviate the salinity effects by upregulation of the stress-responsive CIPK genes. The strain FB-14 was isolated by using nutrient agar medium at 855 mM NaCl concentration and it was taxonomically identified as Achromobacter sp. with more than 99% 16S rRNA gene sequence similarity with many Achromobacter species. The strain FB-14 demonstrated substantial in vitro potential for ACCD activity, synthesis of indole compounds, and phosphate solubilization up to 100 mM NaCl concentration in the culture medium. The gene corresponding to ACCD activity (acdS) was amplified and sequenced in order to confirm the inherent enzyme activity of the strain at a molecular level. The rifampicin-resistant derivative of strain FB-14 was recovered from the rice rhizosphere on antibiotic medium up to 21 days of sowing. Moreover, the strain FB-14 was inoculated on rice plants under salinity and it not only enhanced the growth of rice plants in terms of root and shoot length, and fresh and dry weight, but also upregulated the expression of stress-responsive CIPK genes (OsCIPK03, OsCIPK12, and OsCIPK15) according to the results of qRT-PCR analysis. To the best of our knowledge, this is the first report deciphering the role of plant-beneficial Achromobacter strain relieving the rice plants from salt stress by promoting the growth and enhancing the expression of stress-responsive CIPK genes.
Electronic supplementary material
The online version of this article (10.1007/s42770-019-00199-8) contains supplementary material, which is available to authorized users.
Keywords: ACC deaminase, Gene expression, PGPR, Rice, Salt tolerance
Introduction
Global food demand is increasing because of rapid population growth as well as the alteration in dietary habits [1]. To fulfill this demand, global food production increases by 40% by 2030 and 70% by 2050 is essential. Salt stress is one of the key abiotic stresses limiting plant growth and productivity and is a threat to global food security [2]. According to the report published by the Food and Agricultural Organization (FAO), salinity may destroy almost 50% of agricultural lands around the globe until 2050 [2]. In Pakistan, 6.5 million ha of irrigated land is salt-affected. Geographically, Pakistan is located in the dry and semi-arid regions which favor the accretion of salts on soil surface due to higher rates of water evaporation disrupting the agricultural systems [3, 4]. Recently, global warming intensified the soil salinity issue due to lower precipitation and higher evaporation rates. Collectively, soil salinity stress affected up to 50% of irrigated land and 20% of cultivated land worldwide depending upon numerous ecological factors [5].
Rice (Oryza sativa L.) is cultivated extensively as a staple food to feed billions of people around the world. The rice, being the primary food source for human beings, is an important commodity and 60% of the total rice yield is consumed in Asia [6, 7]. It has been estimated that 6.9 dSm−1 salt levels resulted in a 50% reduction in the yield of rice plants [8]. In 2007, CBL-interacting protein kinases (CIPK) genes (OsCIPK03, OsCIPK12, and OsCIPK15) were reported to improve salt tolerance in rice plants [9]. The results showed that the rice plants overexpressing CIPK genes have more stress tolerance than control plants [9].
Moreover, CIPK genes play a key role in plant growth promotion and alleviation of negative effects of stressful environment especially soil salinity through the increased production of osmolytes [10, 11]. Under such conditions, devising efficient breeding programs to develop salt-tolerant rice is one of many other options to cope with the presence of salts in the soil. Over the years, a few halo-tolerant rice cultivars have been developed, but the yield of these varieties under high salinity conditions is still unsatisfactory [2]. Moreover, many of the soil reclamation strategies do not work efficiently due to being laborious, costly, and non-ecofriendly [12].
Under such circumstances, phytobeneficial bacterial species inhabiting the rhizosphere are biological tools that promote nutrient uptake from soil and salt tolerance induction in plants by utilizing various direct and indirect mechanisms [13–15]. The bacterial 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase synthesis is one of the well-known mechanisms to induce salt stress tolerance in plants by the regulation of cellular ethylene levels [12, 16]. The PGPR harboring ACC deaminase (ACCD) activity acts as a sink of ACC in order to utilize it as a nitrogen source after colonization of plant roots and cause plants to produce more ACC [17]. These bacteria utilize ACC as nitrogen source and convert it into ammonia and α-ketobutyrate by using ACCD activity; thus, the concentration of ACC outside the plant roots decreases causing more ACC to come out of the plant cells. In this way, the equilibrium between the levels of ACC inside and outside the plant roots is maintained [17]. Hence, the ACCD activity prevents the formation of ethylene from ACC (precursor of ethylene) inside the plant cells [18]. Some ACCD-producing PGPR genera such as Azospirillum brasilense, Alcaligenes, Acinetobacter, Agrobacterium, Burkholderia, Achromobacter, Pseudomonas, Bacillus, Enterobacter, Planomicrobium, and Chryseobacterium have been well known to protect different plants from deleterious salinity effects [14, 19–22]. Achromobacter spp. have also been documented as PGPR relieving plants from stress conditions by various mechanisms including ACCD activity [23, 24].
Although the PGPR-mediated stress tolerance induction in plants and the involvement of ACCD in this process is well documented, no research-based evidence is available about the expression dynamics of CIPK genes in plants inoculated with PGPR species. Thus, the objective of the current study was to examine the role of ACCD-producing Achromobacter sp. FB-14 to promote the growth of rice plants and to elucidate the role of CIPK genes in salinity tolerance.
Materials and methods
Bacterial isolation and soil analysis
Achromobacter sp. FB-14 was isolated from a rhizospheric soil sample of rice collected from the healthy rice-growing field of Faisalabad, Punjab, Pakistan (31.5° N, 73° E). One gram of soil was added in 9 mL 0.85% (w/v) sterilized NaCl solution followed by serial dilution as demonstrated by Somasegaran and Hoben [25]. A 100-μL volume from dilution 10−5 and 10−7 was spread on nutrient agar media supplemented with 5% NaCl and plates were kept in an incubator (Memmert, Germany) at 28 ± 2 °C for 24 h. Colonies were purified by repeated-streaking and bacterial cell purity was further confirmed by observing the culture on glass slides under a light microscope (Olympus, Japan). The rhizosphere soil sample was physicochemically analyzed at Ayub Agriculture Research Institute (AARI), Faisalabad, Punjab, Pakistan.
Physiological characterization of bacterial strain for plant-beneficial traits
ACC deaminase activity by estimation of α-ketobutyrate
ACC deaminase activity of Achromobacter sp. strain FB-14 was evaluated by measuring the amount of α-ketobutyrate produced when the enzyme ACCD cleaves ACC. The quantity of α-ketobutyrate produced by this reaction was determined by comparing the absorbance at 540 nm of the bacterial extract of strain FB-14 to a standard curve of α-ketobutyrate (0.1 to 1.0 μM). The culture was grown in DF salt minimal medium [26] containing 0, 25, 50, and 100 mM concentration of NaCl (w/v), and 3 mM ACC to fulfill the nitrogen requirement of bacterial culture followed by incubation for 24 h at 28 ± 2 °C at 150 rpm in an orbital shaker. The ACCD activity was then determined by following a well-described procedure [26] modified from the method of Penrose and Glick [27]. The OD values measured in non-inoculated control were normalized with the sample for accurate calculations. The protein quantity in cell extracts was determined by using the Bradford protein assay [28]. Similarly, the OD values of non-inoculated control were subtracted from the values of the inoculated samples for accurate protein estimation, and finally, nmol of α-ketobutyrate was estimated in mg−1 protein h−1.
Synthesis of indole compounds
Auxin synthesis potential of strain FB-14 was estimated in nutrient broth medium by a method described by Gordon and Weber [29]. The broth culture of the isolated strain was prepared with varying NaCl concentration (w/v) (i.e., 0, 25, 50, and 100 mM) and containing or not 100 mg L−1l-tryptophan as a precursor. The culture was kept under shaking conditions at 28 ± 2 °C at 150 rpm. After 24 h, 5 mL of broth culture was poured in a 15-mL tube and centrifuged at 13,000g for 2 min. A 2-mL volume of supernatant was added in double volume of Salkowskaya’s reagent. To rule out the probability of false-positive results, the experiment was performed with a negative control without inoculation. Tubes were held in dark for 30 min. to produce a pink color, which was then spectrophotometrically measured at 535 nm (Shimadzu UV/VIS, Kyoto, Japan). Measurements were done against a standard curve drawn with a series IAA (Sigma-Aldrich, USA) solutions.
Phosphate Solubilization
The plates were prepared with Pikovskaya’s agar media amended with four salt concentrations (i.e., 0, 25, 50, and 100 mM). The plates were spot-inoculated with strain FB-14 [30] and placed in an incubator (Memmert, Germany) at 28 ± 2 °C for a week followed by visual observations of halo-zones development. The diameter of both colony and zone was measured with the help of measuring scale and the solubilization index was recorded by the formula written below.
where “a” represents colony diameter and “b” represents zone diameter.
Molecular characterization of Achromobacter sp. FB-14
The isolation of the genomic DNA of FB-14 strain was carried out by the CTAB method as reported by Wilson [31] and its concentration was determined through spectrophotometry using Nano Drop™ 2000/2000c (Thermo Fisher Scientific, USA). Amplification of the 16S rRNA gene was accomplished with universal primers pair fD1 (5′ AGAGTTTGATCCTGGCTCAG 3′) and rD1 (5′AAGGAGGTGATCCAGCC 3′) [32]. The amplicon was sent to Macrogen, Korea, for sequencing by Sanger method. The raw sequence was edited and assembled through the CAP3 sequence assembly program. The final sequence was compared with those in the GenBank (NCBI), EzBioCloud, SILVA, and RDP databases in order to determine sequence similarity. The 16S rRNA gene sequence was submitted to NCBI and the accession number (MG547707) was obtained. The phylogenetic analysis was also carried out by neighbor-joining method in order to infer phylogenetic relationships between strain FB-14 and other type strains of genus Achromobacter. The acdS gene encoding the ACCD enzyme of Achromobacter sp. FB-14 was amplified from genomic DNA using the following primers: forward (5′-GCCAARCGBGAVGACTGCAA-3′) and reverse (5′- TGCATSGAYTTGCCYTC-3′). PCR reaction mixture and conditions of thermocycler (advanced Primus 96; PeQLab Biotechnologie, Germany) were programmed according to Li et al. [33]. The amplified gene was sequenced directly from both sides through Macrogen, Korea. The raw sequence was edited and assembled and both DNA and deduced amino acid sequences were compared with the NCBI GenBank database as described in the above section. The final sequence was also deposited in NCBI and accession number (KY305926) was obtained. The final sequence of acdS gene was analyzed phylogenetically and evolutionary relationship between FB-14 strain and closest GenBank matches of genus Achromobacter was inferred using neighbor-joining method.
Pot experiment
Construction of rifampicin-resistant derivatives of strain FB-14
After in vitro characterization, the inoculation experiment was planned with the strain FB-14 under the induced levels of salinity. The rifampicin-resistant derivatives of strain FB-14 were developed in order to recover the bacterial strain from the natural rhizosphere soil of rice plants. A well-grown bacterial culture (100 μL) in nutrient broth medium was spread on rifampicin (50 μg mL−1) amended nutrient agar plates. The plates were placed in an incubator (Memmert, Germany) at 28 ± 2 °C for 48 h, and the colonies harboring the ability to grow in the presence of rifampicin were picked and further subcultured on rifampicin-containing media plates. A comparative fitness study was also conducted between the wild-type strain (FB-14w) and the rifampicin-resistant (FB-14rif) derivatives by placing liquid cultures in an incubator at 28 ± 2 °C for 24 h under constant shaking. The serial dilution was performed after 1, 6, 14, and 24 h of incubation followed by the spreading of serially diluted cultures onto freshly prepared LB agar plates. The relative growth patterns of wild and derivative strains after each time interval was evaluated by counting CFU mL−1. The subsequent data was analyzed to calculate the log values, which were subsequently used for the construction of the growth curve.
Experimental conditions
The surface sterilization of super kernel basmati rice seeds was carried out by immersing them in sodium hypochlorite solution (5% w/v) for 5 min followed by washing five times with sterile distilled water. The inoculum was prepared by culturing the strain FB-14 up to 108 CFU mL−1 in the nutrient broth medium. Pots were filled with pre-characterized non-sterile soil (clay loam, available P 7.4 mg kg−1, total N 0.92 g kg−1, available K 99 mg kg−1, organic matter 19 g kg−1 and pH 7.3) having 700 g soil in each pot. Two ways were used for inoculation; firstly, 7 mL of inoculum or sterile distilled water was mixed per 100 g of soil, and secondly, the seeds were immersed in either inoculum or sterile distilled water for 20 min before sowing. Seven seeds in each pot were sown in their respective pots after inoculation [34]. The four salt levels (i.e., 0, 25, 50, and 100 mM) were supplied to pots (30 mL each) with Hoagland solution. The pots were placed in a plant growth chamber under controlled conditions (i.e., temperature 30 °C, moisture 65%, and day night 16/8 h), and the whole experiment was conducted according to a completely randomized design with three replications of each pot. After 7 days of sowing, thinning was done to maintain five plants of uniform size in each pot. The strain FB-14rif was recovered from the rice rhizosphere on antibiotic media plates at different time intervals and quantified by plate count method both in inoculated and non-inoculated treatments. The plants were harvested after 21 days of sowing to collect the data on growth parameters.
Expression analysis of OsCIPK03, OsCIPK12, and OsCIPK15
Total RNA from 21-day-old rice plants was extracted by JeneJET Plant RNA Purification Kit (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. The cDNA was synthesized by Maxima First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. Three pre-reported primer sets were used for the quantification of the OsCIPK03 (OsCIPK03F, 5′-AGATGCGCATGGAGAACCTG-3′ and OsCIPK03R, 5′-CATTGCGGCATCTGATTGTG-3′), OsCIPK12 (OsCIPK12F, 5′-ACCCTCCCTTGTAGTAGTGG-3′ and OsCIPK12R, 5′-GGAAGCTCCTGTCTCTAGCTC-3′), and OsCIPK15 (OsCIPK15F, 5′-GTTACCACTTCCTATCATATCATC-3′ and OsCIPK15R, 5′-CTAAACATCAACTCTCCAAATAC-3′) gene expression in rice plants by qRT-PCR [10]. The qRT-PCR was performed in an optical 96-well plate (CFX 96 Touch™ Real-Time PCR, Bio-Rad, USA). The reaction mixture with a final volume of 20 μL was prepared by adding 2XSYBR Green Master Mix reagent (10 μL volume) (Applied Biosystems), cDNA samples (6 μL volume), and 200 nM gene-specific primer set. The conditions of thermocycler were programmed as follows: 95 °C for 3 min; 40 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 1 min. For internal control, actin1 gene (accession no. X16280) present in rice was used with following primer set: 5′-TGGCATCTCTCAGCACATTCC-3 and 5′-TGCACAATGGATGGGTCAGA-3′. The relative change in the expression of genes were determined according to Livak, Schmittgen [35].
Statistical analysis
The data collected after experimentation was analyzed by one way of variance (ANOVA) using Statistix 8.1 software package. The least significance difference (LSD) and Tukey’s test was performed to compare treatment means comprised of three replications with a 95% confidence level [36].
Results
Bacterial isolation
The strain Achromobacter sp. FB-14 was selected among nine isolates on the basis of its salt tolerance and in vitro plant growth–promoting attributes in the presence of NaCl in the culture medium. The strain was isolated from sandy clay soil with high electrical conductivity (ECe) and pH and poor organic matter content. The nutritional profile indicated that soil contained a low amount of total N and available P and K (Table S1).
Characterization for plant-beneficial traits
The strain FB-14 was found proficient in terms of ACCD activity, synthesis of indole compounds, and inorganic phosphate solubilization under induced salinity stress (Fig. 1). The strain showed maximum ACCD activity in terms of α-ketobutyrate production at 0 mM (711.38 ± 60.85 nmol mg−1 protein h−1) and followed by at 25 mM (653.61 ± 21.44 nmol mg−1 protein h−1) salt concentration. In contrast, at maximum salt stress (100 mM NaCl), the strain produced 155.26 ± 17.50 nmol mg−1 protein h−1 of α-ketobutyrate. Moreover, the Achromobacter sp. FB-14 strain synthesized indole compounds both in tryptophan amended and the medium without tryptophan addition under salt stress. However, the synthesis of indole compounds was found to be higher in the tryptophan-containing culture as compared with the culture without tryptophan. The maximum amount (17.55 ± 1.06 μg mL−1 and 6.68 ± 0.36 μg mL−1) at 0 mM followed by 14.14 ± 0.53 μg mL−1 and 4.67 ± 0.31 μg mL−1 of indole compounds at 25 mM salt concentration was measured in tryptophan amended and non-tryptophan added culture conditions, respectively. However, indole compound production in tryptophan-containing and non-tryptophan-containing media at 100 mM salt concentration was decreased to 9.50 ± 0.80 μg mL−1 and 1.65 ± 0.94 μg mL−1, respectively. Similarly, the phosphate solubilizing index was found to be maximum at 0 mM (5.12 ± 0.42) followed by 4.57 ± 0.40 at 25 mM salt concentration, which was reduced to 1.47 ± 0.22 at a higher salt concentration of 100 mM.
Fig. 1.
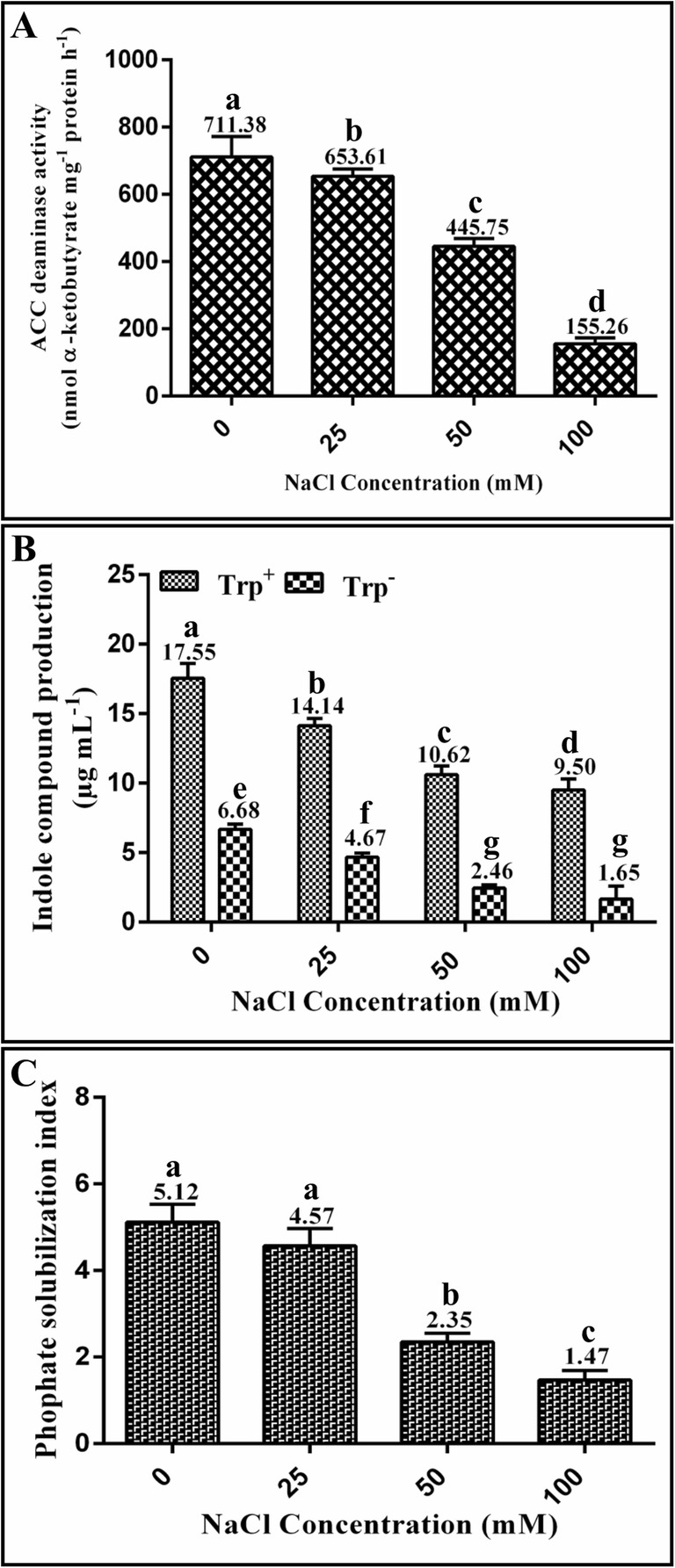
Plant-beneficial properties shown by Achromobacter sp. FB-14 in terms of ACCD (a), synthesis of indole-related compounds (b), and phosphate solubilization (c)
Molecular characterization and phylogenetic analysis
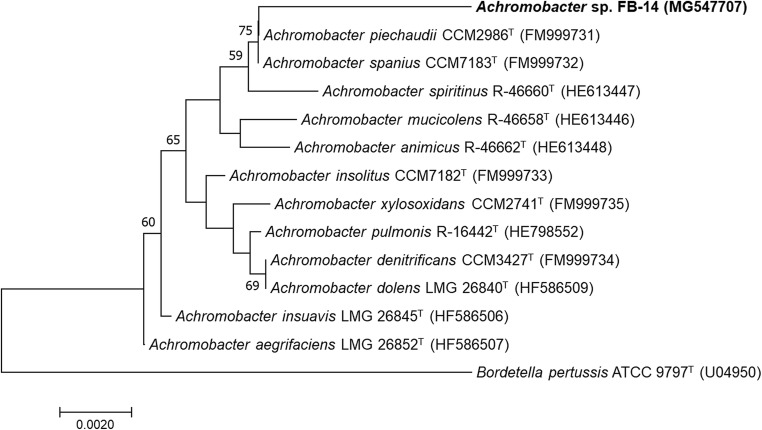
The BLASTn and phylogenetic analysis of the 16S rRNA gene of the strain FB-14 revealed that it belongs to the genus Achromobacter, hence named as Achromobacter sp. FB-14 (accession number, MK630284). The sequence similarity search using BLASTn and EzBioCloud resulted in 99.30% and 99.37% matching of 16S rRNA gene of strain FB-14 with Achromobacter spanius S9–2 (MG547707) and Achromobacter deleyi LMG 3458T (HG324053), respectively. Similarly, SILVA and RDP databases also confirmed the taxonomic rank of FB-14 strain as Achromobacter sp. as the 16S rRNA gene sequence showed similarity with Achromobacter piechaudii (AB681803) and Achromobacter denitrificans DSM30026T (AJ278451), respectively. In the phylogenetic tree, the strain FB-14 formed a cluster with Achromobacter piechaudii CCM2986T (FM999731) and Achromobacter spanius CCM7183T (FM999732) (Fig. 2). Similarly, a 758-bp acdS gene (MK639601) showed 96.45% sequence similarity with Achromobacter sp. SYC-RS-PIA-8 (KY305926) in the GenBank database, while in phylogenetic tree, acdS gene formed cluster with acdS gene sequence of Achromobacter sp. SYC-RS-PIA-9 (KY305927) and Achromobacter sp. SYC-RS-PIA-8 (Fig. S1).
Fig. 2.
Phylogenetic tree of Achromobacter sp. FB-14 with the type strains of genus Achromobacter. The evolutionary history was inferred using the neighbor-joining method. The percentages (≥ 50%) of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown. The evolutionary distances were computed using the maximum composite likelihood method and are in the units of the number of base substitutions per site
Pot experiment
Comparative growth studies and bacterial population density
The log CFU values recorded after the plate count method revealed that the growth patterns of wild-type strain (FB-14w) and its derivative (FB-14rif) are similar (Fig. 3a). Both strains FB-14w and FB-14rif showed a similar growth pattern as depicted by the growth curve. The population density of FB-14rif of 3.71 × 108 CFU g−1 rhizosphere soil was observed on agar plates containing rifampicin prior to seed planting. The FB-14rif strain thrived in the rice rhizosphere up to 21 days after sowing at a population density of 5.22 × 107 CFU g−1 soil (at 0 mM NaCl) and 4.66 × 105 CFU g−1 soil (at 25 mM NaCl). The population density of FB-14rif decreased with increasing salt concentrations (i.e., 50 mM and 100 mM NaCl) up to 3.43 × 105 CFU g−1 soil and 3.54 × 104 CFU g−1, respectively (Fig. 3b).
Fig. 3.
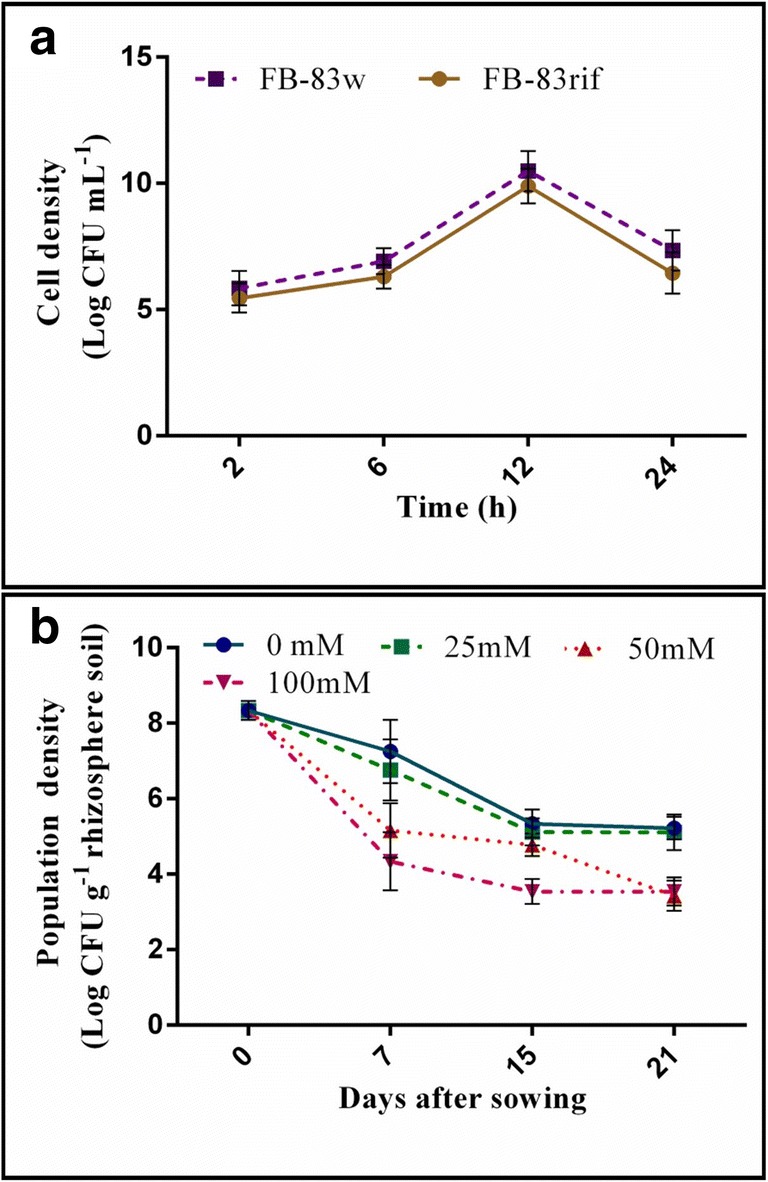
Growth curves of wild-type and derivative Achromobacter sp. FB-14 strains (a) and population density of the derivative strain recovered from rice rhizosphere up to 21 days of growth (b)
Effect of Achromobacter sp. FB-14 inoculation on rice growth under salt stress
The growth of Achromobacter sp. FB-14-treated plants was found significantly higher in contrast to the non-treated plants under salt stress conditions (Fig. 4). Although both inoculated and non-inoculated plants reduced their growth with increasing salt concentration, a better response to inoculation in terms of less decrease in plant biomass was found in the inoculated rice plants. At 25 mM salt concentration, the root and shoot lengths of Achromobacter sp. FB-14 inoculated plants were observed 35.78% and 37.56% higher than non-inoculated plants, respectively. Similarly, the biomass of the inoculated plants was also improved as evident from the increase in the root and shoot fresh and dry weight. At 25 mM salt stress, the root and shoot fresh weight values were measured to be 55.54% and 26.53%, while root and shoot dry weight values were found to be 42.30% and 14.59% greater than the non-inoculated plants, respectively. At 100 mM salt stress, growth parameters of FB-14 inoculated and non-inoculated plants were not found significantly different from each other.
Fig. 4.
Rice growth as influenced by the inoculation of Achromobacter sp. FB-14 at different NaCl concentrations; root length (a), shoot length (b), root fresh wt. (c), shoot fresh wt. (d), root dry wt. (e), and shoot dry wt. (f). Error bars represent the standard deviations (n = 3) and different letters show the significant difference of mean values (P ≤ 0.05)
Expression analysis of OsCIPK03, OsCIPK12, and OsCIPK15
The qRT-PCR analysis of the OsCIPK03, OsCIPK12, and OsCIPK15 genes of the treated and non-treated rice plants revealed the molecular basis of the positive effect of Achromobacter sp. FB-14 on the rice plants under salt stress in terms of growth parameters (Fig. 5). At a lower salt concentration of 25 mM, the three genes, OsCIPK03, OsCIPK12, and OsCIPK15, presented higher expression levels in terms of 1.22-, 1.66-, and 1.27-fold increase in expression levels in inoculated rice plants as compared with non-inoculated ones. On the contrary, expression levels in terms of fold increase of these genes were found similar at higher salt stress levels in rice plants inoculated with Achromobacter sp. FB-14 with respect to non-inoculated plants, which was 1.47-, 1.20-, and 1.16-fold higher at 50 mM and 1.21-, 1.43-, and 1.65-fold higher at 100 mM salt concentration.
Fig. 5.
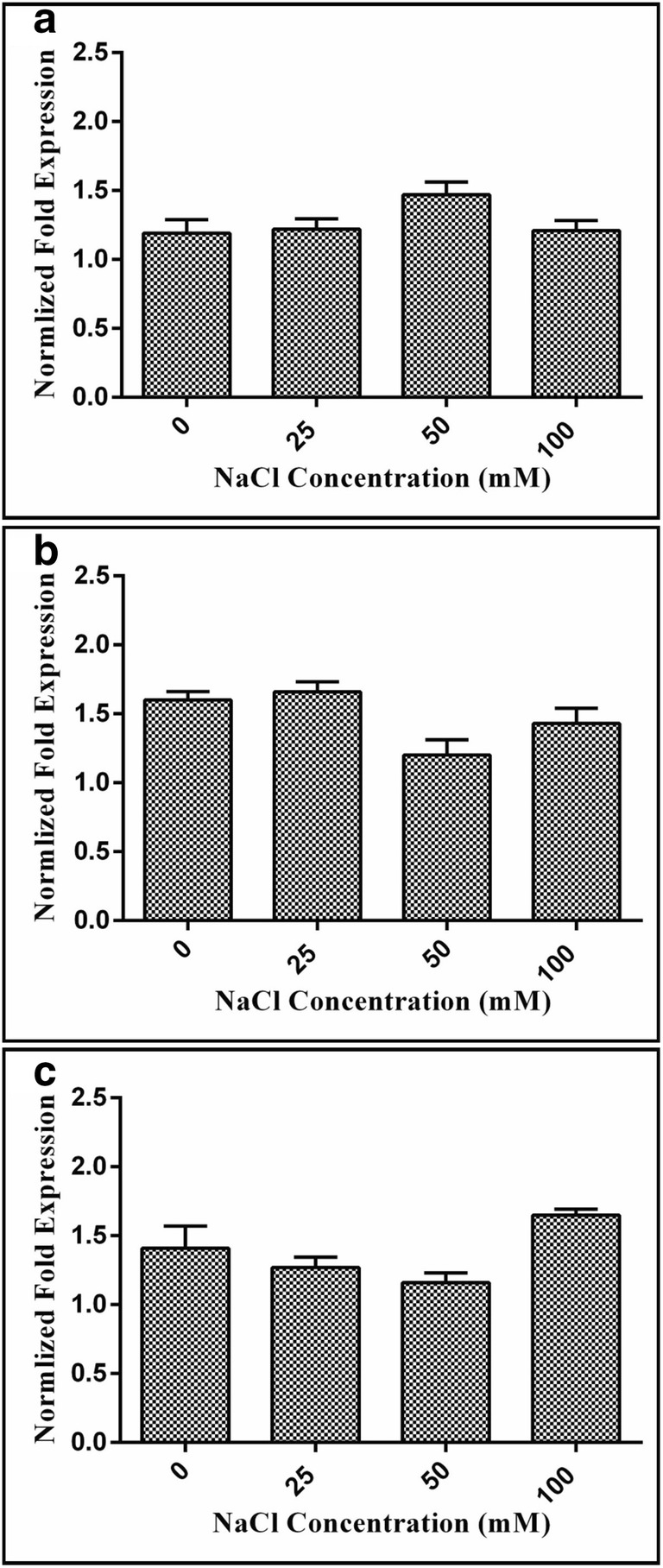
The relative expression of OsCIPK03 (a), OsCIPK12 (b), and OsCIPK15 (c) genes. The expression of these genes was normalized with the housekeeping gene actin1 gene. The fold increase is relative to the expression in the plants inoculated with Achromobacter sp. FB-14rif with the non-inoculated plants
Discussion
The molecular identity of 16S rRNA gene sequence was confirmed by comparing with others in various databases and constructing a phylogenetic tree including the strain FB-14 and type strains of genus Achromobacter using the 16S rRNA gene sequence (Fig. 2). The Achromobacter sp. FB-14 was isolated from the rice plant rhizosphere and was found to tolerate 5% NaCl salt in the culture medium. The phytobeneficial and stress ameliorative role of Achromobacter spp. under saline environment is well documented [37]. Mayak et al. [38] reported that inoculation of Achromobacter piechaudii ARV8 on Solanum lycopersicum plants not only promoted their growth but also relieved them from salinity effects.
The strain FB-14 exhibited the substantial in vitro potential for ACCD activity, which is a key bacterial enzyme regulating plant ethylene levels under stressful environments. The onset of abiotic conditions, especially salinity, boosts the levels of ACC in plants due to elevated activity of ACC synthase. Hence, cellular ethylene concentration exceeds because ACC acts as a precursor of ethylene. In the rhizosphere, bacterial indole molecules, especially IAA, also play a critical role in the synthesis of ACC and its release from roots to be taken up by bacteria as nitrogen source and converting it into ammonia and α-ketobutyrate by ACCD enzyme [17]. Indole-related compounds have a role in initiating the key phenotypic and physiological responses of plants to bacterial association [39]. As per recorded results, it was evident that Achromobacter sp. FB-14 was able to produce indole-related compounds both in the presence and absence of tryptophan. Indole3-acetic acid (IAA), being the most widely studied indole molecule, might be the major constituent of indole compounds synthesized by strain FB-14 in the current study and many phytobeneficial strains have demonstrated the concurrent IAA and ACCD activity in earlier studies [14, 15]. The presence of acdS gene further confirmed the ACCD enzyme activity of strain FB-14(Fig. S1, Table 1 in the ESM). The strain FB-14 was able solubilize phosphate under in vitro conditions (Fig. 1) and this phosphate solubilizing ability of the strain was in line with others reported earlier [15, 40]. The underlying mechanism of phosphate solubilization is the production of organic acids. The PGPR make soil phosphates available for plant uptake by releasing it from the divalent cations (Al2+, Fe2+, and Ca2+) through rhizospheric acidification [41].
For the successful recovery of the strain following the inoculation, rifampicin-resistant derivatives of the strain FB-14 (Achromobacter sp. FB-14rif) were developed. Most of the soil bacteria are susceptible to rifampicin which favors its choice as a selectable marker [42]. The comparative growth patterns of FB-14w and FB-14rif depicted that the resistant strain was suitable to inoculate with rice plants. In the pot experiment, the population density of the derivative strain was found to be 4.66 × 105 CFU g−1 soil up to 21 days after sowing, which revealed that the strain was competent enough to stay alive in the rice rhizosphere [42]. Initially, the cell density of the strain was low, which increased gradually with the passage of time (Fig. 3). Moreover, there was no growth found on antibiotic media in case non-inoculated treatments, which might be due to the susceptibility of indigenous soil bacteria for the exposed concentration of rifampicin. Fischer et al. [43] reported that Pseudomonas sp. SF4crif derivative showed an initial decline in the cell density in the wheat rhizosphere followed by an increase in bacterial cell density and plant growth. Moreover, Akram et al. [15] documented that Staphylococcus sciuri SAT-17rif derivative adapted well with maize rhizosphere and the strain was recovered from plant roots up to 30 days after inoculation.
Various studies reported that PGPR inoculation improved plant biomass and helped plant in ameliorating salt stress [14, 15, 44]. The results of the present study revealed that the treatment of rice plants with strain FB-14rif significantly (P ≤ 0.05) promoted the growth parameters such as root and shoot length (27.63% and 35.99%, respectively) and fresh and dry weights (39.36% and 23.24%, respectively) up to 50 mM salt stress (Fig. 4) and upregulated the expression of the stress-inducible genes (e.g., OsCIPK03, OsCIPK12, and OsCIPK15) up to 100 mM NaCl level (Fig. 5). This growth-promoting effect of the strain might be due to the better root colonization, nutrient mobilization, and synthesis of indole compounds, which might have initiated stress-related responses in rice plants. On the contrary, ACCD activity of strain FB-14 might have converted excessive ACC of rice plants to ammonia and α-ketobutyrate in order to regulate the excessive ethylene levels in rice plants [45, 46]. In addition, IAA might have contributed to more root proliferation, which resulted to better access of roots to water and nutrients. At 100 mM NaCl stress, no significant increase in the plant growth parameters was observed in FB-14rif-treated plants. This might be due to the ion water imbalance, reduced photosynthesis, and limited nutrient uptake [47]. The limited nutrient uptake with increasing salt concentration may be due the fact that salt mobilizes into the plant cell through membrane-embedded channels that normally import the essential nutrients into the cell, thus limiting the plant growth. Xiang et al. [9] reported that OsCIPK03, OsCIPK12, and OsCIPK15 genes play a key role in rice plants under various abiotic stress conditions including salinity, drought, and cold. These genes are known to be differentially induced under various abiotic stresses and, thus, have a role in stress tolerance improvement in rice [9]. In the present study, the rice plants inoculated with strain FB-14rif strain showed enhanced expression OsCIPK03, OsCIPK12, and OsCIPK15 at lower salt stress levels of 25 mM. This enhanced gene expression under salt stress might be associated with the unknown complex signaling pathways triggered as a result of strain FB-14rif inoculation. The strain FB-14 helped the inoculated rice plants to mitigate the negative effects of the saline conditions by increased expression of CIPK genes. These results are similar to Xiang et al. [9] in the case of OsCIPK15 gene whereby the expression of this gene is induced under salinity. In contrast, the current results are not in line with Xiang et al. [9] in the case of OsCIPK03 and OsCIPK12 as these are induced by abiotic stresses other than salinity. Under salt stress conditions, bacterial inoculation might have affected the rice plants with a better nutrient acquisition, phytohormone production, ACCD activity, and ultimately enhanced expression abiotic stress-responsive CIPK genes under salt stress conditions.
Conclusion
The study identified the Achromobacter sp. FB-14 with the potential to synthesize IAA, execute ACC deaminase activity, and solubilize inorganic phosphate in saline conditions. The strain was found as a suitable candidate to inoculate rice plants under salt stress for increased growth and induction of salinity tolerance by upregulating the genetic mechanisms by unknown signaling pathways. To the best of our knowledge, this report deciphered the inoculation effect of Achromobacter sp. with rice plants in terms of enhanced stress-responsive gene expression for the first time.
Electronic supplementary material
Phylogenetic tree of acdS gene sequence of Achromobacter sp. FB-14 with the strains of genus Achromobacter. The evolutionary history was inferred using the Neighbor-Joining method. The percentages (≥50%) of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site (JPG 411 kb).
(DOCX 15 kb).
Funding information
This study is funded by the Start-up Research Grant # 1093/SRGP/R&D/HEC/2016 by the Higher Education Commission (HEC), Pakistan. This study also received financial support from the Annual Departmental Grant by the Government College University, Faisalabad, Pakistan.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Birla DS, Malik K, Sainger M, Chaudhary D, Jaiwal R, Jaiwal PK. Progress and challenges in improving the nutritional quality of rice (Oryza sativa L.) Crit Rev Food Sci Nutr. 2017;57(11):2455–2481. doi: 10.1080/10408398.2015.1084992. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar A, Pramanik K, Mitra S, Soren T, Maiti TK. Enhancement of growth and salt tolerance of rice seedlings by ACC deaminase-producing Burkholderia sp. MTCC 12259. J Plant Physiol. 2018;231:434–442. doi: 10.1016/j.jplph.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Chen CY, Mei HC, Cheng CY, Lin JH, Chung YC. Enhancing the conversion of organic waste into biofertilizer with thermophilic bacteria. Environ Eng Sci. 2012;29(7):726–730. [Google Scholar]
- 4.Zafar S, Ashraf MY, Anwar S, Ali Q, Noman A. Yield enhancement in wheat by soil and foliar fertilization of K and Zn under saline environment. Soil Environ. 2016;35(1):46–55. [Google Scholar]
- 5.Heydarian Z, Yu M, Gruber M, Glick BR, Zhou R, Hegedus DD. Inoculation of soil with plant growth promoting bacteria producing 1-aminocyclopropane-1-carboxylate deaminase or expression of the corresponding acdS gene in transgenic plants increases salinity tolerance in Camelina sativa. Front Microbiol. 2016;7:1966. doi: 10.3389/fmicb.2016.01966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker R, Herdt RW, Rose B (1987) The rice economy of Asia. Johns Hopkins University Press, Washington, DC, pp 224
- 7.Cheeseman J (2016) Food security in the face of salinity, drought, climate change, and population growth. In: Khan MA, Ozturk M (ed) Halophytes for food security in dry lands. Academic Press, Amazon Digital Services LLC, Cambridge, pp 111–123
- 8.Radanielson A, Gaydon D, Li T, Angeles O, Roth C. Modeling salinity effect on rice growth and grain yield with ORYZA v3 and APSIM-Oryza. Eur J Agron. 2018;100:44–55. doi: 10.1016/j.eja.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiang Y, Huang Y, Xiong L. Characterization of stress-responsive CIPK genes in rice for stress tolerance improvement. Plant Physiol. 2007;144(3):1416–1428. doi: 10.1104/pp.107.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Gu Z, Liu F, Ma B, Zhang H. Molecular analysis of rice CIPKs involved in biotic and abiotic stress responses. Chinese J Rice Sci. 2010;24(6):567–574. [Google Scholar]
- 11.Kanwar P, Sanyal SK, Tokas I, Yadav AK, Pandey A, Kapoor S, Pandey GK. Comprehensive structural, interaction and expression analysis of CBL and CIPK complement during abiotic stresses and development in rice. Cell Calcium. 2014;56(2):81–95. doi: 10.1016/j.ceca.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Shahid M, Javed MT, Mushtaq A, Akram MS, Mahmood F, Ahmed T, Noman M, Azeem M (2019) Microbe-mediated mitigation of cadmium toxicity in plants. In: Hasanuzzaman M, Prasad MNV, Fujita M (ed) Cadmium Toxicity and Tolerance in Plants. Elsevier, pp 427–449
- 13.Tariq M, Noman M, Ahmed T, Hameed A, Manzoor N, Zafar M. Antagonistic features displayed by plant growth promoting rhizobacteria (PGPR): a review. J Plant Sci Phytopathol. 2017;1:38–43. [Google Scholar]
- 14.Shahid M, Akram M, Khan M, Zubair M, Shah S, Ismail M, Shabir G, Basheer S, Aslam K, Tariq M. A phytobeneficial strain Planomicrobium sp. MSSA-10 triggered oxidative stress responsive mechanisms and regulated the growth of pea plants under induced saline environment. J Appl Microbiol. 2018;124(6):1566–1579. doi: 10.1111/jam.13732. [DOI] [PubMed] [Google Scholar]
- 15.Akram MS, Shahid M, Tariq M, Azeem M, Javed MT, Saleem S, Riaz S. Deciphering Staphylococcus sciuri SAT-17 mediated anti-oxidative defense mechanisms and growth modulations in salt stressed maize (Zea mays L.) Front Microbiol. 2016;7:867. doi: 10.3389/fmicb.2016.00867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadeem SM, Zahir ZA, Naveed M, Arshad M. Preliminary investigations on inducing salt tolerance in maize through inoculation with rhizobacteria containing ACC deaminase activity. Can J Microbiol. 2007;53(10):1141–1149. doi: 10.1139/W07-081. [DOI] [PubMed] [Google Scholar]
- 17.Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2014;169(1):30–39. doi: 10.1016/j.micres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Jha CK, Saraf M. Plant growth promoting rhizobacteria (PGPR): a review. J Agric Res Dev. 2015;5(2):108–119. [Google Scholar]
- 19.Bharti N, Barnawal D (2019) Amelioration of salinity stress by PGPR: ACC deaminase and ROS scavenging enzymes activity. In: Singh AK, Kumar A, Singh PK (ed) PGPR Amelioration in Sustainable Agriculture. Cambridge Woodhead Publishing, pp 85–106
- 20.Gontia-Mishra I, Sasidharan S, Tiwari S. Recent developments in use of 1-aminocyclopropane-1-carboxylate (ACC) deaminase for conferring tolerance to biotic and abiotic stress. Biotechnol Lett. 2014;36(5):889–898. doi: 10.1007/s10529-014-1458-9. [DOI] [PubMed] [Google Scholar]
- 21.Raghuwanshi R, Prasad JK (2018) Perspectives of rhizobacteria with ACC deaminase activity in plant growth under abiotic stress. In: Giri B, Prasad R, Varma A (ed) Root Biology. Springer International Publishing, pp 303–321
- 22.Sarkar A, Ghosh PK, Pramanik K, Mitra S, Soren T, Pandey S, Mondal MH, Maiti TK. A halotolerant Enterobacter sp. displaying ACC deaminase activity promotes rice seedling growth under salt stress. Res Microbiol. 2018;169(1):20–32. doi: 10.1016/j.resmic.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Abdel-Rahman H, Salem A, Moustafa MM, El-Garhy HA. A novice Achromobacter sp. EMCC1936 strain acts as a plant-growth-promoting agent. Acta Physiol Plant. 2017;39(2):61. [Google Scholar]
- 24.Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res. 2016;184:13–24. doi: 10.1016/j.micres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Somasegaran P, Hoben HJ (1994) Handbook for rhizobia: methods in legume-rhizobium technology. Springer-Verlag, Berlin
- 26.Saravanakumar D, Samiyappan R. ACC deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogea) plants. J Appl Microbiol. 2007;102(5):1283–1292. doi: 10.1111/j.1365-2672.2006.03179.x. [DOI] [PubMed] [Google Scholar]
- 27.Penrose DM, Glick BR. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant. 2003;118(1):10–15. doi: 10.1034/j.1399-3054.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 28.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 29.Gordon SA, Weber RP. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951;26(1):192–195. doi: 10.1104/pp.26.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pikovskaya R. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiol. 1948;17:362–370. [Google Scholar]
- 31.Wilson K. Preparation of genomic DNA from bacteria. Curr Protoc Mol Biol. 2001;56(1):2–4. doi: 10.1002/0471142727.mb0204s56. [DOI] [PubMed] [Google Scholar]
- 32.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173(2):697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Chang S, Ye S, Chen M, Lin L, Li Y, Li S, An Q. Differentiation of 1-aminocyclopropane-1-carboxylate (ACC) deaminase from its homologs is the key for identifying bacteria containing ACC deaminase. FEMS Microbiol Ecol. 2015;91(10):112. doi: 10.1093/femsec/fiv112. [DOI] [PubMed] [Google Scholar]
- 34.Arnon D, Hoagland D. Crop production in artificial culture solutions and in soils with special reference to factors influencing yields and absorption of inorganic nutrients. Soil Sci. 1940;50:463–485. [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Steel R, Torrie J, Dickey D. Principles and procedures of statistics: a biometrical approach. 3. New York: McGraw-Hill; 1997. [Google Scholar]
- 37.Joe MM, Islam MR, Karthikeyan B, Bradeepa K, Sivakumaar PK, Sa T. Resistance responses of rice to rice blast fungus after seed treatment with the endophytic Achromobacter xylosoxidans AUM54 strains. Crop Protect. 2012;42:141–148. [Google Scholar]
- 38.Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol Biochem. 2004;42(6):565–572. doi: 10.1016/j.plaphy.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert S, Xu J, Acosta K, Poulev A, Lebeis S, Lam E. Bacterial production of indole related compounds reveals their role in association between duckweeds and endophytes. Front Chem. 2018;6:265. doi: 10.3389/fchem.2018.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oliveira C, Alves V, Marriel I, Gomes E, Scotti M, Carneiro N, Guimaraes C, Schaffert R, Sa N. Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol Biochem. 2009;41(9):1782–1787. [Google Scholar]
- 41.Akram MS, Shahid M, Tahir M, Mehmood F, Ijaz M (2017) Plant-microbe interactions: current perspectives of mechanisms behind symbiotic and pathogenic associations. In: Singh DP, Singh HB, Prabha R (ed) Plant-Microbe Interactions in Agro-Ecological Perspectives. Springer Nature, pp 97–126
- 42.Shahid M, Hameed S, Imran A, Ali S, van Elsas JD. Root colonization and growth promotion of sunflower (Helianthus annuus L.) by phosphate solubilizing Enterobacter sp. Fs-11. World J Microbiol Biotechnol. 2012;28(8):2749–2758. doi: 10.1007/s11274-012-1086-2. [DOI] [PubMed] [Google Scholar]
- 43.Fischer SE, Jofré EC, Cordero PV, Mañero FJG, Mori GB. Survival of native Pseudomonas in soil and wheat rhizosphere and antagonist activity against plant pathogenic fungi. Antonie Leeuwenhoek. 2010;97(3):241–251. doi: 10.1007/s10482-009-9405-9. [DOI] [PubMed] [Google Scholar]
- 44.Islam F, Yasmeen T, Arif MS, Ali S, Ali B, Hameed S, Zhou W. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Reg. 2016;80(1):23–36. [Google Scholar]
- 45.Couillerot O, Combes ME, Pothier JF, Bellvert F, Challita E, Poirier MA, Rohr R, Comte G, Moënne LY, Prigent Combaret C. The role of the antimicrobial compound 2, 4-diacetylphloroglucinol in the impact of biocontrol Pseudomonas fluorescens F113 on Azospirillum brasilense phytostimulators. Microbiol. 2011;157(6):1694–1705. doi: 10.1099/mic.0.043943-0. [DOI] [PubMed] [Google Scholar]
- 46.Shahid M, Hameed S, Tariq M, Zafar M, Ali A, Ahmad N. Characterization of mineral phosphate-solubilizing bacteria for enhanced sunflower growth and yield-attributing traits. Ann Microbiol. 2015;65(3):1525–1536. [Google Scholar]
- 47.Kumar R, Goyal V, Kuhad M. Influence of fertility-salinity interactions on growth, water status and yield of Indian mustard (Brassica juncea) Indian J Plant Physiol. 2005;10(2):139. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree of acdS gene sequence of Achromobacter sp. FB-14 with the strains of genus Achromobacter. The evolutionary history was inferred using the Neighbor-Joining method. The percentages (≥50%) of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown. The evolutionary distances were computed using the Maximum Composite Likelihood method and are in the units of the number of base substitutions per site (JPG 411 kb).
(DOCX 15 kb).