Abstract
The human T cell lymphotropic virus (HTLV) has a worldwide distribution. HTLV is endemic in some states in the northeastern region of Brazil. This study investigated the prevalence of HTLV-1/2 in 713 pregnant women attended at the Central Laboratory of Public Health of Maranhão (LACEN-MA) between February 2015 and May 2017. Serological screening was performed by chemiluminescent microparticle immunoassay (CMIA), and reactive sera were subsequently confirmed by Western blot (WB) analysis. Five samples were determined to be HTLV-1/2-reactive by CMIA analysis, while in the WB analysis, three sera were positive for HTLV-1, and two were indeterminate. The polymerase chain reaction (PCR) analysis used to detect HTLV-1 proviral DNA showed a specific 336 base pair fragment for HTLV-1 in all CMIA-reactive serum samples. PCR products were purified and sequenced. We observed a 0.7% molecular prevalence of HTLV-1 infection. The average age of the HTLV-1-positive pregnant women was 25.6 ± 8.2 years, and the average age of the HTLV-1-negative pregnant women was 24.3 ± 6.2 (p = 0.60). We observed that there was no association of HTLV-1 infection with age, ethnicity, marital status, educational level, family income, age of first sexual intercourse, previous pregnancy, breastfeeding, intravenous drug use by partner, history of blood transfusions, or use of condoms. The prevalence of HTLV-1 observed in pregnant women demonstrated the need to implement public health policies for the screening of HTLV-1/2 in prenatal care and counseling to avoid breastfeeding by infected women; this approach could control vertical transmission and reduce the spread of this virus in the population.
Keywords: HTLV-1/2, Pregnant woman, Prevalence, Seroprevalence, PCR
Introduction
The human T cell lymphotropic virus (HTLV) belongs to the genus Deltaretrovirus type C, family Retroviridae and subfamily Orthoretrovirinae [1], including four known serotypes, ranging from HTLV-1 to HTLV-4 [2]. Types 1 and 2 are more prevalent and are responsible for human infections. Types 3 and 4 have not been associated with disease in humans, although they have been detected and isolated from Central African populations [3, 4].
Transmission of HTLV-1/2 can occur through infected T lymphocytes via blood transfusion and/or hemoderivatives, transplantation of organs and tissues, sharing of needles and syringes, sexual intercourse, and vertical transmission (mother to child) [5].
Breastfeeding is the most common mode of vertical transmission of HTLV-1. It has been reported that some factors may be associated with the vertical transfer route, including prolonged breastfeeding, high proviral load in breast milk and blood cells, and elevated serum antibody titers [6, 7].
HTLV-1 infections are lifelong, and most infected individuals remain asymptomatic. Approximately 10% of carriers can develop severe clinical complications, such as adult T cell leukemia/lymphoma (ATLL) or HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [2, 8]. Other clinical manifestations related to HTLV-1 infections are uveitis, arthropathies, infective dermatitis, and polymyositis [8], as well as symptoms resulting from coinfection with other infectious agents [9, 10].
Brazil is considered to be the country with the most significant number of individuals affected by HTLV-1 [11], having approximately 800,000 carriers [5], and this virus is present in the populations of all Brazilian regions with varying prevalence, with the highest prevalence being observed in the North and Northeast of Brazil [12].
The prevalence of HTLV-1/2 may vary according to geographical region, ethnicity, and individual risk behavior [13]. Most HTLV-1 endemic areas may be affected by age, socioeconomic, and genetic conditions, with a higher incidence being observed in women [5]. HTLV-1 is endemic among individuals of African origin, and HTLV-2 is endemic among intravenous drug users and native Amazonian populations [14].
Serological tests are the most commonly used method for the laboratory diagnosis of HTLV-1/2 infections, and they are related to the detection of specific components of the viral core and the viral envelope [15]. The two most commonly used techniques are the enzyme-linked immunosorbent assay (ELISA) and chemiluminescent microparticle immunoassay (CMIA), but these assays may present false-positive results and require further confirmation [16]. The confirmatory analysis commonly used is the Western blot (WB) analysis, which can differentiate HTLV-1 from HTLV-2. However, the WB analysis frequently yields indeterminate results and is costly [17].
Polymerase chain reaction (PCR) is an alternative approach for the diagnostic confirmation of HTLV-1/2 infections and has been used qualitatively and quantitatively [15, 16]. PCR is sensitive, specific, and less costly than WB analysis and may be used for the early diagnosis of HTLV infection, especially in cases where serological tests are unable to detect the virus [15].
To date, the Brazilian Ministry of Health has not established a standard protocol for the detection of HTLV-1/2 in pregnant women during prenatal counseling. Therefore, the absence of routine procedures for maternal screening may enable vertical transmission and increase the prevalence of infection with these viruses. The present study employed serological and molecular methods to investigate the prevalence of HTLV-1/2 in pregnant women attended at the Central Laboratory of Public Health of Maranhão (LACEN-MA, Brazil).
Materials and methods
Ethics statement
This study was approved by the Research Ethics Committee of the CEUMA University (CEP-UNICEUMA, n° 372.289/2013) and was conducted in keeping with the ethical precepts specified by the Resolution of the National Health Council of Brazil n° 466/2012. All women who agreed to participate in the study signed an informed consent form (ICF). The anonymity and confidentiality of the data were guaranteed.
Study population, blood sampling collection, and serological screening
A cross-sectional study was conducted to determine the prevalence of HTLV-1 in pregnant women. The sample consisted of 713 pregnant volunteers who were selected by free choice during the prenatal period at the LACEN-MA between February 2015 and May 2017. The pregnant women were aged 15 to 45 years, and all agreed to participate in this study.
Two tubes of venous blood from each pregnant woman were collected by technicians of LACEN-MA according to the standard protocol for the collection of clinical samples. To isolate peripheral blood mononuclear cells (PBMCs), 5 mL of whole blood was collected in a Vacutainer® tube containing ethylenediamine tetraacetic acid (EDTA), and to obtain blood serum, a tube with separator gel was used. Serum samples were screened for HTLV-1/2 by CMIA (Abbott Diagnostics, Germany), and the reactive samples were confirmed by WB analysis (HTLV BLOT 2.4, MP Diagnostics, Singapore). All tests were performed and interpreted according to the manufacturers’ instructions.
Extraction of proviral DNA from PBMCs and PCR reaction conditions
The isolation of PBMCs was performed by Ficoll density gradient centrifugation (Ficoll-Paque Plus, GE Healthcare Life Sciences, USA) according to the manufacturer’s instructions. Proviral DNA extraction from PBMCs was performed using the commercial QIAamp DNA blood kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s instructions. DNA was quantified using a NanoDrop 2000/2000c spectrophotometer (Wilmington, USA).
The PCR analyses were performed in a final volume of 25 μL containing 10 pmol of each specific primer HTLV-1 sense, 5′-CTCCTTCCCCACCCARCGAACYTC-3′, and HTLV-1 antisense, 5′-TAGGGAACATTGGTGAGGAAG-3′, which amplifies a fragment with 336 base pairs (bp). This set of primers was designed specifically for this study from the HTLV-1 tax sequence. Also, 12.5 μL of PCR Master Mix (Promega, USA) (Taq DNA polymerase, dNTPs, MgCl2, PCR buffer [pH 8.5]), 1.5 μL of nuclease-free water, and 300 ng of DNA template were added to each reaction tube. The MyCycler thermal cycler system (Bio-Rad, California, USA) was used for PCR assays under the following conditions: 1 cycle at 95 °C for 5 min (initial denaturation) followed by 35 cycles of 95 °C for 1 min, annealing at 59 °C for 1 min, extension at 72 °C for 1 min, and a final extension at 72 °C for 5 min. Positive controls for HTLV-1 (DNA of infected MT2 cells) and negative controls (nuclease-free water) were also used.
The PCR products were loaded into a 2% agarose gel and subjected to electrophoresis at a voltage of 85 V for 1 h in 1X Tris Borate EDTA (TBE) buffer containing (45 mM Tris Borate, 1-mM EDTA) pH 8.0. After electrophoretic running, the gels were stained with ethidium bromide (0.5 μg/mL) for 15 min [18].
Sensitivity and specificity of the primers evaluated in this study
To analyze the limit of detection of primers designed for this study, our template was the DNA extracted from the PBMCs of the 708 serologically negative pregnant donors by CMIA for HTLV-1. Serial dilutions were performed from 300 ng of proviral DNA as follows: 1:2 (150); 1:4 (75); 1:8 (37.5); and 1:16 (18.75) ng. PCR assays performed in duplicate on alternate days verified the specificity of the primers.
Sequencing of the PCR products and computational analysis
To confirm the identity of the profile obtained by PCR for HTLV-1, the amplified products were purified using the Wizard® SV Gel and PCR Clean-Up System kits (Promega, USA) according to the manufacturer’s instructions. The sequencing of each purified product was performed by the dideoxynucleotide chain termination method [19] in both directions on both strands (sense and antisense) using the ABI Prism BigDye Kit on the ABI 3130 Genetic Analyzer (Applied Biosystems). All sequencing was performed at Myleus Biotecnologia Ltda. (Belo Horizonte, Minas Gerais, Brazil).
The similarity between the sequences was verified by the BLASTn program of the BLAST 2.0 package (Basic Alignment Search Tool – http://www.ncbi.nlm.nih.gov/BLAST/) [20] with HTLV-1 sequences from GenBank selected for comparative analyses by the MEGA program version 6.0 [21].
Statistical analysis
The data were evaluated by the software IBM SPSS Statistics 20.0 for Windows [22]. Quantitative variables were described by means and standard deviation, and the qualitative variables were described by absolute and relative frequencies. Fisher’s exact test was used to analyze the association of the classificatory variables concerning the PCR-determined HTLV-1 groups (positive and negative). The variable “age” was evaluated in two HTLV-1 groups via Student’s t test. The level of significance for the analysis was set at 5%.
Results
Sociodemographic data of pregnant women evaluated
The analysis of the sociodemographic data of the 713 pregnant women showed that the average age was 24.3 ± 6.2 years (15–43), 63.8% were mulatto, 95.2% lived in the metropolitan area of São Luis (São Luis, São José de Ribamar, Paço do Lumiar and, Raposa), 71.8% were either married or in a stable relationship, 61.0% had an education equal to or higher than a high school education, 53.7% were housewives, and 72.0% had a family income of 1 to 3 minimum wages. The other data, such as age, race, residence, marital status, level of education, occupation, previous pregnancies, family income, and number of residents per household, are summarized in Table 1.
Table 1.
Sociodemographic characteristics of 713 pregnant women analyzed in this study
| Characteristics | Number | % |
|---|---|---|
| Age (years) | ||
|
15–30 31–45 |
595 118 |
83.5 16.5 |
|
Ethnicity White Black Mulatto Place of residence Metropolitan area Other municipalities Marital status Single Married/stable union Divorced/separated Educational level < Full high school ≥ Full high school Occupation Unemployed Paid employment Retired/pensioner Works at home Previous pregnancy Yes No Family income (minimum wages) < 1 1–3 > 3 Residents per household 1–3 people 4–6 people > 6 people |
116 142 455 679 34 191 512 10 273 440 140 186 4 383 406 307 185 513 15 354 307 52 |
16.3 19.9 63.8 95.2 4.8 26.8 71.8 1.4 38.3 61.7 19.6 26.1 0.6 53.7 56,9 43,1 25.9 72.0 2.1 49.6 43.1 7.3 |
| Total | 713 | 100.0 |
Serological and molecular assays
Of the 713 clinical samples screened by CMIA, 5 were reactive to HTLV-1/2. After confirmation by WB analysis, only 3 samples were indicative of an HTLV-1 infection, which is equivalent to a seroprevalence of 0.42%.
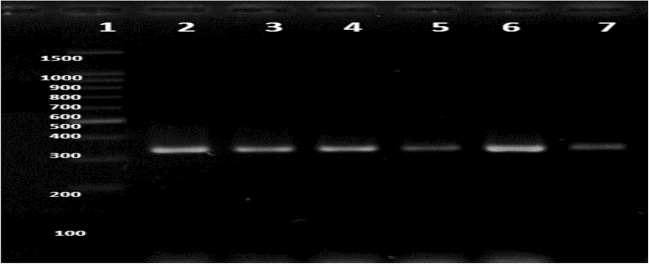
The results of the PCR analyses, for which the template was the proviral DNA obtained from the 713 PBMC samples, showed that all 5 samples determined to be CMIA-positive for HTLV-1 were also PCR-positive and corresponded to a molecular prevalence of infection of 0.7%. The electrophoretic profile of the 336 bp fragment amplified by PCR for each HTLV-1-positive sample is shown in Fig. 1.
Fig. 1.
HTLV-1 profiles obtained by PCR amplification of the proviral DNA extracted from peripheral blood mononuclear cells. Profiles of HTLV-1 in 2% agarose gel stained with ethidium bromide. Line 1, Molecular standard 100 bp DNA ladder (Promega, USA); line 2, HTLV-1 positive control (isolate profile in MT2);Lines 3–7, HTLV-1 profile of clinical samples
The demographic and epidemiological data were compared between the PCR-determined HTLV-1-positive and HTLV-1-negative pregnant women groups. The average age of virus-positive women was 25.6 ± 8,2 years (16–38), and the average age of virus-negative women was 24.4 ± 6.2 years with no significant difference between the ages (p = 0.60). Furthermore, no significant differences were found in any of the other analyzed variables (age, ethnicity, marital status, educational level, family income, first sexual intercourse, prior pregnancy, breastfeeding, use of intravenous drugs by partner, or use of condoms) (Table 2).
Table 2.
Sociodemographic and epidemiological variables of HTLV-1 positive and negative pregnant women in serological and molecular methods
| Variables | HTLV-1 positive n = 5/n (%) | HTLV-1 negative n = 708/n (%) | p value** |
|---|---|---|---|
| Age (years) | 0.33 | ||
| 15–30 | 3 (60.0) | 592 (83.6) | |
| 31–45 | 2 (40.0) | 116 (16.4) | |
| Ethnicity | 0.23 | ||
| White | 0 (0.0) | 116 (16.4) | |
| Black | 2 (40.0) | 140 (19.8) | |
| Mulatto | 3 (60.0) | 452 (63.8) | |
| Marital status | 0.45 | ||
| Single | 2 (40.0) | 189 (26.7) | |
| Married/stable union | 3 (60.0) | 509 (71.9) | |
| Divorced/separated | 0 (0.0) | 10 (1.4) | |
| Educational level | 0.71 | ||
| < Full high school | 2 (40.0) | 275 (38.8) | |
| ≥ Full high school | 3 (60.0) | 433 (61.2) | |
| Family income (MW) | 0.21 | ||
| < 1 | 3 (60.0) | 182 (25.7) | |
| 1–3 | 1 (20.0) | 512 (72.3) | |
| > 3 | 1 (20.0) | 14 (2.0) | |
| First intercourse (years) | 1.00 | ||
| ≤ 16 | 3 (60.0) | 394 (55.6) | |
| > 16 | 2 (40.0) | 314 (44.4) | |
| Previous pregnancy | 0.47 | ||
| Yes | 2 (40.0) | 404 (57.0) | |
| No | 3 (60.0) | 304 (43.0) | |
| Breastfeeding (months)* | 0.75 | ||
| ≤ 6 | 1 (50.0) | 134 (33.2) | |
| > 6 | 1 (50.0) | 270 (66.8) | |
| Partner’s intravenous drug use | 0.76 | ||
| Yes | 0 (0.0) | 26 (3.7) | |
| No | 5 (100.0) | 682 (96.3) | |
| Condom use | 0.14 | ||
| Never | 2 (40.0) | 198 (28.0) | |
| Occasionally | 2 (40.0) | 390 (55.1) | |
| Always | 1 (20.0) | 120 (16.9) |
MW minimum wages. *In the analysis of the variable breastfeeding, only volunteers with previous pregnancies were considered. **Fischer’s exact test. The significance level adopted was 5%
In the PCR-determined HTLV-1-positive group, all women were residents of the metropolitan area of São Luis (4 of São Luís and 1 of Paço do Lumiar). Sociodemographic data showed that most of these women were mulattos (3/5–60.0%) were married or in a stable relationship (3/5–60.0%), possessed formal education equal to or higher than high school (3/5–60.0%), and had a monthly family income of less than 1 minimum wage (3/5–60.0%). We also observed that the women started sexual activities at 16 years of age or younger (3/5–60.0%) and were in their first pregnancy (3/5–60.0%), and they all had a fixed sexual partner. One pregnant woman reported receiving a blood transfusion. Among the HTLV-1-positive pregnant women who had had previous pregnancies, two breastfed their children, and one breastfed for more than 6 months (Table 2).
Sensitivity and specificity tests
The results of the sensitivity test with primers evaluated in the present study showed a detection limit of 37.5 ng of HTLV-1 proviral DNA (data not shown). No viral genetic material was amplified in 708 samples that were determined to be serologically negative by CMIA.
Sequencing of the amplified HTLV-1 fragment
The sequencing of the purified PCR products used to confirm the results showed that all amplified samples were positive for HTLV-1. The analysis of the alignment of the sequences of the 336 bp fragments of the tax gene region assessed in the present study showed 97 to 98% similarity with different nucleotide sequences of HTLV-1 in GenBank databases. These sequences were deposited in GenBank under accession numbers MN309698, MN309699, MN309700, MN309701, and MN309702.
Discussion
The infections caused by HTLV-1/2 remain a largely overlooked public health problem in Brazil, as these infections are usually asymptomatic and may go unnoticed by the carriers of the virus and by some health professionals; thus, HTLV-1/2 may be silently disseminated in the population [23].
Most studies that have been carried out in Brazil employed blood donors as the target population, of which men represent the majority [24]. A seroepidemiological survey carried out in blood banks of the 27 Brazilian capitals showed a regional prevalence of HTLV-1/2 infection ranging from 0.04 to 1.0%; the prevalence was higher in the North and Northeast regions and lower in the South region. Among the capitals analyzed, São Luis (Maranhão) had the highest incidence of HTLV-1/2 (1.0%) followed by Salvador (Bahia) (0.94%), both of which are capitals in the Northeast region [12]. São Luis and Salvador have high numbers of HTLV carriers [12, 25]. The regional variation in the prevalence of HTLV-1/2 in Brazil can be explained by the different ethnic characteristics of its population [14, 26]. The North and Northeast regions have a higher percentage of African and Amerindian descendants than the South region [24].
The prevalence of HTLV-1/2 infection is higher among women [8, 27], with pregnant women being the group that best represents the general population, unlike blood donors [5]. Women have an important role in the transmission chain because they can transmit the virus to their children, mainly through breastfeeding [6]. However, studies in Brazil that assess the infection by HTLV-1/2 in pregnant women are still scarce and isolated [24], and notably few such studies have been conducted in Maranhão.
As with blood donors, there is a significant regional heterogeneity in the seroprevalence of infection by HTLV-1/2 in Brazilian pregnant women, with variations ranging from 0.0% in the western Amazon [28] to approximately 1.0% in the state of Bahia [29, 30]. In the present study, a seroprevalence of HTLV-1 of 0.42% was found by WB analysis, indicating that the metropolitan area of São Luis is the region with the fourth largest seroprevalence among pregnant Brazilian women. This seroprevalence is almost twofold higher than that found among blood donors (0.15%) [31] and is highly similar to that found in a previous study in pregnant women in this region [26]. In Europe, a multinational study observed a seroprevalence of HTLV-1/2 infection six times higher in pregnant women than in blood donors, despite different prevalence rates among countries [32]. Variations of seroprevalence among pregnant women are found even in Africa, which is considered the area most endemic for HTLV-1 worldwide, as well as the place of origin of human retroviruses, with variations from ranging 0.2% in South Africa to 5.5% in Nigeria [5].
In recent years, molecular methods for the diagnosis of different types of infectious agents have revolutionized diagnostic medicine because of their high sensitivity, specificity, and rapidity [33]. This study is the first to use PCR as a molecular diagnostic screening method for HTLV-1 in pregnant Brazilian women, while previous studies have used this technique only as a confirmatory and discriminatory test [26, 30, 34–39]. In the present study, the PCR technique detected a prevalence of 0.7% (5/713) of HTLV-1 in the pregnant women who were analyzed. This prevalence is similar to that found in Bahia, which presents the highest incidence in pregnant women in the Brazilian literature [30, 34].
Only the presence of HTLV-1 was detected in the present study. Previous research performed in pregnant women [26] and blood donors in Maranhão [40] also detected the presence of HTLV-1 and HTLV-2, indicating that HTLV-2 is disseminated in the Maranhão population. It is of fundamental importance to know which type of HTLV is involved in the infection because HTLV-1 is the most pathogenic and is related to severe diseases, such as ATLL and HAM/TSP [2].
We observed that the average age of the 5 pregnant women who tested positive for HTLV-1 by CMIA and PCR was 25.6 years, which is in keeping with the findings reported by other authors [26, 34, 37, 41, 42]. However, a population-based study in Bahia’s capital found a high number of seropositive women older than 50 years of age [25].
Factors related to poverty, such as lower income and lower education levels, may be associated with HTLV-1 infection, suggesting that these factors can influence the transmission and maintenance of the virus in the population [7, 27]. This influence was not observed in the present study, as no association was found between HTLV-1 infection and family income or schooling. These data are consistent with the findings of previous studies conducted in the states of Bahia [29, 30] and Rio de Janeiro [41]. However, Ribeiro et al. [43] and Nunes et al. [25] reported conflicting data.
We found that 60.0% of the HTLV-1-infected women reported beginning sexual intercourse at or below 16 years of age. The early initiation of sexual intercourse among young women may increase the risk of contracting sexually transmitted infections, which also becomes a risk factor for HTLV-1/2 infection [44].
Sexual and vertical transmissions are the main routes of HTLV-1 [45]. A population study carried out in Salvador, Bahia, concluded that sex might have been the main route of HTLV-1 infection in the evaluated population [25]. Thus, the increased risk of women acquiring HTLV infection may be related to the higher effectiveness of transmission from men to women and may be associated with a history of sexually transmitted diseases and mucosal lesions, which would increase the chances of transmission by cell-cell contact [46].
Other high-risk behaviors can increase the likelihood of the sexual transmission of HTLV, such as having multiple sexual partners and not using condoms during sex [47]. This study revealed that all of the infected women had a fixed sexual partner. Although 80.0% of these women affirmed that they occasionally or never used condoms during sex, these data agree with those reported for Rio de Janeiro, where most of the infected pregnant women did not use condoms [41].
HTLV-1 infection was higher among primigravidae (3/5–60.0%), in contrast to data reported by Oliveira and Avelino [35], Figueiró-Filho et al. [36], and Sequeira et al. [47], who observed a higher number of infected pregnant women with multiple pregnancies in Goiás, Mato Grosso, and Pará, respectively.
In the present study, 50% (1/2) of the infected women who had previous pregnancies breastfed their children for more than 6 months. Although no significant differences were observed between the HTLV-1-positive and HTLV-1-negative groups, prolonged breastfeeding for more than 6 months is a risk factor for HTLV-1/2 infection [6, 45] because vertical transmission can occur in approximately 20% of children breastfed by infected women [6]. Additionally, vertical transmission is also a risk factor for the development of HTLV-1-related diseases [27]. In Japan, the vertical transmission of this virus was significantly reduced by prenatal screening for HTLV-1/2 and by counseling seropositive mothers to avoid breastfeeding [6].
Statistical analysis showed no significant difference between the PCR-determined HTLV-1-positive and HTLV-1-negative groups after analysis of the sociodemographic variables and epidemiological data analyzed. These results are in keeping with studies carried out in Bahia [29, 30] and Rio de Janeiro [41]. The high prevalence of HTLV-1 observed in this study, as well as the similarity of sociodemographic and epidemiological profiles between the groups, demonstrates the need for HTLV-1/2 screening in routine prenatal care for pregnant women, as suggested by other researchers [26, 29, 30, 37, 41].
In conclusion, the results obtained through both serological and molecular screening showed a high prevalence of HTLV-1 infection in pregnant women residing in the metropolitan area of São Luis, MA. These data highlight the extent of the problem and demonstrate that this metropolitan area is among the Brazilian capitals with the highest HTLV-1 prevalence in pregnant women. The high prevalence of HTLV-1 infection in the studied pregnant women demonstrates a need for the implementation of public health measures for the control and prevention of this infection in our population. As there is no cure, vaccine, or specific treatment for HTLV infection and its associated diseases to date, prevention is the best option to combat the spread of this virus. However, to this end, it is necessary to adopt effective public policies, including educating the population and health professionals. In addition, it is essential to incorporate HTLV-1/2 screening during the prenatal care of pregnant women, to provide free milk formula to the children of infected mothers and to make notification of this infection compulsory.
Acknowledgments
The authors would like to thank the staff of the LACEN-MA for collecting the blood samples and performing the serological tests for HTLV, and the authors are also grateful to the pregnant women who agreed to participate in this study, making this research possible.
Authors’ contributions
Maria de Fátima Castro Mendes, Bruna de Oliveira de Melo, and Hermerson Sousa Maia extracted the proviral DNA from the peripheral blood mononuclear cells (PBMCs) and performed molecular biology assays. Silvio Gomes Monteiro and Thiago Azevedo Feitosa Ferro performed the statistical analyses of the results. Conceição de Maria Fernandes da Silva Pinto selected the records of the patients of HTLV in the LACEN-MA database. José de Ribamar Oliveira Lima performed the serological tests chemiluminescent microparticle immunoassay (CMIA) and Western blotting (WB) analysis. Maria Rosa Quaresma Bomfim and Edel Figueiredo Barbosa Stancioli designed and conducted the study, analyzed the data, and composed the manuscript. All authors read and approved the final manuscript.
Funding information
This work was supported by the Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA, under grant number 03382/13).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.ICTV (2013) International committee on taxonomy of viruses. Available in: https://talk.ictvonline.org/taxonomy/. Accessed: November 2018
- 2.Szczypinska EM, Wallace MR, Wainscoat B, Salas CM, Rich JD (2015) Human T-cell lymphotropic viruses review. Medscape. Available in: http://emedicine.medscape.com/article/219285-overview#showall. Accessed: October 2018
- 3.Calattini S, Chevalier AS, Duprez R, Bassot S, Froment A, Mahieux R, Gessain A. Discovery of a new human T-cell lymphotropic virus (HTLV-3) in Central Africa. Retrovirology. 2005;2:31–34. doi: 10.1186/1742-4690-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfe ND, Heneine W, Carr JK, Garcia AD, Shanmugam V, Tamoufe U, Torimiro JN, Prosser AT, Lebreton M, Mpoudi-Ngole E, McCutchan F, Birx DL, Folks TM, Burke DS, Switzer WM. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc Natl Acad Sci U S A. 2005;102:7994–7999. doi: 10.1073/pnas.0501734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hino S. Establishment of the milk-borne transmission as a key factor for the peculiar endemicity of human T-lymphotropic virus type 1 (HTLV-1): the ATL prevention program Nagasaki. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:152–166. doi: 10.2183/pjab.87.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carneiro-Proietti AB, Amaranto-Damasio MS, Leal-Horiguchi CF, Bastos RHC, Seabra-Freitas G, Borowiak DR, Ribeiro MA, Proietti FA, Ferreira AS, Martins ML. Mother-to-child transmission of human T-cell lymphotropic viruses-1/2: what we know, and what are the gaps in understanding and preventing this route of infection. J Pediatric Infect Dis Soc. 2014;3(Suppl 1):S24–S29. doi: 10.1093/jpids/piu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonçalves DU, Proietti FA, Ribas JGR, Araújo MG, Pinheiro SR, Carneiro-Proietti ABF. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Reviews. 2010;23:577–589. doi: 10.1128/CMR.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caterino-de-Araujo A, Sacchi CT, Gonçalves MG, Campos KR, Magri MC, Alencar WK, Group of Surveillance and Diagnosis of HTLV of Sa˜o Paulo (GSuDiHTLV-SP) Current prevalence and risk factors associated with HTLV-1 and HTLV-2 infections among HIV/AIDS patients in São Paulo, Brazil. AIDS Res Human Retrovir. 2015;31:543–549. doi: 10.1089/AID.2014.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caterino-de-Araujo A, Alves FA, Campos KR, Lemos MF, Moreira RC. Making the invisible visible: searching for human T-cell lymphotropic virus types 1 and 2 (HTLV-1 and HTLV-2) in Brazilian patients with viral hepatitis B and C. Mem Inst Oswaldo Cruz. 2018;113:130–134. doi: 10.1590/0074-02760170307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carneiro-Proietti AB, Ribas JG, Catalan-Soares BC, Martins ML, Brito-Melo GE, Martins-Filho OA, Pinheiro SR, Araújo Ade Q, Galvão-Castro B, de Oliveira MS, Guedes AC, Proietti FA. Infection and disease caused by the human T cell lymphotropic viruses type I and II in Brazil. Rev Soc Bras Med Trop. 2002;35:499–508. doi: 10.1590/S0037-86822002000500013. [DOI] [PubMed] [Google Scholar]
- 12.Catalan-Soares B, Carneiro-Proietti AB, Proietti FA. Grupo Interdisciplinar de Pesquisas em HTLV. Heterogeneous geographical distribution of human T cell lymphotropic viruses I and II (HTLV-1/2): serological screening prevalence rates in blood donors from large urban áreas in Brazil. Cad Saúde Pública. 2005;21:926–931. doi: 10.1590/S0102-311X2005000300027. [DOI] [PubMed] [Google Scholar]
- 13.Nascimento LB, Carneiro MAS, Teles AS, Lopes CLR, Reis NRSR, Silva AMS, et al. Prevalence of infection due to HTLV-1 in remnant quilombos in Central Brazil. Rev Soc Bras Med Trop. 2009;42:657–660. doi: 10.1590/S0037-86822009000600009. [DOI] [PubMed] [Google Scholar]
- 14.Paiva A, Casseb J. Origin and prevalence of human T-lymphotropic virus type 1 (HTLV-1) and type 2 (HTLV-2) among indigenous populations in the Americas. Rev Inst Med Trop. 2015;57:1–13. doi: 10.1590/S0036-46652015000100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sabino EC, Carvalho SMF (2015). Diagnóstico Laboratorial do HTLV. In: Carneiro-Proietti ABF (Org). Cadernos Hemominas. HTLV. Belo Horizonte, 6ª ed., 16: p.73-83
- 16.Cassar O, Gessain A. Serological and molecular methods to study epidemiological aspects of human T-cell lymphotropic virus type 1 infection. Methods Mol Biol. 2017;1582:3–24. doi: 10.1007/978-1-4939-6872-5-1. [DOI] [PubMed] [Google Scholar]
- 17.Kuramitsu M, Sekizuka T, Yamochi T, Firouzi S, Sato T, Umeki K, Sasaki D, Hasegawa H, Kubota R, Sobata R, Matsumoto C, Kaneko N, Momose H, Araki K, Saito M, Nosaka K, Utsunomiya A, Koh KR, Ogata M, Uchimaru K, Iwanaga M, Sagara Y, Yamano Y, Okayama A, Miura K, Satake M, Saito S, Itabashi K, Yamaguchi K, Kuroda M, Watanabe T, Okuma K, Hamaguchi I. Proviral features of human T cell leukemia virus type 1 in carriers with indeterminate Western blot analysis results. J Clin Microbiol. 2017;55:2838–2849. doi: 10.1128/JCM.00659-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2. N.Y: Cold Spring Harbor Laboratory, Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.Sanger F, Nickelen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.SPSS 20 . IBM SPSS statistics base 20. Chicago: SPSS Inc.; 2011. [Google Scholar]
- 23.Zihlmann KF, Alvarenga AT, Casseb J. Living invisible: HTLV-1-infected persons and the lack of care in public health. PLoS Negl Trop Dis. 2012;6:1–5. doi: 10.1371/journal.pntd.0001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barmpas DBS, Monteiro DLM, Taquette SR, Trajano AJB, Raupp RM, Miranda FRD, et al. HTLV-1/2 infection in Brazilian pregnant women. Rev HUPE. 2014;13:81–88. doi: 10.12957/rhupe.2014.12132. [DOI] [Google Scholar]
- 25.Nunes D, Boa-Sorte N, Grassi MFR, Taylor GP, Teixeira MG, Barreto ML, et al. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. PLoS One. 2017;12:1–10. doi: 10.1371/journal.pone.0171303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Souza VG, Martins ML, Carneiro-Proietti ABF, Januário JN, Ladeira RVP, Silva CMS, et al. High prevalence of HTLV-1 and 2 viruses in pregnant women in São Luis, state of Maranhão, Brazil. Rev Soc Bras Med Trop. 2012;45:159–162. doi: 10.1590/S0037-86822012000200004. [DOI] [PubMed] [Google Scholar]
- 27.Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–6068. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 28.Machado Filho AC, Sardinha JFJ, Ponte RL, Costa EP, da Silva SS, Martinez-Espinosa FE. Prevalence of infection for HIV, HTLV, HBV and of syphilis and chlamydia in pregnant women in a tertiary health unit in the western Brazilian Amazon region. Rev Bras Ginecol e Obstet. 2010;32:176–183. doi: 10.1590/S0100-72032010000400005. [DOI] [PubMed] [Google Scholar]
- 29.Magalhães T, Mota-Miranda AC, Alcantara LC, Olavarria V, Galvão-Castro B, Rios-Grassi MF. Phylogenetic and molecular analysis of HTLV-1 isolates from a medium sized town in northern of Brazil: tracing a common origin of the virus from the most endemic city in the country. J Med Virol. 2008;80:2040–2045. doi: 10.1002/jmv.21278. [DOI] [PubMed] [Google Scholar]
- 30.Mello MAG, Conceição AF, Sousa SMB, Alcântara LC, Marin LJ, Raiol MRS, et al. HTLV-1 in pregnant women from the southern Bahia, Brazil: a neglected condition despite the high prevalence. Virol J. 2014;11:28. doi: 10.1186/1743-422X-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viana GMC, Nascimento MDSB, Oliveira RAS, Santos AC, Galvão CS, Silva MACN. Seroprevalence of HTLV-1/2 among blood donors in the state of Maranhão, Brazil. Rev Bras Hematol Hemoter. 2014;36:50–53. doi: 10.5581/1516-8484.20140013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor GP, Bodeus M, Courtois F, Pauli G, Del Mistro A, Machuca A, et al. The seroepidemiology of human T-lymphotropic viruses: types I and II in Europe: a prospective study of pregnant women. J Acq Immun Def Synd. 2005;38:104–109. doi: 10.1097/00126334-200501010-00018. [DOI] [PubMed] [Google Scholar]
- 33.Messacar K, Parker SK, Todd JK, Dominguez SR. Implementation of rapid molecular infectious disease diagnostics: the role of diagnostic and antimicrobial stewardship. J Clin Microbiol. 2017;55:715–723. doi: 10.1128/JCM.02264-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bittencourt AL, Dourado I, Filho PB, Santos M, Valadão E, Alcantara LC, Galvão-Castro B. Human T-cell lymphotropic virus type 1 infection among pregnant women in northeastern Brazil. J Acquir Immune Defic Syndr. 2001;26:490–494. doi: 10.1097/00126334-200104150-00016. [DOI] [PubMed] [Google Scholar]
- 35.Oliveira SR, Avelino MM. Human T-cell lymphotropic virus type I seroprevalence among pregnant women in Goiânia, GO, Brazil. Rev Bras Ginecol Obstet. 2006;28:467–472. doi: 10.1590/S0100-72032006000800005. [DOI] [Google Scholar]
- 36.Figueiro’-Filho EA, Senefonte FRA, Lopes AHA, De Morais OO, Souza Junior VG, Maia TL, et al. Frequency of HIV-1, rubella, syphilis, toxoplasmosis, cytomegalovirus, simple herpes virus, hepatitis B, hepatitis C, Chagas disease and HTLV I/II infection in pregnant women of state of Mato Grosso do Sul. Rev Soc Bras Med Trop. 2007;40:181–187. doi: 10.1590/S0037-86822007000200007. [DOI] [PubMed] [Google Scholar]
- 37.Dal Fabbro MM, Cunha RV, Bóia MN, Portela P, Botelho CA, Freitas GMB, Soares J, Ferri J, Lupion J. HTLV 1/2 infection: prenatal performance as a disease control strategy in state of Mato Grosso do Sul. Rev Soc Bras Med Trop. 2008;41:148–151. doi: 10.1590/S0037-86822008000200003. [DOI] [PubMed] [Google Scholar]
- 38.Medeiros ACM, Vidal LRR, Von Linsingen R, Ferin NA, Bessani Strapasson T, Almeida SM, et al. Confirmatory molecular method for HTLV-1/2 infection in high-risk pregnant women. J Med Virol. 2017;90:998–1001. doi: 10.1002/jmv.25014. [DOI] [PubMed] [Google Scholar]
- 39.Guerra AB, Siravenha LQ, Laurentino RV, Feitosa RNM, Azevedo VN, Vallinoto ACR, Ishak R, Machado LFA. Seroprevalence of HIV, HTLV, CMV, HBV and rubella virus infections in pregnant adolescents who received care in the city of Belém, Pará, northern Brazil. BMC Pregnancy and Childbirth. 2018;18:1–7. doi: 10.1186/s12884-018-1753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viana GMC, Silva MACN, Souza VL, Lopes NBS, Nascimento MDSB. Endemic transmission of HTLV-2 in blood donors from São Luís do Maranhão, northeastern Brazil: report of two asymptomatic individuals. Rev Bras Hematol Hemoter. 2015;37:130–131. doi: 10.1016/j.bjhh.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Monteiro DLM, Taquette SR, Barmpas DBS, Rodrigues NCP, Teixeira SAM, Villela LHC, et al. Prevalence of HTLV-1/2 in pregnant women living in the metropolitan area of Rio de Janeiro. PLoS Negl Trop Dis. 2014;9(e3146):1–5. doi: 10.1371/journal.pntd.0003146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moura AA, Mello MJG, Correia JB. Prevalence of syphilis, human immunodeficiency virus, hepatitis B virus, and human T-lymphotropic virus infections and coinfections during prenatal screening in an urban northeastern Brazilian population. Int J Infect Dis. 2015;39:10–15. doi: 10.1016/j.ijid.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 43.Ribeiro MA, Proietti FA, Martins ML, Januário JN, Ladeira RV, Oliveira MF, et al. Geographic distribution of human T-lymphotropic virus types 1 and 2 among mothers of newborns tested during neonatal screening, Minas Gerais, Brazil. Rev Panam Salud Publica. 2010;27:330–337. doi: 10.1590/S1020-49892010000500002. [DOI] [PubMed] [Google Scholar]
- 44.Olbrich Neto J, Meira DA. Soroprevalence of HTLV-I/II, HIV, syphilis and toxoplasmosis among pregnant women seen at Botucatu - São Paulo - Brazil: risk factors HTLV-I/II infection. Rev Soc Bras Med Trop. 2004;37:28–32. doi: 10.1590/S0037-86822004000100008. [DOI] [PubMed] [Google Scholar]
- 45.Paiva AM, Assone T, Haziot MEJ, Smid J, Fonseca LAM, Luiz OC, et al. Risk factors associated with HTLV-1 vertical transmission in Brazil: longer breastfeeding, higher maternal proviral load and previous HTLV-1-infected offspring. Scientifc Reports. 2018;8:1–6. doi: 10.1038/s41598-018-25939-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paiva A, Casseb J. Sexual transmission of human T-cell lymphotropic virus type 1. Rev Soc Bras Med Trop. 2014;47:265–274. doi: 10.1590/0037-8682-0232-2013. [DOI] [PubMed] [Google Scholar]
- 47.Sequeira CG, Tamegão-Lopes BP, Santos EJ, Ventura AM, Moraes-Pinto MI, Succi RCM. Descriptive study of HTLV infection in a population of pregnant women from the state of Pará, northern Brazil. Rev Soc Bras Med Trop. 2012;45:453–456. doi: 10.1590/S0037-86822012005000007. [DOI] [PubMed] [Google Scholar]