Abstract
The plant microbiota diversity is often underestimated when approaches developed mainly for the identification of cultivable microorganisms are used. High-throughput sequencing allows a deeper understanding of the microbial diversity associated with plants. The amplification of ITS1 was used to analyze fungal diversity in several plant organs and rhizosphere of three common bean (Phaseolus vulgaris) varieties grown in a greenhouse. The fungal diversity diverged between those plant organs and the rhizosphere, with the highest found in the rhizosphere and the lowest in the stem. In each organ different numbers of genus, OTUs were identified, in a total of 283 OTUs evenly distributed among the varieties. In the co-occurrence network, a larger number of positive interactions were found in the organs of the aerial part in all varieties. We observed that the diversity of the endophytic microbiota differed more between plant organs than between common bean varieties. Our results show that the diversity of endophytic fungi can be efficiently accessed with the sequencing of ITS amplicons and that this diversity may vary among distinct plant organs and the rhizosphere of a single plant variety.
Electronic supplementary material
The online version of this article (10.1007/s42770-019-00217-9) contains supplementary material, which is available to authorized users.
Keywords: Cultivation-independent approach, Endophytic community, ITS amplicon sequencing, Mycobiome, Phaseolus vulgaris
Introduction
Endophytic microorganisms are able to colonize plant species without causing any disease [1]. These microorganisms can be transmitted vertically by plants to their seeds or horizontally by inoculum from other plants or soil [2]. The presence of these microorganisms inside plant organs may even be beneficial to them, promoting growth, tolerance to abiotic stresses, and disease resistance [2]. Besides adaptive advantages they provide to the host plants, these microorganisms can synthesize secondary metabolites with medical, agronomic, industrial, or biotechnological importance [3, 4].
Endophytic microorganisms are usually identified through isolation from the host plant followed by morphological and/or molecular characterization of the cultivated microorganisms [5, 6]. However, one of the limitations in the study of endophytic communities is the methodology used. With the introduction of high-throughput sequencing, it became possible to achieve a deeper understanding of how the endophytic microbiota is composed.
Amplicon sequencing has been widely used to analyze endophytic microorganisms, since it enables to reach a deeper layer of the microbial community colonizing several agriculturally important plant species [7, 8]. It was observed that the endophytic fungal diversity found in plants grown under the same conditions changes during their life cycle [9], in the same way different plant organs of a single individual such as fruit, stem, and leaves can present distinct endophytic compositions [10]. Cultivation practices usually adopted in agricultural systems such as the application of pesticides [10] and the use of different genotypes of a single plant species [11, 12] change the endophytic community structure.
Like many plant species, the common bean (Phaseolus vulgaris) can be colonized by endophytic microorganisms. It was shown that the composition of cultivable endophytic bacteria of three bean varieties (BRS Talismã, Ouro Negro, and Vermelhinho) grown in an experimental field under the same conditions varies depending on the plant variety [13]. In another work, endophytic fungi were isolated and identified in two common bean varieties (Talismã and Ouro Negro) grown in experimental fields as belonging to different genera [14]. Studies using metagenomics evidenced differences between bacterial populations present in the rhizosphere of distinct bean varieties [15, 16].
The limited number of studies carried out on the subject until the present reflects in the poor knowledge about the total diversity of endophytic fungi found in distinct organs and in the rhizosphere of different common bean varieties. Furthermore, it is not known about the composition of endophytic fungi present in resistant and susceptible varieties. Therefore, the objective of the present work was to analyze endophytic fungi communities present in several organs of three common bean genotypes, as well as to draw a comparison between the microbiota of these genotypes.
Material and methods
Experimental design and sample preparation
Three common bean (Phaseolus vulgaris L.) varieties were used in the present study, as follows: BRS Talismã, Ouro Negro, and Rosinha. The seeds were preliminary disinfested in a solution of 2.5% sodium hypochlorite for 30 min followed by three washes in sterile distilled water and then sown in non-sterile field soil. The soil used was from an experimental field at Universidade Federal de Viçosa, MG. To minimize the environmental effects of the field, the plants were maintained in a greenhouse for 23 days and later, four plants of each variety were randomly chosen for DNA extraction. The technical rhizosphere (soil fraction still adhered to the root surface after vigorous agitation) of the selected plants was also collected.
The plants were then separated into organs (root, stem, and leaves) and rhizosphere. Each organ was surface disinfested and the epiphytic microorganisms were removed according to the following protocol: 1 min in 70% ethanol followed by 5 min in 2% sodium hypochlorite and three washes with sterile distilled water for leaves and stem. For the roots, the sodium hypochlorite step was replaced by 3 min in 3% H2O2. A total of 48 samples were collected, 16 of each variety consisting of four samples of each organ (root, stem, and leaves) and four rhizosphere samples.
DNA extraction and sequencing
DNA was extracted from the plant organs using the kit NucleoSpin® Plant II (MACHEREY-NAGEL, Düren, Germany), while the kit NucleoSpin® Soil (MACHEREY-NAGEL, Düren, Germany) was used to extract DNA from the rhizosphere. In both cases, the manufacturer’s instructions were followed. The ITS1 region was amplified using ITS1F [17] and ITS2 [18] primers and the amplicon sequencing were carried out by the company Argonne National Laboratory (Illinois, USA) using the sequencing platform Illumina MiSeq (2 × 250 pb).
Sequence analysis
The sequences were analyzed with the software USEARCH 8.1 [19] and Quantitative Insights Into Microbial Ecology (QIIME) 1.9.1 [20], according to the pipeline developed by the Brazilian Microbiome Project [21] for the analysis of ITS data produced by Illumina sequencing.
The raw forward reads originated from the sequencing were demultiplexed, filtered by quality (reads with an expected error > 0.5 were discarded), and dereplicated. The ITS1 region was isolated using the ITSx software [22] and trimmed to 140 pb. The operational taxonomic units (OTUs) were grouped considering 97% similarity between the sequences obtained with the Uparse method [19]. After chimeras were excluded, the taxonomic annotation of the OTUs was carried out based on the UNITE database [23]. The table showing OTU abundance data was normalized before the subsequent analyses, such as the calculation of alpha and beta diversities. The Venn diagrams were drawn with the online tool Jvenn [24]. The reverse reads were not included in the analyses due to their low quality. Species richness and diversity indexes were calculated with the methodology recommended by Chao [25] using the INEXT package for the R software [26].
A co-occurrence network was constructed to assess the microbial interactions in the different organs and varieties. For this purpose, SparCC correlations were calculated with the SparCC module available in Python using 100 permutations in the OTU abundance matrix [27]. Only the SparCC correlations with absolute values above 0.9 and p values below 0.01 were included in the network. The IGRAPH package [28] for R [26] was used to calculate the network parameters.
Results
A total of 17,313,333 reads were sequenced. The rhizosphere presented the greatest number of reads, followed by leaves, stem, and root. After quality filtering 5,935,695 sequences (34.28%) remained in the analyses. The number of reads for each sample (leaf, steam, root, and rhizosphere for each variety) was normalized to 5440 for the downstream comparative analyses.
Alpha and beta diversities
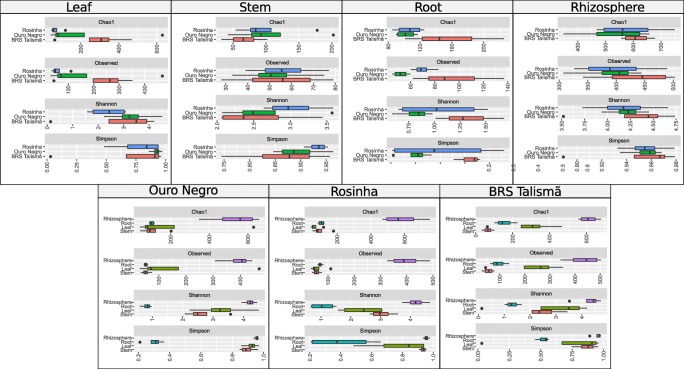
Rarefaction analyses showed that the sampling of the endophytic fungal community was representative and quality would probably have not improved with additional samplings (Fig. S1). The coverage value was higher than 97% for all samples, evidencing that the sampling was good enough to determine most of the fungal diversity existing in the plant organs and in the rhizosphere. The number of OTUs detected in the leaf, steam, root, and rhizosphere for each variety varied from 55 to 428 (Table S1). The rhizosphere presented a significantly higher number of OTUs than stem, leaf, and root samples for all three varieties (Fig. 1). The values of diversity indices were similar among varieties for the same organ and among different organs of the same variety.
Fig. 1.
Species richness and diversity in the different organs and in the rhizosphere of the common bean varieties Ouro Negro, Rosinha and BRS Talismã
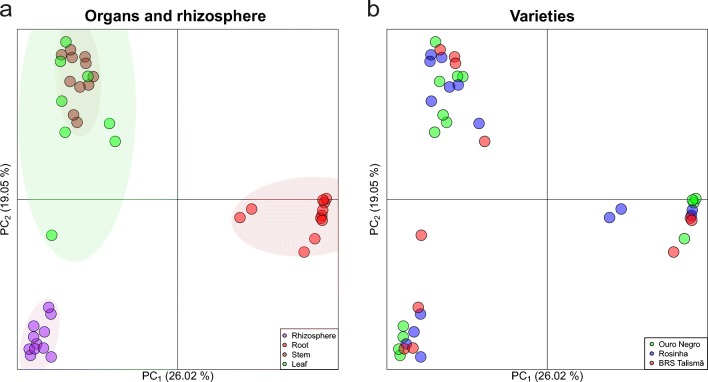
According to the principal coordinates analysis (PCoA), the endophytic fungal community found in the root was similar among the varieties but different from the rhizosphere and other plant organs (Fig. 2a). The same occurred in the rhizosphere. On the other hand, the fungal community found in the leaves was similar to stem. The fungal composition of the plant organs and the rhizosphere did not differ among the common bean varieties (Fig. 2b).
Fig. 2.
Principal coordinates analysis (PCoA) based on the Bray-Cutis distance matrix showing the beta diversity for organs and rhizosphere samples (a) and the different varieties (b). Ellipses represent a confidence interval of 95%
Endophytic fungal communities present in organs and in the rhizosphere of the common bean varieties
Based on the analysis of ITS1 amplicons, the phyla Ascomycota, Basidiomycota, and Mortierellomycota were the most abundant among the common bean endophytes (Fig. S2). In the leaf, stem, and rhizosphere samples, the phylum Ascomycota predominated, comprising between 23 and 74% of the microorganisms found. Only in the stem samples of all three varieties and in the leaf samples of the Rosinha variety, the phylum Basidiomycota was more abundant, representing from 16 to 40% of the total. In turn, in root samples, approximately 90% of the detected sequences belonged to the phylum Mortierellomycota.
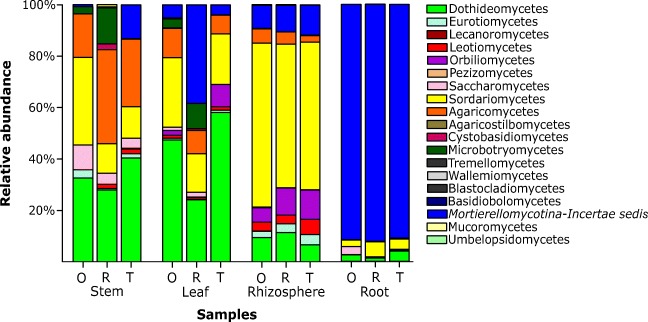
The most abundant Ascomycota classes in the leaf, stem, and rhizosphere samples were Sordariomycetes and Dothideomycetes, while the Basidiomycota was represented mainly by Agaricomycetes (Fig. 3). The class Sordariomycetes was predominant in the rhizosphere, whereas the class Dothideomycetes predominated in stem and leaf samples. The Agaricomycetes class was more abundant in stem samples. In root samples, about 90% of the samples were identified as Mortierellomycotina (Incertae sedis).
Fig. 3.
Relative abundance of the classes detected in the organs and rhizosphere samples of three common bean varieties (O, Ouro Negro; R, Rosinha; T, BRS Talismã)
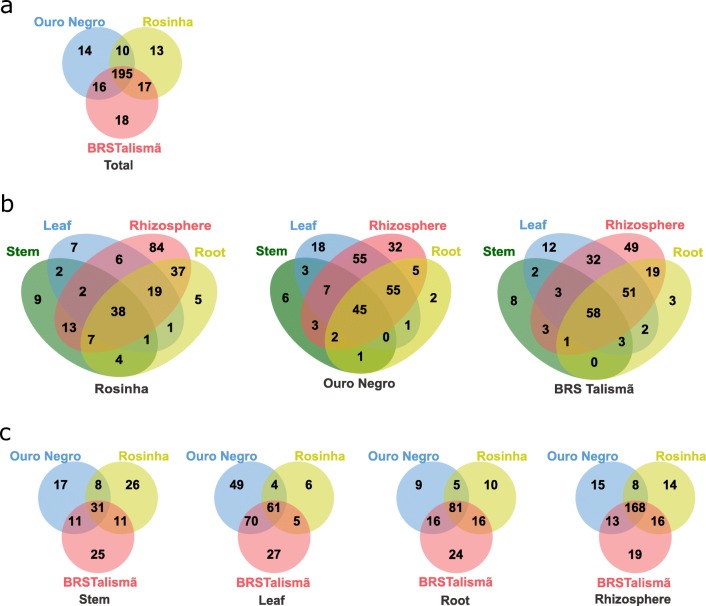
The identified OTUs were attributed to 283 genera. The number of OTUs among the varieties was similar, 195 (69%) were shared among all three varieties and few taxa were unique to each plant variety (Fig. 4a and Table S2). The five most abundant genera present in the plant organs and in the rhizosphere varied between the common bean varieties (Fig. S3). At least 76 genera of fungi were detected in the leaves, with the more abundant being Rhamularia (22%), Mortierella (19%), Fusarium (6%), and Cladosporium (5%). The Colletotrichum OTU found in stem and leaf samples of the Rosinha (susceptible) and in one stem sample of the Ouro Negro (resistant) variety were identified as belonging to C. lindemuthianum, a species known to cause anthracnose in common bean plants. At least 16% of the genera identified in the three varieties were shared among the plant organs and the rhizosphere (Fig. 4b). The rhizosphere of all varieties presented the largest number of unique genera, and roots the smallest. Unique genera were found in the different organs and in the rhizosphere of all three common bean varieties (Fig. 4c).
Fig. 4.
Venn diagrams showing the number of genera shared for the distinct varieties (a), for the plant organs and the rhizosphere of each variety (b), and for the same plant organ or rhizosphere between the different varieties (c)
Structure of the interaction network of endophytic fungi in the different organs
The co-occurrence network was analyzed to evaluate the number of interactions of endophytic fungi present in the plant organs and in the rhizosphere of the distinct plant varieties. This number was larger in the rhizosphere than in the plant organs, except for the leaves of the Ouro Negro variety, which presented the largest number of interactions (Table 1). Curiously, the roots of this same variety exhibited a lower number of interactions compared with the other varieties.
Table 1.
Features of the co-occurrence network in the distinct organs and in the rhizosphere of three common bean varieties
| Parameters | Variety | Samples | |||
|---|---|---|---|---|---|
| Leaf | Stem | Root | Rhizosphere | ||
| Number of nodes | Ouro Negro | 176 | 52 | 14 | 755 |
| Rosinha | 57 | 73 | 40 | 770 | |
| BRS Talismã | 64 | 67 | 46 | 826 | |
| Number of interactions | Ouro Negro | 1347 | 101 | 13 | 1103 |
| Rosinha | 90 | 101 | 116 | 881 | |
| BRS Talismã | 107 | 116 | 96 | 941 | |
| Number of positive interactions | Ouro Negro | 1091 (81%) | 84 (83%) | 6 (46%) | 600 (54%) |
| Rosinha | 78 (87%) | 91 (90%) | 60 (52%) | 460 (52%) | |
| BRS Talismã | 95 (89%) | 105 (90%) | 52 (54%) | 524 (55%) | |
| Number of negative interactions | Ouro Negro | 256 (19%) | 17 (17%) | 7 (54%) | 503 (46%) |
| Rosinha | 12 (13%) | 10 (10%) | 56 (48%) | 421 (48%) | |
| BRS Talismã | 12 (11%) | 11 (10%) | 44 (46%) | 417 (44%) | |
| Modularity | Ouro Negro | 0.62 | 0.67 | 0.56 | 0.57 |
| Rosinha | 0.80 | 0.79 | 0.23 | 0.55 | |
| BRS Talismã | 0.63 | 0.63 | 0.31 | 0.58 | |
| Diameter | Ouro Negro | 8 | 6 | 4 | 8 |
| Rosinha | 7 | 6 | 7 | 9 | |
| BRS Talismã | 8 | 7 | 7 | 8 | |
| Average length | Ouro Negro | 3.22 | 2.28 | 1.98 | 3.66 |
| Rosinha | 2.69 | 1.95 | 2.69 | 3.67 | |
| BRS Talismã | 2.74 | 2.58 | 2.84 | 3.80 | |
| Average degree | Ouro Negro | 7.65 | 1.94 | 0.93 | 1.46 |
| Rosinha | 1.58 | 1.38 | 2.90 | 1.14 | |
| BRS Talismã | 1.67 | 1.73 | 2.09 | 1.14 | |
Positive interactions predominated in the organs of the aerial part in all varieties, whereas in the roots, the number of positive and negative interactions was similar to that observed in the rhizosphere (Table 1). The average length and diameter of the network referent to the organs of the aerial part of the Ouro Negro and BRS Talismã varieties were larger than those of the Rosinha. The modularity of the aerial part organs in the Ouro Negro and BRS Talismã varieties was smaller than that in Rosinha. The average degree, which consists of the average number of interactions established by each OTU, was considerably higher in the Ouro Negro leaves, indicating an elevated interdependency between the OTUs.
Discussion
This work was the first to investigate the endophytic fungal community in different plant organs and rhizosphere of three common bean varieties, where a wide diversity of endophytic fungi was found. The diversity index values varied between the plant organs and rhizosphere. Among the organs, the highest diversity was observed in the leaves. A study evaluating the fungal microbiome of strawberry plants showed similar results, with the highest alpha and beta indexes found in the leaves when compared with the other organs of the aerial part. In addition, the mycobiota composition in leaves was different from other organs of the same plant [10]. The rhizosphere presented the highest indexes, probably due to the fact that soil microbiota is complex and these microorganisms are attracted by root exudates [29]. It has already been shown that the host genotype may affect the endophytic microbiota [30]. In common bean plants, the mycobiota composition seems to vary not only with the variety but also with plant organ. In other studies, difference in mycobiota composition among organs of the same variety and difference in mycobiota composition of a single organ in different genotypes was observed [10, 11]. Endophytes in the roots and in the rhizosphere form groups different than those in the stem and leaves, which share the mycobiota.
In the vegetal samples, we found mainly members of the phyla Ascomycota, Basidiomycota, and Mortierellomycota. Ascomycota representatives were more abundant in the aerial plant organs, corroborating the findings of another work in which endophytic fungi present in the aerial parts of common bean plants also belonged to the referred phylum [14]. Certain classes of endophytic fungi predominate in several plants [5, 31]. Two of them, Dothideomycetes and Sordariomycetes, were the most abundant in the aerial part of the common bean plants. The phylum Basidiomycota has been detected mainly in the stem of common bean varieties. Endophytic fungi belonging to this phylum are more often found in stem tissues, while Ascomycota fungi can be detected both in the leaves and in stem [32]. The phylum Mortierellomycota comprises fungi of the subphylum Mortierellomycotina, which have been described as saprophytic soil inhabitants [33]. Fungi of the Mortierellomycotina genus Mortierella have already been reported in studies on microbiome [34] and as endophytic microorganisms [35, 36]. Although common beans can perform symbiosis with mycorrhizal fungi [37], interestingly, Glomeromycota was detected in none of the rhizosphere or common bean root samples. The same was observed in cormosphere, rhizosphere, and bulk soil during dormant stage and flowering Crocus sativus [9]. A possible cause is the absence of phylum in the samples or the primers used. Primers for the ITS region are not the most suitable and/or used for Glomeromycota, as well as the UNITE database for taxonomic information of this group [38, 39].
In previous studies using isolation to evaluate the common bean endophytic communities, only 27 fungal genera were identified in the leaves of plants at 45 days of cultivation on experimental fields. The genera Colletotrichum, Cochliobolus, Hannaella, and Phomopsis predominated [14]. However, in a cultivation-independent analysis of the common bean microbiome, the abundance of these genera was relatively low (0.1%). In some samples, they could not even be detected. Nevertheless, up to date, there are no reports of endophytic C. lindemuthianum being isolated from common bean plants. Interestingly, an analysis of the microbiome found in strawberry plants revealed a single representative of the Colletotrichum genus, the C. acutatum fungus, which causes anthracnose in that same crop [10]. The pathogenic fungus Ustilago maydis, a causal agent of the corn smut, has been detected in the community of both resistant and susceptible maize plants [40].
The common bean varieties were grown under controlled conditions for the microbiome analysis; therefore, the absence of other genera reported in the literature may have been associated to the place of cultivation. The majority of studies on endophyte diversity, such as that by Gonzaga et al. (2015), are carried out on plants grown under field conditions, in which fungal propagation occurs mainly by rain and wind that carry out the spores. In greenhouses, these factors are absent. Furthermore, the common bean plants collected were young, and studies on leaf endophytes showed that older leaves present a higher diversity of fungal endophytes due to their longer exposition to environmental factors, which increase the chance of spore deposition and the consequent colonization by fungi present in the environment [41]. The endophytic fungal species detected in the present study either already existed on the seeds used or in the soil and then colonized the plants, considering that many endophytic species can also be vertically propagated [2].
The large number of genera identified in our work corroborates the higher capacity of microorganism detection in the endophytic microbiota based on ITS1 amplicons. When isolation techniques are used, the presence and the relative abundance of a certain species may be associated with its ability to grow and develop on specific culture medium or cultivation conditions in the laboratory [42]. The use of the ITS1 or ITS2 region for diversity studies using high-throughput sequencing is widely employed, with either of the two regions being sufficient to study the fungal diversity of environmental samples [43].
Interaction networks of fungal endophytes were constructed for distinct plant organs. These co-occurrence networks evidenced that in the organs of the aerial part, there were more positive than negative interactions, reflecting a possibly stronger functional dependency between the species. Curiously, the Ouro Negro variety, which was resistant to anthracnose, showed a large number of interactions in the leaves when compared with the Rosinha variety (susceptible) considering the same organ. The abundant interactions together with the high fungal diversity may represent crucial features in the resistance of the variety to that disease [44]. However, the average length and the diameter exhibited higher values, and the modularity values were smaller for the interaction network observed in the aerial part organs of the Ouro Negro and BRS Talismã varieties, suggesting a less connected community [16, 45].
The present study on the diversity of endophytic fungi existing in common bean plants evidenced that the presence of certain fungi depends much more on the plant organ than on the variety itself. Microbiome studies are important as they generate knowledge that may help answer questions arising along the study. For example, if in the cases where fungal genera are shared among plant varieties, it means that there exists a minimal mycobiota for a certain species. The same applies to the cases where genera specific of a given plant variety could be involved in the differential response to biotic or abiotic stresses, or even resistance to plant pathogens. Similarly, if the presence of phytopathogenic fungi in plant varieties considered resistant indicate that these fungi are in a latent stage or living as endophyte. This way, we could try to understand and elucidate processes taking place in the interactions among these microorganisms and their host plants as well as the response of these plants to environmental conditions. We can also direct future studies by focusing on these issues that are raised.
Electronic supplementary material
Rarefaction curve. a: For plant organs and rhizosphere samples. b: For different common bean varieties.(PDF 23 kb)
Relative abundance of taxonomic phyla found in organs and rhizosphere samples of three common bean varieties. O: Ouro Negro; R: Rosinha; T: BRS Talismã.(PDF 64 kb)
Five most abundant genera in each variety by organs and rhizosphere.(PDF 141 kb)
(DOCX 14 kb)
(XLSX 11 kb)
Funding information
This research was financially supported by the following Brazilian agencies: Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) - Finance Code 001 and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Petrini O. Microbial Ecology of Leaves. 1991. Fungal endophytes of tree leaves; pp. 179–197. [Google Scholar]
- 2.Rodriguez RJ, White JF, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytol. 2009;182:314–330. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- 3.Hardoim PR, van Overbeek LS, Berg G, Pirttilä AM, Compant S, Campisano A, Döring M, Sessitsch A. The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial Endophytes. Microbiol Mol Biol Rev. 2015;79:293–320. doi: 10.1128/MMBR.00050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porras-Alfaro A, Bayman P. Hidden fungi, emergent properties: endophytes and microbiomes. Annu Rev Phytopathol. 2011;49:291–315. doi: 10.1146/annurev-phyto-080508-081831. [DOI] [PubMed] [Google Scholar]
- 5.Arnold AE, Lutzoni F. Diversity and host range of foliar fungal endophytes: are tropical leaves biodiversity hotspots? Ecology. 2007;88:541–549. doi: 10.1890/05-1459. [DOI] [PubMed] [Google Scholar]
- 6.Ding X, Liu K, Deng B, Chen W, Li W, Liu F. Isolation and characterization of endophytic fungi from Camptotheca acuminata. World J Microbiol Biotechnol. 2013;29:1831–1838. doi: 10.1007/s11274-013-1345-x. [DOI] [PubMed] [Google Scholar]
- 7.Müller H, Berg C, Landa BB, et al. Plant genotype-specific archaeal and bacterial endophytes but similar Bacillus antagonists colonize Mediterranean olive trees. Front Microbiol. 2015;6:1–9. doi: 10.3389/fmicb.2015.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souza RSC, Okura VK, Armanhi JSL, et al. Unlocking the bacterial and fungal communities assemblages of sugarcane microbiome. Sci Rep. 2016;6:28774. doi: 10.1038/srep28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ambardar S, Singh HR, Gowda M, Vakhlu J. Comparative metagenomics reveal phylum level temporal and spatial changes in mycobiome of belowground parts of Crocus sativus. PLoS One. 2016;11:1–17. doi: 10.1371/journal.pone.0163300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdelfattah A, Wisniewski M, Giulia M, Destri L. Metagenomic analysis of fungal diversity on strawberry plants and the effect of management practices on the fungal community structure of aerial organs. PLoS One. 2016;11:1–18. doi: 10.1371/journal.pone.0160470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bálint M, Tiffin P, Hallström B et al (2013) Host genotype shapes the foliar fungal microbiome of balsam poplar (Populus balsamifera). PLoS One 8. 10.1371/journal.pone.0053987 [DOI] [PMC free article] [PubMed]
- 12.Mashiane RA, Ezeokoli OT, Adeleke RA. Metagenomic analyses of bacterial endophytes associated with the phyllosphere of a Bt maize cultivar and its isogenic parental line from South Africa. World J Microbiol Biotechnol. 2017;33:1–12. doi: 10.1007/s11274-017-2249-y. [DOI] [PubMed] [Google Scholar]
- 13.Costa LE de O, de Queiroz MV, Borges AC, et al (2012) Isolation and characterization of endophytic bacteria isolated from the leaves of the common bean (Phaseolus vulgaris). Braz J Microbiol 43:1562–1575 . doi: 10.1590/S1517-838220120004000041 [DOI] [PMC free article] [PubMed]
- 14.Gonzaga LL, Costa LEO, Santos TT, et al. Endophytic fungi from the genus Colletotrichum are abundant in the Phaseolus vulgaris and have high genetic diversity. J Appl Microbiol. 2015;118:485–496. doi: 10.1111/jam.12696. [DOI] [PubMed] [Google Scholar]
- 15.Pérez-jaramillo JE, Carrión VJ, Bosse M, et al (2017) Linking rhizosphere microbiome composition of wild and domesticated Phaseolus vulgaris to genotypic and root phenotypic traits. Nat Publ gr 1–14 . doi: 10.1038/ismej.2017.85 [DOI] [PMC free article] [PubMed]
- 16.Mendes LW, Raaijmakers JM, De Hollander M, et al. Influence of resistance breeding in common bean on rhizosphere microbiome composition and function. ISME J. 2018;12:212–224. doi: 10.1038/ismej.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GARDES M, BRUNS TD. ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2:113–118. doi: 10.1111/j.1365-294X.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 18.White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols. A Guide to Methods and Applications, Academic Press, pp 315-322. 10.1016/b978-0-12-372180-8.50042-1
- 19.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 20.Caporaso JG, Kuczynski J, Stombaugh J, et al. correspondEnce QIIME allows analysis of high- throughput community sequencing data intensity normalization improves color calling in SOLiD sequencing. Nat Publ Group. 2010;7:335–336. doi: 10.1038/nmeth0510-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pylro VS, Roesch LFW, Ortega JM, do Amaral AM, Tótola MR, Hirsch PR, Rosado AS, Góes-Neto A, da Costa da Silva AL, Rosa CA, Morais DK, Andreote FD, Duarte GF, de Melo IS, Seldin L, Lambais MR, Hungria M, Peixoto RS, Kruger RH, Tsai SM, Azevedo V, Brazilian Microbiome Project Organization Committee Brazilian microbiome project: revealing the unexplored microbial diversity-challenges and prospects. Microb Ecol. 2014;67:237–241. doi: 10.1007/s00248-013-0302-4. [DOI] [PubMed] [Google Scholar]
- 22.Bengtsson-Palme J, Ryberg M, Hartmann M, et al. Improved software detection and extraction of ITS1 and ITS2 from ribosomal ITS sequences of fungi and other eukaryotes for analysis of environmental sequencing data. Methods Ecol Evol. 2013;4:914–919. doi: 10.1111/2041-210X.12073. [DOI] [Google Scholar]
- 23.Koljalg U, Nilsson RH, Abarenkov K, et al. Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol. 2014;22:5271–5277. doi: 10.1111/mec.12481. [DOI] [PubMed] [Google Scholar]
- 24.Bardou P, Mariette J, Escudié F, et al (2014) Jvenn: an interactive Venn diagram viewer. BMC bioinformatics 15:293 . doi: 10.1186/1471-2105-15-293 [DOI] [PMC free article] [PubMed]
- 25.Chao A, Gotelli NJ, Hsieh TC, et al. Rarefaction and extrapolation with hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr. 2014;84:45–67. doi: 10.1890/13-0133.1. [DOI] [Google Scholar]
- 26.R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing [Internet], Vienna, Austria Available from: https://www.r-project.org
- 27.Friedman J, Alm EJ. Inferring correlation networks from genomic survey data. PLoS Comput Biol. 2012;8:1–11. doi: 10.1371/journal.pcbi.1002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Csárdi G, Nepusz T. The igraph software package for complex network research. Int J Complex Syst. 2006;1695:1–9. doi: 10.3724/SP.J.1087.2009.02191. [DOI] [Google Scholar]
- 29.Huang X-F, Chaparro JM, Reardon KF, et al. Rhizosphere interactions: root exudates, microbes, and microbial communities 1. Botany. 2014;92:267–275. doi: 10.1139/cjb-2013-0225. [DOI] [Google Scholar]
- 30.Wagner MR, Lundberg DS, Del Rio TG, et al. Host genotype and age shape the leaf and root microbiomes of a wild perennial plant. Nat Commun. 2016;7:1–15. doi: 10.1038/ncomms12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins KL, Arnold AE, Miadlikowska J, Sarvate SD, Lutzoni F. Phylogenetic relationships, host affinity, and geographic structure of boreal and arctic endophytes from three major plant lineages. Mol Phylogenet Evol. 2007;42:543–555. doi: 10.1016/j.ympev.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Arnold AE. Understanding the diversity of foliar endophytic fungi: progress, challenges, and frontiers. Fungal Biol Rev. 2007;21:51–66. doi: 10.1016/j.fbr.2007.05.003. [DOI] [Google Scholar]
- 33.Hoffmann K, Voigt K, Kirk PM (2011) <I>Mortierellomycotina</I> subphyl. Nov., based on multi-gene genealogies. Mycotaxon 115:353–363 . doi: 10.5248/115.353
- 34.Almario J, Jeena G, Wunder J et al (2017) Root-associated fungal microbiota of nonmycorrhizal Arabis alpina and its contribution to plant phosphorus nutrition. Proc Natl Acad Sci:201710455. 10.1073/pnas.1710455114 [DOI] [PMC free article] [PubMed]
- 35.Peršoh D. Factors shaping community structure of endophytic fungi-evidence from the Pinus-Viscum-system. Fungal Divers. 2013;60:55–69. doi: 10.1007/s13225-013-0225-x. [DOI] [Google Scholar]
- 36.Melo IS, Santos SN, Rosa LH, Parma MM, Silva LJ, Queiroz SC, Pellizari VH. Isolation and biological activities of an endophytic Mortierella alpina strain from the Antarctic moss Schistidium antarctici. Extremophiles. 2014;18:15–23. doi: 10.1007/s00792-013-0588-7. [DOI] [PubMed] [Google Scholar]
- 37.Abdel-Fattah GM, El-Haddad SA, Hafez EE, Rashad YM. Induction of defense responses in common bean plants by arbuscular mycorrhizal fungi. Microbiol Res. 2011;166:268–281. doi: 10.1016/j.micres.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Lee S, Young JPW. Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol Ecol. 2008;65:339–349. doi: 10.1111/j.1574-6941.2008.00531.x. [DOI] [PubMed] [Google Scholar]
- 39.Lindahl BD, Nilsson RH, Tedersoo L, Abarenkov K, Carlsen T, Kjøller R, Kõljalg U, Pennanen T, Rosendahl S, Stenlid J, Kauserud H. Fungal community analysis by high-throughput sequencing of amplified markers - a user’s guide. New Phytol. 2013;199:288–299. doi: 10.1111/nph.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan JJ, Baumgarten AM, May G. Effects of host plant environment and Ustilago maydis infection on the fungal endophyte community of maize (Zea mays) New Phytol. 2008;178:147–156. doi: 10.1111/j.1469-8137.2007.02350.x. [DOI] [PubMed] [Google Scholar]
- 41.López-González RC, Gómez-Cornelio S, De la Rosa-García SC, et al. The age of lima bean leaves influences the richness and diversity of the endophytic fungal community, but not the antagonistic effect of endophytes against Colletotrichum lindemuthianum. Fungal Ecol. 2017;26:1–10. doi: 10.1016/j.funeco.2016.11.004. [DOI] [Google Scholar]
- 42.Hyde KD, Soytong K. The fungal endophyte dilemma. Fungal Divers. 2008;33:163–173. [Google Scholar]
- 43.Monard C, Gantner S, Stenlid J. Utilizing ITS1 and ITS2 to study environmental fungal diversity using pyrosequencing. FEMS Microbiol Ecol. 2013;84:165–175. doi: 10.1111/1574-6941.12046. [DOI] [PubMed] [Google Scholar]
- 44.Latz E, Eisenhauer N, Rall BC, et al. Plant diversity improves protection against soil-borne pathogens by fostering antagonistic bacterial communities. J Ecol. 2012;100:597–604. doi: 10.1111/j.1365-2745.2011.01940.x. [DOI] [Google Scholar]
- 45.Newman MEJ. Modularity and community structure in networks. Proc Natl Acad Sci. 2006;103:8577–8582. doi: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rarefaction curve. a: For plant organs and rhizosphere samples. b: For different common bean varieties.(PDF 23 kb)
Relative abundance of taxonomic phyla found in organs and rhizosphere samples of three common bean varieties. O: Ouro Negro; R: Rosinha; T: BRS Talismã.(PDF 64 kb)
Five most abundant genera in each variety by organs and rhizosphere.(PDF 141 kb)
(DOCX 14 kb)
(XLSX 11 kb)