Abstract
This study was conducted to characterize the immunological parameters of chickens vaccinated with two formulated inactivated vaccines, water in oil (WO) and water in oil in water (WOW), prepared from velogenic Newcastle disease virus (vNDV) genotype VIIj isolated from outbreak among vaccinated chickens. Six groups (G1–G6) of commercial broiler chickens were established (n = 20). The G1–G3 were received homologous (WO and WOW) and heterologous (LaSota) inactivated vaccines, respectively. The G4 was vaccinated with live heterologous (LaSota) vaccine, while G5 and G6 were kept as control positive and control negative non-vaccinated groups. The antibody titers were measured against vNDV and LaSota antigens using hemagglutination inhibition (HI) test, the cytokine gene expressions of IFNγ, IL1β, IL4, IL6, IL8, and IL18 were quantified using real-time RT-PCR, and the virus shedding was titrated on chicken embryo fibroblast cells after challenging by vNDV. The classical clinical signs and 100% mortality were observed only in G5 after vNDV challenging. The highest HI titers were detected in G1, G2, and G3 using NDV/168 antigen with no significant differences among them. These groups showed higher HI titer than G4 (2-4log2). Cytokine gene expression of IFNγ, IL1, IL6, IL8, and IL18 were significantly downregulated in vaccinated chickens with upregulation of IL4 than non-vaccinated challenge group. Viral shedding titers were significantly (0.0001, p ≤ 0.001) reduced in all samples form vaccinated chickens. In conclusion, the prepared vaccines produced highly efficient immunological responses and could be used for controlling the NDV infection.
Keywords: NDV, Vaccine formulation, Cytokines, Real-time RT-PCR, Chickens
Introduction
Newcastle disease (ND) has harmful economic losses on poultry industry worldwide. It is caused by Avian orthoavulavirus 1 (AAvV-1), an enveloped, negative single-stranded, non-segmented RNA virus, belonging to the genus Orthoavulavirus, subfamily Avulavirinae, family Paramyxoviridae, order Mononegavirales [1]. Its genome contains six open reading frames (ORFs) that encode nucleoprotein (NP), phosphoprotein (P), matrix (M), fusion (F), hemagglutinin-neuraminidase (HN), and large polymerase (L) proteins [2]. The HN protein is an immunogenic and virulence factor for ND virus (NDV) [3], while the F protein cleavage site is considered the main determinant of NDV virulence [4]. Genetically, NDVs are categorized into class І and class ІІ, which include 21 genotypes) І–XXІ); the majority of them are virulent and some avirulent NDVs [1]. Genotype VII is the most common genotype of class II which has been associated to many outbreaks [1, 5–8]. Recently, genotype VII has been re-classified into 2 sub-genotypes (VII.1 and VII.2); sub-genotype VII.1 is clustered into clade VII.1.1 that combines VIIb, VIId, VIIe, and VIIj and clade VII.1.2 that contains VIIf, while VIIh, VIIi, and VIIk are located into sub-genotype VII.2 [9]. The NDV can be distinguished biologically into three distinct pathotypes based on mean death time (MDT) of embryonated chicken eggs (ECEs); velogenic, mesogenic, and lentogenic types cause embryo fatality within 40–60 h, 60–90 h, and 90–150 h, respectively [10].
Vaccination is the most effective method to combat ND in domestic poultry; therefore, innovative vaccines, vaccination regimes, and their routes of administration are necessary for the virus control [11, 12]. Until now, live vaccines prepared from lentogenic strains such as LaSota and Hitchner B1 are commonly used because of their high efficacy and availability under optimal conditions. However, under the field conditions with mass application, their protection reaches as little as 53% and 60% through the spray and drinking water, respectively, for the receiving flocks [13]. On other hand, inactivated oil emulsion vaccines of the same viruses have been used for enhancing and maintaining the immunity against ND [14–16]. Serological evidences indicated that oil emulsion–inactivated NDV vaccines induced higher hemagglutination inhibiting (HI) antibody titer as well as more persistent immunity [17, 18]. The oil emulsions, water in oil (WO) and water in oil in water (WOW) vaccines, are the most forms used for inactivating avian vaccines. WO emulsion vaccines are potent that induce long-term efficacy in poultry [19]. Also, WOW-inactivated vaccine had been developed by using a subunit of the virus by complete or partial disruption using Tween 80, as an antigen [20]. It possesses low viscosity, great stability, and decreased percentage of mineral oil that facilities its practical application and cleaning of vaccination materials as well as it causes fewer tissue reactions than the WO vaccine [21, 22].
The defense against infection with pathogens can be classified into innate and adaptive mechanisms. Several forms of innate defense exist to prevent the entrance of pathogens. Adaptive defense mechanisms or specific immunity can be sorted into humoral immunity (antibodies produced by B lymphocytes and plasma cells) and cellular immunity (helper and cytotoxic T lymphocytes). Both cellular and humoral immune responses have been suggested to play an important role in the host’s defense against NDV infection [23, 24].
Cytokines are soluble, low molecular weight polypeptides and glycopeptides produced by a broad range of cell types of hematopoietic and non-hematopoietic origin that have suppressive or enhancive effects on cellular proliferation, differentiation, activation, and motility [25]. Classically, cytokines are classified into multiple categories; pro-inflammatory/innate cytokines (IL-1β and IL-6) are mainly involved in innate immune response and are known to stimulate sickness behavior (anorexia, lethargy, and fever). T helper cell type 1 cytokines (IFNγ) is mainly involved in the proliferation of cell-mediated immunity; T helper cell type 2 cytokines (IL-4) are responsible for inducing a humoral immune response by inducing antigen-specific B cells to proliferate and secrete antibody. The IL8 and IL18 act as chemoattractant cytokines [26].
In this study, inactivated oil emulsion vaccines were prepared from Egyptian velogenic NDV VIIj (VII 1.1) isolated from outbreak among vaccinated chickens and their efficacy was compared with commercially available lentogenic ND vaccines. Also, serological and molecular evaluation of the immunological potency of the prepared vaccines in chickens was carried out.
Materials and methods
Viruses
The NDV used in this study was designated as CK/EG/NDV/168/2012 (NDV/168) which was isolated from an outbreak among vaccinated chickens in Esna City, Luxor, Egypt, during 2012. It was propagated and titrated in 10-day-old embryonated specific pathogen free (SPF) chicken eggs (Kom Oshim, EL-Fayoum Governorate) via allantoic route. After that, the allantoic fluid was collected and clarified at 5000 rpm/15 min, and virus supernatant was stored at − 70 °C until use [27]. Its virulence was determined biologically by mean death time (MDT) that classified the NDV strains based on the required duration to produce fatality in chicken embryos, and the egg infectious dose 50 (EID50) was determined according to [28].
Genetic characterization of the NDV/168 strain
Sequence analysis and phylogenetic relationship of the F and HN genes of the NDV/168 were conducted using NDV strain sequence available in the public database (BLAST, NCBI, USA). Phylogeny was constructed via neighbor-joining method; bootstrapping at 1000 repeats using Mega10 software [29] and Bioedit software (version 7.1) was used for alignment of the nucleotide and amino acid sequences.
Preparation of the inactivated vaccines from NDV/168 strain
The inactivation of NDV was done by 0.1% formalin at 37 °C/18 h, and two successive passages of the inactivated vaccine in the SPF egg were performed to confirm that vaccine is free from live virus according to [30]. The inactivated fluids were used for preparing WO vaccine according to [31] with a ratio of the aqueous phase to oil phase at 30:70 and WOW vaccine according to [20]. In addition, the hydrophilic-lipophilic balance (HLB) values were adjusted to 7 as described by [32].
The physical properties of the prepared inactivated vaccines from NDV/168 strain
The type of the emulsion (drop test), relative viscosity, stability, sterility, and tissue reaction of the vaccines were performed according to [33, 34]. In brief, the drop test was performed by mixing one drop of the emulsion (WO or WOW) with one drop water and placed on a clean glass microscope slide; WO emulsion should not mix with water [33]. Relative viscosity was conducted by flow time (mean of 3 replicates) for discharge of emulsion from vertical serological pipettes (1.0 ml) to move from zero to 0.4-ml mark at 24 °C [35]. The emulsion stability was assessed by incubating the inactivated vaccines at different temperatures (4 °C and 37 °C) and observing the emulsion homogeneity for a period of 1 month [19], while the sterility test was confirmed by inoculating a loopful of the prepared vaccines on different bacteriological culture media and incubating them at 37 °C for detecting any contaminated pathogen [33]. Finally, the tissue reaction was determined by observing the injection site of the breast muscle of 5 chickens per treatment 12 days after the primary vaccination. The lesion has been scored into 3 levels. The mild level (pale muscles around 1 cm and no vaccine left at the injection site), the intermediate level (pale to red muscles around 1–3 cm in the superficial muscles at the injection site and the small droplets of vaccine left), and the severe level (red and inflammable muscles around 3–4 cm in the superficial and deep muscles at the injection site and vaccine) were observed when the muscle was dissected) [33].
Experimental design
One-hundred-and-twenty-day-old commercial white chicks (Arbo Acres), obtained from a known source (Cairo Poultry Company), were randomly divided into 6 groups (n = 20) designated as G1–G6 as shown in Table 1. Each group was housed in a separate place with feed and water ad libitum. All chicks were kept free from any bacteria or protozoan infection throughout the experiment. Also, random cloacal swabs were collected from chicks and were screened for NDV and avian influenza virus (AIV) infection by real-time RT-PCR before starting the experiment. All groups except negative and positive control groups (G5 and G6) were received vaccination regimes as in Table 1. At 48 days old, all groups except G6 (negative control) were challenged with 0.1 ml 108.15EID50 of NDV/168 strain by oculonasal route. The clinical signs, mortality, and morbidity rates of the chickens were recorded for 14 days post challenge (dpc). Serum samples were collected every week for measuring NDV antibodies by HI test using 1% chicken red blood cells and 4 hemagglutination unit (HAU) of NDV antigens (LaSota and NDV/168) according to [36].
Table 1.
Timeline schedule of immunization and challenge process for different bird groups during the experiment
| Age | Vaccination programs for different bird groups | |||||
|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G5 | G6 | |
| 7th day | LaSota (clone 79) eye drop | LaSota (clone 79) eye drop | LaSota (clone 79) eye drop | LaSota (clone 79) eye drop | None | None |
| 12th day | Infectious bursal disease vaccine (Cevac bursal L) (Intermediate strain) by drinking water | None | None | |||
| 19th day | WO (0.5 ml) S/C | WOW (0.5 ml) S/C | Inactivated LaSota S/C | Volvac LaSota eye drop | None | None |
| 22nd day | Infectious bursal disease vaccine (Cevac bursal L) (Intermediate strain) by drinking water | None | None | |||
| 33rd day | WO (0.5 ml) S/C | WOW (0.5 ml) S/C | Inactivated LaSota S/C | LaSota Clone 79 eye drop | None | None |
| 49th day | Challenge with vVNDV (NDV/168) strain | None | ||||
WO water in oil formed a vaccine from local vVNDV (NDV/168) strain, WOW water in oil in water formed vaccine from local vVNDV (NDV/168) strain
Detection of cytokine gene expression by real-time RT-PCR among different chicken groups
Spleen tissues were collected for detection of total RNA using the RNeasy mini RNA extraction kit (Qiagen Inc., Valencia, CA) with DNase treatment (Qiagen Inc., Valencia, CA). At 3, 5, 7, and 9 days post challenge (dpc), parts of the spleens were kept in RNA later solution according to the manufacturer’s instruction (Ambion, USA) and stored at − 20 °C until use. The mRNA expression of the IFNγ, IL-1β, IL-4, IL-6, IL-8, IL-18, and 28SrRNA was determined by quantitative real-time RT-PCR using a QuantiTect probe RT-PCR mini kit (Qiagen Inc., Valencia, CA) and specific primers for each gene are shown in Table 2. The 28SrRNA gene was used as an internal control gene for normalization of cytokine gene amplification data. Real-time RT-PCR was performed using Stratagene MX3005P and thermal conditions are as follows: 30 min at 50 °C, 94 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. Gene expression fold change was determined by the ΔΔCt method [40].
Table 2.
The oligonucleotide primers and probes for real-time RT-PCR
| Quantitative gene | Sequence (5′–3′) | Reference |
|---|---|---|
| IFN-γ | AAACAACCTTCCTGATGGCGT | [37] |
| CTGGATTCTCAAGTCGTTCATCG | ||
| (FAM)TGAAAGATATCATGGACCTGGCCAAGCTC(TAMRA) | ||
| IL-1β | GCTCTACATGTCGTGTGTGATGAG | [38] |
| TGTCGATGTCCCGCATGA | ||
| (FAM) CCACACTGCAGCTGGAGGAAGCC (TAMRA) | ||
| IL-4 | AACATGCGTCAGCTCCTGAAT | [39] |
| TCTGCTAGGAACTTCTCCATTGAA | ||
| (FAM) AGCAGCACCTCCCTCAAGGCACC (TAMRA) | ||
| IL-6 | GCTCGCCGGCTTCGA | |
| GGTAGGTCTGAAAGGCGAACAG | ||
| (FAM) AGGAGAAATGCCTGACGAAGCTCTCCA (TAMRA) | ||
| IL-8 | GCCCTCCTCCTGGTTTCAG | |
| TGGCACCGCAGCTCATT | ||
| (FAM) TCTTTACCAGCGTCCTACCTTGCGACA (TAMRA) | ||
| IL-18 | AGGTGAAATCTGGCAGTGGAAT | |
| ACCTGGACGCTGAATGCAA | ||
| (FAM) CCGCGCCTTCAGCAGGGATG (TAMRA) | ||
| 28SrRNA | GGCGAAGCCAGAGGAAACT | |
| GACGACCGATTTGCACGTC | ||
| (FAM) AGGACCGCTACGGACCTCCACCA (TAMRA) |
Cross hemagglutination inhibition test
To measure the antigenic differences between the LaSota and NDV/168 strains, the cross HI test was conducted as described by [41, 42]. The antigenic relatedness between strains was expressed in R value, as described by [43]. The following formula was used: R = r1 × r2, where r1 is the titer of strain A with antiserum B divided by the titer of strain A with antiserum A and r2 is the titer of strain B with antiserum A divided by the titer of strain B with antiserum B. The result of 0.67 ≤ R ≤ 1.5 indicates no significant antigenic differences between the two strains, whereas 0.5 ≤ R ≤ 0.67 indicates a minor difference between the two strains while the R value of R < 0.5 indicates a major difference between the two strains [42].
Virus shedding titration among challenged chickens
The organs such as the spleen, lung, thymus, and bursa as well as cloacal and tracheal swabs were collected in 1 ml virus transport media at 3, 5, 7, and 9 dpc for determining the viral load of NDV by tissue culture infectious dose 50 (TCID50). The sample titration was carried out on chicken embryo fibroblast cells (CEF) for calculation of TCID50 by [28]. The samples were clarified by centrifugation at 3000×g for 10 min. After that, 100-μl aliquot of the supernatant was serially diluted 10-fold and inoculated onto monolayers of primary CEF in 96-well microtiter tissue culture plates (Griener bioone, Germany). The cells were incubated in CO2 incubator at 37 °C for 5 days and observed daily for cytopathic effects, which are characterized by scattered focal areas of cell rounding and syncytia formation.
Statistical analysis
The HI titers are presented as geometric mean titers (±) standard error (GM ± SE). Statistical differences between different groups were determined using the analysis of variance (ANOVA) with Tukey’s posttest method followed by pair Student’s t test analyzed by SPSS 24 software. The probability (p) values ≤ 0.05 and 0.001 were considered statistically significant and highly significant, respectively.
Accession numbers
The nucleotide sequences of F and HN genes were submitted to the GenBank under the accession numbers MN381174 and MN381175, respectively.
Results
Characterization and phylogenetic analysis of the NDV/168 strain
The biological characterization of the ND/168 strain by MDT revealed that it has a MDT value of 39.2 h, which characterizes velogenic strain (< 60 h). The phylogenetic analysis of the complete F gene sequence of the NDV/168 strain showed that it belongs to class II genotype VIIj (VII.1.1) as shown in Fig. 1a and the deduced amino acid (aa) sequence possess 553 aa. The F gene cleavage site aa motifs 112RRQKR*F117 revealed its velogenic in nature. Several amino acid substitutions were recognized in comparison with the vaccine strains ( LaSota, Clone 79, Clone 30 and Ulster2C/67) as 14, 2, 3, 10, and 4 in the signal peptide, cleavage site, fusion peptide, heptad repeat region (HRa, HRb, and HRb), and transmembrane domain, respectively (Table 3). The phylogenetic analysis of the HN protein gene (528 aa) of NDV/168 strain was presented in Fig. 1b. The deduced aa sequence of HN protein gene revealed 2 amino acid substitutions at receptor recognition and N-linked glycosylation sites (Table 4). Also, 3 aa substitutions in the neutralizing epitopes were identified at positions E347K, R353Q, and I514V in the HN protein gene and the K78R substitution in the F gene protein neutralizing epitopes (Tables 3 and 4).
Fig. 1.
The phylogenetic analysis of vNDV (NDV/168) of F (a) and HN (b) genes. The trees are constructed with multiple alignments of 553 and 528 amino acid sequences of F and HN genes, respectively, using neighborhood joining method in MEGA10 with bootstrap values calculated for 1000 replicates. The NDV/168 was indicated by solid triangle
Table 3.
The mutated amino acids in different regions of the NDV/168 F gene in comparison with the vaccine strains
| The F gene regions | Virus strain | ||||
|---|---|---|---|---|---|
| Amino acid position | LaSota | Ulster 2C/67 | Clone 79/30 | NDV/168 | |
| Signal peptide (1–31) | 4 | R | -* | - | K |
| 5 | P | S | - | - | |
| 8 | K | R | - | R | |
| 9 | N | I | - | I | |
| 11 | A | V | - | A | |
| 13 | M | L | - | L | |
| 16 | T | - | - | I | |
| 17 | I | V | - | T | |
| 19 | V | - | - | I | |
| 20 | A | - | - | M | |
| 22 | V | E | - | T | |
| 26 | I | V | - | - | |
| 27 | C | - | - | R | |
| 28 | P | - | - | L | |
| 29 | A | T | - | T | |
| 30 | N | S | - | S | |
| Cleavage site (112-117) | 112 | G | - | - | R |
| 113 | R | K | - | - | |
| 115 | G | - | - | K | |
| Fusion peptide (117–141) | 117 | L | - | - | F |
| 121 | I | - | - | V | |
| 124 | G | - | - | S | |
| 125 | V | A | - | - | |
| 139 | A | S | - | - | |
| HRa (143–185) | 145 | K | N | - | - |
| 176 | A | - | - | X | |
| HRb (268–299) | 272 | N | - | - | Y |
| 279 | Q | - | - | H | |
| 288 | T | - | - | N | |
| HRc (471–500) | 479 | N | D | - | D |
| 480 | K | - | - | R | |
| 482 | E | - | - | A | |
| 486 | R | S | - | S | |
| 489 | D | - | - | E | |
| 494 | K | - | - | R | |
| Transmembrane domain (501–521) | 509 | I | V | - | V |
| 513 | V | - | - | I | |
| 514 | F | C | - | - | |
| 516 | I | - | A | ||
| 520 | I | V | - | A | |
*Identical to the LaSota amino acid
Table 4.
The amino acid substitutions in the HN protein gene of NDV/168 in comparison with vaccine strains
| The HN gene regions | Virus strain | ||||
|---|---|---|---|---|---|
| Amino acid position | LaSota | Ulster 2C/67 | Clone 79 | NDV/168 | |
| Receptor Recognition site | 547 | L | -* | - | Q |
| N-linked glycosylation site | 538 | N | L | - | L |
| Functional (neutralizing ) site | 347 | E | - | - | K |
| 353 | R | - | - | Q | |
| 514 | I | - | - | V | |
*Identical to the LaSota amino acid
Physical properties, sterility, stability and tissue reaction of inactivated vaccines
The physical properties of WO and WOW emulsion–inactivated NDV vaccines are milky white appearance, with WO emulsion not mixed in the water and mixed properly in the oil, and stable at 4 °C and 37 °C for more than 1 month. Also, WO is more viscous than WOW and commercial LaSota-inactivated vaccines. Furthermore, both formulas (WO and WOW) showed no growth on various bacteriological media. The evaluation of tissue reaction revealed that WO-inactivated vaccine, intermediate type, produced high tissue reaction than WOW and commercial inactivated vaccines (mild type).
NDV antibody response profiles of different chickens groups
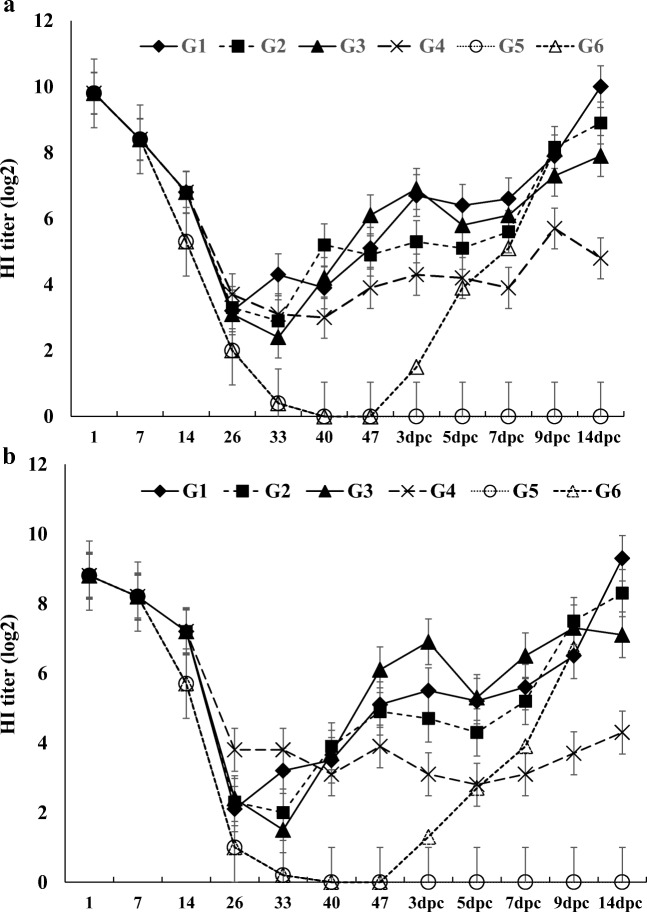
The maternal antibody titers of chickens in unvaccinated control groups (G5 and G6) steadily declined and completely diminished at 40 days old. It was noticed that serum HI titers of chickens in the other groups (G1–G4) against NDV/168 and LaSota antigens were steadily increased to 47 days old post vaccination (dpv) as shown in Fig. 2a, b.
Fig. 2.
The geometric mean of HI titer of serum samples obtained from bird groups (G1–G4) receiving various vaccination programs as well as non-vaccinated groups (G5 and G6) before and after challenge with vNDV (NDV/168) that determined by HI test using a LaSota antigen b Velogenic (NDV/168) antigen. The significant difference of HI titers among various groups was calculated by geometrical means
The HI titers of the prepared inactivated vaccines (WO and WOW) were higher than the live vaccine by 2-4log2 while the formulated WO-inactivated vaccine was as efficient as commercial inactivated LaSota vaccine or significantly higher (value = 0.047, p ≤ 0.05) at 33 dpv using NDV/168 antigen (Fig. 2a). On the other hand, the HI antibody profile showed that LaSota-inactivated vaccine produced higher HI titer than other formulated inactivated vaccines using LaSota antigens without improvement in the titer induced by live LaSota vaccine (Fig. 2a). Interestingly, the antibody titers continuously increased later on in all vaccinated groups receiving inactivated vaccines (G1, G2, and G3) compared with that receiving live vaccine (G4) with significant difference (value = 0.48, p ≤ 0.5) as shown in the Fig. 2a, b.
No overt clinical signs of ND were observed in any birds prior to the challenge. Protection from virulent NDV challenge was determined by observing the challenged birds during a period of 14 dp challenge (dpc). No clinical signs, morbidity, or mortality in vaccinated groups were observed in the course of 14 dpc. In contrast, 100% of the unvaccinated challenged chickens developed the classical ND clinical signs including depression, anorexia, greenish diarrhea, and neurological signs. All birds in G6 died within 9 dpc while non-vaccinated unchallenged birds remained normal during the period of 14 dpc.
Cross hemagglutination inhibition test and antigenic relatedness analysis
To assess the antigenic difference between the vaccine and NDV/168 strains, the cross HI assay was carried out to calculate the R value as described by [43]. The cross HI titer of two strains, NDV/168 and LaSota, showed that the prepared vaccine gives more antibody titer against the NDV/168 antigen than the LaSota antigen. The R values between two strains were 0.67 ≤ R ≤ 1.5 (R = 0.75–1.16) indicating that no significant antigenic difference existed between the vaccine strain (LaSota) and the NDV/168 strain.
Cytokine gene expression profiles of the different chicken groups
At 3, 5, 7, and 9 dpc, 3 birds per treatment were euthanized and their spleens were collected to determine the alteration in mRNA expression of the IFN-γ, IL1β, IL4, IL6, IL8, and IL18 as shown in the Fig. 3a–f. The IFN-γ gene expression illustrated that G2 was increased slightly than G1 and G3 at 3, 5, 7, and 9 dpc (Fig. 3a). Although the fold change value of IL1β of G2 was the same as G1 and G3 at 3 dpc, the mRNA gene expression of IL1β of G2 was slightly higher than G1 and G3 in 5 and 9 dpc (Fig. 3b). Among the vaccinated challenged groups, G4 showed a significant increase in the expression of IL1β (value = 0.0071, 0.0096, and 0.0061 at p ≤ 0.05) to G1, G2, and G3, respectively (Fig. 3b). Interestingly, The IL4 was gradually increased during 3–9 dpc in all vaccinated groups (G1–G4). The IL4 increase in G3, G1, and G2 was 2.6–9.3-, 2–6- and 1.7–1.8-fold change, respectively, compared with that in G4; furthermore, the IL4 fold change was statistically significant in all vaccinated groups (value = 0.006, 0.0035, 0.01, and 0.01 at p ≤ 0.05, respectively) compared with G5 (Fig. 3c). The IL6 was significantly decreased in G3 (value = 0.03, p ≤ 0.05) from 3–9 dpc compared with that in G4 and no significant change in G1 and G2 (Fig. 3d). The fold change of IL8 in G2 was higher than in G1, G3, and G4 at 3–9 dpc (Fig. 3e). Finally, the gene expression of IL18 was decreased in G3 than in the other vaccinated groups (G1, G2, and G4) at 3–9 dpc (Fig. 3f).
Fig. 3.
Cytokine gene expression. a IFNγ, b IL-1β, c IL-4, d IL-6, e IL-8, and f IL-18 among different vaccinated (G1–G4) and non-vaccinated groups (G5 and G6) at 3, 5, 7, and 9 dpc. The expressed fold change value of cytokines was calculated by the ΔΔCt method and normalized by 28SrRNA gene expression value. The significant difference between various vaccinated groups and control group was indicated by an asterisk (*p ≤ 0.05), and between inactivated vaccines (G1–G3) and live vaccine (G4) was indicated by double asterisks (**p ≤ 0.05)
Virus shedding titration among chicken groups
The results of virus shedding titer on CEF are presented in Fig. 4. The challenged birds in G5 possess significantly higher viral shedding titer started after the virus was challenged until all birds died at 9 dpc. The high virus titer shedding in this group was detected at 7 and 9 dpc, while the NDV was not detected from any of the samples which were collected from control negative (G6) unchallenged birds. Among the vaccinated groups (G1–G4), there was a highly significant decrease (value = 0.0001, p ≤ 0.001) in viral shedding from the swabs and organs compared with that in G5 at 3 dpc (Fig. 4) and it was undetectable at 5, 7, 9, and 14 dpc. In this study, the viral titers in tracheal swabs were high in G1 and G4 than in G2 and G3. Also, in G2 as well as in G3, the viral shedding in cloacal swabs was higher than in G1 and G4, respectively (Fig. 4). The viral titers in the lung were high for almost all groups except G3 at 3 dpc (Fig. 4). However, the viral load of G3 was high in the spleen, bursa, and thymus (Fig. 4).
Fig. 4.
Viral shedding of NDV from the various organs (the lung, spleen, bursa, and thymus) as well as tracheal and cloacal swabs at 3 dpc after challenge with vNDV (NDV/168) was titrated in CEF and TCID50 was expressed as log10. The significant difference between various vaccinated groups and control group was indicated by an asterisk (*p < 0.05)
Discussion
The raised calls of NDV outbreaks among vaccinated chickens enhanced the significance of thinking about vaccination efficacy [24, 44, 45]. The phylogenetic and sequence analysis of F and HN genes of the NDV/168 indicated that it belongs to class II genotype VIIj (VII.1.1) and possesses the multibasic amino acids and MDT value of the velogenic strain. Its genetic distance from vaccine strains indicates the potential evolution of virulent NDV in Egyptian poultry [46, 47].
The amino acid substitutions at the fusion peptide and the HR regions of NDV/168 F protein were accounted to affect the NDV fusion activity [48, 49]. Also, the mutations in the N-glycosylation sites of HN protein were reported to affect virus attachment, neuraminidase, and fusion promotion activities [50, 51]. Several amino acid substitutions in the neutralizing epitopes of the F (K78R) and HN (E347K, R353Q, and I514V) proteins of the NDV/168 were also observed [52]. Those amino acid substitutions in the neutralizing epitopes have a vital role in neutralizing escape variants [53].
Montanide ISA 71 and tween 80 were used in the composition of the prepared inactivated vaccines. The Montanide ISA 71 has been shown to be safe, efficient, and able to stimulate both humoral and cellular immune response in poultry [19, 54]. In addition to this, Tween 80 improves the stability, increases the immune response, and prolongs the immunity time of NDV vaccines [54–56]. The prepared WO- and WOW-inactivated vaccines were physically good vaccines. Also, they produced protective homologous antibody titers in vaccinated birds higher than those produced by commercial inactivated and live vaccines (Fig. 2a). Controlling ND is based mainly on good management, biosafety, and vaccination of flock. Current vaccine strategies can be effective in controlling serious illness and death in infected birds and reduced the viral shedding [57]. Furthermore, the NDV vaccine homologous to the field isolate protects and reduces the viral shedding compared with the heterologous vaccines. The HI titer profiles against homologous antigen (NDV/168) were higher than those produced by heterologous antigen (LaSota) and vice versa. This result agrees with [58, 59] who reported that the genotype-matched vaccines produced better humoral immune response and minimized the clinical signs, death rate, and virus shedding than heterologous vaccines. Also, the prepared vaccine HI titers were more efficient (2–4log2 differences) than that produced by live vaccine which is the most commonly used vaccine among poultry farms to combat ND (Fig. 2a, b).
In this study, for the cytokines IFNγ, IL-1β, IL-4, IL-6, IL-8, and IL-18, gene expressions were used to evaluate the immune response of the chickens to prepared vaccines (WO and WOW), commercial inactivated vaccines, and live LaSota vaccines. The upregulation of IFNγ, IL-1β, IL-6, IL-8, and IL-18 gene expression and downregulation of IL-4 gene expression were observed in non-vaccinated challenged chickens. However, IFNγ, IL-1β, IL-6, IL-8, and IL-18 gene expressions in vaccinated groups were more counteracted than that in non-vaccinated challenge group (Fig. 3a–f). Also, the homologous inactivated vaccine (G1 and G2) gives a better decrease in cytokines gene expressions than in the live vaccine (G4) group. Thus, the decline expression of cytokines in vaccinated birds could reduce rapid inflammatory response that responsible for tissue damage, sickness behavior, and mortality. Also, it prevented the challenge virus to penetrate the mucosa in a sufficient amount to cause a systemic immune response [26, 60]. This is obvious in vaccinated groups (G1–G4) compared with the non-vaccinated challenge group (G5) which showed speedy increase in the HI titer, severe illness, and pronounced mortality among chickens after challenge with NDV/168 (Fig.2).
As shown in Fig. 3c, the fold change of the IL-4 were significantly higher in G1, G2, and G3 than G4 and it reach up to 9-fold change in chicken receiving inactivated vaccine than that of the live vaccine. The IL-4 plays a critical role in the differentiation, the maturation of B lymphocytes, and the synthesis of immunoglobulins. It is a strong cytokine in the inhibition of the inflammatory activities of stimulated macrophages. Besides that, it modulates the balance between inflammatory and immune responses [61]. This can be accounted for the high HI titers produced in these groups than live vaccine receiving group.
Conclusion
In this study, the prepared inactivated vaccines from NDV/168 VIIj (VII 1.1) are efficient in protecting chickens against NDV challenge and reducing the virus shedding titers. Also, they produced higher neutralization antibodies against the homologous antigen than the commercial inactivated and live LaSota vaccines. The cytokine gene expression profiles of the prepared vaccine revealed their good configuration to reduce the illness stimulating cytokines and enhance the immune improving cytokines than the live vaccines.
Compliance with ethical standards
Ethical approval
All procedures performed in studies involving chickens were in accordance with the ethical standards of the Ethics committee of Faculty of Veterinary Medicine, South Valley University, Qena, Egypt.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Amarasinghe A, Abdul-Cader MS, Almatrouk Z, et al. Induction of innate host responses characterized by production of interleukin (IL)-1β and recruitment of macrophages to the respiratory tract of chickens following infection with infectious bronchitis virus (IBV) Vet Microbiol. 2018;215:1–10. doi: 10.1016/j.vetmic.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Ganar K, Das M, Sinha S, Kumar S. Newcastle disease virus: current status and our understanding. Virus Res. 2014;184:71–81. doi: 10.1016/j.virusres.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu M, Qu Y, Wang F, Liu S, Sun H. Genotypic and pathotypic characterization of Newcastle disease virus isolated from racing pigeons in China. Poult Sci. 2015;94:1476–1482. doi: 10.3382/ps/pev106. [DOI] [PubMed] [Google Scholar]
- 4.Connolly SA, Leser GP, Jardetzky TS, Lamb RA. Bimolecular complementation of paramyxovirus fusion and hemagglutinin-neuraminidase proteins enhances fusion: implications for the mechanism of fusion triggering. J Virol. 2009;83:10857–10868. doi: 10.1128/JVI.01191-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi J, Liu C, Chen B, Wu S. Molecular characterization of a virulent genotype VIId strain of Newcastle disease virus from farmed chickens in Shanghai. Avian Dis. 2011;55:279–284. doi: 10.1637/9383-042710-Reg.1. [DOI] [PubMed] [Google Scholar]
- 6.Abolnik C, Gerdes GH, Kitching J, Swanepoel S, Romito M, Bisschop SP. Characterization of pigeon paramyxoviruses (Newcastle disease virus) isolated in South Africa from 2001 to 2006. Onderstepoort J Vet Res. 2008;75:147–152. doi: 10.4102/ojvr.v75i2.13. [DOI] [PubMed] [Google Scholar]
- 7.Irvine RM, Aldous EW, Manvell RJ, Cox WJ, Ceeraz V, Fuller CM, Wood AM, Milne JC, Wilson M, Hepple RG, Hurst A, Sharpe CE, Alexander DJ, Brown IH. Outbreak of Newcastle disease due to pigeon paramyxovirus type 1 in grey partridges (Perdix perdix) in Scotland in October 2006. Vet Rec. 2009;165:531–535. doi: 10.1136/vr.165.18.531. [DOI] [PubMed] [Google Scholar]
- 8.Sultan S, Osman N, Ahmed A, et al (2014) Phylogenetic characterization of velogenic newcastle virus isolates from field outbreaks among vaccinated chickens in the southern part of Egypt. 33rd Annual Meeting of the American Society for Virology at Colorado State University, June 20 -25, in Fort Collins
- 9.Dimitrov KM, Abolnik C, Afonso CL et al (2019) Updated unified phylogenetic classification system and revised nomenclature for Newcastle disease virus. Infect Genet Evol:74. 10.1016/j.meegid.2019.103917 [DOI] [PMC free article] [PubMed]
- 10.Hanson RP, Brandly CA. Identification of vaccine strains of Newcastle disease virus. Science. 1955;122:156–157. [PubMed] [Google Scholar]
- 11.OIE (2009) Biotechnology in the diagnosis of infectious diseases and vaccine development. Africa (Lond):1–25
- 12.Kapczynski DR, King DJ (2005) Protection of chickens against overt clinical disease and determination of viral shedding following vaccination with commercially available Newcastle disease virus vaccines upon challenge with highly virulent virus from the California 2002 exotic Newcastle disease outbreak. Vaccine. 10.1016/j.vaccine.2005.01.140 [DOI] [PubMed]
- 13.Degefa T, Dadi L, Yami A, et al. Technical and economic evaluation of different methods of newcastle disease vaccine administration. J Vet Med Ser A Physiol Pathol Clin Med. 2004;51:365–369. doi: 10.1111/j.1439-0442.2004.00658.x. [DOI] [PubMed] [Google Scholar]
- 14.Borland LJ, Allan WH. Laboratory tests for comparing live lentogenic newcastle disease vaccines. Avian Pathol. 1980;9:45–59. doi: 10.1080/03079458008418385. [DOI] [PubMed] [Google Scholar]
- 15.Senne DA, King DJ, Kapczynski DR. Control of Newcastle disease by vaccination. Dev Biol (Basel) 2004;119:165–170. [PubMed] [Google Scholar]
- 16.Dimitrov KM, Afonso CL, Yu Q, Miller PJ. Newcastle disease vaccines—a solved problem or a continuous challenge? Vet Microbiol. 2017;206:126–136. doi: 10.1016/j.vetmic.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Box PG, Furminger IG. Newcastle disease antibody levels in chickens after vaccination with oil emulsion adjuvant killed vaccine. Vet Rec. 1975;96:108–111. doi: 10.1136/vr.96.5.108. [DOI] [PubMed] [Google Scholar]
- 18.Stone HD, Brugh M, Erickson GA, Beard CW. Evaluation of inactivated Newcastle disease oil-emulsion vaccines. Avian Dis. 1980;2499-111(24):99–111. doi: 10.2307/1589770. [DOI] [Google Scholar]
- 19.Stone HD, Brugh M, Beard CW. Influence of formulation on the efficacy of experimental oil-emulsion Newcastle disease vaccines. Avian Dis. 1983;27:688–697. doi: 10.2307/1590311. [DOI] [PubMed] [Google Scholar]
- 20.Cajavec S, Bidin Z, Sladic D, et al. Tween 80-solubilized Newcastle disease virus prepared as a water-in-oil-in-water vaccine. Avian Dis. 1996;40:193–201. doi: 10.2307/1592389. [DOI] [PubMed] [Google Scholar]
- 21.Wanasawaeng W, Tawatsin A, Sasipreeyajan J, et al. Development of inactivated newcastle disease vaccine using palm oil as an adjuvant. Thai J Vet Med. 2009;39:9–16. [Google Scholar]
- 22.Fukanoki SI, Iwakura T, Iwaki S, et al. Safety and efficacy of water-in-oil-in-water emulsion vaccines containing Newcastle disease virus haemagglutinin-neuraminidase glycoprotein. Avian Pathol. 2001;30:509–516. doi: 10.1007/s12263-011-0257-3. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds DL, Maraqa AD. Protective immunity against Newcastle disease: the role of cell-mediated immunity. Avian Dis. 2000;44:145–154. doi: 10.2307/1592518. [DOI] [PubMed] [Google Scholar]
- 24.Sultan S, Osman N, Mohamed MA, et al. Infectious bursal disease vaccine ameliorates velogenic Newcastle disease virus infection in immunopotentiated chickens. Comp Clin Pathol. 2016;25:91–100. doi: 10.1007/s00580-015-2145-5. [DOI] [Google Scholar]
- 25.Balkwill FR, Burke F. The cytokine network. Immunol Today. 1989;10:299–304. doi: 10.1016/0167-5699(89)90085-6. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser P, Rothwell L, Galyov EE, et al. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology. 2000;146(Pt 12):3217–3226. doi: 10.1099/00221287-146-12-3217. [DOI] [PubMed] [Google Scholar]
- 27.OIE . Manual of diagnostic tests and vaccines for terrestrial animals: (mammals, birds and bees) Paris: Office International des Epizooties; 2004. [PubMed] [Google Scholar]
- 28.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Epidemiol. 1938;27:493–497. doi: 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
- 29.Kumar S, Stecher G, Li M, et al. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hofstad MS, Picken JCJ, Collins KE, Yoder HWJ. Immunogenicity of inactivated Newcastle disease virus preparations. Avian Dis. 1963;7:435–445. doi: 10.2307/1587880. [DOI] [PubMed] [Google Scholar]
- 31.Stone HD. Newcastle disease oil emulsion vaccines prepared with animal, vegetable, and synthetic oils. Avian Dis. 1997;41:591–597. doi: 10.2307/1592149. [DOI] [PubMed] [Google Scholar]
- 32.Schick MJ. Nonionic surfactants. New York: Marcel Dek- Ker, Inc; 1966. pp. 609–611. [Google Scholar]
- 33.Stone HD, Brugh M, Hopkins SR, et al. Preparation of inactivated oil-emulsion vaccines with avian viral or Mycoplasma antigens. Avian Dis. 1978;22:666–674. doi: 10.2307/1589643. [DOI] [PubMed] [Google Scholar]
- 34.Stone HD, Xie ZX. Efficacy of experimental Newcastle disease water-in-oil oil-emulsion vaccines formulated from squalane and squalene. Avian Dis. 1990;34:979–983. doi: 10.2307/1591392. [DOI] [PubMed] [Google Scholar]
- 35.Xie ZX, Stone HD. Immune response to oil-emulsion vaccines with single or mixed antigens of Newcastle disease, avian influenza, and infectious bronchitis. Avian Dis. 1990;34:154–162. doi: 10.1016/j.intermet.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 36.OIE . Manual of diagnostic tests and vaccines for terrestrial animals. 7 2012. [Google Scholar]
- 37.Markowski-Grimsrud CJ, Schat KA. Infection with chicken anaemia virus impairs the generation of pathogen-specific cytotoxic T lymphocytes. Immunology. 2003;109:283–294. doi: 10.1046/j.1365-2567.2003.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samy AA, El-Enbaawy MI, El-Sanousi AA, et al. Different counteracting host immune responses to clade 2.2.1.1 and 2.2.1.2 Egyptian H5N1 highly pathogenic avian influenza viruses in naïve and vaccinated chickens. Vet Microbiol. 2016;183:103–109. doi: 10.1016/j.vetmic.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K, Okada H, Itoh T, Tada T, Mase M, Nakamura K, Kubo M, Tsukamoto K. Association of increased pathogenicity of Asian H5N1 highly pathogenic avian influenza viruses in chickens with highly efficient viral replication accompanied by early destruction of innate immune responses. J Virol. 2009;83:7475–7486. doi: 10.1128/JVI.01434-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Cho S-H, Kim S-J, Kwon H-J. Genomic sequence of an antigenic variant Newcastle disease virus isolated in Korea. Virus Genes. 2007;35:293–302. doi: 10.1007/s11262-007-0078-z. [DOI] [PubMed] [Google Scholar]
- 42.Li ZJ, Li Y, Chang S, et al. Antigenic variation between Newcastle disease viruses of goose and chicken origin. Arch Virol. 2010;155:499–505. doi: 10.1007/s00705-010-0610-7. [DOI] [PubMed] [Google Scholar]
- 43.Archetti I. Persistent antigenic variation of influenza A viruses after incomplete neutralization in ovo with heterologous immune serum. J Exp Med. 2004;92:441–462. doi: 10.1084/jem.92.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Umali DV, Ito H, Suzuki T, Shirota K, Katoh H, Ito T. Molecular epidemiology of Newcastle disease virus isolates from vaccinated commercial poultry farms in non-epidemic areas of Japan. Virol J. 2013;10:330. doi: 10.1186/1743-422X-10-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang B, Chen Y, Mu C, Su Y, Liu R, Huang Z, Li Y, Yu Q, Chang G, Xu Q, Chen G. Identification and expression analysis of the interferon-induced protein with tetratricopeptide repeats 5 (IFIT5) gene in duck (Anas platyrhynchos domesticus) PLoS One. 2015;10:e0121065. doi: 10.1371/journal.pone.0121065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller PJ, Kim LM, Ip HS, Afonso CL (2009) Evolutionary dynamics of Newcastle disease virus. Virology. 10.1016/j.virol.2009.05.033 [DOI] [PubMed]
- 47.Miller PJ, Afonso CL, El Attrache J, et al. Effects of Newcastle disease virus vaccine antibodies on the shedding and transmission of challenge viruses. Dev Comp Immunol. 2013;41:505–513. doi: 10.1016/j.dci.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 48.McGinnes LW, Sergel T, Chen H, Hamo L, Schwertz S, Li D, Morrison TG. Mutational analysis of the membrane proximal heptad repeat of the Newcastle disease virus fusion protein. Virology. 2001;289:343–352. doi: 10.1006/viro.2001.1123. [DOI] [PubMed] [Google Scholar]
- 49.Gravel KA, McGinnes LW, Reitter J, Morrison TG. The transmembrane domain sequence affects the structure and function of the Newcastle disease virus fusion protein. J Virol. 2011;85:3486–3497. doi: 10.1128/jvi.02308-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGinnes L, Sergel T, Morrison T. Mutations in the transmembrane domain of the hn protein of newcastle disease virus affect the structure and activity of the protein. Virology. 1993;196:101–110. doi: 10.1006/viro.1993.1458. [DOI] [PubMed] [Google Scholar]
- 51.McGinnes LW, Morrison TG. The role of individual oligosaccharide chains in the activities of the HN glycoprotein of Newcastle disease virus. Virology. 1995;212:398–410. doi: 10.1006/viro.1995.1497. [DOI] [PubMed] [Google Scholar]
- 52.Umali DV, Ito H, Shirota K, Katoh H, Ito T. Characterization of complete genome sequence of genotype VI and VII velogenic Newcastle disease virus from Japan. Virus Genes. 2014;49:89–99. doi: 10.1007/s11262-014-1075-7. [DOI] [PubMed] [Google Scholar]
- 53.Choi K-S, Kye S, Kim J-Y, Lee H-S. Genetic and antigenic variation of shedding viruses from vaccinated chickens after challenge with virulent Newcastle disease virus. Avian Dis. 2013;57:303–306. doi: 10.1637/10379-092112-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 54.Stone HD. Optimization of hydrophile-lipophile balance for improved efficacy of Newcastle disease and avian influenza oil-emulsion vaccines. Avian Dis. 1988;32:68–73. doi: 10.2307/1590950. [DOI] [PubMed] [Google Scholar]
- 55.Scheid A, Caliguiri LA, Compans RW, Choppin PW. Isolation of paramyxovirus glycoproteins. Association of both hemagglutinating and neuraminidase activities with the larger SV5 glycoprotein. Virology. 1972;50:640–652. doi: 10.1016/0042-6822(72)90418-7. [DOI] [PubMed] [Google Scholar]
- 56.Pokric B, Juros S, Hlavaty H, Cajavec S. Determination of size of antigenic fragments after treatment of enveloped viruses with non-ionic detergents. Biologicals. 1993;21:157–162. doi: 10.1006/biol.1993.1068. [DOI] [PubMed] [Google Scholar]
- 57.Cornelissen LAHM, de Leeuw OS, Tacken MG, Klos HC, de Vries RP, de Boer-Luijtze EA, van Zoelen-Bos D, Rigter A, Rottier PJ, Moormann RJ, de Haan CA. Protective efficacy of Newcastle disease virus expressing soluble trimeric hemagglutinin against highly pathogenic H5N1 influenza in chickens and mice. PLoS One. 2012;7:e44447. doi: 10.1371/journal.pone.0044447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller PJ, King DJ, Afonso CL, Suarez DL. Antigenic differences among Newcastle disease virus strains of different genotypes used in vaccine formulation affect viral shedding after a virulent challenge. Vaccine. 2007;25:7238–7246. doi: 10.1016/j.vaccine.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 59.Hu Z, Hu S, Meng C, Wang X, Zhu J, Liu X. Generation of a genotype VII Newcastle disease virus vaccine candidate with high yield in embryonated chicken eggs. Avian Dis. 2011;55:391–397. doi: 10.1637/9633-122410-Reg.1. [DOI] [PubMed] [Google Scholar]
- 60.Ecco R, Brown C, Susta L, Cagle C, Cornax I, Pantin-Jackwood M, Miller PJ, Afonso CL. In vivo transcriptional cytokine responses and association with clinical and pathological outcomes in chickens infected with different Newcastle disease virus isolates using formalin-fixed paraffin-embedded samples. Vet Immunol Immunopathol. 2011;141:221–229. doi: 10.1016/j.vetimm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 61.Zhou Y, Lin G, Baarsch MJ, Scamurra RW, Murtaugh MP. Interleukin-4 suppresses inflammatory cytokine gene transcription in porcine macrophages. J Leukoc Biol. 1994;56:507–513. doi: 10.1002/jlb.56.4.507. [DOI] [PubMed] [Google Scholar]