Abstract
Objectives
This study aimed to review and report the serotype distribution and antimicrobial resistance patterns of invasive pneumococcal disease (IPD) isolates, as this information is important for policy making since China has not adopted any pneumococcal vaccines in the national immunization schedule.
Methods
A systematic review of the published literature from January 2000 to December 2018 was performed to identify articles that describe the serotype and/or antimicrobial resistance patterns of IPD cases in children in mainland China. Analysis of the extracted data was performed with the Microsoft Excel spreadsheet program. The percentage of the serotypes was calculated by dividing the number of isolates for each serotype with the total number of isolates included in all the studies. The theoretical impact of the vaccine was estimated by calculating the percentage of isolates that exhibited the serotypes included in the vaccines. The prevalence of antimicrobial resistance was defined as the number of isolates that were resistant divided by the total number of isolates tested for resistance to the specific antimicrobial agent.
Results
Forty-two articles were screened in the preliminary search, of which sixteen fulfilled inclusion criteria and were included in the final analysis. The predominant serotypes were 19A, 19F, 14, 23F, and 6B, and the estimated impact of PCV13 was 90.4%. The isolates exhibited a high frequency of resistance to cefuroxime, cefaclor, and erythromycin.
Conclusions
It is necessary for Chinese children to receive PCV13. Clinical workers should pay attention to the high frequency of resistance to antimicrobial agents.
Keywords: Antibiotic resistance, Children, Invasive pneumococcal disease, Pneumococcal conjugate vaccine, Serotype
Introduction
Streptococcus pneumoniae is the leading cause of a wide spectrum of serious invasive diseases, such as bacteremia, sepsis, and meningitis that are responsible for the life-threatening morbidity and mortality in children worldwide. Invasive pneumococcal disease (IPD), diagnosed as isolation of S. pneumoniae from a normally sterile body fluid, is a significant threat to children’s health, especially those infections caused by antibiotic-resistant strains.
There are more than 90 serotypes of S. pneumoniae. The 7-valent pneumococcal conjugate vaccine (PCV7, covering serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) was licensed in the USA in 2000. By 2010, PCV7 had been replaced with the higher valent vaccines PCV10 (PCV7 + 1, 5, and 7F) and PCV13 (PCV10 + 19A, 6A, and 3), which were introduced in over 130 countries [1]. Countries that introduced PCVs observed large reductions of IPD cases [2–8] and changes in serotype [9–11] and drug resistance patterns [12–15] of IPD strains.
China has not adopted any pneumococcal vaccines in the national immunization schedule. PCV7 was licensed in China in 2008, but the immunization rate was low, as only 10% of children had received PCV7 in 2011 [16]. PCV13 was approved in China in November 2016, but it has not been widely used due to its high price. Another 23-valent pneumococcal polysaccharide vaccine (PPV23: 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F) is available in China for children older than 2 years. The uptake of the vaccines is concentrated in urban areas and available only to those whose parents can afford to pay for them. Reports on vaccination data are scarce, and the vaccine coverage is not clear.
In China, data for IPD and studies on IPD isolates are rare because of the low positive culture rate caused by inappropriate use of antibiotics and difficulties in culturing [17–19]. The present study aimed to review and summarize the serotype distribution and antimicrobial resistance patterns of IPD strains from children in mainland China to fill in the knowledge gaps and provide basic information for the pneumococcal vaccination strategy.
Methods
Literature search
We performed a systematic literature review of the published literature from January 2000 to December 2018 to identify articles that describe the serotype and/or antimicrobial resistance patterns of IPD cases in children in mainland China. Articles published in English or Chinese were included, with the following literature databases searched: PubMed, Google Scholar, China National Knowledge Internet (CNKI), and Wan Fang database. The search terms were “invasive pneumococcal disease” or “invasive pneumococcal isolates/strains” or “IPD” and “serotype” or “antimicrobial resistance/susceptibility” and “China” or “Chinese” and “child” or “children.”
Inclusion and exclusion criteria
We included observational hospital-based studies (prospective and retrospective) reporting data on serotypes and/or antimicrobial resistance patterns of pediatric IPD strains from mainland China.
Inclusion criteria: (a) Study sites were in mainland China (Hong Kong, Macao, and Taiwan were not included); (b) the population was children, or data for adults and children could be separated; (c) the sources of the specimens had to be clearly stated and had to be invasive S. pneumoniae strains; (d) Quellung reaction alone was used for serotyping; and (e) antimicrobial resistance testing method and the susceptibility judgment criteria used had to be clearly described.
Exclusion criteria: (a) The article type was a review or case report; (b) the isolates were duplicates; (c) only a portion of the strains were tested for serotypes or antibiotic susceptibility; (d) studies that provided data on only serogroups instead of serotypes; (e) only frequency of nonsusceptibility was reported without a resistance rate; and (f) the antibiotic resistance judgment criteria were not based on those developed by the CLSI in or after 2008.
Data collection and extraction
The full texts included were read, and structured model tables were developed in Microsoft Excel spreadsheet and used to record the extracted information for the following variables: study period, geographical region, age of the children, number of strains, specimen sources, serotypes isolated, coverage rate of vaccines, judgment criteria, and frequency of resistance to common antimicrobial agents (penicillin, amoxicillin, amoxicillin-clavulanic acid, cefepime, cefotaxime, cefuroxime, ceftriaxone, cefaclor, imipenem, meropenem, and erythromycin).
Data analysis
Analysis of the extracted data was performed with the Microsoft Excel spreadsheet program. The percentage of the serotypes was calculated by dividing the number of isolates for each serotype by the total number of isolates included in all the studies. A predominant serotype was defined as the serotype that had a distribution percentage greater than 5% of the total isolates. The theoretical impacts of PCV13 and PPV23 were estimated by calculating the percentage of isolates that expressed the serotypes included in these two vaccines. Serotype coverage calculation was performed whenever possible by using only the serotypes within the vaccine and without taking into account potential serogroup cross-protection as the basecase. The prevalence of antimicrobial resistance was defined as the percentage of isolates that were resistant divided by the total number of isolates tested for resistance to the specific antimicrobial agent.
Results
Data review
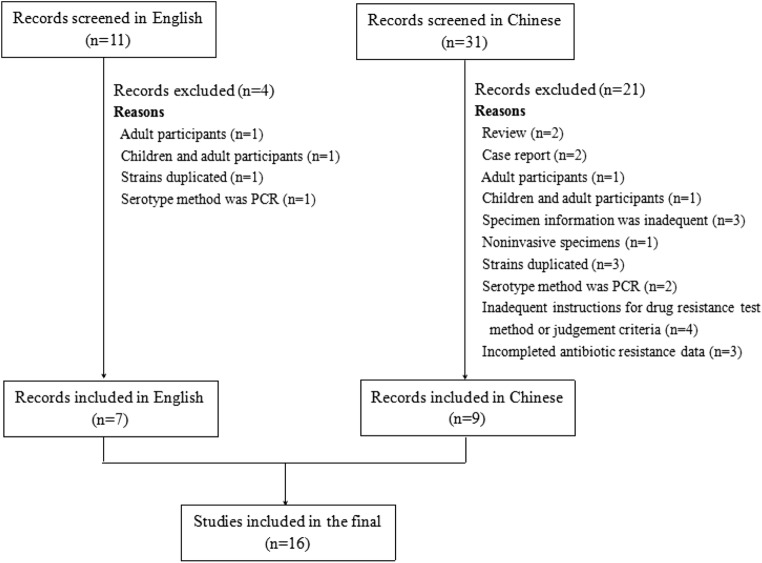
We screened 42 articles in our preliminary search, of which 16 studies [17, 20–34] fulfilled inclusion criteria and were included in the final analysis (Fig. 1). Among them, 4 studies only focused on only serotypes [20–23], 6 reported only antibiotic resistance patterns [29–34], and the other 6 recorded both serotypes and antibiotic resistance profiles [17, 24–38]. Of these, 7 articles [17, 20, 24–27, 29] were published in English, and 10 [21–23, 28, 30–34] were reported in Chinese language journals.
Fig. 1.
Selection process for inclusion of articles in the final analysis of the review
All of the studies used culturing for isolation of the organism from blood, CSF, and other sterile body fluid samples. The basic information of the 16 articles is listed in Table 1. Table 2 summarizes the serotype characteristics and the vaccine coverage rates of the literature, and Table 3 summarizes the reported frequency of resistance to tested drugs. Resistance data in reference [27] were not included in the analysis because CLSI 2006 recommendations were used as the antibiotic resistance judgment criteria in this paper.
Table 1.
Basic information of the studies included
| References | Study period | Location in China | Age (years or months) | No. of strains | Specimen sources |
|---|---|---|---|---|---|
| [17] | 2006–2008 | Multi-lefta | ≤ 14 years | 171 | Blood, CSFd, PLe, arthroedema |
| [20] | 2000.01–2014.08 | Shenyang, Liaoning | ≤ 14 years | 256 | Blood, CSF, PL, ascites, arthroedema |
| [21] | 2007.01–2010.12 | Nanjing, Jiangsu | NRc | 48 | Blood, CSF, PL, fester, ascites |
| [22] | 2010.02–2013.08 | Shenzhen, Guangdong | ≤ 14 years | 76 | Blood, CSF, PL, fester |
| [23] | 2009.01–2013.12 | Nanjing, Jiangsu | NR | 51 | Blood, CSF, PL, arthroedema, hydropericardium |
| [24] | 2011.10–2014.05 | Wenling, Zhejiang | 0–107 months | 67 | Blood, CSF, PL, ascites |
| [25] | 2012.04–2017.03 | Beijing | ≤ 14 years | 111 | Blood, CSF, PL, fester, subdural effusion, bone marrow |
| [26] | 2009.01–2012.08 | Shenzhen | ≤ 14 years | 87 | Blood, CSF, PL, fester, ascites, arthroedema, broncho-alveolar lavage |
| [27] | 2005.01–2006.12 | Multi-leftb | < 5 years | 31 | Blood, CSF, PL |
| [28] | 2013.01–2016.04 | Beijing | ≤ 14 years | 30 | Blood, CSF, PL, fester, ascites, bone marrow |
| [29] | 2008.01–2017.12 | Shanghai; Lanzhou, Gansu | < 14 years | 123 | Blood, CSF, PL |
| [30] | 2008.06–2014.03 | Chengdu, Sichuan | NR | 14 | CSF |
| [31] | 2010.10–2014.10 | Chongqing | 3 months–14 years | 102 | Blood, CSF, PL, subdural effusion |
| [32] | 2007.04–2011.06 | Shenzhen, Guangdong | NR | 63 | Blood |
| [33] | 2005.01–2011.12 | Shanghai | NR | 62 | Blood, CSF, PL, ascites |
| [34] | 2004.01–2011.03 | Suzhou, Jiangsu | 2 months–12 years | 38 | Blood, CSF, PL |
aThis multi-left study involved 11 cities in 10 provinces: Beijing; Tianjin; Shenyang, Liaoning; Shanghai; Nanjing, Jiangsu; Suzhou, Jiangsu; Wenzhou, Zhejiang; Hefei, Anhui; Shenzhen, Guangdong; Xinjiang; Chongqing
bThis multi-left study involved 8 cities in 8 provinces: Beijing; Shanghai; Shenzhen, Guangdong; Chengdu, Sichuan; Nanjing, Jiangsu; Wuhan, Hubei; Shenyang, Liaoning; Hangzhou, Zhejiang
cNR, pediatric patients, but the accurate age information was not reported
dCSF, cerebrospinal fluid
ePL, pleural fluid
Table 2.
Summary of serotype characteristics of studies related to serotype researches
| References | Serotypes (%) | Vaccine serotypes (%) | |
|---|---|---|---|
| PCV13 | PPV23 | ||
| [17] | 14 (20.5%), 19A (19.3%), 19F (17.0%), 6B (9.4%), 23F (7.6%), 18C (4.1%), 1 (3.5%), 6A (2.9%), 5 (2.3%), 9V (1.8%), 3 (1.2%), 8 (1.2%), 18a (1.2%), 34 (1.2%), 11A (0.6%), 11B (0.6%), 12A (0.6%), 15B (0.6%), 17A (0.6%), 18F (0.6%), 24F (0.6%), 7C (0.6%), 7F (0.6%), 9N (0.6%), 16 (0.6%), 29 (0.6%) | 90.1% | 90.1% |
| [20] | 19A (35.9%), 14 (17.2%), 19F (16.0%), 6B (9.0%), 23F (8.2%), othersb (13.7%) | 93.8% | - |
| [21] | 19F (27.1%), 19A (22.8%), 14 (18.7%), 9V (8.3%), 6B (6.3%), 23F (6.3%), 7F (4.2%), 8 (2.1%), NTc (4.2%) | 93.7% | 95.8% |
| [22] | 19F (31.58%), 19A (22.37%), 14 (15.79%), 9V (7.89%), 6B (6.59%), 23F (6.59%), 7F (3.95%), 8 (2.63%), NT (2.63%) | 94.7% | 97.4% |
| [23] | 19F (27.45%), 19A (19.61%), 14 (17.65%), 9V (9.81%), 6B (7.84%), 23F (7.84%), 7F (5.88%), 8 (1.96%), NT (1.96%) | 96.1% | 98.0% |
| [24] | 23F (22.4%), 14 (20.9%), 6B (17.9%), 19F (9.0%), 19A (9.0%), 5 (7.5%), 9V (4.5%), 19B (3.0%), UTd (6.0%) | 91.0% | 91.0% |
| [25] | 19F (22.5%), 19A (17.1%), 14 (16.2%), 23F (13.5%), 6B (9.0%), 6A (4.5%), 9V (3.6%), 5 (1.8%), 15B (1.8%), 24B (1.8%), 6C (1.8%), 3 (0.9%), 4 (0.9%), 8 (0.9%), 11A (0.9%), 15C (0.9%), 19B (0.9%), 24F (0.9%) | 90.1% | 89.2% |
| [26] | 19F (28.7%), 14 (25.3%), 23F (11.5%), 19A (9.2%), 6B (6.9%), others (18.4%) | 89.7% | - |
| [27] | 19A (29.0%), 19F (22.5%), 14 (12.9%), 5 (9.7%), 11A (6.5%), 23F (3.2%), others (16.2%) | 77.4% | 83.9% |
| [28] | 19F (36.7%), 19A (33.3%), 14 (13.3%), 23F (6.7%), 6A (6.7%), 11A (3.3%) | 96.7% | 93.3% |
aThe isolates were identified as serogroup 18, but could not be confirmed as one of type 18A, 18B, and 18D in that study
bOthers, the list and the number of the serotypes were not reported
cNT, non-typable serotype
dUT, these isolates were undifferentiated types as this study only identified serotypes covered by PPV23, 6A, and 23A
Table 3.
Summary of reported frequency of resistance to common antimicrobial agents
| References | Judgment criteria | Resistance rate to common antibioticsa (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PEN | AMX | AMC | FEP | CTX | CXM | CRO | CEC | IPM | MEM | ERY | ||
| [17] | CLSI 2008 |
Nonmeningitis isolates 0 Meningitis isolates 76.6 |
- | 0.6 | - | - | 62.0 |
Nonmeningitis isolates 0 Meningitis isolates 29.8 |
- | 0 | - | 95.9 |
| [24] | CLSI 2014 |
Nonmeningitis isolates 0 Meningitis isolates 50.0 |
- | 0 | - | - | - |
Nonmeningitis isolates 0 Meningitis isolates 2.9 |
- | - | - | 100 |
| [25] | CLSI 2017 |
Nonmeningitis isolates 0 Meningitis isolates 95.7 |
- | 0 | - | - | 75.7 |
Nonmeningitis isolates 1.1 Meningitis isolates 13.0 |
- | 0 | - | 99.1 |
| [26] | CLSI 2010 |
Nonmeningitis isolates 1.3 Meningitis isolates 100 |
- | 0 | - | - | 79.3 |
Nonmeningitis isolates 3.8 Meningitis isolates 33.3 |
81.6 | 0 | - | 96.6 |
| [28] | CLSI 2013 | 53.3 | 46.6 | - | 40.0 | 26.7 | - | - | - | - | 63.3 | 96.7 |
| [29]b | CLSI 2008 | 20.58 | 5.88 | - | - | 11.76 | - | 11.76 | - | - | 11.76 | 95.58 |
| [29]c | CLSI 2008 | 36.36 | 5.45 | - | - | 19.09 | - | 16.36 | - | - | 12.72 | 98.18 |
| [30] | CLSI 2013 | 71 | 7 | - | - | 29 | - | - | - | - | - | 100 |
| [31] | CLSI 2009 | 39.21 | 34.31 | - | 15.96 | 13.83 | - | - | - | - | 40.43 | 100 |
| [32] | CLSI 2008 | 53.97 | 14.29 | - | - | 9.52 | - | 9.52 | - | 3.85 | - | 85.71 |
| [33] | CLSI 2012 | 43.6 | - | 19.4 | - | - | 63.3 | 27.4 | - | - | - | 95.2 |
| [34] | CLSI 2008 | 42.11 | - | - | - | 23.53 | - | - | - | - | - | 94.74 |
aAbbreviations for common antibiotics: PEN, penicillin; AMX, amoxicillin; AMC, amoxicillin-clavulanic acid; FEP, cefepime; CTX, cefotaxime; CXM, cefuroxime; CRO, ceftriaxone; CEC, cefaclor; IPM, imipenem; MEM, meropenem; ERY, erythromycin
bIsolates included in reference [11] contained two parts, and these parts were from Shanghai
cIsolates included in reference [12] contained two parts, and these parts were from Lanzhou, Gansu
Serotype distribution and proportion of vaccine serotypes
A total of 942 isolates were included in the 10 studies reporting serotypes. The predominant serotypes were 19A (215, 22.8%), 19F (195, 20.7%), 14 (185, 19.6%), 23F (89, 9.4%), and 6B (79, 8.4%).
The theoretical impact of PCV13 was 90.4% (852/942). As shown in Table 2, only 8 studies involving 585 isolates reported the PPV23 coverage rate, and the proportion of PPV23 serotypes was 92.0% (538/585).
Antimicrobial resistance
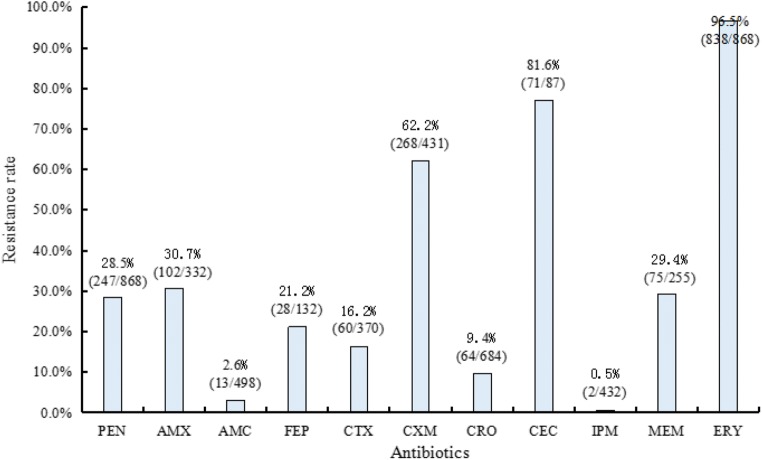
The 11 articles reporting antibiotic resistance patterns involved 868 isolates, and all of the isolates were tested for resistance rates to penicillin and erythromycin. The number of isolates tested for resistant to amoxicillin, amoxicillin-clavulanic acid, cefepime, cefotaxime, cefuroxime, ceftriaxone, cefaclor, imipenem, and meropenem were 332 (5 articles), 498 (5 articles), 132 (2 articles), 370 (6 articles), 431 (4 articles), 684 (7 articles), 87 (1 article), 432 (4 articles), and 255 (3 articles), respectively. Figure 2 shows the resistance frequency profiles for these common antibiotics.
Fig. 2.
Frequency of resistance to common antimicrobial agents used in the reviewed articles among invasive pneumococcal isolates in China. PEN penicillin, AMX amoxicillin, AMC amoxicillin-clavulanic acid, FEP cefepime, CTX cefotaxime, CXM cefuroxime, CRO ceftriaxone, CEC cefaclor, IPM imipenem, MEM meropenem, ERY erythromycin
Discussion
In this review, we have shown that the most frequent serotypes obtained from Chinese children with IPD were 19A, 19F, 14, 23F, and 6B. Together, these five serotypes constituted 81.0% of all strains. The result was in congruence with the previously published systematic reviews conducted in China, focusing on IPD and non-IPD strains [35, 36]. Four of the five predominant serotypes reported were also found in other regions, with differences in the order of prevalence and in a few serotypes. In a systematic review implemented in India, serotypes 14, 1, 19F, 6B, 5, 6A, 9V, and 23F were the most common serotypes, accounting for 65.5% of all IPD strains from children ≤ 5 years old [37]. The result from India was consistent with systematic reviews, in which authors have also identified seven serotypes (1, 5, 6A, 6B, 14, 19F, 23F) to be the most common worldwide and in the South Asian region [38, 39].
The effectiveness of PCV13 in reducing the morbidity and mortality of IPD has already been demonstrated in either high-income countries [5, 40, 41] or middle- and low-income regions [6, 7, 42]. This review revealed that the theoretical impact of PCV13 on IPD strains from Chinese children was as high as 90.4%. Serotype 19A, which emerged as a predominant serotype in countries where PCV7 or PCV10 was adopted for routine children vaccination [43–45], was the most important serotype in Chinese children, accounting for nearly one-fifth of the IPD strains. In general, countries that later included PCV13 into their national immunization program were able to reduce diseases caused by serotype 19A [45, 46]. All of these results highlighted the importance and necessity of vaccination with PCV13 for Chinese children.
Worldwide, reports on antibiotic resistance of S. pneumoniae isolates have been increasing sharply in recent decades. Prior antibiotic exposure among young children is a dominant risk factor associated with antibiotic resistance. The CLSI revised the breakpoints for penicillin in 2008. Before 2008, the breakpoints were sensitive, ≤ 0.06 μg/mL; intermediate, 0.12~1.00 μg/mL; and resistant: ≥ 2.00 μg/mL [47]. After 2008, the breakpoints for nonmmeningitis isolates were sensitive, ≤ 2 μg/mL; intermediate, 4 μg/mL; and resistant, ≥ 8 μg/mL; and for meningitis isolates, sensitive, ≤ 0.06 μg/mL and resistant, ≥ 0.12 μg/mL [48]. In the Asian Network for Surveillance of Resistant Pathogens (ANSORP) report [49], following the revised CLSI breakpoints, the prevalence of penicillin resistance for nonmmeningitis strains was 0.7% and for meningitis isolates was 57.7%. In the included twelve articles [17, 24–34], only four separately reported penicillin resistance rates of meningitis and nonmmeningitis isolates [17, 24–26] and the data were consistent with the results from ANSORP [49]. Actually, it is important for researchers and clinical workers to distinguish specimen types and resistance patterns for meningitis and nonmmeningitis isolates.
Among the β-lactam drugs, third-generation cephalosporins are considered to be the best alternative for the treatment of infections caused by penicillin-resistant S. pneumoniae. However, the present review showed a high resistance rate to cefaclor, which is a third-generation cephalosporin commonly used in China. Our review also highlighted the high resistance of pneumococci to cefuroxime and erythromycin, although the resistance rate varied slightly by region. The serious resistance phenomenon may be related to the abuse of antibiotics. In China, a campaign to overhaul the clinical use of antibiotics around the country has been initiated since 2011, and the government has made great efforts to control the abuse of antibiotics. However, the abuse of antibiotics is still serious, and antimicrobial resistance remains high. Therefore, it is critical for China to implement more effective policies to control the abuse of antibiotics.
Our review is an integrated summary of the IPD isolates collected from children in mainland China, which involves studies from 2000 to 2018. Some limitations should be realized for further studies. Only 16 articles were qualified for review, which is a small number compared with the vast land area and huge population base. Furthermore, among these 16 articles, not all of them analyzed the antimicrobial resistance data as expected. However, given that these studies were conducted in cities with large populations in China, the results are of great value for vaccine policy decisions.
Conclusions
The present review summarized the distribution of serotypes, theoretical impact of the vaccine, and resistance profiles to common antibiotics of IPD isolates from children in mainland China. Our results showed that a large proportion of IPD cases are caused by serotypes that are included in PCV13, so it is necessary to introduce PCV13 into the Chinese national immunization plan. Our review also highlights the high resistance of pneumococci to cefuroxime, cefaclor, and erythromycin, although the frequency of resistance varied slightly by region.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Weidong Men and Qiaoli Dong contributed equally to this work.
References
- 1.World Health Organization (2019) WHO vaccine-preventable diseases: monitoring system. 2018 global summary. 2018; http://apps.who.int/immunization_monitoring/globalsummary. Accessed 01 April 2019
- 2.Whitney CG, Pilishvili T, Farley MM, Schaffner W, Craig AS, Lynfield R, Nyquist AC, Gershman KA, Vazquez M, Bennett NM, Reingold A, Thomas A, Glode MP, Zell ER, Jorgensen JH, Beall B, Schuchat A. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495–1502. doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]
- 3.Weatherholtz R, Millar EV, Moulton LH, Reid R, Rudolph K, Santosham M, O’Brien KL. Invasive pneumococcal disease a decade after pneumococcal conjugate vaccine use in an American Indian population at high risk for disease. Clin Infect Dis. 2010;50:1238–1246. doi: 10.1086/651680. [DOI] [PubMed] [Google Scholar]
- 4.Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, Reingold A, Thomas A, Schaffner W, Craig AS, Smith PJ, Beall BW, Whitney CG, Moore MR, Active Bacterial Core Surveillance/Emerging Infections Program Network Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 5.Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Holtzman C, Harrison LH, Zansky SM, Rosen JB, Reingold A, Scherzinger K, Thomas A, Guevara RE, Motala T, Eason J, Barnes M, Petit S, Farley MM, McGee L, Jorgensen JH, Whitney CG. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med. 2016;4:399–406. doi: 10.1016/S2213-2600(16)00052-7. [DOI] [PubMed] [Google Scholar]
- 6.Cohen C, von Mollendorf C, de Gouveia L, Lengana S, Meiring S, Quan V, et al. Effectiveness of the 13-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in South Africa children: a case-control study. Lancet Glob Health. 2017;5:e359–e369. doi: 10.1016/S2214-109X(17)30043-8. [DOI] [PubMed] [Google Scholar]
- 7.Kambiré D, Soeters HM, Ouédraogo-Traoré R, Medah I, Sangaré L, Yaméogo I, et al. Early impact of 13-valent pneumococcal conjugate vaccine on pneumococcal meningitis-Burkina Faso, 2014-2015. J Inf Secur. 2018;76:270–279. doi: 10.1016/j.jinf.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitney CG. Examining duration of protection: should a booster dose be part of all infant pneumococcal conjugate vaccine programs? Clin Infect Dis. 2018;67:375–377. doi: 10.1093/cid/ciy135. [DOI] [PubMed] [Google Scholar]
- 9.Kent A, Makwana A, Sheppard CL, Collins S, Fry NK, Heath PT, Ramsay M, Ladhani SN. Invasive pneumococcal disease in UK children under 1 year of age in the post-PCV13 era: what are the risks now? Clin Infect Dis. 2019;69:84–90. doi: 10.1093/cid/ciy842. [DOI] [PubMed] [Google Scholar]
- 10.Arushothy R, Ahmad N, Amran F, Hashim R, Samsudin N, Azih CRC. Pneumococcal serotype distribution and antibiotic susceptibility in Malaysia: a four-year study (2014-2017) on invasive paediatric isolates. Int J Infect Dis. 2019;80:129–133. doi: 10.1016/j.ijid.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Perniciaro S, Imöhl M, Fitzner C, van der Linden M. Regional variations in serotype distribution and vaccination status in children under six years of age with invasive pneumococcal disease in Germany. PLoS One. 2019;14(1):e0210278. doi: 10.1371/journal.pone.0210278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens DS, Zughaier SM, Whitney CG, Baughman WS, Barker L, Gay K, Jackson D, Orenstein WA, Arnold K, Schuchat A, Farley MM, Georgia Emerging Infections Program Incidence of macrolide resistance in Streptococcus pneumoniae after introduction of the pneumococcal conjugate vaccine: population-based assessment. Lancet. 2005;365:855–863. doi: 10.1016/S0140-6736(05)71043-6. [DOI] [PubMed] [Google Scholar]
- 13.von Gottberg A, de Gouveia L, Tempia S, Quan V, Meiring S, von Mollendorf C, et al. Effects of vaccination on invasive pneumococcal disease in South Africa. N Engl J Med. 2014;371:1889–1899. doi: 10.1056/NEJMoa1401914. [DOI] [PubMed] [Google Scholar]
- 14.Kim L, McGee L, Tomczyk S, Beall B. Biological and epidemiological features of antibiotic-resistant Streptococcus pneumoniae in pre- and post-conjugate vaccine eras: a United States perspective. Clin Microbiol Rev. 2016;29:525–552. doi: 10.1128/CMR.00058-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder MR, Chancey ST, Thomas S, Kuo WH, Satola SW, Farley MM, Stephens DS. A population-based assessment of the impact of 7- and 13-valent pneumococcal conjugate vaccines on macrolide-resistant invasive pneumococcal disease: emergence and decline of Streptococcus pneumoniae serotype 19A (CC320) with dual macrolide resistance mechanisms. Clin Infect Dis. 2017;65:990–998. doi: 10.1093/cid/cix446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng JS, Cao L, Guo SC, A KZ, Wang L, Yu WZ, et al. Survey on the immunization status of category B vaccine among children aged 1 to 2 years in China. Chin J Vaccine Immunization. 2012;18:233–237. [Google Scholar]
- 17.Xue L, Yao K, Xie G, Zheng Y, Wang C, Shang Y, Wang H, Wan L, Liu L, Li C, Ji W, Xu X, Wang Y, Xu P, Liu Z, Yu S, Yang Y. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates that cause invasive disease among Chinese children. Clin Infect Dis. 2010;50:741–744. doi: 10.1086/650534. [DOI] [PubMed] [Google Scholar]
- 18.Kan QC, Wen JG, Liu XH, Li ZZ. Inappropriate use of antibiotics in children in China. Lancet. 2016;387:1273–1274. doi: 10.1016/S0140-6736(16)30019-8. [DOI] [PubMed] [Google Scholar]
- 19.Yao KH, Wang LB, Zhao GM, Zheng YJ, Deng L, Huang JF, Wang JX, Zhao RZ, Deng QL, Hu YH, Yu SJ, Yang YH, Young M. Pneumococcal serotype distribution and antimicrobial resistance in Chinese children hospitalized for pneumonia. Vaccine. 2011;29:2296–2301. doi: 10.1016/j.vaccine.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 20.Zhou X, Liu J, Zhang Z, Liu Y, Wang Y, Liu Y. Molecular characteristics of penicillin-binding protein 2b, 2x and 1a sequences in Streptococcus pneumoniae isolates causing invasive diseases among children in Northeast China. Eur J Clin Microbiol Infect Dis. 2016;35:633–645. doi: 10.1007/s10096-016-2582-3. [DOI] [PubMed] [Google Scholar]
- 21.Xu F, Chi FL, Tan H, Liu XM, Cao T, Pan W, et al. Study on serotype distribution in 48 isolates of invasive Streptococcus pneumoniae with which children infected. Chin J Biochem Pharm. 2012;33:909–912. [Google Scholar]
- 22.Lu C. Investigation on serotype distribution of invasive pneumococcal disease isolates from children. Int J Lab Med. 2015;36:990–992. [Google Scholar]
- 23.Zhou K, Xie GJ, Wang XW, Xu F, Yao KH. Clinical characteristics of invasive pneumococcal disease and its serotype distribution. Chin J Nosocomiol. 2015;25:3392–3394. [Google Scholar]
- 24.Wang J, Liu F, Ao P, Li X, Zheng H, Wu D, et al. Detection of serotype distribution and drug resistance of Streptococcus pneumoniae isolated from pediatric patients. Lab Med. 2017;48:39–45. doi: 10.1093/labmed/lmw059. [DOI] [PubMed] [Google Scholar]
- 25.Shi W, Li J, Dong F, Qian S, Liu G, Xu B, Zhou L, Xu W, Meng Q, Wang Q, Shen K, Ma L, Yao K. Serotype distribution, antibiotic resistance pattern, and multilocus sequence types of invasive Streptococcus pneumoniae isolates in two tertiary pediatric hospitals in Beijing prior to PCV13 availability. Expert Rev Vaccines. 2019;18:89–94. doi: 10.1080/14760584.2019.1557523. [DOI] [PubMed] [Google Scholar]
- 26.Ma X, Zhao R, Ma Z, Yao K, Yu S, Zheng Y, et al. Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates causing invasive diseases from Shenzhen Children’s Hospital. PLoS One. 2013;8:e67507. doi: 10.1371/journal.pone.0067507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Wang H, Chen M, Sun Z, Zhao R, Zhang L, et al. Serotype distribution and antimicrobial resistance patterns of Streptococcus pneumoniae isolated from children in China younger than 5 years. Diagn Microbiol Infect Dis. 2008;61:256–263. doi: 10.1016/j.diagmicrobio.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Wu J, Liu J, Dong F, Yao KH, Shen KL, et al. Clinical features and outcomes of invasive pneumococcal disease in pediatric intensive care unit. Chin J Appl Clin Pediatr. 2016;31:1400–1404. [Google Scholar]
- 29.Cai K, Wang Y, Guo Z, Xu X, Li H, Zhang Q. Clinical characteristics and antimicrobial resistance of pneumococcal isolates of pediatric invasive pneumococcal disease in China. Infect Drug Resist. 2018;11:2461–2469. doi: 10.2147/IDR.S183916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su M, Chang L, Zhou W, Mu LY, Kuang LH. Clinical characteristics of children with meningitis caused by Streptococcus pneumoniae and antibiotic resistance of Streptococcus pneumoniae strains. Chin J Contemp Pediatr. 2015;17:706–709. [PubMed] [Google Scholar]
- 31.Zou ZP. Clinical features and antimicrobial resistance patterns of 102 pediatric cases with invasive pneumococcal disease. Shenzhen J Integr Tradit Chin W Med. 2015;25:188–190. [Google Scholar]
- 32.Wang HM, Ma DL, Quan JL, Huang BX, Zhao RZ, Chen HY. Drug-resistance of Streptococcus pneumoniae isolated from children. Chin J Infect Control. 2012;11(221–222):220. [Google Scholar]
- 33.Zhang TD, Kong Q, Wang C, Qin HH, Zhang H. Resistant mechanism of β-lactam antibiotic of invasive Streptococcus pneumoniae. Chin J Lab Med. 2014;37:748–752. [Google Scholar]
- 34.Wang ML, Yan YD, Huang L, Zhu CH, Wang YQ, Chen ZR, et al. Clinical features and antimicrobial resistance of invasive pneumococcal disease in 38 children. Chin J Pract Pediatr. 2012;27:604–607. [Google Scholar]
- 35.Lyu S, Hu HL, Yang YH, Yao KH. A systematic review about Streptococcus pneumoniae serotype distribution in children in mainland of China before the PCV13 was licensed. Expert Rev Vaccines. 2017;16:997–1006. doi: 10.1080/14760584.2017.1360771. [DOI] [PubMed] [Google Scholar]
- 36.Chen K, Zhang X, Shan W, Zhao G, Zhang T. Serotype distribution of Streptococcus pneumoniae and potential impact of pneumococcal conjugate vaccines in China: a systematic review and meta-analysis. Hum Vaccin Immunother. 2018;14:1453–1463. doi: 10.1080/21645515.2018.1435224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jyotsana Singh J, Sundaresan S, Manoharan A, Shet A. Serotype distribution and antimicrobial susceptibility pattern in children ≤ 5 years with invasive pneumococcal disease in India-a systematic review. Vaccine. 2017;35:4501–4509. doi: 10.1016/j.vaccine.2017.06.079. [DOI] [PubMed] [Google Scholar]
- 38.Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7:e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaiswal N, Singh M, Das RR, Jindal I, Agarwal A, Thumburu KK, et al. Distribution of serotypes, vaccine coverage, and antimicrobial susceptibility pattern of Streptococcus pneumoniae in children living in SAARC countries: asystematic review. PLoS One. 2014;9:e108617. doi: 10.1371/journal.pone.0108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harboe ZB, Dalby T, Weinberger DM, Benfield T, Mølbak K, Slotved HC, et al. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumoccal disease incidence and mortality. Clin Infect Dis. 2014;59:1066–1073. doi: 10.1093/cid/ciu524. [DOI] [PubMed] [Google Scholar]
- 41.Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccination on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15:535–543. doi: 10.1016/S1473-3099(15)70044-7. [DOI] [PubMed] [Google Scholar]
- 42.Mackenzie GA, Hill PC, Jeffries DJ, Hossain I, Uchendu U, Ameh D, Ndiaye M, Adeyemi O, Pathirana J, Olatunji Y, Abatan B, Muhammad BS, Fombah AE, Saha D, Plumb I, Akano A, Ebruke B, Ideh RC, Kuti B, Githua P, Olutunde E, Ofordile O, Green E, Usuf E, Badji H, Ikumapayi UNA, Manjang A, Salaudeen R, Nsekpong ED, Jarju S, Antonio M, Sambou S, Ceesay L, Lowe-Jallow Y, Jasseh M, Mulholland K, Knoll M, Levine OS, Howie SR, Adegbola RA, Greenwood BM, Corrah T. Effect of the introduction of pneumococcal conjugate vaccination on invasive pneumococcal disease in The Gambia: a population-based surveillance study. Lancet Infect Dis. 2016;16:703–711. doi: 10.1016/S1473-3099(16)00054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann D, Willis J, Moore HC, Giele C, Murphy D, Keil AD, Harrison C, Bayley K, Watson M, Richmond P. The changing epidemiology of invasive pneumococcal disease in aboriginal and non-aboriginal western Australians from 1997 through 2007 and emergence of nonvaccine serotypes. Clin Infect Dis. 2010;50:1477–1486. doi: 10.1086/652440. [DOI] [PubMed] [Google Scholar]
- 44.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;2011(11):760–768. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 45.Knol MJ, Wagenvoort GHJ, Sanders EAM, Elberse K, Vlaminckx BJ, de Melker HE, et al. Invasive pneumococcal disease 3 years after introduction of 10-valent pneumococcal conjugate vaccine, the Netherlands. Emerg Infect Dis. 2015;2015(21):2040–2044. doi: 10.3201/eid2111.140780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galanis I, Lindstrand A, Darenberg J, Browall S, Nannapaneni P, Sjöström K, et al. Effects of PCV7 and PCV13 on invasive pneumococcal disease and carriage in Stockholm, Sweden. Eur Respir J. 2016;2016(47):1208–1218. doi: 10.1183/13993003.01451-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing; 20th informational supplement. Wayne: Clinical and Laboratory Standards Institute; 2006. pp. M100–MS16. [Google Scholar]
- 48.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing; 20th informational supplement. Wayne: Clinical and Laboratory Standards Institute; 2010. pp. M100–MS20. [Google Scholar]
- 49.Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56:1418–1426. doi: 10.1128/AAC.05658-11. [DOI] [PMC free article] [PubMed] [Google Scholar]