Abstract
The validation of chromatographic methods is a costly process, however necessary, especially with regard to the validation of methods that accurately determine concentrations of pesticides in different environmental compartments. This research aimed at the development and validation of a simple and fast method for the determination of chlorpyrifos concentrations in water by means of a gas chromatograph with electron capture detection (GC/ECD), and to investigate chlorpyrifos dynamics of adsorption in a Rhodic Ferralsol in Southern Brazilian conditions. The developed chromatographic method was based in EPA 8141 method. Parameters to be checked for method validation were: Selectivity/specificity, linearity, precision, accuracy, robustness, limit of detection (LOD) and limit of quantitation (LOQ). Were employed the following methodologies for the validation process: ANVISA Resolution 899, DOQ-CGCRE-008 and FDA Bioanalytical Method Validation Guide. Also, through laboratory tests, the sorption dynamics of chlorpyrifos in Rhodic Ferralsol was evaluated. Thus, the soil was contaminated with increasing concentrations of chlorpyrifos, which were subjected to solid-liquid extraction with SPE cartridge Chromabond® C18 ec. The obtained results were submitted to the models of Langmuir, Freundlich, Dubinin-Radushkevich and Sips. By this method, chlorpyrifos peaks are obtained at 16.9 min, demonstrating practicality and low cost. This method exhibits precision and sensitivity, with satisfactory LQ and LQ values. The models of Langmuir, Freundlich, Dubinin-Radushkevich and Sips suggest the occurrence of simultaneous adsorption in mono and multilayer of chlorpyrifos in Rhodic Ferralsol colloids, as well as the predominance of a chemical, high energy binding process (irreversible). However, the chemisorption of chlorpyrifos is more related to the good fit found for Dubinin-Radushkevich sorption energy values (9.861 and 11.079 KJ mol−1) and Qm values estimated by Langmuir (485.55 and 389.61 μg g−1 for linear and nonlinear model).
Keywords: Organophosphorus, Pesticide dynamics, Insecticide adsorption, Soil contamination
Introduction
The increase in agricultural activities, generated by the demographic growth in the terrestrial globe, is directly related to the increase in the use of pesticides in these activities. These products have increased agricultural productivity.
However, the disorderly and excessive use of pesticides has had several impacts on the environment. Among the harmful effects on the environment are the presence of pesticide residues in soil, water, air, plants and animals [1].
Agrochemicals can reach aquatic environments through intentional application, drift and surface runoff from areas where applications have occurred [2].
Leaching of agrochemicals through the soil profile can lead to the contamination of groundwater and therefore, in addition to affecting surface watercourses themselves, agrochemicals can reach groundwater whose decontamination presents great difficulty [3].
In this scenario, persistent Organic Pollutants (POPs) are organic substances that have as main characteristic persistence in the environment, having a long half-life in soils, sediments, air and biota. They are lipophilic compounds, which make them bioaccumulative in the food chain [1]. Pesticides are among these POPs.
The environmental impact generated by these pollutants is very large and in recent years, there is a greater concern with these environmental pollutants. This care is denoted by more severe environmental policies, regulated by government agencies and pressured by non-governmental organizations (NGOs) [4].
Pesticides can reach aquatic environments through rainfall and leaching in the areas where applications occur, and their uncontrolled use leads to an increase in risks, making populations not directly linked to the production chain of these substances are also exposed due to environmental contamination and food, making agro-toxicology an even more serious public health issue [5].
To prevent adverse health effects, pesticide residue determinations on agricultural waste are required. However, this requires the development of convenient, accredited and economical analytical methods, in a way that the determination of these in agricultural products and environmental compartments is inexpensive [4].
Several studies have reported on analytical methods for the determination of pesticides such as chlorpyrifos (CPF), to quote: Javaroni et al. [6], Sharma et al. [4], Barchańska et al. [5], Comber [7], Vonberg et al. [8], Wang et al. [9], EPA 551.1 [11], EPA 505 [12], EPA 525.2 [13], EPA 8141B [14], among others, which in the vast majority of cases present a certain complexity and high cost in determining, besides the use of sophisticated equipment, mainly because these are methods that determine dozens of pesticides simultaneously.
However, when it comes to the steps prior to chromatographic analysis, numerous techniques are reported in the literature, such as liquid-liquid solvent extraction, cartridge solid phase extraction (SPE), or solid phase micro extraction. (SPME). Each of these techniques may have its advantages or disadvantages, depending on the other procedures and analyte involved.
Kin and Huat [15], in determining residues of eight organochlorine and organophosphate pesticides in food matrices (including chlorpyrifos), concluded that HS-SPME and SPE are more efficient than HS-SDME in the proposed system because it has better linearity, precision, LOD, and LOQ. However, the HS-SDME was, according to the authors, simpler to perform, being free from memory effects and is cost effective.
Dalvie et al. [16], when comparing the cost analysis of ELISA, SPE and SPME for the monitoring of pesticides in water (including chlorpyrifos), found out that the cost analysis per sample of the SPME method was lower than that ELISA and SPE.
In this scenario, chlorpyrifos, a phosphorothioate ester, is produced by the reaction of 3,5,6-trichloropyridin-2-ol (TCP) with O,O-diethyl phosphorochlorodithioate. The hydrophobicity of the CPF molecule results in extensive partitioning into the organic fractions of environmental matrices [17].
Insecticides such as CPF (CAS number 2912-88-2) are inhibitors of the enzyme acetylcholinesterase (AChE). This pesticide is absorbed by the digestive, respiratory and dermal routes, due to its high liposolubility. After being absorbed, they are rapidly distributed through various tissues and organs [18].
Experiments conducted in pregnant rats reveals mother-to-offspring transmission of CPF as also chronic oral exposure to this pesticide during early adulthood. Besides, chronic life-time exposure to CPF was associated with an elevated sleep apnea index, greater diaphragmatic contraction and thus greater fatigability [18].
The occurrence of residues of CPF in natural waters, soils or even humans is not new, according to findings of several authors: waters of the municipality of Agudo, Brazil [19]; CPF and other pesticides also in waters of the municipality Agudo, Brazil [20], CPF residues in nut-planted soils of China [21], residues of CPF in humans - rice farmers - in Vietnam [22], in surface waters of the Central Velley, California [23], among other reports in many parts of the globe.
In this scenario, Miclean et al. [24] presented a method for the determination of CPF concentrations in surface waters, however these authors do not perform a validation study based on international standards such as: EPA 551.1 [11], EPA 505 [12], EPA 525.2 [13], EPA 8141B [14]. In addition, the aforementioned study, like many others, do not report the interactions of CPF in water with its sorption on soil, thus CPF is a molecule with high soil-affinity, by organic and inorganic colloids and there are very few data regarding this subject.
Considering all the aforementioned, the objective of this research was to validate an analytical chromatographic method for the specific determination of CPF in water and soils samples by GC/ECD, and to investigate chlorpyrifos dynamics of adsorption in a Rhodic Ferralsol in Southern Brazilian conditions.
Material and methods
This research was developed at the Laboratory of Environmental Chemistry and Instrumental of the State University of Western Paraná, Brazil.
Equipment and chromatographic conditions
A Thermo Scientific 1310 gas chromatograph with electron capture detection (GC/ECD) and automatic sampling AS 1310 was used. The analysis and determination of peaks areas used the software Chromeleon 7.1.
The developed chromatographic method was based on EPA 8141 [14]. A capillary column was used (TR-5MS, 5% diphenyl and 95% dimethylpolysiloxane with 30 m × 0.25 mm internal diameter × 0.25 μm film thickness). The chromatographic conditions were: splitless injector temperature of 250 °C, initial oven temperature of 120 °C with heating ramp of 5 °C min−1 to 200 °C and ECD at 300 °C, make up gas (N2 of 99,999% of purity) of 30.0 mL min−1 and split-stream of 2:5 in a run time of 20 min.
Preparation of standard solutions
All solutions were prepared from certified chlorpyrifos standards (PESTANAL, 99.99% purity). A stock solution of 1.000 mg L−1, which was used for the preparation of 2.000 μg L−1 solutions employed in the other studies, was prepared.
Chlorpyrifos curves and water sample preparation
For the construction of the analytical reference curves, dilutions of the solid CPF standards in MTBE (Methyl Tertiary Butyl Ether) were performed, with subsequent GC/ECD injection.
Already for the construction of recovery curves, water samples were contaminated with a solution of the standard 1.000 mg L−1 CPF diluted in acetone. In this way, the different solutions contaminated with the pesticide were prepared. All the reagents used during the analytical procedures presented high purity. For all analysis, including the validation of CPF determinations, type-1 water used, which was obtained by an ultra-purification system (Puritech PT0021 - Permution).
The chemical and physical characteristics of chlorpyrifos are exhibited in Table 1.
Table 1.
Some important characteristics of chlorpyrifos
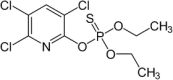 |
IUPAC name: O,O-Diethyl O-3,5,6-trichloropyridin-2-yl phosphorothioate |
|---|---|
| CAS Number | 2921-88-2 |
| Chemical formula | C9H11Cl3NO3PS |
| Molar mass | 350.57 g mol−1 |
| Density | 1.398 g cm−3 (43.5 °C) |
| Melting point | 43 °C (109 °F; 316 K) |
| Boiling point | 160 °C; 320 °F; 433 K (decomposes) |
| Solubility in water | 2 mg L−1 |
| Octanol-Water Partition Coefficient (Kow) | 4.70 |
| Soil Sorption Coefficient (Koc) | 360 to 31,000 depending on soil type and environment. |
Source: National Pesticide Information Center (NPIC, [25])
Construction of the analytical reference curve
The analytical reference curve is important to discover the expected response in terms of peak area for samples of known concentration of CPF. To do this, the chromatographic analysis must be performed with defined concentrations of CPF.
Thus, 2.000 μg L−1 standard solution was prepared in a 100 mL flask from chlorpyrifos standard (PESTANAL) diluted directly in MTBE in order to prepare the following concentrations of CPF for the construction of the reference curve (Table 2).
Table 2.
Reference and recovery curves for the determination of CPF
| Concentrations for CPF reference curve (μg L−1) | Concentrations for CPF recovery curve (μg L−1) |
|---|---|
| 100.00 | 10.00 |
| 150.00 | 15.00 |
| 200.00 | 20.00 |
| 300.00 | 30.00 |
| 350.00 | 35.00 |
| 400.00 | 40.00 |
| 500.00 | 50.00 |
| 600.00 | 60.00 |
It should also be noted that the points of the reference curve are 10 times higher than the stipulated points for the recovery curve, due to liquid extraction occurring in a ratio of 10:1 (10 mL water +1 mL MTBE).
Determination of the recovery curve
The recovery curve is used to know the response obtained in terms of peak area for samples of known concentration of the pesticide in the aqueous matrix. This step aims to calibrate the analytical method to determine the studied pesticide in water samples.
For the present study, a standard solution of 2000 μg L−1 of CPF in acetone was used for the contamination of water. The other concentrations were obtained by using this solution. After the artificial contamination of the water, samples were taken and submitted to liquid-liquid extraction using MTBE in the ratio 1:10 v/v.
For this purpose, 1 mL of MTBE was added to 10 mL of contaminated water +3% NaCl in tubes for liquid-liquid extraction at 1000 rpm in Vibrax VXR IKA for 2 h.
At the end of this process, the supernatant composed of MTBE + CPF was sampled and sent to GC for determination by ECD. This methodological process was performed to obtain the recovery curve, as shown in Table 2.
For both stages, 2.2 and 2.3, evaluations were carried out to validate the proposed analytical method according to the validation figures proposed by Resolution 899 of 2003 of the National Agency of Sanitary Surveillance [26], National Institute of Metrology, Quality and Technology - DOQ-CGCRE-008 (INMETRO [27]), and Bioanalytical Method Validation (FDA, 2018): Specificity and Selectivity, Linearity, Interval, Accuracy (Limits of detection and quantification), Accuracy and Robustness.
Specificity and selectivity
It is the ability of the method to accurately measure a compound in the presence of other components such as impurities, degradation products and matrix components. For this, CPF was evaluated in contrast with atrazine, by the same method.
Linearity and interval (working range and linear working range)
It is the ability of an analytical methodology to demonstrate that the results obtained are directly proportional to the concentration of the analyte in the sample, within a specified range. In this study, CPF was evaluated from 100 to 600 μg L−1.
Precision
Precision is the evaluation of the proximity of the results obtained in a series of measurements of a multiple sampling of a same sample. In this research an intermediary precision test was conducted, i.e., when the results have agreement from the same laboratory, but obtained on different days, with different analysts. In the current research the analysis CPF were performed with five days of difference and with two operators.
Detection Limit (DL or LD); Quantification Limit (QL or LQ)
The DL is the smallest amount of the analyte exhibit in a sample that can be detected, but not necessarily quantified under the established experimental conditions.
According to ANVISA [26], in the case of instrumental methods (HPLC, GC, atomic absorption), the detection limit estimation can be determined by the eq. 1.
| 1 |
Where: DPa is the standard deviation of the intercept with the Y-axis of at least 3 calibration curves constructed containing drug concentrations close to the assumed limit of quantification. IC is the slope of the calibration curve.
Detection Limit (DL or LD); Quantification Limit (QL or LQ)
It is the smallest amount of analyte in a sample that can be determined with acceptable accuracy and accuracy under established experimental conditions. The quantification limit is a parameter mainly determined for quantitative assays of impurities, pesticide degradation products and is expressed as analyte concentration (e.g., percentage w/w or w/v, parts per million) in the sample ([26]; FDA, 2018).
The quantification limit as established by analyzing solutions containing decreasing pesticide concentrations to the lowest determinable level with acceptable accuracy and accuracy. It can be calculated by the eq. 2 [26].
| 2 |
where: DPa is the standard deviation of the intercept with the Y-axis of at least 3 calibration curves constructed containing pesticide concentrations close to the assumed limit of quantification. IC is the slope of the calibration curve.
Accuracy
The accuracy was calculated as a percentage of recovery of the known amount of analyte added to the sample, or as the percentage difference between the means and the accepted true value, plus the confidence intervals.
The method accuracy must be determined after the establishment of linearity, linear range and specificity. The accuracy was found by verifying 8 (eight) determinations contemplating the linear interval of the procedure ([26]; FDA, 2018).
Thus, for the calculus of the accuracy, solutions containing CPF were prepared in the same conditions predicted by the analytical curve. The accuracy was expressed by the relationship between the experimentally determined average concentration and the corresponding theoretical concentration (Eq. 3).
| 3 |
Robustness
The robustness of an analytical method is the measure of its ability to withstand small, deliberate variations in analytical parameters. Indicates your confidence during normal use [26].
In view of the susceptibility of the method to variations in the analytical conditions, these should be controlled and precautions should be included in the procedure [4].
According to ANVISA [26], for the validation of analytical methods in GC, it is possible to verify the robustness of the method by means of variations in different batches or manufacturers of columns, temperature (furnace heating ramp), as well as speed and flow of the entrainment gas by the chromatographic system.
To evaluate the robustness of the proposed method, the oven temperature was varied during the heating ramp as well as the detector temperature (ECD). In this way, the following changes were caused to the method: The initial oven temperature passed at 100 °C, with a heating ramp of 5 °C min−1 to 200 °C. In addition, the detector temperature was increased to 280 °C. After these changes, a three-fold determination of the CPF concentration were performed from samples with 100 μg L−1.
Adsorption studies of chlorpyrifos in Rhodic Ferralsol
The soil samples (Rhodic Ferralsol) were obtained in the State University of Western Paraná farm, located in Marechal Cândido Rondon, State of Paraná, Brazil, from the 0–20 cm arable layer, in an area formerly cultivated with soybean in succession to corn (summer/winter), during February of 2018.
For an evaluation of CPF sorption in Rhodic Ferralsol, soil samples were taken from 0 to 20 cm depth (arable layer), these were dried at a temperature of 45 °C and ground. After the preparation, the samples were analyzed in order to determine their chemical and physical characteristics, by the following parameters: P, OM (organic matter), pH (CaCl2), H + Al, Al3+, K+, Ca2+, Mg2+, SB, CEC, V%, Al%, levels of Cu, Zn, Mn and Fe [28], total levels of Cd, Pb and Cr [29, 30], content of clay, silt and sand [31].
In addition, soil samples were evaluated for adsorption equilibrium studies. For this purpose, 5 g of soil were added in Erlenmeyer’s flasks of 125 mL. In these erlenmeyers were added 1 mL of increasing concentrations of CPF in ethanol of 90, 150, 300, 420 and 600 μg L−1. These increasing doses of CPF remained for 5 min for the occurrence of sorption in soil particles.
After that, 20 mL of an extractor solution methanol/water (4:1 v/v) were added into the Erlenmeyers flaks, these were stirred for 1 h in dubnoff system at 200 rpm at 25 °C. Then, the samples were centrifuged at 1500 rpm for 15 min. 3 mL of the obtained extract were taken for SPE extraction (Chromabond® C18 ec), MTBE was used for the elution and later injection in GC/ECD for pesticide determination. This procedure of solid-liquid extraction was performed according to the established procedures of EPA Method 525.2, Revision 2.0 [11].
Chromabond® C18 ec SPE cartridge has a nonpolar solid phase, with hydrophobic interactions with a wide variety of organic compounds, including chlorpyrifos and other many organochlorine and organophosphate pesticides.
The results were used for the construction of adsorption isotherms, which were evaluated by the models of Langmuir [32], Freundlich [33], Dubinin-Radushkevich [34], Sips [35] and Temkin [36], besides the estimation of KD and KOC coefficients.
Results and discussion
Specificity and selectivity
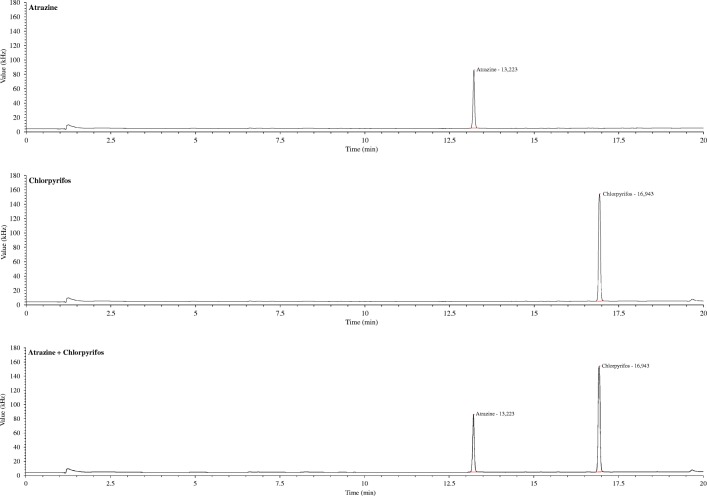
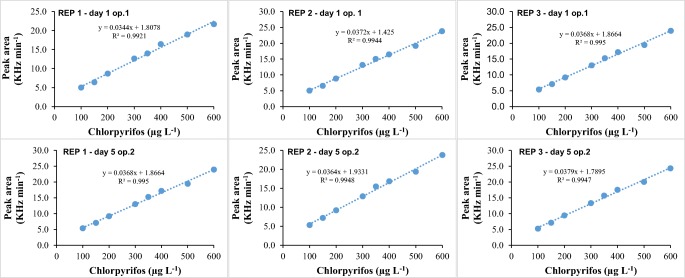
The specificity and selectivity refer to the ability of the method to accurately measure a compound in the presence of other components. By means of injections of pure atrazine and CPF (2000 μg L−1 in MTBE), with the proposed method it is possible to observe the CPF peak at 16.9 min, atrazine at 13.2 min, as well as both, together as shown in Fig. 1a, b, and c. These results demonstrate the selectivity of the proposed method against other pesticides.
Fig. 1.
Specificity and selectivity of CPF determinations using GC/ECD and the proposed method. Atrazine and CPF concentration of 1000 μg L−1
Linearity and construction of the analytical reference curve
The linearity study refers to the ability of an analytical methodology to demonstrate that the results obtained are directly proportional to the analyte concentration in the sample within a specified range.
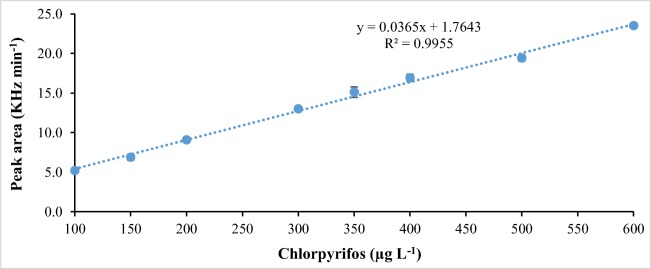
For this purpose, concentrations of CPF of 100, 150, 200, 300, 350, 400, 500 and 600 μg L−1 were prepared. Such solutions were taken to the GC/ECD to determine the analytical response. The results are shown in Fig. 2.
Fig. 2.
Analytical reference curves of chlorpyrifos (n = 6)
It is observed in Fig. 2 increasing concentrations of CPF and proportionally response in peak area. Anvisa Resolution n. 899 [26] mentions that the minimum acceptable criterion for R2 is 0.99, being the obtained result satisfactory.
Study of linearity and liquid-liquid extraction for determination of chlorpyrifos in water samples
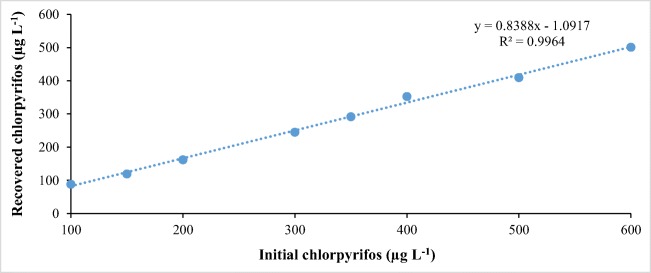
After liquid-liquid extraction mentioned above, samples of MTBE containing CPF were injected into GC/ECD under the same chromatographic conditions. The results obtained are shown in Fig. 3.
Fig. 3.
Recovery curve for chlorpyrifos in water matrix (n = 6)
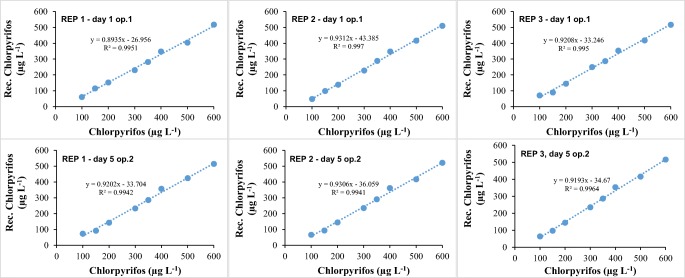
It is observed in Fig. 3 that by liquid-liquid extraction, linearity is maintained for CPF. Thus, both the proposed method and the extraction process are efficient for the determination of these compounds in the water matrix.
In general, the above study demonstrates an average recovery of 86% for the proposed liquid-liquid extraction.
Accuracy of the method – Analytical reference curves
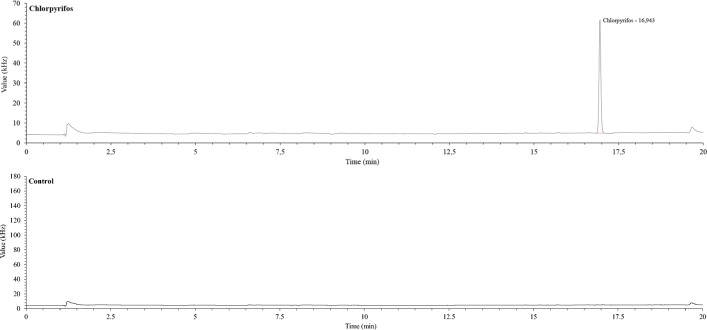
The accuracy tests were performed with a time interval of five days between determinations and with change of operator. The following chromatograph (Fig. 4) evidence a peak of chlorpyrifos (around 17 min.) with a water sample of 600 μg L−1.
Fig. 4.
Determination example of CPF in water samples of 600 μg L−1 in accuracy tests (above) and the “matrix effect” of a sample without CPF (bellow)
For this validation were prepared solutions with CPF concentrations of 100, 150, 300, 350, 400, 500 and 600 μg L−1 in MTBE, with 3 replicates for each concentration (Fig. 5). It is observed that the analytical reference curves present excellent linearity, with R2 values higher than 0.99, i.e., in accordance with ANVISA, FDA and INMETRO.
Fig. 5.
Accuracy of the method – analytical reference curves (n = 3). Notes: REP 1 or 2: replicate 1 or 2. Analysis were conducted in 5 days intervals (day 1 or day 5). Two different operators were employed in the analysis (op. 1 or op. 2)
Accuracy of the method – Determination of chlorpyrifos in water samples
Also for the recovery of CPF in water, the accuracy test was performed with a time interval of five days between determinations and with change of operator (Fig. 5).
It is observed in Fig. 6 that the proposed liquid-liquid extraction (Water: MTBE v/v 10:1) was successful, extracting on average 84% of the and CPF from water. In addition, good determination coefficients above 0.99 are observed in the first and second evaluations (with a five-day interval and operator change), as stipulated in resolutions from ANVISA, INMETRO and FDA. These results suggest that the proposed method is sufficiently efficient for the determination of CPF of contaminated water.
Fig. 6.
Accuracy of the method – recovery curves (n = 3). Notes: REP 1 or 2: replicate 1 or 2. Analysis were conducted in 5 days intervals (day 1 or day 5). Two different operators were employed in the analysis (op. 1 or op. 2)
Limit of detection (LD) and limit of quantification (LQ) for analytical reference curves and recovery curves
In relation to analytical reference curves for CPF the value of LD = 0.108 μg L−1, LQ = 0.361 μg L−1. For the recovery curves, LD = 0.494 μg L−1 and LQ = 1.648 μg L−1.
Accuracy of the method for analytical reference curves and recovery curves
Three analytical curves were constructed to evaluate the accuracy of the method, according to the aforementioned graphs, obtaining the following values for method accuracy (Table 3).
Table 3.
Obtained values for the accuracy of the proposed analytical method
| Accuracy of the proposed method (%) | Chlorpyrifos (%) |
|---|---|
| Analytical Reference Curve | 96.80 |
| Recovery Curve (liquid-liquid extraction) | 84.24 |
n = 3.
Robustness
To evaluate the robustness of the proposed method, the oven temperature was varied during the heating ramp (initial temperature decreased to 100 °C) as well as the detector temperature (ECD temperature decreased to 280 °C).
After these changes, a three-fold determination of the concentration of CPF in samples of 100 μg L−1 in MTBE. The results are exhibited in Table 4.
Table 4.
Robustness test for chlorpyrifos
| Replicates | CPF | CPF | Deviation | CV (%) |
|---|---|---|---|---|
| (μg L−1) | (μg L−1) | |||
| With alteration | Without alteration | |||
| Rep 1 | 97.42 | 99.060 | 1.356 | 1.382 |
| Rep 2 | 97.416 | 94.129 | 2.325 | 2.427 |
| Rep 3 | 91.937 | 97.142 | 3.681 | 3.893 |
| Deviation | 3.088 | 0.153 | 2.603 | – |
| CV (%) | 3.23 | 0.16 | – | 2.708 |
It is observed that even with the changes in the method, with variations in the initial temperature of the heating ramp, or with variations in the temperature of the detector ECD, the deviations obtained for the concentrations are insignificant.
Adsorption of Chlorpyrifos by a Rhodic Ferralsol
The adsorption is considered to be one of the main processes that affect the interaction that occurs between the pesticide and the solid phase of the soil. The main constituents that represent the solid phase in the soil are clay, minerals, organic matter, oxides and hydroxides of aluminum and iron and silica [37].
According to Table 5, the evaluated soil (a Rhodic Ferralsol) have medium levels of organic matter (range of 15 to 30 g dm−3), medium CEC (range of 5 to 15 cmolc dm−3), and medium base saturation (V% among 50 and 70%).
Table 5.
Physical and chemical attributes of Rhodic Ferralsol
| P | OM | pH CaCl2 | H + Al | Al3+ | K+ | Ca2+ | Mg2+ | SB | CEC | V | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg dm−3 | g dm−3 | 0,01 mol L−1 | ----------------------------- cmolc dm−3 ----------------------------- | --------------%-------------- | |||||||
| 18.03 | 17.08 | 5.50 | 3.36 | 0.05 | 0.63 | 3.94 | 1.52 | 6.09 | 9.45 | 64.46 | 0.81 |
| Cu | Zn | Mn | Fe | Cd | Pb | Cr | Clay | Silt | Sand | ||
| ------------------------------------- mg dm−3 ------------------------------------- | ------------------------- g kg−1------------------------- | ||||||||||
| 11.60 | 4.60 | 116.00 | 33.50 | <LQ | 26.00 | <LQ | 477 | 314 | 209 | ||
P: Phosphorous (Mehlich-1); OM: Organic Matter (Walkley-Black); CEC: Cations-Exchange-Capacity; V: Base saturation; Al: aluminum saturation; LQ: limit of quantification.
Besides, the V% value also states that the studied Rhodic Ferralsol is in eutrophic conditions (V% > 50%). It is also important to point out that this particular soil exhibit clay texture (clay level among entre 350 and 600 g Kg−1), being this clay predominantly highly weathered iron oxides.
The diagnostic horizon is called the oxic horizon or the oxic B-horizon, and is defined by FAO “Guidelines for soil description” as argillic and natric horizons which show significant enrichment in clay which has migrated from overlying horizons. They have usually a blocky structure, a clear upper boundary, and show illuviatlon cutans (clay skins) on horizontal and vertical ped surfaces [38].
Ferralsols are mineral soils (the thickness of the organic horizons does not exceed 40 cm), that have an oxic horizon. The upper boundary of the oxic horizon occurs at less than 125 cm depth. They may not show between 25 and 100 cm of the surface intersecting slickensides 1/ or wedgeshaped structural aggregates, and cracks which are at least 1 cm wide at a depth of 50 cm. They should not have a spodic horizon [38].
The dynamics of pesticides in soil can be influenced by some factors such as adsorption, movement and decomposition. Adsorption directly influences the magnitude of the effect of other factors such as biodegradability, bioaccumulation and others [39].
The movement of pesticides in the soil can occur through leaching, runoff and volatilization. Information on the movement of pesticides is useful in forecasting their chemical efficacy. The decomposition processes perform an important performance in the dissipation of many pesticides in the soil. The disappearance of a pesticide from the soil can also occur through several chemical processes, including photodecomposition and chemical reactions [39].
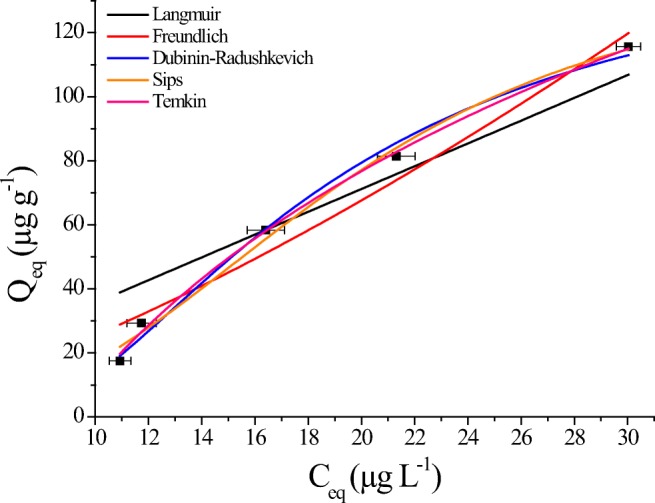
The results shown in Fig. 7 demonstrate that most of the CPF in contact with Rhodic Ferralsol colloids are chemically adsorbed, since desorption rates are very low.
Fig. 7.
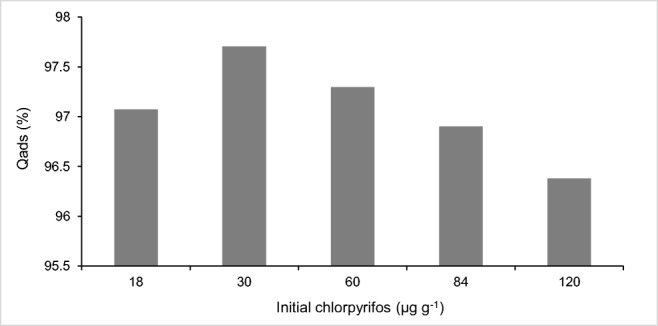
Percentage of adsorption of chlorpyrifos in Rhodic Ferralsol (n = 5)
The applied models illustrate a good mathematical fit for sorption of CPF (Table 6). This may indicate that the sorption process of this pesticide in Rhodic Ferralsol colloids is a complex process, since the models suggest the occurrence of mono and multilayer sorption (Langmuir, Freundlich and Sips) [1, 40].
Table 6.
Parameters that elucidate the sorption of chlorpyrifos in Rhodic Ferralsol
| Linear | Langmuir |
Freundlich |
Dubinin and Radushkevich lnQeq = ln Qd − Bdε2 |
Sips |
Temkin qeq = B ln At + B ln Ceq |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qm (μg g−1) |
485.554 | Kf (μg g−1) |
1.816 | Qd (mol L−1) |
0.0005 | n | 0.493 | B (J mol−1) | 57.611 | ||||||
| KL (L μg−1) |
0.001 | n | 1.031 | E (KJ mol−1) |
9.861 | Ks (L μg−1) |
0.026 | bt | 43.237 | ||||||
| RL | 0.595 | R2 | 0.980 | R2 | 0.990 | R2 | 0.980 | At (L g−1) | 0.084 | ||||||
| R2 | 0.994 | R2 | 0.996 | ||||||||||||
| Non-linear |
Langmuir
|
Freundlich
|
Dubinin and Radushkevich
|
Sips
|
Temkin
|
||||||||||
| Values | SSE | Values | SSE | Values | SSE | Values | SSE | Values | SSE | ||||||
|
Qm (μg g−1) |
389.61 | 102.83 |
Kf (μg g−1) |
2.035 | 0.617 |
Qsat (μg g−1) |
4.5E-04 | 6.0E-05 | n | 1.610 | 0.322 | B (J mol−1) | 57.302 | 1.670 | |
|
KL (L μg−1) |
0.006 | 0.0019 | n | 1.076 | 0.088 |
Bd (mol2 J−2) |
4.1E-03 | 3.4E-04 |
Ks (L μg−1) |
1.8E-03 | 1.1E-03 | bt | 43.237 | 1.670 | |
| RL | 0.188 | R2 | 0.985 |
E (KJ mol−1) |
11.079 |
Qsat (μg g−1) |
182.340 | 55.437 | At (L g−1) | 0.084 | 0.004 | ||||
| R2 | 0.990 | R2 | 0.984 | R2 | 0.989 | R2 | 0.988 | ||||||||
n = 5; SSE: Error sum of squares.
In addition, the values obtained by Dubinin-Radushkevich E > 8 KJ mol−1 suggest the occurrence of chemisorption [40] of CPF into colloids of Rhodic Ferralsol.
Temkin isotherm contains a factor that is explicitly entered into the adsorbent–adsorbate interactions. By ignoring the extremely low and large value of concentrations, the model assumes that heat of adsorption (function of temperature) of all CPF in the layer would decrease linearly rather than logarithmic with coverage [41].
For Temkin isotherm, the At, bt, R, T are equilibrium binding constant (L g−1) Temkin isotherm constant, universal gas constant (8.314 J mol−1 K−1) and temperature at 298 K, respectively. B is constant related to the heat of sorption (J mol−1) obtained by the expression B = RT/bt (Fig. 8).
Fig. 8.
Isotherms of adsorption for chlorpyrifos in Rhodic Ferralsol (n = 5)
It is important to highlight that the Langmuir maximum capacity of adsorption were 485 and 389 μg g−1 (for linear and nonlinear models), suggesting a high monolayer sorption of CPF by the Rhodic Ferralsol colloids. These results suggests a higher monolayer than a multilayer affinity, once that Freundlich maximum adsorption capacity remained around 1.816 and 2.035 μg g−1. However, the good fitting for Sips model suggests that as the soil is a complex matrix, mono and multilayer of CPF seems to occur [40].
However, the good fittings with Langmuir and the E values of D-R higher than 8 KJ mol−1, indicate that predominantly CPF is chemisorbed in monolayers by the soil colloids.
It is also emphasized that the obtained results (Qm, KL, Kf, n, Ks, E and others) by the linear and non-linear models were close, indicating the accuracy of the results. However, the use of nonlinear models is usually a better way to obtain the isotherm parameters [42].
According to Sahin and Tapadia [42], the linear least-square model with linearly transformed isotherm equations has been widely applied to confirm the experimental results. Nevertheless, conversion of non-linear isotherm equations to linear forms unconditionally modify their error structure and may violate the error variance and normality assumption of standard least squares [42].
Given its hydrophobic characteristics, the sorption of CPF is recognized as closely linked to soil organic matter content. However, in this particular Rhodic Ferralsol the clay content (mainly composed by iron oxides) reaches 477 g Kg−1 (almost 50% of soil mass), and organic matter represent only 17.08 g Kg−1 (1,7%), what suggest that the most part of interactions between the pesticide and the soil occurs with the clay content.
When investigating the adsorption of CPF on Almeria soils (Spain), Garcia et al. [43], have not found significant correlation of this molecule with the organic matter, however good correlations where found with CPF sorption and the clay content.
In the experimental conditions, values for Kd and Koc of 1566 L Kg−1 and 1581 Kg−1, respectively, were obtained, again suggesting a high affinity of CPF with Rhodic Ferralsol colloids, i.e., strong chemical interactions between CPF and iron oxides, mainly hematite.
These values are very close to Parolo et al. [44], when characterizing the soil organic matter by FT-IR spectroscopy and its relationship with chlorpyrifos sorption. With distinct soils, like since loam, clay loam, loam-silt loam, sandy loam, soils with OM content from 2.13% to 8.34, these authors found KOC values from 11,220 to 9192 L Kg−1. Acording to Parolo et al. [44], the higher adsorption of CPF occurs in soils with lower pH values and with aliphatic and hydrophilic fractions of the SOM.
Goethite (α-FeOOH) and hematite (α-Fe2O3) are the most common pedogenic iron oxides, accompanied by maghemite and ferrihydrite in small amounts, typically in high quantities in soils such subtropical Rhodic Ferralsol [45].
The (SSA) of natural soils hematite can reach 90 m2 g−1 [46]. These soil minerals have incredible high affinity with phosphates [47]; what may influence the sorption activity of the organophosphorus chlorpyrifos.
However, this is not a general rule, once Vagi et al. [48] schown that Dimethoate was weakly adsorbed on three different studied soils and thus suspected for leaching, while in other hand Fenthion was strongly bonded onto soil and therefore characterized by these authors as immobile.
Or Alfonso et al. [49] which found in Yucaan soils rich in montmorillonite (Leptosols), susceptibility of leaching of Diazinon, Dimethoate, Methyl Parathion, and Sulfotep in function of their low affinity with soil colloids.
It is worth noting that although the OM is considered one of the most important soil component in organic pesticide retention, other factors, like pH, humidity, temperature, quantity and quality of clay minerals can be very important in pesticide retention [48].
According to Gebremariam et al. [39], chlorpyrifos adsorption can partly by correlated with trace levels of organic carbon associated with soil minerals. However, when soils have low quantities of OM, CPF may undergo preferential adsorption to the clay fractions in soils.
It is notable that not all clay minerals have a strong affinity for CPF however, there are a few available studies indicated that CPF shows a relatively higher affinity for aquatic sediments than soils [39].
When studying sorption of chlorpyrifos in different smectites, Wu and Laird [52] found that this pesticide is strongly adsorbed on Ca-humate and not desorbed from this fraction, suggesting strong chemical bonds between adsorbent/adsorbate. According to the authors, CPF was moderately adsorbed by river sediments. This result implies that organic and/or inorganic materials suspended in sediments may influence the sorption/desorption process of chlorpyrifos in aqueous medium.
Schmidt et al. [37] when studying the sorption of Tiametoxam and Atrazine in Humic Ferralsol, found values of Kf and n by the Freundlich linear model, similar to those found in the present study.
Although CPF is a poor water-soluble pesticide, highly soluble in fats, and therefore easily absorbed in environmental matrices, it is observed that the quality of substrate also plays a fundamental role in its sorption.
By comparing the obtained coefficients with other researches, as can be seen in Table 7, all values found for sorption of CPF were high, however there is a great variation, like Koc values of 8200 in Humic Ferralsol [50] up to 30,381 L Kg−1 for pre-amended clay loam [51].
Table 7.
Comparative of adsorption results
| Soil/Sufrace | Kf (μg g−1) (Freundlich) | n (Freundlich) |
Kd (L Kg−1) |
Koc (L Kg−1) |
Pesticide | Author |
|---|---|---|---|---|---|---|
| Rhodic Ferralsol |
(Linear model) 1.816 |
(Linear model) 1.031 |
1566 | 1581 | Chlorpyrifos | Current research |
|
(Non-Linear model) 2.035 |
(Non-Linear model) 1.076 |
|||||
| Humic Ferralsol | – | – | – | 8200 | Chlorpyrifos | Soares et al. [50] |
| Humic Ferralsol | – | – | – | 20 | 2,4-D | Soares et al. [50] |
| Humic Ferralsol | – | – | – | 2.7 | Acefato | Soares et al. [50] |
| Humic Ferralsol | – | – | – | 240,000 | Bifentrina | Soares et al. [50] |
| Humic Ferralsol | – | – | – | 12,000 | Endosulfam | Soares et al. [50] |
| Humic Ferralsol | – | – | – | 22,000 | Glifosato | Soares et al. [50] |
| Humic Ferralsol | 1.10 | 0.67 | – | 24 to 178 | Tiametoxam | Schmidt et al. [37] |
| Humic Ferralsol | 1.94 | 0.89 | – | 37 to 104 | Atrazina | Schmidt et al. [37] |
| Sand, OH | 5609* | 0.82 | 22.6 | 7931 | Chlorpyrifos | Spieszalski et al. [51] |
| Wooster silt loam, OH | 62,405* | 0.93 | 190.2 | 17,272 | Chlorpyrifos | Spieszalski et al. [51] |
| Wooster silt loam, OH | 291,690* | 0.84 | 190.2 | 17,272 | Chlorpyrifos | Spieszalski et al. [51] |
| Pre-amended clay loam (9:1 (v/v), OH | 291,690* | 0.84 | 1036 | 30,381 | Chlorpyrifos | Spieszalski et al. [51] |
*Transformed values for the units used in this research
Conclusion
A rapid and practical method of liquid-liquid extraction and determination of chlorpyrifos by gas chromatography – electron capture detection, has been described. By this method, chlorpyrifos peaks are obtained at 16.9 min, demonstrating that the proposed method is practical and present low cost.
The selectivity, linear range, recovery, accuracy and limit of quantification were all evaluated and verified, thus this method presents precision and sensitivity, with satisfactory LQ and LD values. The results demonstrate the ability of the method and are satisfactory for routine laboratory needs regarding the determination of chlorpyrifos in water.
The adsorption of chlorpyrifos on Rhodic Ferralsol appears to be chemical in nature, with sorption energy (estimated from Dubini-Radushkevic linear and nonlinear model) higher than 8 KJ mol−1, and adsorption rates higher 90%.
The models of Langmuir, Freundlich, Dubinin-Radushkevich and Sips suggest the occurrence of simultaneous adsorption in mono and multilayer of chlorpyrifos in Rhodic Ferralsol colloids, as well as the predominance of a chemical, high energy binding process (irreversible). However, the chemisorption of chlorpyrifos is more related to the good fit found for Dubinin-Radushkevich sorption energy values (9.861 and 11.079 KJ mol−1) and Qm values estimated by Langmuir (485.55 and 389.61 μg g−1 for linear and nonlinear model).
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alves TC, Pinheiro A, Schwantes D, Gonçalves AC., Jr Organic micropollutant adsorption in chemically modified forestry Pinus elliotti spp barks. The Journal of Solid Waste Technology and Management. 2018;44:142–152. doi: 10.5276/JSWTM.2018.142. [DOI] [Google Scholar]
- 2.Martins CX, Salvador P de M, Jesus JD de, Ferreira LFR, Américo JHP, Torres NH (2014). Análise de atrazina em amostras de água e solo por cromatografia gasosa (GC-ECD). Bioenergia em revista: diálogos, 3(1):128–138. Available in: http://fatecpiracicaba.edu.br/revista/index.php/bioenergiaemrevista/article/view/124/80. Access in January 2019.
- 3.Abdulkareem JH, Abdulkadir A, Abdu N. A review of different types of lysimeter used in solute transport studies. International Journal of Plant & Soil Science. 2015;8(3):1–14. doi: 10.9734/IJPSS/2015/18098. [DOI] [Google Scholar]
- 4.Sharma DK, Kumar A, Mahender A simple and fast solid-phase extraction GC-ECD method for the routine assessment of atrazine residues in agricultural produces. J Chromatogr Sep Tech. 2017;8(1):1–4. doi: 10.4172/2157-7064.1000353. [DOI] [Google Scholar]
- 5.Barchańska H, Czaplicka M, Giemza A. Simultaneous determination of selected insecticides and atrazine in soil by MAE–GC–ECD. Archives of Environmental Protection. 2013;39(1):27–40. doi: 10.2478/aep-2013-0003. [DOI] [Google Scholar]
- 6.Javaroni CA, Landgraf MD, Rezende MOO. Comportamento dos herbicidas atrazina e alaclor aplicados em solo preparado para o cultivo de cana-de-açúcar. Quim Nova. 1999;22(1):58–64. doi: 10.1590/S0100-40421999000100012. [DOI] [Google Scholar]
- 7.Comber SDW. Abiotic persistence of atrazine and simazine in water. Pestic Sci. 1999;55:696–702. doi: 10.1002/(SICI)1096-9063(199907)55:7<696::AID-PS11>3.0.CO;2-7. [DOI] [Google Scholar]
- 8.Vonberg D, Vanderborght J, Cremer N, Pütz T, Herbst M, Vereecken H. 20 years of long-term atrazine monitoring in a shallow aquifer in western Germany. Water Res. 2014;50:294–306. doi: 10.1016/j.watres.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Li J, Xing H, Xu S. Review of toxicology of atrazine and chlorpyrifos on fish. J Northeast Agric Univ. 2011;18(4):88–92. doi: 10.1016/S1006-8104(12)60031-2. [DOI] [Google Scholar]
- 10.USEPA - United States Environmental Protection Agency (1992). Method 619: The determination of triazine pesticides in municipal and industrial Wastewater. Available in: https://www.epa.gov/sites/production/files/2015-10/documents/method_619_1992.pdf. Access in January 2019.
- 11.USEPA - United States Environmental Protection Agency (1995a). Method EPA 505: Analysis of organohalide pesticides and commercial polychlorinated biphenyl (PCB) products in water by microextraction and gas chromatography. Available in: https://www.o2si.com/docs/epa-method-505.pdf. Access in January 2019.
- 12.USEPA - United States Environmental Protection Agency (1995b). Method EPA 525.2: Determination of organic compounds in drinking water by liquid-solid extraction and capillary column Gas Chromatography/Mass Spectrometry. Available in: https://www.epa.gov/sites/production/files/2015-10/documents/method_525-2_rev-2_1995.pdf. Access in January 2019.
- 13.USEPA - United States Environmental Protection Agency (1995c). Method 551.1: Determination of chlorination disinfection byproducts, chlorinated solvents, and halogenated pesticides/herbicides in drinking water by liquid-liquid extraction and gas chromatography with electron-capture detection. Available in: https://www.epa.gov/sites/production/files/2015-06/documents/epa-551.1.pdf. Access in January 2019.
- 14.USEPA - United States Environmental Protection Agency (2007). Method EPA 8141B: Organophosphorus compounds by gas chromatography. Available in: https://www.epa.gov/sites/production/files/2015-12/documents/8141b.pdf. Access in January 2019.
- 15.Kin CM, Huat TG (2009). Comparison of HS-SDME with SPME and SPE for the determination of eight Organochlorine and Organophosphorus pesticide residues in food matrices. J Chromatogr Sci, 47:694–699. https://academic.oup.com/chromsci/article-abstract/47/8/694/285339 [DOI] [PubMed]
- 16.Dalvie MA, Sinanovic E, London L, Cairncross E, Solomon A, Adam H. Cost analysis of ELISA, solid-phase extraction, and solid-phase microextraction for the monitoring of pesticides in water. Environ Res. 2005;98:143–150. doi: 10.1016/j.envres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Simon D, Helliwell S, Robards K. Analytical chemistry of chlorpyrifos and diuron in aquatic ecosystems. Anal Chim Acta. 1998;360(1–3):1–16. doi: 10.1016/S0003-2670(97)00680-6. [DOI] [Google Scholar]
- 18.Darwiche W, Gay-Quéheillard J, Delanaud S, Sabbouri HEKE, Khachfe H, Joumaa W, et al. Impact of chronic exposure to the pesticide chlorpyrifos on respiratory parameters and sleep apnea in juvenile and adult rats. PLoS One. 2018. 10.1371/journal.pone.0191237. [DOI] [PMC free article] [PubMed]
- 19.Bortoluzzi EC, Rheinheimer DS, Gonçalves CS, Pelegrini JBR, Zanella R, Copetti ACC. Contaminação de águas superficiais por agrotóxicos em função do uso do solo numa microbacia hidrográfica de Agudo, RS. Rev. bras. eng. agríc. Campina Grande. 2006;10(4):881–887. doi: 10.1590/S1415-43662006000400015. [DOI] [Google Scholar]
- 20.Marchesan E, Zanella R, de Avila LA, Camargo ER, Machado SLO, Macedo VRM. Rice herbicide monitoring in two brazilian rivers during the growing season. Sci Agric. 2007;64(2):131–137. doi: 10.1590/S0103-90162007000200005. [DOI] [Google Scholar]
- 21.Han Y, Mo R, Yuan X, Zhong D, Tang F, Caifen Y, Yihua L. Pesticide residues in nut-planted soils of China and their relationship between nut/soil. Chemosphere. 2017;180:42–47. doi: 10.1016/j.chemosphere.2017.03.138. [DOI] [PubMed] [Google Scholar]
- 22.Phung DT, Connell D, Miller G, Hodge M, Patel R, Cheng R, Abeyewardene M, Chu C. Biological monitoring of chlorpyrifos exposure to rice farmers in Vietnam. Chemosphere. 2012;87(4):294–300. doi: 10.1016/j.chemosphere.2011.11.075. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Singhasemanon N, Goh KS. A statistical assessment of pesticide pollution in surface waters using environmental monitoring data: Chlorpyrifos in Central Valley, California. Sci Total Environ. 2016;571:332–341. doi: 10.1016/j.scitotenv.2016.07.159. [DOI] [PubMed] [Google Scholar]
- 24.Miclean M, Şenilă L, Cadar O, Roman M, Levei E, Tănăselia C, Török A, Majdik C. Determination of chlorpyrifos in surface water using SPE-DI-SPME/GC-ECD. Stud U Babes-Bol Che. 2014;59(3):43–48. [Google Scholar]
- 25.National Pesticide Information Center. Accessed in August 2018, Available at: http://npic.orst.edu/
- 26.ANVISA (Agência Nacional de Vigilância Sanitária) - Resolução n°899 de 29 de Maio de 2003 (2003). Guia para validação de métodos analíticos e bioanalíticos métodos analíticos. Available in: http://portal.anvisa.gov.br/documents/10181/2718376/RE_899_2003_COMP.pdf/ff6fdc6b-3ad1-4d0f-9af2-3625422e6f4b. Access in January 2019
- 27.INMETRO - Instituto Nacional de Metrologia, Normalização e Qualidade Industrial (2016) - Orientações sobre validação de métodos e ensaios químicos, DOQ-CGCRE-008. Available in: http://www.inmetro.gov.br/Sidoq/Arquivos/CGCRE/DOQ/DOQ-CGCRE-8_05.pdf. Access in January 2019
- 28.Pavan MA, Bloch MF, Zempulski HC, Miyazawa M, Zocoler DC. Manual de análises químicas de solo e controle de qualidade. Londrina: IAPAR; 1992. [Google Scholar]
- 29.AOAC - Association of Official Analytical Chemist. (2016). Official methods of analysis. Maryland: Association of Official Agricultural Chemists.
- 30.Welz B, Sperling M. Atomic absorption spectrometry. Weinheim: Wiley-VCH; 1999. [Google Scholar]
- 31.EMBRAPA - Empresa Brasileira de Pesquisa Agropecuária . Manual de Métodos de Análise de Solo. Rio de Janeiro: EMBRAPA-CNPS; 1997. [Google Scholar]
- 32.Langmuir I. The constitution and fundamental properties of solids and liquids. J Am Chem Soc. 1916;38(11):2221–2295. doi: 10.1021/ja02268a002. [DOI] [Google Scholar]
- 33.Freundlich HMF. Over the adsorption in solution. J Phys Chem. 1906;657:385–471. [Google Scholar]
- 34.Dubinin MM, Radushkevich LV. The equation of the characteristic curve of the activated charcoal, proceedings of the National Academy of Sciences. USSR physical chemistry section. 1947;55:331–337. [Google Scholar]
- 35.Sips R. Combined form of Langmuir and Freundlich equations. J Chem Phys. 1948;16:490–495. doi: 10.1063/1.1746922. [DOI] [Google Scholar]
- 36.Tempkin MI, Pyzhev V. Kinetics of ammonia synthesis on promoted iron catalyst. Acta Phys Chim USSR. 1940;12:327–356. [Google Scholar]
- 37.Schmidt TD, Salton JC, Júnior RPS. Sorption and desorption of thiamethoxam and atrazine in soil under different management systems. Rev bras eng agríc. 2015;19(6):613–618. doi: 10.1590/1807-1929/agriambi.v19n6p613-618. [DOI] [Google Scholar]
- 38.FAO – Food and Agriculture Organization of the United Nations. Guidelines for soil description. Fourth edition. Rome, 2006. Available at: http://www.fao.org/3/a-a0541e.pdf
- 39.Gebremariam SY, Buetel MW, Yonge DR, Flury M, Harsh JB. Adsorption and desorption of Chlorpyrifos to soils and sediments. Rev Environ Contam Toxicol. 2012;215:123–175. doi: 10.1007/978-1-4614-1463-6_3. [DOI] [PubMed] [Google Scholar]
- 40.Gonçalves AC Jr, Schwantes D, Campagnolo MA, Dragunski DC, Tarley CRT, Silva AKS. Removal of toxic metals using endocarp of açaí berry as biosorbent. Wat Sci Tec. 2018;77(60):1547–57. 10.2166/wst.2018.032. [DOI] [PubMed]
- 41.Balarak D, Mostafapour FK, Azarpira H, Joghataei A. Langmuir, Freundlich, Temkin and Dubinin–radushkevich isotherms studies of equilibrium sorption of Ampicilin unto Montmorillonite nanoparticles. Journal of Pharmaceutical Research International. 2017;20(2):1–9. doi: 10.9734/JPRI/2017/38056. [DOI] [Google Scholar]
- 42.Sahin R, Tapadia K. Comparison of linear and non-linear models for the adsorption of fluoride onto geo-material: limonite. Water Sci Technol. 2015;72(12):2262–2269. doi: 10.2166/wst.2015.449. [DOI] [PubMed] [Google Scholar]
- 43.Garcia AV, Viciana MS, Pradas EG, Sánchez MV. Adsorption of chlorpyrifos on Almeria soils. Sci Total Environ. 1992;123–124:541–549. doi: 10.1016/0048-9697(92)90176-S. [DOI] [Google Scholar]
- 44.Parolo ME, Savini MC, Loewy RM. Characterization of soil organic matter by FT-IR spectroscopy and its relationship with chlorpyrifos sorption. J Environ Manag. 2017;196:316–322. doi: 10.1016/j.jenvman.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Fink JR, Inda AV, Tiecher T, Barrón V. Iron oxides and organic matter on soil phosphorus availability. Ciênc agrotec. 2016;40(4):369–379. doi: 10.1590/1413-70542016404023016. [DOI] [Google Scholar]
- 46.Dannenberg A, Pehkonen SO. Investigation of the heterogeneously catalyzed hydrolysis of Organophosphorus pesticides. J. Agric. Food Chem. 1998;46:325–334. doi: 10.1021/jf970368o. [DOI] [PubMed] [Google Scholar]
- 47.Yaghi N, Hartikainen H. Enhancement of phosphorus sorption onto light expanded clay aggregates by means of aluminum and iron oxide coatings. Chemosphere. 2013;93(9):1879–1886. doi: 10.1016/j.chemosphere.2013.06.059. [DOI] [PubMed] [Google Scholar]
- 48.Vagi MC, Petsas AS, Kostopoulou MN, Lekkas TD. Adsorption and desorption processes of the organophosphorus pesticides, dimethoate and fenthion, onto three Greek agricultural soils. Int J Environ Anal Chem. 2010;90(3):369–389. doi: 10.1016/10.1080/03067310903194980. [DOI] [Google Scholar]
- 49.Alfonso L-F, Germán GV, Carmen PCM, Hossein G. Adsorption of organophosphorus pesticides in tropical soils: the case of karst landscape of northwestern Yucatan. Chemosphere. 2017;166:292–299. doi: 10.1016/j.chemosphere.2016.09.109. [DOI] [PubMed] [Google Scholar]
- 50.Soares DF, Faria AM, Rosa AH. Risk analysis of groundwater contamination by pesticide residue in campo novo do Paracis (MT) Brazil Eng Sanit Ambient. 2017;22(2):277–284. doi: 10.1590/S1413-41522016139118. [DOI] [Google Scholar]
- 51.Spieszalski WW, Niemczyk HD, Shetlar DJ. Sorption of chlorpyrifos and fonofos on four soils and turfgrass thatch using membrane filters. J Environ Sci Health, Part B. 1994;29(6):1117–1136. doi: 10.1080/03601239409372919. [DOI] [Google Scholar]
- 52.Wu J, Laird DA. Interactions of chlorpyrifos with colloidal materials in aqueous systems. J Environ Qual. 2004;33(5):1765–1770. doi: 10.2134/jeq2004.1765. [DOI] [PubMed] [Google Scholar]