Abstract
In 2010, the 10-valent (PCV10) and 13-valent (PCV13) pneumococcal conjugate vaccines were introduced in Brazil to immunize children, resulting in serotype replacement. We analyzed 253 carriage isolates recovered from children aged <6 years in Brazil, including 124 and 129 isolates from the pre-PCV10/13 (December 2009–July 2010) and post-PCV10/13 (September–December 2014) periods, respectively, to investigate the prevalence of PspA families and pilus islets, potential vaccine candidates. Serotypes and resistance profiles were previously characterized. We used PCR to type PspA families (Fam1-3) and pilus islets (PI-1 and PI-2). We identified the PspA family of 130 (51.4%) isolates. PspA families 1, 2, and 3 were identified in 12.2%, 38.7%, and 0.4% of the isolates, respectively. Eighteen (58.1%) Fam1 isolates were serogroup 6. Nine (81.8%) of 11 serotype 14 isolates were Fam2. Fam1 isolates resistant to penicillin (50%), erythromycin (43.7%), clindamycin (31.2%), and chloramphenicol (6.2%) were only found after PCV10/13 introduction. Resistance among Fam2 isolates was higher in the post-PCV10/13 period to erythromycin (1.8% vs. 18.6%), clindamycin (0 vs. 13.9%), and tetracycline (10.9% vs. 16.3%). PI-I was detected in 42 (16.6%) isolates. Fourteen (56%) of 25 serotype 15B/C and nine (81.8%) of 11 serotype 14 isolates had PI-1 (p < 0.01). Eight (3.2%) isolates had PI-2, and six (75%) were serogroup 19. Five (2%) serogroup 19 isolates had both PI-1 and PI-2. We found associations between serogroups/serotypes, PspA families, and pilus islets, but distribution of PspA families and pilus islets was similar in both periods. After universal vaccination, we observed higher antimicrobial resistance frequencies, regardless PspA or pilus types.
Keywords: Streptococcus pneumoniae, PspA, Pilus, Vaccine
Introduction
Streptococcus pneumoniae causes severe diseases, such as community-acquired pneumonia (CAP), bacteremia, and meningitis [1]. In 2015, about 393,000 children < 5 years old died of pneumococcal pneumonia in 195 countries [2]. In Brazil, CAP represented the third leading cause of mortality between 1990 and 2015 among all age groups [3]. For pneumococcal meningitis, the average mortality rate was around 30% between 2010 and 2018 [4].
The capsule is the target of all pneumococcal vaccines currently available, including the 23-valent polysaccharide vaccine (PPV23), as well as the 7-valent (PCV7), 10-valent (PCV10), and 13-valent (PCV13) conjugate vaccines [5]. However, more than 90 pneumococcal capsular serotypes have already been described [6]. In addition, the PPV23 is not recommended for children under 2 years old, due to the poor immunogenicity of the polysaccharide antigens [7].
The widespread use of pneumococcal conjugate vaccines (PCV) among children resulted in the serotype replacement phenomenon in both colonization and diseases [8]. Capsule-unrelated vaccine targets are, therefore, urgently required. The pneumococcal surface protein A (PspA) and pilus antigens have been considered potential vaccine candidates alone or in combination with other antigens [9, 10]. To date, two types of pilus have been described in pneumococcus, codified by two different pilus islets (PI): PI-1 and PI-2 [11]. In turn, PspA is almost universally distributed among pneumococci and is divided into three families and six clades [12]; it presents high immunogenicity and has been shown to protect against both colonization and disease [13, 14].
In the early 2000s, the PCV7 was made available in Brazil free of charge via National Immunization Program (NIP) only for children at high risk for invasive pneumococcal diseases (IPD). At the same time, private immunization clinics started marketing PCV7 for childhood vaccination. Consequently, only a small portion of the population was immunized with PCV7 [15, 16]. In 2010, the PCV10 was introduced into the Brazilian NIP for all children aged less than 5 years, and the PCV13 replaced the PCV7 in private immunization clinics. Here, we investigate the prevalence of PspA families and pilus islets among pneumococcal carriage isolates recovered from children before and after universal use of PCV, mostly PCV10, in a major metropolitan area in Southeastern Brazil.
Material and methods
Bacterial isolates
We analyzed 253 pneumococcal carriage isolates, including 124 isolates obtained in the pre-PCV10/13 period (December 2009 to July 2010) and 129 isolates in the post-PCV10/13 period (September to December 2014). The isolates were recovered from nasopharynx of children under 6 years old in Niterói City, a major metropolitan area of Rio de Janeiro, Brazil. Isolates from the pre-PCV10/13 period were obtained from children who attended two public childcare centers (n = 61) and the emergency room of one public hospital (n = 63). The isolates recovered in the post-PCV10/13 period were obtained from children attending one public clinic (n = 72) and two private pediatric clinics (n = 57). All the isolates from the pre-PCV10/13 period and 76 (59.9%) isolates from the post-PCV10/13 period were recovered from children presenting with symptoms, mostly associated with upper respiratory infections, at the moment of the specimen collection. In the area investigated, PCV10 and PCV13 became available in October 2010. Capsular types and resistance profiles to nine antimicrobial agents were previously determined [15, 16].
DNA preparation
DNA of all isolates were obtained by thermal lysis as previously described [17].
PspA typing
The identification of PspA families was performed by PCR using primers previously described. We used the primer sets LSM12/SKH63, LSM12/SKH52 [18, 19], and SKH41/SKH42 [20] to identify families 1 (Fam1), 2 (Fam2), and 3 (Fam3), respectively. Primers LSM12/SKH2 were used to detect all PspA families [12, 18].
Detection and typing of pilus islets
We used the primers pQE30C5/pQE30C3 [21] and sipA_up_F/sipA_do_R [22] to identify PI-1 and PI-2, respectively.
Statistical analysis
We used the Fisher exact test to investigate association of PspA families and types of pilus islets with serotypes, resistance profile, and isolation period. We used tools available in https://www.graphpad.com/quickcalcs/contingency1/. Statistical significance was considered when p value was < 0.05.
Ethical consideration
This study was approved by the Ethics Committee of the Universidade Federal Fluminense (CAAE 26823614.2.0000.5243 and CAAE 26823614.2.0000.5243).
Results
PspA typing
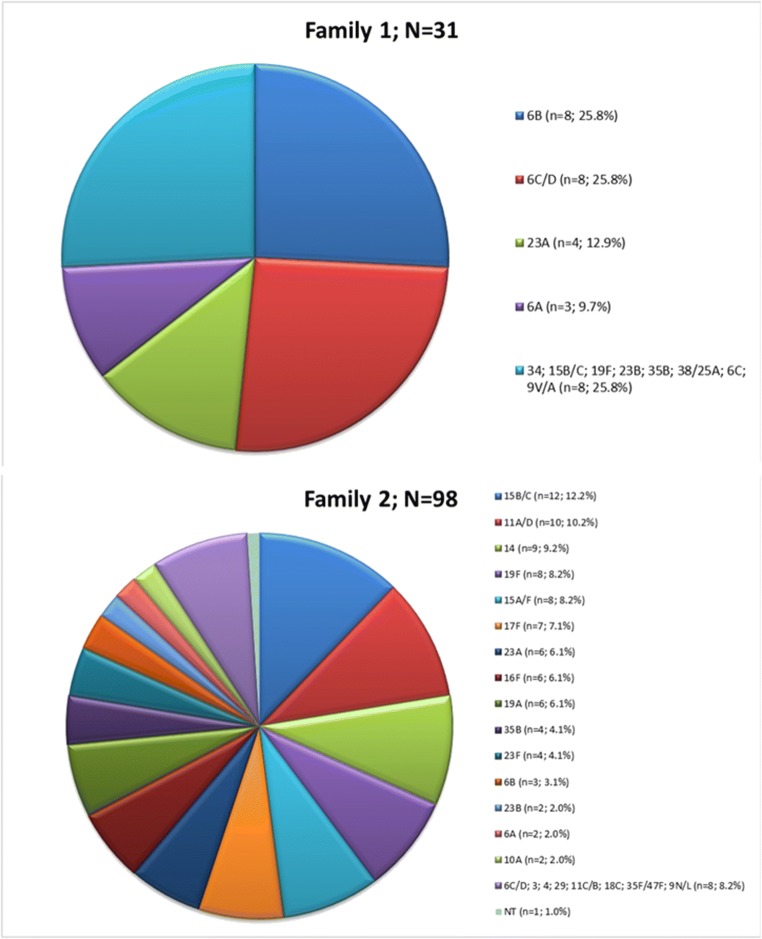
All the 253 pneumococcal isolates had the PspA gene, and we were able to determine the PspA family of 130 (51.3%) isolates. Ninety-eight (38.7%), 31(12.2%), and one (0.4%) isolates had the PspA families 2, 1, and 3, respectively. Of note, nine (81.8%) of 11 serotype 14 isolates, seven (70%) of ten serotype 17F isolates, and six (66.7%) of nine serotype 16F isolates were Fam2. Figure 1 shows the correlation between capsular types and PspA families.
Fig. 1.
Distribution of PspA families among carriage isolates according to the capsular types. The single PspA Fam3 isolate was non-typeable. NT non-typeable
Fifteen (48.4%) and 16 (51.6%) of 31 PspA Fam1 isolates were recovered from children in the pre- and post-PCV10/13 periods, respectively, corresponding to 12.1% and 12.4% of the total isolates from each period. For PspA Fam2, we detected 55 (56.1%) isolates in the pre-PCV10/13 period and 43 (43.9%) in the post-PCV10/13 period, corresponding to 44.3% and 33.3% of the total isolates of each period, respectively. The single PspA Fam3 isolate is non-encapsulated and was obtained in the post-PCV10/13 period.
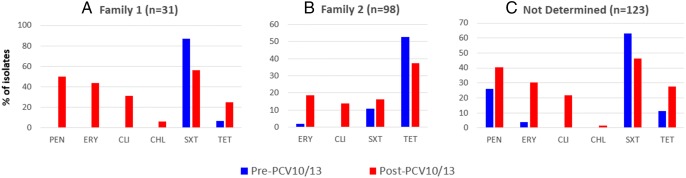
In general, non-susceptibility frequencies to the majority of the antimicrobial agents tested were higher among isolates recovered after PCV10/13 introduction, regardless the PspA family (Fig. 2). The single PspA Fam3 isolate was non-susceptible to penicillin and intermediate to tetracycline. None of the isolates was resistant to levofloxacin, rifampicin, and vancomycin.
Fig. 2.
Frequencies of isolates non-susceptible (resistant + intermediate) to antimicrobial agents according to the PspA family and the isolation period. CHL chloramphenicol, CLI clindamycin, ERY erythromycin, PEN penicillin, TET tetracycline, SXT sulfamethoxazole/trimethoprim
Prevalence and types of pilus islets
We detected pilus islets in 45 (17.8%) isolates. The PI-1 was found in 42 (16.6%) isolates. Of them, 15 (35.7%) isolates were classified as serotype 15B/C; nine (21.4%) as serotype 14; six (14.3%) as serotype 19F; four (9.5%) as serotype 19A; three (7.1%) as serotype 35B; and only one (2.4% each) as serotypes 4, 15A/F, 16F, 23A or 6C/D. Fourteen (56%) of 25 serotype 15B/C isolates (p < 0.01) and nine (81.8%) of 11 serotype 14 isolates had the PI-1 (p < 0.01).
Seventeen (40.5%) of 42 PI-1 isolates were non-susceptible to penicillin, eight (19%) were resistant to erythromycin, and five (11.9% each) were resistant to clindamycin and tetracycline. Furthermore, 26 (61.9%) and four (9.5%) isolates were resistant and intermediate to sulfamethoxazole/trimethoprim, respectively. Eight (19%) isolates were susceptible to all antimicrobial agents tested. All nine serotype 14 isolates with PI-1 had the PspA Fam2 and eight (88.9%) of them were non-susceptible to penicillin and resistant to sulfamethoxazole/trimethoprim.
Eight (3.2%) isolates had the PI-2. Six (75%) of them belonged to serogroup 19, including three serotype 19A and three serotype 19F isolates. The remaining two (25%) isolates were serotype 11A/D. Five (2%) isolates, including three serotype 19A and two serotype 19F, harbored both the PI-1 and PI-2.
Non-susceptibility to penicillin and resistance to erythromycin, clindamycin, and tetracycline were found in three (37.5%) of eight PI-2 isolates, all identified as serotype 19A. In addition, six (75%) isolates, all serogroup 19, were non-susceptible to sulfamethoxazole/trimethoprim. The two (25%) serotype 11A/D isolates were susceptible to all antimicrobial agents tested. Table 1 shows the correlation between PspA families, pilus islets, and the isolation period.
Table 1.
Frequency of PspA families and pilus islet in nasopharyngeal pneumococcal isolates obtained in the pre- and post-PCV10/13 periods.
| PspA family | Pre-PCV10/13 (n = 124) | Post-PCV10/13 (n = 129*) | ||||
|---|---|---|---|---|---|---|
| Fam1 (n = 15) | Fam2 (n = 55) | ND (n = 54) | Fam1 (n = 16) | Fam2 (n = 43) | ND (n = 69) | |
| PI-1 | 1 (6.7%) | 17 (30.9%) | 3 (5.5%) | 0 | 6 (13.9%) | 10 (14.5%) |
| PI-2 | 0 | 0 | 1 (1.9%) | 0 | 2 (4.7%) | 0 |
| PI-1 + 2 | 1 (6.7%) | 0 | 1 (1.9%) | 0 | 1 (2.3%) | 2 (2.9%) |
aThe single PspA Fam3 isolate did not present any pilus islet
ND not determined
Discussion
After universal childhood vaccination in Brazil with pneumococcal conjugate vaccines, mostly PCV10, Fam2 remained the prevalent PspA family among pneumococci recovered from children’s nasopharynx, representing almost 40% of the isolates. Pilus islets were found in about 20% of the isolates, with the predominance of PI-1. PI-2, although less common, was strongly associated with serogroup 19, especially with the MDR serotype 19A, that emerged in the post-PCV10/13 period.
PspA Fam2 has been usually reported as the most common type of PspA in many countries, such as China (120/171; 70.1%) [10], Canada (91/148; 61.5%), France (159/215; 73.9%), Spain (90/150; 60.0%), Sweden (39/67; 58.2%), the USA (539/930; 57.9%) [20], and even in Brazil (81/183; 44.3%) [23]. However, studies in Australia (54/10; 54.0%) and the UK (120/237; 50.6%) found Fam1 as the prevalent one [20]. Family 3 is usually rare [10, 20, 23, 24], and, in the present study, it was associated with a non-encapsulated isolate.
Among PspA Fam1 isolates, the most common serotypes were 6B and 6C/D. For PspA Fam2 isolates, we observed a greater diversity of serotypes, but serotype 15B/C prevailed. Of these serotypes, only 6B is covered by the PCV10, which is available free of charge via the Brazilian Unified Health System (SUS, Sistema Único de Saúde) for the pediatric population. Serotype 6C, in turn, emerged in colonization and disease in children and adults after the universal childhood use of PCV10 in Brazil [16, 25, 26]. PCV13 appears to offer cross-protection against serotype 6C due to its similarity to serotype 6A [27]. However, serotype 15B/C is only present in the VPP23 and has been associated with colonization and non-invasive diseases in the pre- and post-PCV10/13 era [15, 16, 28].
We observed strong associations of serotypes 14, 17F, and 16F with PspA Fam2. Association of serotype 14 (78%) with PspA Fam2 has been previously observed [29]. On the other hand, we found no evident association of serotypes with PspA Fam1. However, most (58%) Fam1 isolates belonged to serogroup 6.
There was no significant variation in the prevalence of PspA families according to the isolation period. This suggests pneumococcal conjugate vaccines had little or no influence on the distribution of PspA families. This finding can be largely explained by the fact that these vaccines are based on capsular polysaccharides, and, in general, we found no strong association of PspA families with specific serotypes. Additionally, antimicrobial resistance seems to be more associated with PspA Fam1 isolates, since 16% of Fam1 and 38% of Fam2 isolates were susceptible to all the antimicrobial agents tested. However, regardless the PspA family, higher non-susceptibility frequencies were observed among isolates obtained in the post-PCV10/13 period.
Epidemiological studies on the distribution of pilus islets in pneumococci are still uncommon. In a study conducted with invasive isolates recovered from adults in Portugal before the introduction of PCV13, PI-1 and PI-2 made up about 10% and 20% of the isolates, respectively [30]. Here, PI-1 was more frequent. These data may indicate a more important role of PI-2 in the invasive process of the pathogen.
Indeed, in the present study, the association between PI-2 and serogroup 19 isolates, including serotypes 19A and 19F, was evident. Of note, 19F was an important serotype associated with IPD in the pre-PCV10/13 period. After universal vaccination in Brazil, mostly with PCV10, serotype 19F became rare, but serotype 19A emerged as a major cause of IPD among all age groups [26, 31]. Similar findings had been observed in high-income countries after the introduction of PCV7 and before its replacement by PCV13 [32, 33]. This fact becomes even more worrying due to the strong association of serotype 19A with multidrug resistance [26, 31–33]. Despite the low prevalence of pilus islets in the population investigated, the inclusion of the PI-2 in a vaccine formulation would theoretically prevent infections caused by the main emerging serotype associated with multidrug resistance. Additionally, the high frequency of antimicrobial-resistant isolates carrying the PI-2 can be explained by its association with serogroup 19, especially serotype 19A.
We also observed a strong association of serotypes 15B/C and 14 with PI-1. We only detected serotype 14 isolates in the pre-PCV10/13 period, but we did not observe significant variation in the prevalence of pili regarding the isolation period. A previous study found association of PI-2 with serotypes 1, 7F, 11A, 19A, and 19F in 1999, with a prevalence of 3.6%, rising to 21% after the PCV7 introduction in the USA, due to the expansion of serotypes 19A and 7F [22]. Another study in the same country observed a significant reduction from 42.8 to 21.3% in the occurrence of PI-1-related genes following PCV7 introduction [21].
Isolates carrying only PI-1 or PI-1 plus PI-2 showed similar frequencies of non-susceptibility to penicillin (approximately 40%) and sulfamethoxazole/trimethoprim (approximately 60%), while those with only PI-2 had a higher frequency of resistance to erythromycin, clindamycin, and tetracycline. Approximately 42% of the PI-1 isolates were non-susceptible to penicillin and about half of them was resistant to erythromycin and multidrug resistant. Interestingly, correlation between the presence of PI-1 and antimicrobial resistance in clinical isolates from acute otitis media had already been described in Israel [34]. It is noteworthy that serotype 14 isolates, a major cause of pneumococcal diseases in the pre-PCV10/13 period, was associated with PspA Fam2, presence of PI-1, and high frequency of non-susceptibility to penicillin and sulfamethoxazole/trimethoprim.
The major limitation of the present study is the analysis of isolates exclusively associated with colonization. Additionally, we did not determine the clades of PspA families. Comparisons with invasive isolates and the definition of PspA clades would result in a more robust analysis.
In conclusion, we found associations between serogroups/serotypes, PspA families, and pilus islets. We observed higher resistance frequencies to antimicrobial agents, regardless PspA or pilus types, after universal vaccination with pneumococcal conjugate vaccines, mostly PCV10. Our data may help select targets for new anti-pneumococcal vaccine formulations.
Acknowledgments
The authors thank all healthcare institutions and professionals that contributed to this study, especially Dr. Carlos Campbell and Dr. Paulo Monnerat.
Financial information
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) via Science without Borders program (grant number 234873/2014-0); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)–Finance Code 001; Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ)–grant number E-26/203.164/2017; and Pró-Reitoria de Pesquisa, Pós-Graduação e Inovação da Universidade Federal Fluminense (PROPPi/UFF).
Compliance with ethical standards
This study was approved by the Ethics Committee of the Universidade Federal Fluminense (CAAE 26823614.2.0000.5243 and CAAE 26823614.2.0000.5243).
Conflict of interests
The authors declare that they have no conflict of interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lynch J, Zhanel G. Streptococcus pneumoniae: epidemiology, risk factors, and strategies for prevention. Semin Respir Crit Care Med. 2009;30(2):189–209. doi: 10.1055/s-0029-1202938. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Naghavi M, Allen C, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corrêa RA, José BPS, Malta DC, Passos VMA, França EB, Teixeira RA, Camargos PAM. Carga de doença por infecções do trato respiratório inferior no Brasil, 1990 a 2015: estimativas do estudo Global Burden of Disease 2015. Rev Bras Epidemiol. 2017;20(suppl 1):171–181. doi: 10.1590/1980-5497201700050014. [DOI] [PubMed] [Google Scholar]
- 4.Ministério da Saúde (2019) Portal da Saúde, Tabela de casos confirmados, óbitos, incidência e letalidade por tipo de meningite de 2010 a 2018. Publishing Brazilian Ministry of Health. http://portalarquivos2.saude.gov.br/images/pdf/2019/abril/25/tabela-dados-2010-2018-site.pdf. Accessed 29 Apr 2019
- 5.Feldman C, Anderson R. Review: current and new generation pneumococcal vaccines. J Inf. 2014;69(4):309–325. doi: 10.1016/j.jinf.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Konradsen HB, Nahm MH. Pneumococcal capsules and their types: past, present, and future. Clin Microbiol Rev. 2015;28(3):871–899. doi: 10.1128/CMR.00024-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Westerink MA, Schroeder HW, Jr, Nahm MH. Immune responses to pneumococcal vaccines in children and adults: rationale for age-specific vaccination. Aging Dis. 2012;3(1):51–67. [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378(9807):1962–1973. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogunniyi AD, Grabowicz M, Briles DE, Cook J, Paton JC. Development of a vaccine against invasive pneumococcal disease based on combinations of virulence proteins of Streptococcus pneumoniae. Infect Immun. 2006;75(1):350–357. doi: 10.1128/IAI.01103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian J, Yao K, Xue L, Xie G, Zheng Y, Wang C, Shang Y, Wang H, Wan L, Liu L, Li C, Ji W, Wang Y, Xu P, Yu S, Tang Y-W, Yang Y. Diversity of pneumococcal surface protein A (PspA) and relation to sequence typing in Streptococcus pneumoniae causing invasive disease in Chinese children. Eur J Clin Microbiol Infect Dis. 2012;31(3):217–223. doi: 10.1007/s10096-011-1296-9. [DOI] [PubMed] [Google Scholar]
- 11.Bagnoli F, Moschioni M, Donati C, Dimitrovska V, Ferlenghi I, Facciotti C, Muzzi A, Giusti F, Emolo C, Sinisi A, Hilleringmann M, Pansegrau W, Censini S, Rappuoli R, Covacci A, Masignani V, Barocchi MA. A second pilus type in Streptococcus pneumoniae is prevalent in emerging serotypes and mediates adhesion to host cells. J Bacteriol. 2008;190(15):5480–5492. doi: 10.1128/JB.00384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollingshead SK, Becker R, Briles DE. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect Immun. 2000;68(10):5889–5900. doi: 10.1128/iai.68.10.5889-5900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniels CC, Coan P, King J, Hale J, Benton KA, Briles DE, Hollingshead SK. The proline-rich region of pneumococcal surface proteins A and C contains surface-accessible epitopes common to all pneumococci and elicits antibody-mediated protection against sepsis. Infect Immun. 2010;78(5):2163–2172. doi: 10.1128/IAI.01199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schachern PA, Tsuprun V, Goetz S, Cureoglu S, Juhn SK, Briles DE, Paparella MM, Ferrieri P. Viability and virulence of pneumolysin, pneumococcal surface protein A, and pneumolysin/pneumococcal surface protein A mutants in the ear. JAMA Otolaryngol Head Neck Surg. 2013;139(9):937–943. doi: 10.1001/jamaoto.2013.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neves FPG, Pinto TC, Corrêa MA, Barreto RA, Moreira LSG, Rodrigues HG, Cardoso CA, Barros RR, Teixeira LM. Nasopharyngeal carriage, serotype distribution and antimicrobial resistance of Streptococcus pneumoniae among children from Brazil before the introduction of the 10-valent conjugate vaccine. BMC Infect Dis. 2013;13(1):318–324. doi: 10.1186/1471-2334-13-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neves FPG, Cardoso NT, Snyder RE, Marlow MA, Cardoso CAA, Teixeira LM, Riley LW. Pneumococcal carriage among children after four years of routine 10-valent pneumococcal conjugate vaccine use in Brazil: the emergence of multidrug resistant serotype 6C. Vaccine. 2017;35(21):2794–2800. doi: 10.1016/j.vaccine.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues HG, Pinto TCA, Barros RR, Teixeira LM, Neves FPG. Pneumococcal nasopharyngeal carriage among children in Brazil prior to the introduction of the 10-valent conjugate vaccine: a culture- and PCR-based survey. Epidemiol Infect. 2017;145(8):1720–1726. doi: 10.1017/S0950268817000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swiatlo E, Brooks-Walter A, Briles DE, McDaniel LS. Oligonucleotides identify conserved and variable regions of pspA and pspA-like sequences of Streptococcus pneumoniae. Gene. 1997;188(2):279–284. doi: 10.1016/s0378-1119(96)00823-2. [DOI] [PubMed] [Google Scholar]
- 19.Vela Coral MC, Fonseca N, Castañeda E, Di Fabio JL, Hollingshead SK, Briles DE. Pneumococcal surface protein A of invasive Streptococcus pneumoniae isolates from Colombian children. Emerg Infect Dis. 2001;7(5):832–836. doi: 10.3201/eid0705.017510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollingshead SK, Baril L, Ferro S, King J, Coan P, Briles DE, Pneumococcal Proteins Epi Study Group Pneumococcal surface protein A (PspA) family distribution among clinical isolates from adults over 50 years of age collected in seven countries. J Med Microbiol. 2006;55(2):215–221. doi: 10.1099/jmm.0.46268-0. [DOI] [PubMed] [Google Scholar]
- 21.Basset A, Trzcinski K, Hermos C, O’Brien KL, Reid R, Santosham M, McAdam AJ, Lipsitch M, Malley R. Association of the pneumococcal pilus with certain capsular serotypes but not with increased virulence. J Clin Microbiol. 2007;45(6):1684–1689. doi: 10.1128/JCM.00265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zähner D, Gudlavalleti A, Stephens DS. Increase in pilus islet 2-encoded pili among Streptococcus pneumoniae isolates, Atlanta, Georgia, USA. Emerg Infect Dis. 2010;16(6):955–962. doi: 10.3201/eid1606.091820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pimenta FC, Dias FR, Brandileone MCC, Miyaji EN, Leite LCC, Andrade ALSS. Genetic diversity of PspA types among nasopharyngeal isolates collected during an ongoing surveillance study of children in Brazil. J Clin Microbiol. 2006;44(8):2838–2843. doi: 10.1128/JCM.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan F, Khan MA, Ahmed N, Khan MI, Bashir H, Tahir S, Zafar AU. Molecular characterization of pneumococcal surface protein A (PspA), serotype distribution and antibiotic susceptibility of Streptococcus pneumoniae strains isolated from Pakistan. Infect Dis Ther. 2018;7(2):277–289. doi: 10.1007/s40121-018-0195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandileone M-CC, Zanella RC, Almeida SCG, Brandao AP, Ribeiro AF, Carvalhanas T-RMP, Sato H, Andrade A-L, Verani JR. Effect of 10-valent pneumococcal conjugate vaccine on nasopharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae among children in São Paulo, Brazil. Vaccine. 2016;34(46):5604–5611. doi: 10.1016/j.vaccine.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Organização Panamericana de Saúde (OPAS) (2016) Informe regional de SIREVA II, 2014: datos por país y por grupos de edad sobre las características de los aislamientos de Streptococcus pneumoniae, Haemophilus influenzae y Neisseria meningitidis, en procesos invasivos bacterianos. Publishing OPAS. http://iris.paho.org/xmlui/handle/123456789/33875. Accessed 23 Jul 2019.
- 27.Cooper D, Yu X, Sidhu M, Nahm MH, Fernsten P, Jansen KU. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to Streptococcus pneumoniae serotypes 6C and 7A. Vaccine. 2011;29(41):7207–7211. doi: 10.1016/j.vaccine.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawaguchiya M, Urushibara N, Aung MS, Morimoto S, Ito M, Kudo K, Sumi A, Kobayashi N. Emerging non-PCV13 serotypes of noninvasive Streptococcus pneumoniae with macrolide resistance genes in northern Japan. New Microbe New Infect. 2015;9:66–72. doi: 10.1016/j.nmni.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brandileone MCC, Andrade ALSS, Teles EM, Zanella RC, Yara TI, Di Fabio JL. Typing of pneumococcal surface protein A (PspA) in Streptococcus pneumoniae isolated during epidemiological surveillance in Brazil: towards novel pneumococcal protein vaccines. Vaccine. 2004;22(29-30):3890–3896. doi: 10.1016/j.vaccine.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Horácio NA, Silva-Costa C, Diamantino-Miranda J, Lopes JP, Ramirez M, Melo-Cristino J, Portuguese Group for the Study of Streptococcal Infections Population structure of Streptococcus pneumoniae causing invasive disease in adults in Portugal before PCV13 availability for adults: 2008-2011. PLoS One. 2016;11(5):e0153602. doi: 10.1371/journal.pone.0153602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassiolato AP, Almeida SCG, Andrade AL, Minamisava R, Brandileone MCC. Expansion of the clonal multidrug-resistant complex 320 among invasive Streptococcus pneumoniae serotype 19A after the introduction of a ten-valent pneumococcal conjugate vaccine in Brazil. PLoS One. 2018;13(11):e0208211. doi: 10.1371/journal.pone.0208211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cho EY, Kang HM, Lee J, Kang JH, Choi EH, Lee HJ. Changes in serotype distribution and antibiotic resistance of nasopharyngeal isolates of Streptococcus pneumoniae from children in Korea, after optional use of the 7-Valent conjugate vaccine. J Korean Med Sci. 2012;27(7):716–722. doi: 10.3346/jkms.2012.27.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis. 2010;14(3):e197–e209. doi: 10.1016/j.ijid.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Moschioni M, De Angelis G, Melchiorre S, Masignani V, Leibovitz E, Barocchi MA, Dagan R. Prevalence of pilus-encoding islets among acute otitis media Streptococcus pneumoniae isolates from Israel. Clin Microbiol Infect. 2010;16(9):1501–1504. doi: 10.1111/j.1469-0691.2009.03105.x. [DOI] [PubMed] [Google Scholar]