Abstract
Scedosporium spp. and Lomentospora prolificans are filamentous fungi that emerged as human pathogens; however, their mechanisms of virulence/pathogenesis are still largely unknown. In the present work, we have evaluated the interaction of S. apiospermum, S. minutisporum, S. aurantiacum, and L. prolificans with lung epithelial cells (A549 line). The results showed that conidia were able to interact with A549 cells, displaying association indexes of 73.20, 117.98, 188.01, and 241.63 regarding S. apiospermum, L. prolificans, S. minutisporum, and S. aurantiacum, respectively. Light microscopy images evidenced morphological changes in epithelial cells, including rounding and detachment, especially during the interaction with L. prolificans. Plasma membrane injuries were detected in A549 cells after 1 h of co-culturing with S. aurantiacum and S. minutisporum and after 4 h with S. apiospermum and L. prolificans, as judged by the passive incorporation of propidium iodide. After 24 h of fungi-epithelial cells interaction, only mycelia were observed covering the A549 monolayer. Interestingly, the mycelial trap induced severe damage in the A549 cells, culminating in epithelial cell death. Our results demonstrate some relevant events that occur during the contact between lung epithelial cells and Scedosporium/Lomentospora species, including conidial adhesion and hyphal growth with consequent irreversible injury on A549 cells, adding light to the infection process caused by these opportunistic and multidrug-resistant fungi.
Keywords: Scedosporium, Lomentospora, Cellular interaction, Lung epithelial cells, Adhesion, Injury
Introduction
In the last years, in parallel to increased number of immunocompromised individuals (e.g., human immunodeficiency virus (HIV) infection, neutropenia, and solid organ transplant recipients) as well as recurrent natural disasters caused by climatic instabilities (e.g., tsunami), some saprophytic filamentous fungi have emerged as etiologic agents of human infections, including species belonging to the Scedosporium and Lomentospora genera [1–3]. In immunocompromised patients, the respiratory tract is the main portal of entry for disseminated infections of Scedosporium/Lomentospora species. Similarly, in immunocompetent individuals, the invasive cases usually occur as a consequence of massive aspiration of polluted water containing conidia in near-drowning events or due to a traumatic inoculation somewhere near the brain, as in paranasal sinuses [2, 4]. Conidia enter via the respiratory tract and, if pulmonary alveolar macrophages and mucociliary system are unable to destroy them, the germination event takes place and culminates in tissue injuries, angioinvasion, and dissemination of fungal propagules to eventually any human anatomical site. In addition, unchecked hyphae proliferation by polymorphonuclear cells results in a hematogenous dissemination that can reach the central nervous system [4, 5]. In those cases, the infections are usually refractory to the conventional treatment options and have fatal outcomes [4].
The initial interaction between fungal propagules and host is a crucial step for the success of colonization as well as dissemination [6–8]. For filamentous fungi, some features are well known for the establishment of a successfully infectious process, such as cell wall composition, conidial germination, and secretion of metabolites and hydrolytic enzymes [8, 9]. Some of those fungal structures have been studied on Scedosporium/Lomentospora species, such as (i) surface molecules (e.g., peptidorhamnomannans, glucosylceramides, and α-glucan) capable of interacting with host receptors as well as modulating the host immunity responses, (ii) proteases able to cleave key host proteins, including antimicrobial peptides, extracellular matrix proteins, and humoral immune components, (iii) high capacity of conidia to germinate under diverse environmental conditions, and (iv) biofilm formation on a variety of abiotic surfaces and also over mammalian cells [7, 8, 10–18].
In order to add more light on the fungal-host interaction events, a fundamental pathogenicity step, in the present work, we have investigated the capacity of S. apiospermum, S. aurantiacum, S. minutisporum, and L. prolificans (formerly S. prolificans) to interact with and to induce damages in lung epithelial cells (A549 line).
Materials and methods
Fungi and growth conditions
Scedosporium apiospermum (strain RKI07_0416) was provided by Dr. Bodo Wanke (Evandro Chagas Hospital, Oswaldo Cruz Institute, FIOCRUZ, Rio de Janeiro, Brazil); S. minutisporum (strain FMR 4072), S. aurantiacum (strain FMR 8630), and L. prolificans (strain FMR 3569) were kindly given by Dr. Josep Guarro (Microbiology Unit, Medical School and Institute of Advanced Studies, Reus, Spain). The fungi were maintained on Sabouraud (2% glucose, 1% peptone, 0.5% yeast extract) liquid culture medium for 7 days at room temperature with orbital shaking. Conidia were grown at room temperature on Petri dishes containing potato dextrose agar (PDA; Difco Laboratories, USA). After 7 days in culture, conidial cells were obtained by washing the plate surface with phosphate-buffered saline (PBS; 10 mM NaH2PO4, 10 mM Na2HPO4, 150 mM NaCl, pH 7.2) and filtering them through a 40-μm nylon cell strainer (BD Falcon, USA) to remove hyphal fragments. The conidial cells were counted in a Neubauer chamber.
Lung epithelial cell culture
A549 cells (ATCC CCL-185; alveolar basal epithelial cell of human adenocarcinoma) were cultivated in Dulbecco’s modified Eagle medium (DMEM; Sigma-Aldrich, USA) supplemented with both 10% of heat-inactivated fetal bovine serum (FBS; Invitrogen, USA) and 2 mM l-glutamine (Sigma-Aldrich, USA) at 37 °C in an atmosphere containing 5% CO2 in a sterile culture bottle of 75 cm2. The passages and the expansions were made every 72 h, releasing the confluent cell monolayer with 4 mL of trypsin (2.5 g/L) (Sigma-Aldrich, USA) per bottle. For the experiments described below, 105 epithelial cells were incubated in 24-well plates containing (per well) 0.5 mL of DMEM with 2% of penicillin and streptomycin for at least 12 h for cell adhesion to glass coverslips at 37 °C, 5% CO2.
Interaction between conidia and A549 cells
For determination of association index and for light microscopy analyses of the interaction between fungi and A549 cells, 105 epithelial cells were incubated with 106 conidia of S. apiospermum, S. aurantiacum, S. minutisporum, and L. prolificans in DMEM (FBS-free) on glass coverslips for 1, 4, and 24 h at 37 °C, 5% CO2. After the interaction periods, the wells were washed three times with DMEM to discard conidia unattached, fixed in Bowin’s solution, stained with Giemsa, and then observed and counted in a light microscope (Axioplan 2, Zeiss, Germany). The percentage of infected cells after 4 h of interaction was determined by randomly counting 200 of A549 cells on each triplicate coverslip and the adhesion index was calculated by multiplying the mean number of attached fungi in human cells by percentage of infected cells. Light micrographs were obtained at ×40 after 1, 4, and 24 h in the same microscope as mentioned above.
Fluorescence analyses of the interaction process
Localization of conidia in A549 cells was performed after 4 h of interaction with 105 human cells and 106 conidia in DMEM (PBS-free) on glass coverslips at 37 °C, 5% CO2. Initially, conidia of S. apiospermum, S. aurantiacum, S. minutisporum, and S. prolificans were stained with 40 μg/mL of 5/6-carboxy-tetramethyl-rhodamine succinimidyl ester (NHS-rhodamine; Sigma-Aldrich, USA) for 1 h at room temperature in the absence of light. The conidia were washed three times with PBS and then incubated with the A549 cells. After the interaction period, the wells were once washed to discard conidia unattached and the systems were fixed with paraformaldehyde (4%) for 30 min and A549 cells were stained with membrane and nuclear markers: cholera toxin subunit B (CT-B; Sigma-Aldrich, USA) in a concentration of 1 μg/mL for 45 min and 4′,6-diamidine-2′-phenylindole dihydrochloride (DAPI; Sigma-Aldrich, USA), at 10 μg/mL for 10 min, respectively. The systems were washed three times with PBS and taken for viewing on a Zeiss fluorescence microscope (Axioplan2). A549 cells incubated without fungi and labeled with both dyes (membrane and nuclear markers) were used as negative control.
Analysis of host cell damage
In order to analyze possible injury on the plasma membrane of A549 cells, the interaction was processed in the same way as described above. After 1, 2, 3, 4, and 24 h of interaction, the systems were once washed to discard conidia unattached and then incubated with 5 μg/mL of propidium iodide (PI; Sigma-Aldrich, USA) at room temperature for 10 min in the absence of light. Epithelial cells incubated only in the culture medium were used as negative control for PI staining. A positive control for cell membrane damage utilizing PI staining was included which corresponds to A549 cells treated with paraformaldehyde at 4% for 30 min. The 24 h of fungi-epithelial cells interplays was also subjected to labeling with PI and Calcofluor white (CW; Sigma-Aldrich, USA) in order to disclosure the formation of the mycelium and cell membrane damage. CW was used in a final concentration of 5 μg/mL for 1 h at room temperature in the absence of light. Staining with PI was processed in the same manner as previously described. All systems mentioned above were washed three times with PBS after staining and taken immediately for viewing on a Zeiss epifluorescence microscope (Axioplan 2).
Statistics
All experiments were performed in triplicate, in three independent experimental sets and data were expressed as mean ± standard deviation (SD).
Results and discussion
The most severe infection cases caused by Scedosporium and Lomentospora species usually start with the inhalation of conidia, which adhere and germinate inside the lung environment [5]. Conidial germination culminates in invasion of the lower respiratory tract; therefore, the fungal adhesion to lung cells/tissues is one of the most crucial steps on the pathogenesis of scedosporiosis [5]. There are few studies about the interaction process between Scedosporium/Lomentospora species and human cell lines. Pinto et al. [11] reported that the interaction dialogue between larynx carcinoma cells (HEp2) and conidia of Scedosporium boydii (formerly Pseudallescheria boydii) occurred, at least in part, via peptidorhamnomannan (PRM) molecules, which are peptideopolysaccharides located at the fungal cell wall, and a 25-kDa surface polypeptide on HEp2 plasma membrane. Furthermore, those authors described that after 2 h of co-culturing of S. boydii and HEp2 cells, the conidia started to germinate and, subsequently, to penetrate into the plasma membrane of epithelial cells [11]. The PRMs also mediate the in vitro adhesion between L. prolificans conidia and mouse peritoneal macrophages [16]. The conidial adhesion followed by its germination was also observed during the in vitro interaction events of S. apiospermum, S. boydii, and S. aurantiacum with lung epithelial cells (A549), lung fibroblasts (MRC-5), and mouse macrophages (RAW 264.7 line) [7, 14, 19, 20]. Interesting studies were also conducted with peritoneal macrophages and polymorphonuclear cells in an effort to investigate the immune responses against Scedosporium/Lomentospora species [10, 12, 15]. For instance, the cytokine supplementation, such as interferon-γ and granulocyte-macrophage colony-stimulating factor, induced the production of superoxide anion by polymorphonuclear leukocytes, which promoted a more intense damage on hyphae of S. apiospermum and L. prolificans than without cytokine stimulation [10]. The α-glucan chemically extracted from S. boydii cell wall partially mediated the conidia phagocytosis and also stimulated the secretion of inflammatory cytokines by macrophages and dendritic cells in a mechanism involving Toll-like receptor 2, CD14, and MyD88 [12]. Other molecules that participate in the phagocytosis are glucosylceramides described at the surface of S. apiospermum. A monoclonal antibody against the glucosylceramides enhanced the phagocytosis and killing of S. apiospermum conidial cells by peritoneal macrophages [15].
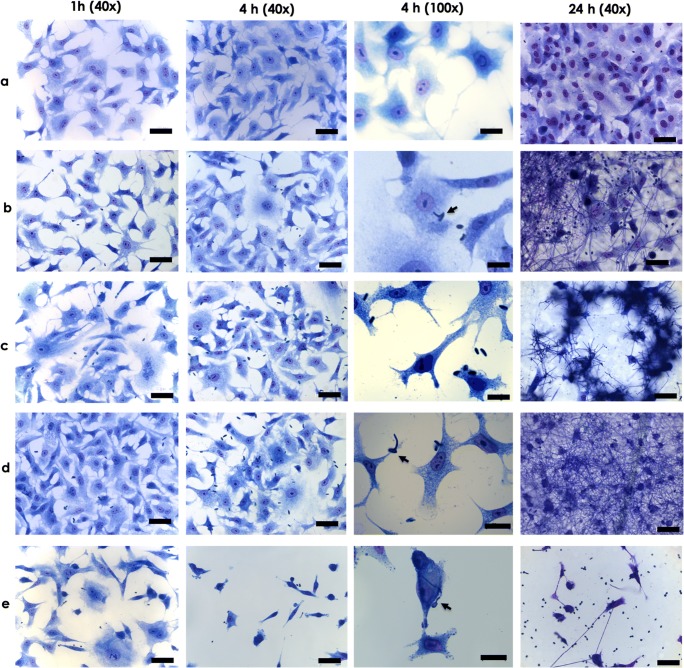
In the present study, we selected 4 representative species belonging to the Scedosporium/Lomentospora genera and a lung cell line (A549), which is the primary target for these fungal pathogens, in order to firstly investigate the association indexes. After 4 h of conidia-A549 cells co-culturing, the association indexes were calculated as 73.20 for S. apiospermum, 117.98 for L. prolificans, 188.01 for S. minutisporum, and 241.63 for S. aurantiacum (Fig. 1). Nevalainen et al. [19] also described a faster attachment of S. aurantiacum to A549 cells compared with other species, like A. fumigatus and S. boydii, with 75–80% of conidia bound to A549 cells after 4 h. In addition, our results revealed that after 4 h of interaction, there was a vast predominance of conidia and a small percentage, approximately 15%, of germinated conidia interacted to A549 cells (Fig. 2). Our result is in accordance with previous works reported for S. boydii, S. apiospermum, and S. aurantiacum, in which after 4 h of interaction with different mammalian cells, non-germinated conidia were the predominantly adhered fungal morphotypes [7, 11, 14, 19]. Conidia of A. fumigatus also started to germinate after approximately 4 h of in vivo infection using a murine experimental model [21]; whereas in in vitro contact with endothelial cell lines, resting conidia of A. fumigatus only start to germinate after 8 h [22]. The conidial differentiation is an essential step during the infectious progress, because the germination leads to the increase fungal adhesive properties [23] as well as it causes damages through host cell/tissue penetration and permits the invasion of the lung parenchyma [24]. Our group have previously described that S. apiospermum, S. minutisporum, S. aurantiacum, and L. prolificans conidia had high ability of germination after 4 h of incubation under several culture conditions, such as different growth media (Sabouraud, DMEM, and fetal bovine serum), pH levels (5.0, 7.0, and 9.0), and temperatures (21 °C and 37 °C) [17]. Interestingly, the conidial differentiation in Scedosporium/Lomentospora species is mainly modulated by the CO2 tension, showing high rates of germination in CO2 concentration found in mammalian tissues (5%) compared with atmospheric concentration (0.033%) [17]. Contrary to the low rates of germination observed in the present study, when conidial cells were incubated in culture medium, at the same environmental conditions (37 °C with and atmosphere containing 5% of CO2), it was observed about 75% of conidia of S. apiospermum, S. minutisporum, S. aurantiacum, and L. prolificans in germinated status [17].
Fig. 1.
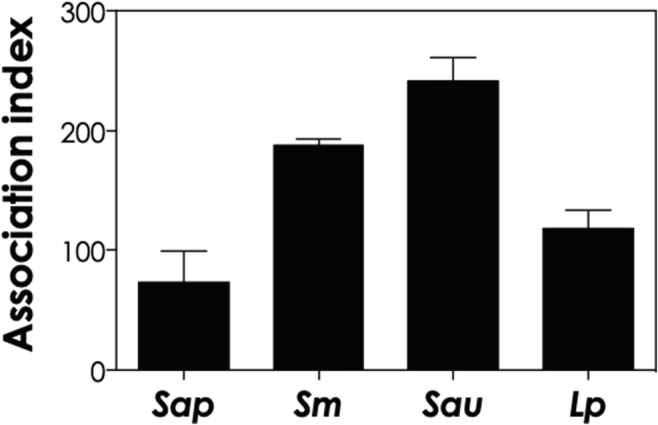
In vitro co-culturing of human lung epithelial cells (A549 line) with S. apiospermum (Sap), S. minutisporum (Sm), S. aurantiacum (Sau), and L. prolificans (Lp). Conidia were interacted with epithelial cells in a ratio of 10:1. After 4 h, the association index was calculated as described in the “Materials and methods” section. The data are expressed as the mean ± standard deviation of three independent experiment sets performed in triplicate. All the systems were significantly different from each other
Fig. 2.
Light micrographs showing the interaction events of A549 cells and Scedosporium/Lomentospora species. A549 cells were cultured in the absence (a) or in the presence of conidia of S. apiospermum (b), S. minutisporum (c), S. aurantiacum (d), and L. prolificans (e) for 1, 4, and 24 h. After 4 h of interaction, at ×100 magnification, it is possible to clearly detect morphological changes in A549 cells, except on S. apiospermum. Detachment of mammalian cells was seen mainly after the interaction with L. prolificans. The black arrows show the germinated conidia. Note the presence of a mycelial network covering the A549 cells after 24 h. Bars, 50 μm
In the last decades, many studies demonstrated that lung epithelial cells are not only physical barriers against inhaled pathogens, they are also able to secrete several bioactive molecules such as cytokines, chemokines, growth factors, lipid mediators, reactive oxygen/nitrogen species, and peptide mediators [25]. In this sense, the whole genome transcriptional profiling of A549 cells after 8 h of interaction with S. aurantiacum and A. fumigatus showed several genes that are differentially expressed in comparison with non-infected cells. This highlights the increase in levels of transcripts from genes associated with wound healing, cell repair, and protective response, like genes that encode the chemokines IL-6, IL-8, IL-11, TNF-α, and MUC5, a gene involved in mucin production that indicates the initiation of mucociliary clearance [19, 20, 26]. In addition, a fibrous-like network secreted by A549 cells surrounding S. apiospermum conidia was previously observed through scanning electron microscopy [7]. The type II alveolar epithelial cells, as the A549 cell line, are well known as being able to secrete a lipoprotein material into the lungs that acts as a surfactant [27]. In this context, the agglutination of A. fumigatus conidia by surfactant protein A or D enhances their phagocytosis by alveolar macrophages [28], demonstrating the indirect role of lung epithelial cells also in phagocytosis. In this manner, the capacity to germinate during interaction with cells and/or tissues depends on the balance between signals that induces germination and mediators produced by host cells against pathogen.
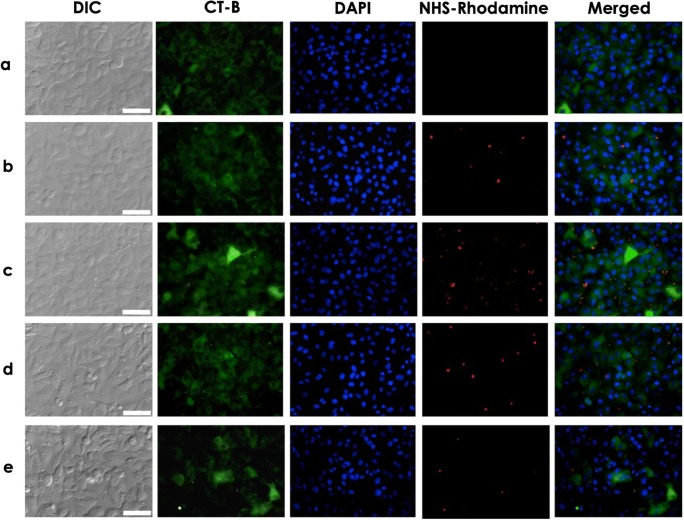
Our results also revealed that conidia of all studied species adhere to A549 cells in a random pattern, at least in the early stages of interaction, as demonstrated by both light and fluorescence microscopies (Figs. 2 and 3). Similarly, this adhesive non-specific pattern was already visualized during the interaction process of S. apiospermum-A549 cells [7] and A. fumigatus-A549 cells as registered by scanning electron microscopy [29]. In another study, Kogan et al. [30] demonstrated that the focal contact between A. fumigatus conidia and A549 cells occurred at the ends of actin stress fibers as observed by fluorescence microscopy using anti-vinculin antibodies. Interestingly, after 2 h of A. fumigatus conidia-A549 cells interplay, those focal contacts were disrupted by factors secreted by the fungus [30]. Two evidences of possible epithelial damage are the alterations in cell morphology and adhesiveness. Through light microscopy, we identified different degrees of morphological changes in A549 cells varying according to the fungal species studied, in which the most observed alteration was rounding the epithelial cells with subsequent detachment of monolayer (Fig. 2). The detachment of monolayer was observed mainly after interaction with L. prolificans, which could explain its low associate index (Figs. 1 and 2). The detachment of epithelial cells from the surface could be due to the extracellular proteases produced by fungal pathogens able to degrade proteins that constitute extracellular matrix components. The effects of different proteases secreted by S. aurantiacum on A549 cells were recently described and, in this context, the elastase-like protease was able to reduce the A549 viability, chymotrypsin-like protease was associated with A549 detachment, and cysteine protease altered both A549 attachment and viability [31]. Degradation of purified extracellular matrix proteins by Scedosporium species was also previously reported by different research groups. In S. apiospermum, a 33-kDa serine protease able to degrade human fibrinogen was identified, while in S. boydii, two metallo-type proteases (28 and 35 kDa) were able to cleave fibronectin and laminin [13, 32]. The degradation of extracellular matrix components helps the dissemination of fungal pathogens into deeper tissues and also assists them to escape from host effector cells, such as fibronectin-activated macrophages [5].
Fig. 3.
Fluorescence micrograph images showing the co-culture of Scedosporium/Lomentospora conidia and A549 cells. NHS-rhodamine-stained conidia and A549 cells (10:1) were interacted for 4 h at 37 °C. Subsequently, the plasma membrane and nucleus of A549 cells were stained with CT-B and DAPI, respectively. A control system containing only lung epithelial cells was used for comparison (a). Note that conidia of S. apiospermum (b), S. minutisporum (c), S. aurantiacum (d), and L. prolificans (e) randomly adhered to A549 cells. Bars, 50 μm
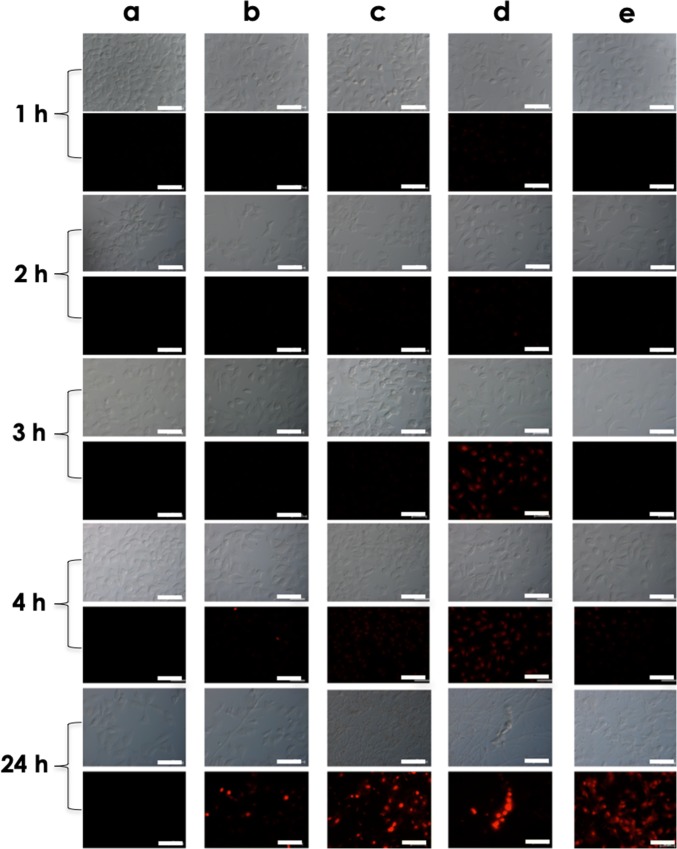
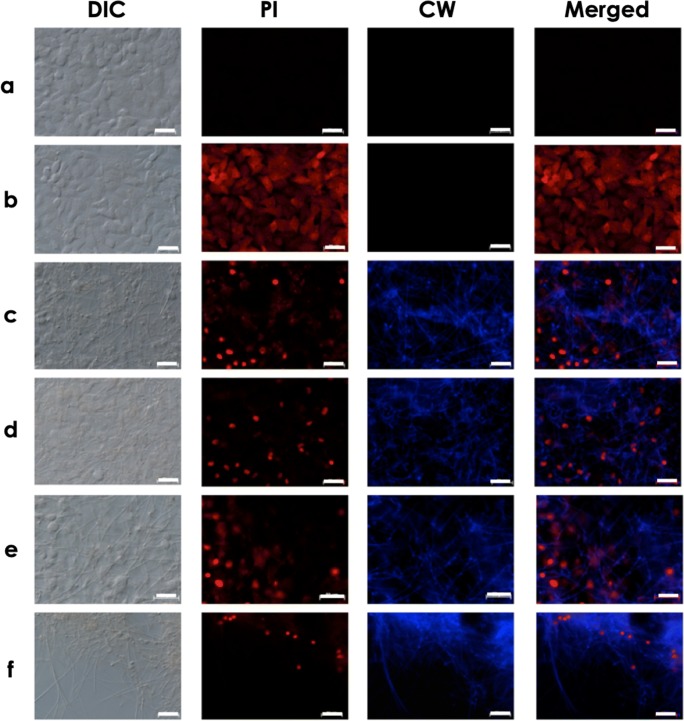
After 24 h of co-culturing of Scedosporium/Lomentospora and A549 cells, all conidia have differentiated into hyphae and, consequently, it was not possible to determine the association index (Fig. 2). The complete conidial differentiation after 24 h is consistent with our earlier published work about biofilm formation in Scedosporium and Lomentospora species over lung epithelial monolayer [33]. Utilizing a dual staining with CW and PI, it was possible to observe the mycelial trap over and among the epithelial cells (Figs. 4 and 5). Moreover, the mycelia induced irreversible damage on plasma membrane of A549 cells, as judged by the passive incorporation of PI (Figs. 4 and 5). In fact, the plasma membrane damages of lung epithelial cells started to be detected earlier, after only 1 h in contact with S. aurantiacum and S. minutisporum, and after 4 h with S. apiospermum and L. prolificans, as judged by the passive incorporation of PI (Figs. 2 and 4). As previously proposed, plasma membrane injury observed after interaction with filamentous fungi is mainly caused by the apical growth of mycelial cells, which are able to penetrate biological membranes, leading to host cell death [6]. In addition, fungal cell during its apical growth is able to secrete a large variety of hydrolytic enzymes, including proteases and lipases, which are able to mediate epithelial damage by cleaving the most abundant biomolecules, proteins and lipids, which form this essential cellular structure [14, 34].
Fig. 4.
Scedosporium/Lomentospora conidia induce plasma membrane damage in lung epithelial cells. A549 cells were cultured in the absence (a) or in the presence of conidia of S. apiospermum (b), S. minutisporum (c), S. aurantiacum (d), and L. prolificans (e) for 1, 2, 3, and 4 h. After each time point, the interaction systems were incubated with propidium iodide (PI), to evidence the A549 plasma membrane injury, and then visualized using phase-contrast (DIC) and fluorescence microscopy. Bars, 50 μm
Fig. 5.
Microscopic analyses showing the mycelial trap covering the monolayer formed by lung epithelial cells. A549 cells were cultured in the absence (a) or in the presence of conidia of S. apiospermum (b), S. minutisporum (c), S. aurantiacum (d), and L. prolificans (e) after 24 h. Subsequently, the interaction systems were incubated with Calcofluor white (CW), to localize the mycelial cells, and propidium iodide (PI), to evidence the A549 plasma membrane injury. Note that after 24 h of co-culturing of fungal conidia and A549 cells, only mycelia were observed using phase-contrast (DIC) and fluorescence microscopy. Bars, 50 μm
In summary, our results show that S. apiospermum, S. minutisporum, S. aurantiacum, and L. prolificans conidia readily adhere in a random pattern on epithelial lung cells with different avidity. After 4 h of interaction, some conidia start to germinate and with additional 20 h, all fungal species form a monolayer of mycelia over the A549 cells, leading to epithelial cell death. Our results add information about the interaction mechanisms of Scedosporium and Lomentospora species, mainly demonstrating the ability to differentiate into hyphae and cause irreversible damage on host cell.
Funding information
This work was financially supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – Finance code 001).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Thaís Pereira de Mello and Ana Carolina Aor contributed equally to this work.
References
- 1.Benedict K, Park BJ. Invasive fungal infections after natural disasters. Emerg Infect Dis. 2014;20:349–355. doi: 10.3201/eid2003.131230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramirez-Garcia A, Pellon A, Rementeria A, Buldain I, Barreto-Bergter E, Rollin-Pinheiro R, de Meirelles JV, Xisto MIDS, Ranque S, Havlicek V, Vandeputte P, Govic YL, Bouchara JP, Giraud S, Chen S, Rainer J, Alastruey-Izquierdo A, Martin-Gomez MT, López-Soria LM, Peman J, Schwarz C, Bernhardt A, Tintelnot K, Capilla J, Martin-Vicente A, Cano-Lira J, Nagl M, Lackner M, Irinyi L, Meyer W, de Hoog S, Hernando FL. Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med Mycol. 2017;56:S101–D125. doi: 10.1093/mmy/myx113. [DOI] [PubMed] [Google Scholar]
- 3.McCarthy MW, Denning DW, Walsh TJ. Future research priorities in fungal resistance. J Infect Dis. 2017;216:S484–S492. doi: 10.1093/infdis/jix103. [DOI] [PubMed] [Google Scholar]
- 4.Lackner M, Guarro J. Pathogenesis of Scedosporium. Curr Fungal Infect Rep. 2013;7:326–333. doi: 10.1007/s12281-013-0157-7. [DOI] [Google Scholar]
- 5.Cortez KJ, Roildes E, Quiroz-Telles F, Meletiadis J, Antachopoulos C, Knudsen T, Buchanan W, Milanovich J, Sutton DA, Fothergill A, Rinaldi MG, Shea YR, Zaoutis T, Kottilil S, Walsh TJ. Infections caused by Scedosporium spp. Clin Microbiol Rev. 2008;21:157–197. doi: 10.1128/CMR.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filler SG, Sheppard DC. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog. 2006;2:e129. doi: 10.1371/journal.ppat.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aor AC, Mello TP, Sangenito LS, Fonseca BB, Rozental S, Lione VF, Veiga VF, Branquinha MH, Santos ALS. Ultrastructural viewpoints on the interaction events of Scedosporium apiospermum conidia with lung and macrophage cells. Mem Inst Oswaldo Cruz. 2018;113:e180311. doi: 10.1590/0074-02760180311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mello TP, Bitencourt VC, Liporagi-Lopes L, Aor AC, Branquinha SALS. Insights into the social life and obscure side of Scedosporium/Lomentospora species: ubiquitous, emerging and multidrug-resistant opportunistic pathogens. Fungal Biol Rev. 2019;33:16–46. doi: 10.1016/j.fbr.2018.07.002. [DOI] [Google Scholar]
- 9.Escobar N, Ordonez SR, Wösten HAB, Haas PJA, Cock H, Haagsman HP. Hide, keep quiet, and keep low: properties that make Aspergillus fumigatus a successful lung pathogen. Front Microbiol. 2016;7:438. doi: 10.3389/fmicb.2016.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gil-Lamaignere C, Winn RM, Simitsopoulou M, Maloukou A, Walsh TJ, Roilides E. Interferon gamma and granulocyte-macrophage colony-stimulating fator augment the antifungal activity of human polymorphonuclear leukocytes against Scedosporium spp.: comparision with Aspergillus spp. Med Mycol. 2005;43:253–260. doi: 10.1080/13693780412331271072. [DOI] [PubMed] [Google Scholar]
- 11.Pinto MR, Sá ACM, Limongi CL, Rozental S, Santos ALS, Barreto-Bergter Involvement of peptidorhamnomannan in the interaction of Pseudallescheria boydii and HEp2 cells. Microbes Infect. 2004;6:1259–1267. doi: 10.1016/j.micinf.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Bittencourt VCB, Figueiredo RT, Silva RB, Mourão-Sá DS, Fernandez PL, Sassaki GL, Mulloy D, Bozza MT, Barreto-Bergter E. An alfa-glucan of Pseudallescheria boydii is involved in fungal phagocytosis and Toll-like receptor activation. J Biol Chem. 2006;281:22614–22623. doi: 10.1074/jbc.M511417200. [DOI] [PubMed] [Google Scholar]
- 13.Silva BA, Pinto MR, Soares RM, Barreto-Bergter E, Santos ALS. Pseudallescheria boydii releases metallopeptidases capable of cleaving several proteinaceous compounds. Res Microbiol. 2006;157:425–432. doi: 10.1016/j.resmic.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Santos ALS, Bittencourt VCB, Pinto MR, Silva BA, Barreto-Bergter E. Biochemical characterization of potential virulence markers in the human fungal pathogen Pseudallescheria boydii. Med Mycol. 2009;47:375–386. doi: 10.1080/13693780802610305. [DOI] [PubMed] [Google Scholar]
- 15.Rollin-Pinheiro R, Liporagi-Lopes LC, Meirelles JV, Souza LM, Barreto-Bergter E. Characterization of Scedosporium apiospermum glucosylceramides and their involvement in fungal development and macrophage functions. PLoS One. 2014;9:e98149. doi: 10.1371/journal.pone.0098149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xisto MI, Bittencourt VC, Liporagi-Lopes LC, Haido RMT, Mendonça MSA, Sassaki G, Figueiredo RT, Romanos MT, Barreto-Bergter E. O-glycosylation in cell wall proteins in Scedosporium prolificans is critical for phagocytosis and inflammatory cytokines production by macrophages. PLoS One. 2015;10(4):e0123189. doi: 10.1371/journal.pone.0123189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mello TP, Aor AC, Oliveira SS, Branquinha MH, Santos ALS. Conidial germination in Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium minutisporum and Lomentospora prolificans: influence of growth conditions and antifungal susceptilibility profiles. Mem Inst Oswaldo Cruz. 2016;111(7):484–494. doi: 10.1590/0074-02760160200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mello TP, Oliveira SSC, Frasés S, Branquinha MH, Santos ALS. Surface properties, adhesion and biofilm formation on different surfaces by Scedosporium spp. and Lomentospora prolificans. Biofouling. 2018;34:800–814. doi: 10.1080/08927014.2018.1503652. [DOI] [PubMed] [Google Scholar]
- 19.Nevalainen H, Kaur J, Han Z, Kautto L, Rampserger M, Meywer W, Chen SCA. Biological, biochemical and molecular aspects of Scedosporium aurantiacum, a primary and opportunistic fungal pathogen. Fungal Biol Rev. 2018;32:156–165. doi: 10.1016/j.fbr.2018.03.001. [DOI] [Google Scholar]
- 20.Kaur J, Kautto L, Penesyan A, Meyer W, Elbourne LDH, Paulsen IT, Nevalainen H. Interactions of an emerging fungal pathogen Scedosporium aurantiacum with human lung epithelial cells. Sci Rep. 2019;9:5035. doi: 10.1038/s41598-019-41435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopes-Bezerra LM, Filler SG. Interactions of Aspergillus fumigatus with endothelial cells: internalization, injury, and stimulation of tissue factor activity. Blood. 2004;103:2143–2149. doi: 10.1182/blood-2003-06-2186. [DOI] [PubMed] [Google Scholar]
- 22.Kalleda N, Amich J, Arslan B. Dynamic immune cell recruitment after murine pulmonary Aspergillus fumigatus infection under different immunosuppressive regimens. Front Microbiol. 2016;7:1107. doi: 10.3389/fmicb.2016.01107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osherov N, May GS. The molecular mechanisms of conidial germination. FEMS Microbiol Lett. 2001;199:153–160. doi: 10.1111/j.1574-6968.2001.tb10667.x. [DOI] [PubMed] [Google Scholar]
- 24.Kamai Y, Lossinsky AS, Liu H, Sheppard DC, Filler SG. Polarized response of endothelial cells to invasion by Aspergillus fumigatus. Cell Microbiol. 2009;11:170–182. doi: 10.1111/j.1462-5822.2008.01247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proud D, Leigh R. Epithelial cells and airway disease. Immunol Rev. 2011;242:186–204. doi: 10.1111/j.1600-065X.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen F, Zhang C, Jia X, Wang S, Chen Y, Zhao J, Tian S, Han X, Han L. Transcriptome profiles of human lung epithelial cells A549 interacting with Aspergillus fumiagtus by RNA-Seq. PLoS One. 2015;10:e0135720. doi: 10.1371/journal.pone.0135720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- 28.Madan T, Eggleton P, Kishore U, Strong P, Aggrawal SS, Sarma PU, Reid KB. Binding of pulmonary surfactant proteins A and D to Aspergillus fumigatus conidia enhances phagocytosis and killing by human neutrophils and alveolar macrophages. Infect. Immunol. 1997;65:3171–3179. doi: 10.1128/IAI.65.8.3171-3179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeHart DJ, Agwu DE, Julian NC, Washburn RG. Binding and germination of Aspergillus fumigatus conidia on cultured A549 pneumocytes. J Infetc Diseas. 1997;175:146–150. doi: 10.1093/infdis/175.1.146. [DOI] [PubMed] [Google Scholar]
- 30.Kogan TV, Jadoun J, Mittelman L, Hirschberg K, Osherov N. Involvement of secreted Aspergillus fumigatus proteases in disruption of the actin fiber cytoskeleton and loss of focal adhesion sites in infected A549 lung pneumocytes. J Infect Dis. 2004;189:1965–1973. doi: 10.1086/420850. [DOI] [PubMed] [Google Scholar]
- 31.Han Z, Kautto L, Meyer W, Chen SC, Nevalainen H (2019) Effect of protease secreted by the opportunistic pathogen Scedosporium aurantiacum on human epithelial cells. Can J Microbiol Epub ahead of print. 10.1139/cjm-2019-0212 [DOI] [PubMed]
- 32.Larcher G, Cimon B, Symoens F, Tronchin G, Chabasse D, Bouchara JP. A 33 kDa serine proteinase from Scedosporium apiospermum. Biochem J. 1996;315:119–126. doi: 10.1042/bj3150119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mello TP, Aor AC, Gonçalves DS, Seabra SH, Branquinha MH, Santos ALS. Assessment of biofilm formation by Scedosporium apiospermum, Scedosporium aurantiacum, Scedosporium minutisporum and Lomentospora prolificans. Biofouling. 2016;32:737–749. doi: 10.1080/08927014.2016.1192610. [DOI] [PubMed] [Google Scholar]
- 34.Croft CA, Culibrk L, Moore MM, Tebbutt SJ. Interactions of Aspergillus fumigatus conidia with airway epithelial cells: a critical review. Front Microbiol. 2016;7:472. doi: 10.3389/fmicb.2016.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]