Abstract
A new strain of Trichoderma reesei (teleomorph Hypocrea jecorina) with high cellulase production was obtained by exposing the spores from T. reesei QM9414 to an ultraviolet light followed by selecting fast-growing colonies on plates containing CMC (1% w/v) as the carbon source. The mutant T. reesei RP698 reduced cultivation period to 5 days and increased tolerance to the end-products of enzymatic cellulose digestion. Under submerged fermentation conditions, FPase, CMCase, and Avicelase production increased up to 2-fold as compared to the original QM9414 strain. The highest levels of cellulase activity were obtained at 27 °C after 72 h with Avicel®, cellobiose, and sugarcane bagasse as carbon sources. The temperature and pH activity optima of the FPase, CMCase, and Avicelase were approximately 60 °C and 5.0, respectively. The cellulase activity was unaffected by the addition of 140 mM glucose in the enzyme assay. When T. reesei RP698 crude extract was supplemented by the addition of β-glucosidase from Scytalidium thermophilum, a 2.3-fold increase in glucose release was observed, confirming the low inhibition by the end-product of cellulose hydrolysis. These features indicate the utility of this mutant strain in the production of enzymatic cocktails for biomass degradation.
Keywords: Cellulase production, CMCase, Trichoderma reesei, Mutation, Cellulose hydrolysis
Introduction
Cellulose is the most abundant renewable biopolymer found in nature [1, 2], and its complete enzymatic hydrolysis to glucose is catalyzed by the synergistic action of endoglucanases (endo-1,4-β-D-glucanase; EG; EC 3.2.1.4), exoglucanases (cellobiohydrolases, CBH, 1,4-β-D-glucan cellobiohydrolase; EC 3.2.1.91), β-glucosidases (cellobiase; BG; β-D-glucoside-glucohydrolase; EC 3.2.1.21) [1, 3–6], and some lytic polysaccharide monooxygenases (LPMO; lytic cellulose monooxygenase (C1-hydroxylating); EC 1.14.99.54 and lytic cellulose monooxygenase (C4-dehydrogenating); EC 1.14.99.56) [7]. Cellulolytic enzymes can also be applied in many industrial processes, for example in the textile, brewing and paper industries, waste water treatment, and in the production of bioethanol [1, 3, 8, 9]. Because of these diverse applications, there is interest in obtaining cellulases with high efficiency, thermostability, stability to a broad range of pH, product tolerance, and low production cost [1, 8].
In recent years, new fungal and bacterial cellulases have been isolated and characterized [10], and the regulation of cellulase production by these microorganisms has also been extensively studied. Furthermore, progress has been made in reducing the cost of cellulase production, particularly in the case of non-complexed cellulases. For example, cellulases from the fungus Trichoderma reesei (teleomorph Hypocrea jecorina) have been the focus of research over the last 50 years and are widely used to release glucose from cellulose [11–13]. Since the production of β-glucosidase by T. reesei is low, the addition of β-glucosidase to the culture extract is required to completely hydrolyze cellulose [14, 15]. Cellobiose is a potent inhibitor of many cellulases, and therefore, the level of β-glucosidases in cellulase preparations is critical, not only for complete cellulose degradation to glucose, but also to avoid the inhibition of cellulases [5, 12, 16].
Random mutagenesis can increase the production of cellulolytic activity of an isolated strain, and the mutant T. reesei QM9414 is a well-studied strain generated from T. reesei QM6a by random mutagenesis using ultraviolet radiation. Although the nature of the mutations remain to be fully elucidated, the mutant produces four to six-fold more cellulases than the wild-type QM6a [17]. The aim of this study was to further mutate the T. reesei QM9414 using an ultraviolet light, with the aim of generating a strain showing high levels of extracellular cellulases and improved biochemical characteristics such as reduced enzyme inhibition by glucose as well as to evaluate its application in enzyme mixtures for cellulose hydrolysis.
Materials and methods
Random mutagenesis by UV light
Ten milliliters of spore suspension (107 spores/mL) from T. reesei QM9414 ATCC 46480 were distributed on Petri dishes and exposed to ultraviolet light (Mineralight Lamp, 50–60 CVC, 60 amp.) at a distance of 12.5 cm from the light source. The samples were irradiated at different times (5, 10, 15, and 20 min) and plated on solid Mandels’ medium [18] with carboxymethyl cellulose (CMC) (1% w/v) as carbon source and incubated in the dark at 27 °C. After 48 h, the fastest growing colonies were collected with an inoculation loop and transferred to glass slants containing solid potato dextrose agar (PDA). About 1000 colonies were isolated in this mutagenic procedure and the strain that showed faster growth in Mandels’ medium containing CMC (1% w/v) and high cellulase activity in liquid Mandels’ medium was selected and named T. reesei RP698. The RP698 strain was maintained on PDA slants at 27 °C for 4–10 days. Samples of T. reesei RP698 were deposited in the Filamentous Fungi Collection of the Laboratory of Microbiology and Cell Biology of the Faculty of Philosophy, Sciences and Letters of Ribeirão Preto-USP.
Growth of T. reesei strains
A suspension containing 107spores/mL of QM9414 and RP698 was obtained by the addition of 10 mL of distilled water and scraping the slant surface with an inoculation loop. One milliliter of these cultures was inoculated into 25 mL of liquid Mandels’ medium with 1% of microcrystalline cellulose (Avicel®) as carbon source. The nutrient concentrations of Mandels’ medium were (g/L): 0.3 urea, 1.4 (NH4)2SO4, 2.0 KH2PO4, 0.3 CaCl2.2.H2O, 0.3 MgSO4.7.H2O, 0.75 proteose peptone, and 7.5 of Avicel®, supplemented by the following trace elements (mg/mL): 5 FeSO4.7.H2O, 1.6 MnSO4.H2O, 1.4 ZnSO4.7.H2O, and 2.0 CoCl2. The initial pH was adjusted to 6.0 and the Erlenmeyer flasks containing the media were previously autoclaved at 121 °C for 20 min prior to inoculation. For the growth in Mandels’ medium, the inoculum of the conidia was directly made in the production medium and incubated with agitation (110 rpm) at 27 °C for determined periods.
Preparation of the crude enzyme extracts
All the cultures were filtered through synthetic foam with a Büchner funnel to recover the crude enzyme extract from the culture media. The crude filtrate was maintained on ice and the mycelial mass was discarded. These filtrates were the source of crude extracellular cellulolytic activity and were used for further assays without further treatment.
Effect of different carbon sources on cellulase production in T. reesei mutant RP698
The effect of different carbon sources on cellulase production in T. reesei RP698 was evaluated using Mandels’ medium. The carbon sources used were Avicel®, filter paper, carboxymethyl cellulose (CMC), cellobiose, steam-exploded sugarcane bagasse, wheat bran, sawdust, xylan, sucrose, starch, lactose, and glucose. All carbon sources were added to Mandels’ medium at a concentration of 1% (w/v). The cultures were maintained at 27 °C, with agitation at 110 rpm in an orbital shaker for 72 h.
Catabolic repression
To check the effect of catabolic repression by glucose on cellulase production, the RP698 strain was inoculated in Mandels’ medium containing 0.75% Avicel® at initial glucose concentrations ranging from 10 to 50 mM at 27 °C, with agitation at 110 rpm in an orbital shaker for 72 h. To remove initial glucose content for enzymatic assays, the extracellular filtrates were repeatedly diluted with distilled water and concentrated using Vivaspin® 20, 10 kDa MWCO Polyethersulfone (GE Healthcare, USA) at 6000×g and 4 °C until no reducing sugar was detected in the DNS assay.
Enzymatic assays
Endoglucanase activity (CMCase) was assayed by incubating 0.5 mL of diluted enzyme samples with 0.5 mL of 100 mM sodium acetate buffer (pH 4.0) containing 1 % (w/v) carboxymethyl cellulose CMC (Sigma Chem. Co., USA). Exoglucanase activity (Avicelase) was assayed by incubating 0.5 mL of diluted enzyme samples with 0.5 mL of 100 mM sodium acetate buffer (pH 4.0) containing 1 % (w/v) microcrystalline cellulose (Avicel®) as substrate (Sigma Chem. Co., USA). Filter paper activity (FPase) was assayed by a strip of Whatman No.1 filter paper (25 mg, 1 × 3 cm) to 0.5 mL of sodium acetate buffer 100 mM pH 5.0 and 0.5 mL of suitably diluted enzyme (modified from Ghose) [19]. The assays were performed at 60 °C for 30 min (Avicelase and FPase) or 60 °C for 10 min (CMCase). The concentration of the released reducing sugars was determined by the 3,5-dinitrosalicylic acid (DNS) method [20] using d-(+)-glucose as standard. The β-glucosidase(BG) activity was assayed in sodium acetate buffer 50 mM, pH 5.0, at 50 °C for 10 min, using p-nitrophenyl-β-D-glucopyranoside (pNPG) as substrate (Sigma Chem. Co., USA), at final concentration of 8 mM. The reaction was stopped by adding two volumes of saturated sodium tetraborate solution, and the sample absorbance was measured at 410 nm.
Controls with heat inactivated enzyme were included in all enzymatic assays to quantify the non-enzymatic hydrolysis of the substrates. The experimental conditions (assay times, enzymatic units) employed were adjusted to guarantee the estimation of initial velocities (linear response of product formation in respect to assay time). One enzyme unit (U) was defined as the amount of enzyme that releases 1 μmol of product per minute, under the assay conditions.
Protein measurements
Extracellular protein concentration was determined by the Lowry method [21] using bovine serum albumin as standard.
Effect of temperature and pH on enzyme activity and stability
The effect of temperature on cellulase activity was determined over the temperature range from 30 to 80 °C, in 50 mM in sodium acetate buffer, pH 5.0. The enzyme thermostability was determined by measuring the residual activity after incubation of crude filtrate in the absence of substrate at 40, 50, 60, and 70 °C for 5, 15, 30, 45, and 60 min. The effect of pH on cellulase activity was determined at 60 °C using 50 mM citric acid buffer ranging from pH 2.5 to 7.5.
Effect of glucose and cellobiose on cellulase activity
Assays to evaluate effect of glucose and cellobiose on cellulase activity were performed using Cellulose Azure® 20 mg/ml (Sigma Chem. Co., USA) as substrate in sodium acetate buffer 100 mM pH 5.0. One milliliter of this solution was mixed with previously diluted crude extracellular extracts from T. reesei QM9414 or T. reesei RP698 plus glucose and cellobiose concentrations ranging from 0 to 2.5% (w/v) to final assay volume of 2 mL. After incubation for 60 min at 60 °C, the assays were stopped with 4 mL of alcohol reagent solution (0.5 mL of saturated calcium chloride solution in methanol, diluted in ethanol to a final volume of 100 mL), and centrifuged at 1500xg for 20 min as described by Lai et al. [22]. The sample absorbance was measured at 575 nm.
Cellulose hydrolysis by enzymatic mixtures
Two assays were carried out with enzyme mixtures in a final volume of 5 mL of 50 mM sodium acetate buffer (pH 5.0), 10 mM of sodium azide (to avoid bacterial contamination), containing 50 mg of Whatman No. 1 filter paper, 10 FPU per gram of substrate of RP698 extract, and 10 U per gram of substrate of β-glucosidase from two extracts of Scytalidium thermophilum. One assay used the intracellular extract rich in a glucose-stimulated β-glucosidase, and the other assay used the extracellular extract rich in a glucose-tolerant β-glucosidase. Both S. thermophilum crude β-glucosidase rich extracts were prepared and collected from the same strain medium according to Zanoelo et al. [23] and Silva et al. [24]. The assays were incubated over the time range of 0–6 h at 50 °C with agitation at 300 rpm in a benchtop orbital shaker (VorTemp 1550, Labnet). Assays with enzymatic samples previously inactivated by heating at 100 oC were used as controls. At different times, the reducing sugar concentration was determined by the DNS method and glucose release was quantified by the glucose oxidase method [25]. All experiments were performed at least in triplicate, and the results were expressed as mean values ± SD.
Results
Growth and production of cellulases
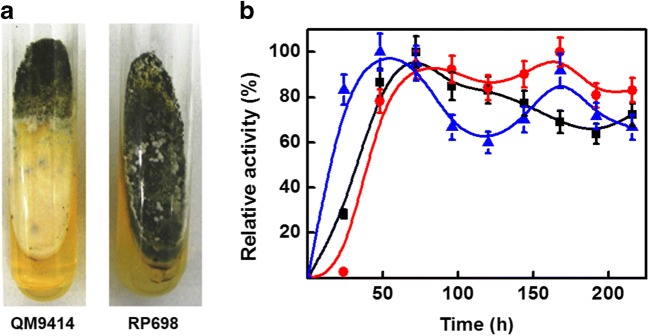
The mutant strain RP698 showed faster growth and sporulation on PDA-medium than the QM9414 when both strains were maintained under the same conditions (Fig. 1a). With the aim of determining the optimum time for cellulase production, the cultures of RP698 were maintained for up to 216 h at 27 °C (110 rpm) on Mandels’ medium. The highest value for CMCase activity was 11.8 U/mL at 168 h of culture, while a FPase activity of 0.72 U/mL was obtained after 48 h and an Avicelase activity of 0.78 U/mL was observed after 72 h growth (Fig. 1b). Although the maximal CMCase and FPase activities were observed at 168 h and 48 h, respectively, 95% of maximum activity was obtained for both enzymes at 72 h. At this time, the activities for Avicelase, CMCase, and FPase were 0.78, 11.3, and 0.7 U/mL, respectively.
Fig. 1.
a Growth of T. reesei QM9414 and T. reesei RP698 on PDA slants after 4 days at 27 °C. b Time course of cellulase production by T. reesei RP698. Culture conditions: Mandels’ medium containing Avicel 1% (w/v), 27 °C, pH 6.0, and 110 rpm. The black-filled square represents the Avicelase activity (100% is 0.78 ± 0.04 U/mL). The red-filled circle represents the CMCase activity (100% is 11.8 ± 0.92 U/mL). The blue up-pointing arrow represents the FPase activity (100% is 0.72 ± 0.06 U/mL). All data are the means ± SD of three experiments (n = 3)
The production of cellulases by QM9414 and RP698 cultured in Mandels’ medium with Avicel® for 3 days at 27 °C and 110 rpm was analyzed (Table 1). Cellulase activity in units per milliliter (U/mL) by the RP698 strain was higher than that observed for the QM9414 strain in Mandels’ medium cultured for 3 days. The CMCase, Avicelase, and FPase activities (U/ml) were 2-, 2.1- and 1.9-fold higher than those observed for QM9414 strain, respectively. A comparison of three cellulase activities in units per total protein (U/mg of total protein) revealed similar values from RP698 and QM9414 strains (Table 1) with CMCase showing the major activity in both strains. Table 1 also shows that despite the approximate 2-fold increase in overall cellulase activity of the RP698 as compared to QM9414, the U/mg of total protein remains almost the same. These data show the greater production of total proteins from RP698 (2-folds more) as compared to QM9414, suggesting that the RP698 strain has a greater capacity to produce cellulases.
Table 1.
Production of Avicelase, CMCase, FPase, and total proteins by T. reesei QM9414 and T. reesei RP698 cultured on Mandels’ medium for 3 days, using Avicel® as the carbon source
| T. reesei QM9414 | T. reesei RP698 | |||
|---|---|---|---|---|
| Enzyme activity | U/mL | U/mg of total proteins | U/mL | U/mg of total proteins |
| Avicelase | 0.35 ± 0.02 | 5.14 ± 0.35 | 0.75 ± 0.03 | 5.75 ± 0.23 |
| CMCase | 5.90 ± 0.44 | 86.76 ± 7.85 | 11.8 ± 0.92 | 90.1 ± 7.02 |
| FPase | 0.37 ± 0.02 | 5.44 ± 0.34 | 0.72 ± 0.06 | 5.49 ± 0.46 |
The protein concentration was 0.068 ± 0.01 for QM9414 and 0.131 ± 0.02 for RP 698. All data are the means ± SD of three experiments (n = 3)
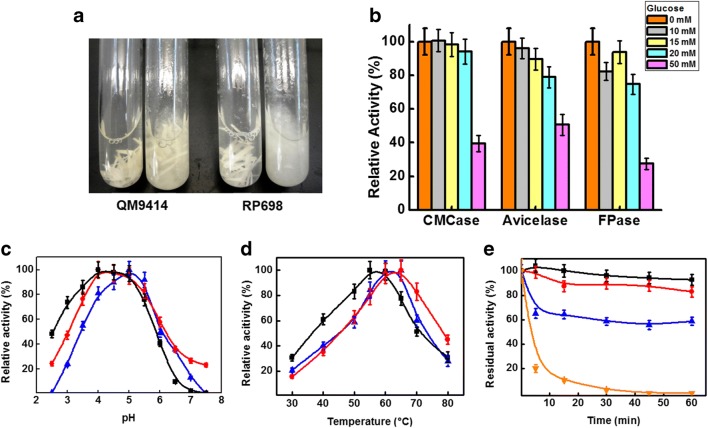
The effects of different carbon sources on the cellulase production by T. reesei RP698 are presented in Table 2. The highest production of CMCase (12.52 U/mL) and FPase (0.7 U/mL) was obtained in the medium supplemented with Avicel®, followed by cellobiose and steam-exploded sugarcane bagasse (SESB). The production of CMCase activity using Avicel® was 3.6-fold higher than that obtained in presence of glucose, which is generally considered as an easily assimilated carbon source. The highest level of Avicelase production was obtained using cellobiose as carbon source and was 36% greater compared to conditions using Avicel® as carbon source. Qualitative results of experiments with Whatman No. 1 filter paper as substrate for the crude extract filtrates indicated a more complete disintegration of the filter paper by the RP698 extract assays as compared to the extract from the parental QM9414 (Fig. 2a).
Table 2.
Effect of different carbon sources (1% w/v) on cellulase production by T. reesei RP698. The cultures were maintained at 27 °C, 110 rpm, for 72 h
| Carbon source | CMCase (U/mL) | Avicelase (U/mL) | FPase (U/mL) |
|---|---|---|---|
| Avicel | 12.52 ± 1.3 | 0.81 ± 0.05 | 0.7 ± 0.05 |
| Filter paper | 6.56 ± 0.41 | 0.69 ± 0.04 | 0.2 ± 0.01 |
| CMC | 1.32 ± 0.12 | 0.70 ± 0.06 | ND |
| Cellobiose | 11.12 ± 1.06 | 1.10 ± 0.08 | 0.4 ± 0.03 |
| SESB | 8.78 ± 0.92 | 0.92 ± 0.08 | 0.4 ± 0.04 |
| Wheat bran | 0.80 ± 0.05 | 0.69 ± 0.06 | ND |
| Sawdust | 0.20 ± 0.01 | 0.50 ± 0.04 | ND |
| Xylan | 0.70 ± 0.04 | 0.50 ± 0.05 | 0.1 ± 0.01 |
| Sucrose | 0.10 ± 0.07 | 0.53 ± 0.04 | ND |
| Starch | 0.10 ± 0.08 | 0.61 ± 0.05 | ND |
| Lactose | 4.24 ± 0.37 | 1.02 ± 0.08 | 0.1 ± 0.01 |
| Glucose | 3.43 ± 0.26 | 0.75 ± 0.06 | ND |
All data are the means ± SD of three experiments (n = 3)
ND not detectable in these conditions
Fig. 2.
a Disintegration of Whatman No. 1 filter paper after 1 h, at 50 °C, pH 5.0, with crude cellulases (10 FPU/g of substrate) from T. reesei QM9414 and T. reesei RP698. The final enzymatic assay volume was 5 mL, and the first and third tubes are control assays with crude cellulase previously inactivated by heating. b Influence of glucose as carbon source on the production of cellulases by T. reesei RP698. One hundred percent of the activities are Avicelase 0.46 ± 0.03 U/mL; CMCase 11.60 ± 0.92 U/mL; FPase 0.43 ± 0.03 U/mL. c pH optima and d temperature optima of cellulase activity from T. reesei RP698. Symbols: The black-filled square represents the Avicelase activity (100% was 0.81 ± 0.05 U/mL). The red-filled circle represents the CMCase activity (100% was 12.52 ± 1.3 U/mL). The blue up-pointing arrow represents the FPase activity (100% was 0.78 ± 0.06 U/mL). e Thermal stability of CMCase activity from T. reesei RP698. The crude filtrate was heated at 40 °C (black-filled square), 50 °C (red-filled circle), 60 °C (blue up-pointing arrow), and 70 °C (orange down-pointing arrow) without substrate and tested for residual CMCase activity. One hundred percent of CMCase activity was 12.30 ± 0.98 U/mL. All data are the means ± SD of three experiments (n = 3)
Catabolic repression
The addition of 50 mM of glucose to cultures of RP698 substantially decreased the production of all three classes of cellulases (Fig. 2b). Under these conditions, the production of CMCase, Avicelase, and FPase decreased by 60%, 50%, and 73%, respectively, as compared to the control without glucose addition. Glucose concentrations below 50 mM affect the production of the enzymes retaining 75% of relative activity for Avicelase and FPase. CMCase production presented the lowest degree of repression, where 95% of the relative activity was retained at a glucose concentration of 20 mM (Fig. 2b).
Effects of pH and temperature
The pH optima were pH 5.0 for Avicelase activity and pH 4.0–5.0 for the CMCase and FPase activities (Fig. 2c), with all activities above 95% of maximum at pH 5.0. The profile of cellulase activity in the crude filtrate obtained from RP698 as a function of the reaction temperature (Fig. 2d) revealed that the optimum temperature was 55–60 °C for Avicelase activity and 60–65 °C for CMCase and FPase activities. At 60 °C, all activities were above 95% of the maximum. As the major cellulase activity observed in the crude filtrate from RP698, the CMCase thermal stability was tested after different incubation times (Fig. 2e), and the activity half-life (T½) at 70 °C was 3 min. After 1 h at 60 °C, approximately 60% of the initial activity remained, and at 40–50 °C, there was no significant change in the activity values even after 1-h incubation.
Effect of hydrolysis end-products on enzyme activity
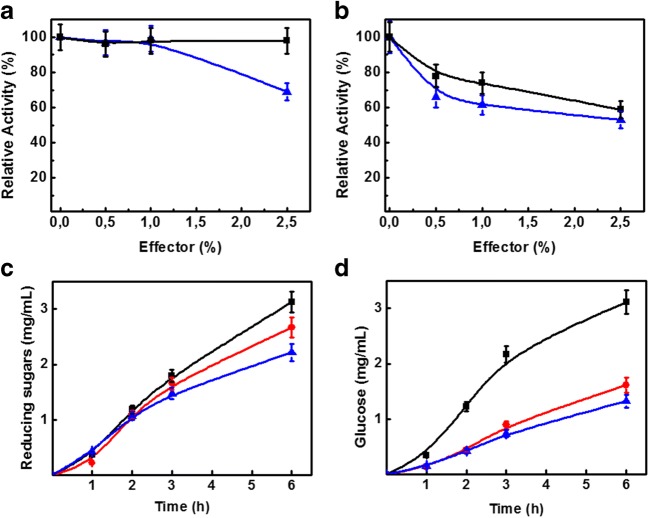
Cellulase activity of RP698 was not significantly affected by low glucose concentrations using Cellulose-Azure® as substrate, maintaining 98% of the control activity at concentrations of < 2.5% (w/v) added glucose (Fig. 3a). RP698 showed a reduction of up to 30% after the addition of 2.5 % cellobiose, whereas QM9414 showed a reduction of 40% and 47% activity in the presence of glucose and cellobiose, respectively, under the same conditions (Fig. 3b).
Fig 3.
Effect of the addition of glucose (black-filled square) and cellobiose (blue up-pointing arrow) on cellulase activity of crude filtrate from T. reesei RP698 (a) and T. reesei QM9414 (b). The assay was performed using Cellulose-Azure® as substrate, at 60 °C, pH 5.0 for 1 h. One hundred percent of cellulose-azure activity was 0.6 ± 0.09 U/mL. Reducing sugars (c) and glucose (d) released in the filter paper assay by enzymatic mixtures. All the enzymes were applied at 10 FPU/g of substrate and assayed at 50 °C. Symbols (for c and d): The blue up-pointing arrow represents the T. reesei RP698 cellulase extract. The red-filled circle represents the T. reesei RP698 cellulase extract plus crude StGTBG. The black-filled square represents the T. reesei RP698 cellulase extract plus crude StGSBG. All data are the means ± SD of three experiments (n = 3)
Hydrolysis of cellulose in enzymatic mixtures
According to Zanoelo et al. [23] and Silva et al. [24], the same strain of the fungus S. thermophilum produces an intracellular glucose-stimulated-β-glucosidase(StGSBG) and an extracellular glucose-tolerant β-glucosidase (StGTBG) concomitantly in the same growth conditions. Taking into consideration the high glucose tolerance of the cellulolytic system from RP698 as demonstrated by the previous results, enzymatic mixtures for cellulose hydrolysis were prepared using cellulase rich extracts obtained from RP698 and the two crude filtrates rich in β-glucosidases from the same strain of S. thermophilum (as described in the Section 2.10). Both types of β-glucosidases added led to an increase in the release of total reducing sugars and glucose from Whatman No. 1 filter paper (Fig. 3c, d). The mixture with StGSBG produced about 130% more glucose than the crude cellulase of RP698 and 110% more than the mixture with StGTBG (Fig. 3d).
Discussion
The mutant strain RP698 was obtained by the selection of fast-growing colonies on solidified Mandels’ medium. The difference in RP698 growth rate can be easily observed on PDA slants when the mutant was cultured under the same conditions as the QM9414 (Fig. 1a). In liquid cultures, cellulase production by RP698 was also observed at shorter times as compared to the QM9414 strain. The optimum period for cellulase production by T. reesei RP698 using Mandels’ medium was 72 h (Fig. 1b), which is considerably shorter than the 5–14 days for Trichoderma lineages reported in the literature [18, 26–28].
Cellulase production by RP698 was increased by up to 2-fold as compared to the QM9414 when both strains were grown in Mandels’ medium (Table 1), even under non-optimized conditions. These results can be compared with previous reports in the literature, where Jiang et al. [28] have described a Trichoderma mutant strain that showed a 1.67- and 2.17-fold increase in CMCase and FPase activities, respectively, relative to QM9414. Li et al. [29] reported the results of microwave and ultraviolet radiation mutagenesis experiments using a strain of T. viride, which presented an increased cellulase production by 7.7% compared to the wild-type. Shafique et al. [30] reported another T. reesei mutant that exhibited a 1.5- to 2-fold increase in cellulase production when compared to the parental strain.
The highest cellulase activities from RP698 were obtained using Avicel®, steam-exploded sugarcane bagasse, and cellobiose as carbon sources (Table 2). It is known that sophorose is a strong inducer of cellulase expression in T. reesei and is synthesized from glucose in a transglycosylation reaction by BGLI/Cel3A during cellulose hydrolysis. Therefore, the accumulation of sophorose in the medium by transglycosylation could be the reason for the high levels of cellulase expression [31, 32].
Low levels of catabolic repression of the production of CMCases, Avicelase, and FPase by RP698 were also observed (Fig. 2b). The role of catabolic repression is important in the natural environment because it prevents the fungus from unnecessarily over-producing cellulases [33]. However, from a biotechnological perspective, the repression of enzyme production is undesirable and the observation that glucose affected the production of the three enzymes by RP698 only at 50 mM is an important characteristic of industrially relevant strains of T. reesei. Persson et al. [34] compared the production of cellulases among several strains of T. reesei and found mutant strains resistance to catabolic repression in T. reesei CL-847, T. reesei Rut C30, and T. reesei L27. In T. reesei, the cellulase genes cbh1, cbh2, egl1, egl2, and egl5 are repressed by glucose at the transcriptional level [35].
The optimum catalytic pH values (Fig. 2c) of the CMCase and Avicelase activities were similar to those previously described in the literature for other fungal cellulases [3, 36, 37]. The optimum temperature for enzyme activity (Fig. 2d) was higher than that observed by Ghose [19] for Trichoderma cellulases. Cellulases from other mesophilic fungi such as A. terreus, Neurospora crassa, T. viride, and several isolates of T. reesei present similar optimum temperatures [38–41]. The thermostability observed for the CMCase produced by T. reesei RP698 was particularly interesting for the application in simultaneous saccharification and fermentation (SSF) processes that reduces end-product enzyme inhibition and investment costs [42]. Some yeast strains have been described that are able to ferment lignocellulosic hydrolysates at temperatures ranging from 37–42 °C with good ethanol yields [43, 44].
Initial glucose concentrations of up to 2.5% (w/v) in the culture medium did not affect the levels of of cellulase activity in RP698 extracts; however, the addition of cellobiose at a concentration of 2.5% (w/v) reduced the enzyme activity for both strains. Analysis of cellulase production by QM9414 under the same conditions demonstrated a greater inhibition of enzyme activity in the presence of glucose (40%) and cellobiose (47%) as compared with the mutant strain RP698. Tolerance to final hydrolysis products is a characteristic that is highly desirable for industrial applications, since many cellulolytic enzymes are inhibited by the end-products of cellulose saccharification [14, 45, 46]. Holtzapple et al. [45] evaluated the effect of sugars and solvents on the activity of a commercial preparation of T. reesei cellulase and demonstrated an inhibitory effect due to the addition of glucose and cellobiose, in which the effect of glucose was lower than the inhibition caused by cellobiose. There are only few reports in the literature of cellulases showing glucose and cellobiose tolerance. Chandra et al. [47] reported a mutant of T. citrinoviride exhibiting low enzyme inhibition in the presence of 30 mM glucose. Since the inhibition of cellulases by glucose and cellobiose is a common feature in Trichoderma species [16], the glucose tolerance observed for the cellulase produced by T. reesei RP698 is an uncommon characteristic of interest for biomass saccharification applications.
Since CMCases are responsible for the random cleavage of the amorphous regions and Avicelase are responsible for the random cleavage of the crystalline regions in cellulose chains, the 2-fold increase in CMCase and Avicelase activity found in the T. reesei RP698 filtrate (Table 1) may explain the greater effect against filter paper (FPase) than that presented by QM9414 CMCase (Fig. 2a). In addition, Saloheimo et al. [48] described a protein with expansin-like function in T. reesei, which was termed “swollenin.” Expansins are proteins found in plants, which act by breaking the hydrogen bonds between cellulose microfibrils and other polysaccharides without hydrolytic action, thereby permitting sliding between cellulose fibers and cell wall expansion [49]. Chen et al. [49] expressed the gene for swollenin from Aspergillus fumigatus in Aspergillus oryzae and found that simultaneous incubation of this protein with a mixture of cellulases increased Avicel® saccharification. Andberg et al. [50] reported that swollenin from T. reesei also has a hydrolytic activity, showing a unique mode of action with similarities to the action of endoglucanases and cellobiohydrolases. It is possible that swollenin production in RP698 was increased in comparison to QM9414, since there was more pronounced filter paper disintegration in the assay with RP698.
An ensemble of different cellulolytic enzymes is required for the enzymatic cocktail to efficiently release glucose from cellulose. The low-level production of β-glucosidases by T. reesei strains is well described in the literature [14]. Zanoelo et al. [23] have described a glucose stimulated mycelial β-glucosidase of S. thermophilum (StGSBG), and the mixture between T. reesei RP698 cellulase and StGSBG released more glucose when compared to the crude T. reesei RP698 cellulase. This mixture also produced more glucose than the mixture with StGTBG, indicating that a high conversion of cellobiose to glucose and suggesting greater possibilities of application of this enzymatic mixture in cellulose hydrolysis applications. Cao et al. [9] observed an increase in the hydrolysis yield using NaOH pretreated sugarcane bagasse when using β-glucosidases derived from a metagenomic library with high glucose tolerance and stimulation to supplement the commercial enzyme cocktail Celluclast 1.5 L (a commercial blend of T. reesei cellulases). Under these conditions, an increase in glucose release was observed that was correlated to a 14–35% increase in the conversion of pre-treated sugarcane bagasse. All these characteristics indicate the potential use of the T. reesei RP698 mutant strain for biotechnological applications, in particular for the formulation of enzymatic cocktails for cellulose hydrolysis aiming the production of cellulosic ethanol.
Acknowledgments
This work was supported by research grants from CNPq, Brazil (Conselho de Desenvolvimento Científico e Tecnológico), CAPES, Brazil (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and FAPESP, Brazil (Fundação de Amparo à Pesquisa do Estado de São Paulo). J.C.R.S. received a Ph.D. scholarship from FAPESP, J.C.S.S. and A.C.V. received a post-doctoral scholarship from CAPES, and R.J.W., M.L.T.M.P., R.P.M.F., and J.A.J. are CNPq research fellows. This work was part of J.C.R.S Doctoral thesis (Departamento de Bioquímica da Faculdade de Medicina de Ribeirão Preto-USP). We thank Ricardo F. Alarcon, Mariana Cereia, and Mauricio de Oliveira for their technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Percival Zhang Y-H, Himmel ME, Mielenz JR. Outlook for cellulase improvement: screening and selection strategies. Biotechnol Adv. 2006;24:452–481. doi: 10.1016/J.BIOTECHADV.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Ummartyotin S, Manuspiya H. A critical review on cellulose: from fundamental to an approach on sensor technology. Renew Sustain Energy Rev. 2015;41:402–412. doi: 10.1016/j.rser.2014.08.050. [DOI] [Google Scholar]
- 3.Ögel ZB, Yarangümeli K, Dündar H, Ifrij İ (2001) Submerged cultivation of Scytalidium thermophilum on complex lignocellulosic biomass for endoglucanase production. Enzyme Microb Technol 28:689–695. 10.1016/S0141-0229(01)00315-5 [DOI] [PubMed]
- 4.Singhvi MS, Chaudhari S, Gokhale DV. Lignocellulose processing: a current challenge. RSC Adv. 2014;4:8271. doi: 10.1039/c3ra46112b. [DOI] [Google Scholar]
- 5.Meleiro LP, Salgado JCS, Maldonado RF, Alponti JS, Zimbardi ALRL, Jorge JA, Ward RJ, Furriel RPM (2015) A Neurospora crassa ß-glucosidase with potential for lignocellulose hydrolysis shows strong glucose tolerance and stimulation by glucose and xylose. J Mol Catal B Enzym 122:131–140. 10.1016/j.molcatb.2015.09.003
- 6.Salgado JCS, Meleiro LP, Carli S, Ward RJ. Glucose tolerant and glucose stimulated β-glucosidases– a review. Bioresour Technol. 2018;267:704–713. doi: 10.1016/J.BIORTECH.2018.07.137. [DOI] [PubMed] [Google Scholar]
- 7.Tandrup T, Frandsen KEH, Johansen KS, Berrin JG, Lo Leggio L. Recent insights into lytic polysaccharide monooxygenases (LPMOs) Biochem Soc Trans. 2018;46(6):1431–1447. doi: 10.1042/BST20170549. [DOI] [PubMed] [Google Scholar]
- 8.Jørgensen H, Olsson L. Production of cellulases by Penicillium brasilianum IBT 20888—effect of substrate on hydrolytic performance. Enzyme Microb Technol. 2006;38:381–390. doi: 10.1016/J.ENZMICTEC.2005.06.018. [DOI] [Google Scholar]
- 9.Cao L-C, Wang Z-J, Ren G-H, Kong W, Li L, Xie W, Liu YH. Engineering a novel glucose-tolerant β-glucosidase as supplementation to enhance the hydrolysis of sugarcane bagasse at high glucose concentration. Biotechnol Biofuels. 2015;8:202. doi: 10.1186/s13068-015-0383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Obeng EM, Adam SNN, Budiman C, Ongkudon CM, Maas R, Jose J. Lignocellulases: a review of emerging and developing enzymes, systems, and practices. Bioresour Bioprocess. 2017;4:16–22. doi: 10.1186/s40643-017-0146-8. [DOI] [Google Scholar]
- 11.Gusakov AV. Alternatives to Trichoderma reesei in biofuel production. Trends Biotechnol. 2011;29:419–425. doi: 10.1016/j.tibtech.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Singhania RR, Patel AK, Sukumaran RK, Larroche C, Pandey A. Role and significance of beta-glucosidases in the hydrolysis of cellulose for bioethanol production. Bioresour Technol. 2013;127:500–507. doi: 10.1016/J.BIORTECH.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Bischof RH, Ramoni J, Seiboth B. Cellulases and beyond: the first 70 years of the enzyme producer Trichoderma reesei. Microb Cell Fact. 2016;15:106. doi: 10.1186/s12934-016-0507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berlin A, Maximenko V, Gilkes N, Saddler J. Optimization of enzyme complexes for lignocellulose hydrolysis. Biotechnol Bioeng. 2007;97:287–296. doi: 10.1002/bit.21238. [DOI] [PubMed] [Google Scholar]
- 15.Tiwari P, Misra BN, Sangwan NS (2013)β-Glucosidases from the Fungus Trichoderma: An efficient Cellulase Machinery in Biotechnological Applications. Biomed Res Int 2013:203735. 10.1155/2013/203735 [DOI] [PMC free article] [PubMed]
- 16.Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol Mol Biol Rev. 2002;66:506–577. doi: 10.1128/MMBR.66.3.506-577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dashtban M, Buchkowski R, Qin W. Effect of different carbon sources on cellulase production by Hypocrea jecorina (Trichoderma reesei) strains. Int J Biochem Mol Biol. 2011;2:274–286. [PMC free article] [PubMed] [Google Scholar]
- 18.Mandels M, Sternberg D. Recent advances in cellulase technology. Ferment Technol. 1975;54:267–286. [Google Scholar]
- 19.Ghose TK. Measurement of cellulase activities. Pure Appl Chem. 1987;59:257–268. doi: 10.1351/pac198759020257. [DOI] [Google Scholar]
- 20.Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Lai TE, Pullammanappallil PC, Clarke WP. Quantification of cellulase activity using cellulose-azure. Talanta. 2006;69(1):68–72. doi: 10.1016/j.talanta.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 23.Zanoelo FF, Polizeli MLTM, Terenzi HF, Jorge JA (2004)Beta-Glucosidase activity from the thermophilic fungus Scytalidium thermophilum is stimulated by glucose and xylose. FEMS Microbiol Lett 240:137–143. 10.1016/j.femsle.2004.09.021 [DOI] [PubMed]
- 24.Bergmeyer HU, Bernt E. Determination of glucose with glucose oxidase and peroxidase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. pp. 1206–1215. [Google Scholar]
- 25.Silva JCR, Guimarães LHS, Salgado JCS, Furriel RPM, Polizeli MLTM, Rosa JC, Jorge JA. Purification and biochemical characterization of glucose-cellobiose-tolerant cellulases from Scytalidium thermophilum. Folia Microbiol (Praha) 2013;58:561–568. doi: 10.1007/s12223-013-0245-7. [DOI] [PubMed] [Google Scholar]
- 26.Mandels M, Weber J, Parizek R. Enhanced cellulase production by a mutant of Trichoderma viride. Appl Microbiol. 1971;21:152–154. doi: 10.1128/AEM.21.1.152-154.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J, Wang Y-H, Chu J, Zhuang YP, Zhang SL, Yin P. Identification and purification of the main components of cellulases from a mutant strain of Trichoderma viride T 100-14. Bioresour Technol. 2008;99:6826–6833. doi: 10.1016/J.BIORTECH.2008.01.077. [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Geng A, He N, Li Q. New isolate of Trichoderma viride strain for enhanced cellulolytic enzyme complex production. J Biosci Bioeng. 2011;111:121–127. doi: 10.1016/J.JBIOSC.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Yang H, Roy B, Park EY, Jiang LJ, Wang D, Miao YG. Enhanced cellulase production of the Trichoderma viride mutated by microwave and ultraviolet. Microbiol Res. 2010;165:190–198. doi: 10.1016/J.MICRES.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Shafique S, Bajwa R, Shafique S. Molecular characterisation of UV and chemically induced mutants of Trichoderma reesei FCBP-364. Nat Prod Res. 2010;24:1438–1448. doi: 10.1080/14786410903132399. [DOI] [PubMed] [Google Scholar]
- 31.Kubicek CP. Involvement of a conidial endoglucanase and a plasma-membrane-boundbeta-glucosidase in the induction of endoglucanase synthesis by cellulose in Trichoderma reesei. J Gen Microbiol. 1987;133:1481–1487. doi: 10.1099/00221287-133-6-1481. [DOI] [PubMed] [Google Scholar]
- 32.Castro LDS, Pedersoli WR, Antoniêto ACC, et al. Comparative metabolism of cellulose, sophorose and glucose in Trichoderma reesei using high-throughput genomic and proteomic analyses. Biotechnol Biofuels. 2014;7:41. doi: 10.1186/1754-6834-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suto M, Tomita F (2001) Induction and catabolite repression mechanisms of cellulase in fungi. J Biosci Bioeng 92:305–311. 10.1016/S1389-1723(01)80231-0 [DOI] [PubMed]
- 34.Persson I, Tjerneld F, Hahn-Hägerdal B (1991) Fungal cellulolytic enzyme production: a review. Process Biochem 26:65–74. 10.1016/0032-9592(91)80019-L
- 35.Ilmén M, Saloheimo A, Onnela ML, Penttilä ME. Regulation of cellulase gene expression in the filamentous fungus Trichoderma reesei. Appl Environ Microbiol. 1997;63:1298–1306. doi: 10.1128/AEM.63.4.1298-1306.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arifoǧlu N, Ögel ZB (2000)Avicel-adsorbable endoglucanase production by the thermophilic fungus Scytalidium thermophilum type culture Torula thermophila. Enzyme Microb Technol 27:560–569. 10.1016/S0141-0229(00)00241-6 [DOI] [PubMed]
- 37.Kaur J, Chadha BS, Saini HS (2006) Regulation of cellulase production in two thermophilic fungi Melanocarpus sp. MTCC 3922 and Scytalidium thermophilum MTCC 4520. Enzyme Microb Technol 38:931–936. 10.1016/J.ENZMICTEC.2005.08.036
- 38.Garg SK, Neelakantan S. Studies on the properties of cellulase enzyme from Aspergillus terreus GN1. Biotechnol Bioeng. 1982;24:737–742. doi: 10.1002/bit.260240316. [DOI] [PubMed] [Google Scholar]
- 39.Beldman G, Searle-Van Leeuwen MF, Rombouts FM, Voragen FGJ. The cellulase of Trichoderma viride purification, characterization and comparison of all detectable endoglucanases, exoglucanases and β-glucosidases. Eur J Biochem. 1985;146:301–308. doi: 10.1111/j.1432-1033.1985.tb08653.x. [DOI] [PubMed] [Google Scholar]
- 40.Busto MD, Ortega N, Perez-Mateos M. Characterization of microbial endo-β-glucanase immobilized in alginate beads. Acta Biotechnol. 1998;18:189–200. doi: 10.1002/abio.370180303. [DOI] [Google Scholar]
- 41.Kadowaki MAS, Camilo CM, Muniz AB, Polikarpov I. Functional characterization and low-resolution structure of an endoglucanase Cel45A from the filamentous fungus Neurospora crassa OR74A: thermostable enzyme with high activity toward lichenan and β-glucan. Mol Biotechnol. 2015;57:574–588. doi: 10.1007/s12033-015-9851-8. [DOI] [PubMed] [Google Scholar]
- 42.Olofsson K, Bertilsson M, Lidén G. A short review on SSF – an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol Biofuels. 2008;1:7. doi: 10.1186/1754-6834-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costa DA, de Souza CJA, Costa PS, Rodrigues MQ, dos Santos A, Lopes MR, Genier HL, Silveira WB, Fietto LG. Physiological characterization of thermotolerant yeast for cellulosic ethanol production. Appl Microbiol Biotechnol. 2014;98:3829–3840. doi: 10.1007/s00253-014-5580-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Narra M, James JP, Balasubramanian V. Simultaneous saccharification and fermentation of delignified lignocellulosic biomass at high solid loadings by a newly isolated thermotolerant Kluyveromyces sp. for ethanol production. Bioresour Technol. 2015;179:331–338. doi: 10.1016/J.BIORTECH.2014.11.116. [DOI] [PubMed] [Google Scholar]
- 45.Holtzapple M, Cognata M, Shu Y, Hendrickson C. Inhibition of Trichoderma reesei cellulase by sugars and solvents. Biotechnol Bioeng. 1990;36:275–287. doi: 10.1002/bit.260360310. [DOI] [PubMed] [Google Scholar]
- 46.Kumar R, Singh S, Singh OV. Bioconversion of lignocellulosic biomass: biochemical and molecular perspectives. J Ind Microbiol Biotechnol. 2008;35:377–391. doi: 10.1007/s10295-008-0327-8. [DOI] [PubMed] [Google Scholar]
- 47.Chandra M, Kalra A, Sangwan NS, Gaurav SS, Darokar MP, Sangwan RS. Development of a mutant of Trichoderma citrinoviride for enhanced production of cellulases. Bioresour Technol. 2009;100:1659–1662. doi: 10.1016/J.BIORTECH.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Saloheimo M, Paloheimo M, Hakola S, Pere J, Swanson B, Nyyssönen E, Bhatia A, Ward M, Penttilä M. Swollenin, a Trichoderma reesei protein with sequence similarity to the plant expansins, exhibits disruption activity on cellulosic materials. Eur J Biochem. 2002;269:4202–4211. doi: 10.1046/j.1432-1033.2002.03095.x. [DOI] [PubMed] [Google Scholar]
- 49.Chen X, Ishida N, Todaka N, Nakamura R, Maruyama J, Takahashi H, Kitamoto K. Promotion of efficient Saccharification of crystalline cellulose by Aspergillus fumigatus Swo1. Appl Environ Microbiol. 2010;76:2556–2561. doi: 10.1128/AEM.02499-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andberg M, Penttilä M, Saloheimo M. Swollenin from Trichoderma reesei exhibits hydrolytic activity against cellulosic substrates with features of both endoglucanases and cellobiohydrolases. Bioresour Technol. 2015;181:105–113. doi: 10.1016/J.BIORTECH.2015.01.024. [DOI] [PubMed] [Google Scholar]