Abstract
Introduction
H1N1 influenza virus, as an indoor/outdoor pathogen in air, can cause the flu-like illness and respiratory complication. The aim of this study was to evaluate the H1N1 influenza virus replication in pancreas and investigate the immune response against infected pancreas.
Material and methods
First, mouse pancreas cell line was infected by H1N1 influenza A virus using intranasally and intravenously infection methods, and then the pancreas tissue was collected and pathology experiment was carried out. Next, the protein and genome of influenza virus were detected using immunocytochemistry and real-time PCR, respectively. In addition, serum cytokines and serum lipase were investigated using ELISA.
Result
The in-vitro results proved that the mouse pancreatic cell line can support influenza virus replication. The result also proved that influenza virus is capable to infect pancreas and induce pancreas damage. Further, the immune response in mice with infected pancreas exhibited a completely different pattern with that of mice infected through intranasal method.
Conclusion
It can be concluded that influenza virus can infect pancreas and change the influenza disease pathway, which might result in a pancreatic injury.
Keywords: Influenza A, Airborne pathogen, Pancreas, H1N1
Introduction
Lower respiratory infectious diseases are consisting 5% of global disability adjusted life years (DALY) as reported by world health organization in Global health estimate 2016. One of the most important related pathogens is H1N1 influenza A (IAV). As reported by the united states’ Centre for diseases control (CDC), H1N1 influenza A was positive in 68.1% (10,439 cases) of hospitalized patients due to influenza between October 1, 2019 and February 22, 2020 in the united states. H1N1 influenza A is an airborne viral pathogen in both outdoor and especially indoor. Sneezing is a common sign of H1N1 influenza A. thus is can be spread everywhere by fine droplet and is a an easy-transmission airborne pathogen. According to this reasons, H1N1 influenza A is one the high priority air pollutant and it has been diagnosed as the most common infectious etiology in human respiratory disease. As such, H1N1 influenza was identified as the most circulating virus in the world at 2009 (previously referred as novel influenza A (H1N1). The previous study reported that the severe lung injury was observed after cytokine storm diagnosis, which was identified as an excessive inflammatory response [1]. In addition to the respiratory systems, IAV infection can also affect other organs including the spleen, liver, small intestine, and brain. Several case studies reported that the various complicated pancreatitis were observed in patients after infected by IAV, which might cause acute pancreatitis and pancreatic injury in human [2, 3]. Similar studies dealing with patients with type 1 diabetes (T1D) have also reported that other viral infections such as Coxsackievirus B, measles, congenital rubella, mumps, cytomegalovirus, and influenza B can be one of the important factors in acute pancreatitis and pancreatic injury [4–6]. In addition, similar studies reported some cases involved T1D after influenza virus infection [7–9]. Notably, a recent study showed an increase in risk of T1D after H1N1 influenza A virus infection [10]. Histopathological and immunohistochemical investigations on bird pancreas indicated the high tropism and the excellent replication of avian influenza in the pancreas [11].
Human Influenza virus can initiate an infection by binding to sialic acids (SA) and human influenza virus through α-2,6-SA and avian influenza virus attachment with α-2,3-SA receptors (located on the surface of cells), [12, 13]. Therefore, the type of SA receptor on cellular surface plays a key role in determining the host tropism of influenza virus. Further, some study indicated that both α-2,3-SA and α-2,6-SA receptors were expressed on the surface of pancreas cells [14].
As stated earlier, influenza virus infection is a specific respiratory disease, thus how this virus spreads to other tissues in systemic infections still needs further researches. It is important to note that the trypsin is needed to break the HA0 protein into HA1 and HA2, thereby converting the non-infectious virus into the infectious virus. Therefore, the trypsin enzyme in the pancreas tends to active an infection following by a systemic influenza infection. As such, some studies reported that presence of trypsinogen in pancreas led to the activation of acute pancreatitis. In addition, these studies have also indicated that the immune system, particularly neutrophils, is the main factor for activation of trypsinogen to trypsin in the pancreas [15, 16].
In this study, the mouse adapted influenza A virus (H1N1/PR8 mouse-adapted) was selected, and the ability of influenza virus replication in mouse pancreas cell line and the ability to induce pancreas damage were investigated. In addition, the potential role of the immune system in pancreatic injury in mice was evaluated.
Material and methods
Ethical considerations
Ethical Committee of Tehran University of Medical Science, approval ID (IR.TUMS.SPH.REC.1397.005), was followed for all experiments involving mouse and chicken embryos.
Virus propagation
Influenza A/H1N1/ PR8 (mouse adapted virus) was supplied by National Laboratory Influenza at Iran. First, the virus was propagated in Specific-Pathogen-Free (SPF) eggs, and then amniotic fluid was harvested. Next, the virus stock was purified and concentrated through centrifugation in 1500×g for 7 min at 4 °C, and the resultants overlaid onto 15% sucrose in Dulbecco’s phosphate-buffered saline (DPBS; Sigma-Aldrich). Samples were centrifuged again in 110,000×g for 2.5 h at 4 °C, followed by pellet suspending in the PBS, and storing at −80 °C prior to further analysis. Titration of virus was carried out using tissue culture infective dose (TCID50) assay through MDCK cell culture in 96 well plate [17].
Cell culture
Cell lines used in this study are as follows: (i) Mouse Insulinoma cell line (MIN6) (Iranian resource centre, Tehran, Iran) cultured in DMEM containing 2 mM L-glutamine,15% fetal bovine serum, 100 μg/ml streptomycin, and 100 U/ml penicillin, (ii) Madin-Darby canine kidney (MDCK-SIAT) cell line (obtained from National Laboratory Influenza, Tehran, Iran) cultured in a mixture of DMEM 10% fetal bovine serum, 100 μg/ml streptomycin, and 100 U/ml penicillin. Both cells were incubated at 37 °C in a humidified atmosphere with 5% CO2.
Cell infection
First, MDCK, MIN6 were cultured in six wells microplate. Once cells received 80% confluence, the cells were washed two times with phosphate buffer saline (PBS). Next, cells were infected with 0.1, 1, and 10 multiplicities of infection (MOIs) of Influenza A/H1N1/ PR8 virus, and incubated at 33 °C for 1 h at 5% CO2. The plates slowly shacked every 10 min to achieve the best adsorption. One of six wells in each plate was considered as control. The confluent cells were washed two times with PBS for removing unadsorbed viruses. Finally, 4 ml media containing 1 μg/ml tosyl phenylalanyl chloromethyl ketone (TPCK)-Tripsin was added into the samples. The virus titration was carried out to measure the virus yield.
Animal model
Eight-week-old female balb/c mice were purchased from Razi Vaccine and Serum Research Institute (Karaj, Iran). All experiments were conducted using live virus, which were performed under laminar hood. The Animal Ethics Committee at the Tehran University of Medical Sciences were followed during animal experiments. All challenged were carried out under anaesthesia by ketamine 50 mg/kg and xylazine 5 mg/kg, IP. Tree groups of balb/c mice were formed and treated as follows:
Group 1 [control]: mice were intranasally mock challenged with 50 mL of sterile PBS-diluted.
Group 2 [H1N1PR8 mouse-adapted virus]: mice were intranasally (IN) infected with 50 mL of viral suspension containing 103 TCID50/50 mL of the H1N1 PR8 mouse-adapted virus.
Group 3 [H1N1PR8 mouse-adapted virus]: mice were intravenously (IV) infected through tail vein with 20 mL of viral suspension containing 102 TCID50/50 mL of the H1N1PR8 mouse-adapted virus.
The daily weight was recorded after infection in which the mice with 25% weigh loss were euthanized. To nest experiment, the blood samples of tail vein were collected from all the mice before infection. Such an experiment was also performed every day after infection until the end of study. The glucose level of blood samples was measured using the Easygluco (South Korea), by which the >250 mg/mL non-fasting blood sugar after two consecutive tests indicated the diabetes in mice. At various days after infection, pancreas tissue samples were collected from mice for detecting virus using RT-PCR, and then samples were fixed in 4% phosphate buffered formalin for histopathological and immunohistochemistry tests.
Cytokine ELISA
The concentrations of serum lipase using sandwich enzyme-linked immunosorbent assay (ELISA) kit (casubio, China), IL-6, IL-17a, IL-33, IL-10, and Granulocyte colony stimulating factor (G-CSF) in mice, in which samples were collected at 1, 3, 5 and 7 days after infection. The cytokine value was investigated using ELISA kit at the same sampling times (Invitrogen, ebioscience, USA) according to the manufacture instruction.
RT-PCR
Total RNA was extracted from samples (MIN6 cell line and pancreas tissue). The cDNA was synthesized from mRNA using random primers and reverse transcriptase (GeNet Bio South Korea). The cDNA of target gens was quantified through Real-time quantitative PCR analysis using applied biosystems advice. Amplification of GAPDH was performed as an internal control. Influenza A virus matrix 2 (M2): sense, 5′-GAGGTCGAAACGCCT-3′, antisense, 5′-CTGTTCCTTTCGATATTCTTCCC-3′. Quantitative Real-Time PCR was performed using 5x evagreen qPCR mix (Solis BioDyne, Estonia). The relative expression of mRNA was determined using of 2-ΔΔCt assay.
Histopathological assay
Pancreatic tissue samples were fixed in 10% formalin and embedded in paraffin. Samples were sectioned and mounted on a slide and stained with haematoxylin and eosin (H&E) before evaluation by light microscopic.
Immunocytochemistry
MIN6 cell line (5 × 107 cell/ml) were transferred into each of six wells cell culture. Influenza A/H1N1 1 MOI was added into cell culture plate, but RPMI was added solely into one of wells, which was considered as control sample, and then plate was incubated for 1 h at 37 °C. The cells were washed two times with PBS for removing unattached virus, and RPMI added into cell culture. At 48 h post-infection time, cells were fixed with 4% paraformaldehyde for 10 min at 25 °C. Next, to permeabilization, cells were added into Triton X100(CMG, IRAN) (0.5%) in PBS at 25 °C for 5 min. Cells were washed two times with PBS and blocked with bovine serum albumin (BSA) (Gipco, USA) (1%) at 25 °C for 1 h. cells were stained with mouse anti-nucleoprotein H1N1 influenza A as primary antibody (final concentration 4 μg/ml in BSA) (Sinobiological, China), and then incubated at 37 °C for 1 h or 4 °C for overnight. Cells were washed again three times with PBS. The secondary antibody, rabbit anti-mouse antibody labelled with FITC (final concentration 1–2 μg/ml), (Sinobiological, China) was added and incubated at room temperature for 1 h at dark condition. Cells were washed three times with PBS, and nuclear cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) (vector labs, UK). Cells were imaged on Olympus ix71 fluorescence microscope and scanning with IX2-BSW (Ver. 01.03) software.
Statistical analysis
Statistical analysis was conducted using Prism (Graphpad Prism 6.1 software, La Jolla. CA, USA). All experiments were analysed by Student t-test, tow-way ANOVA, and Tukey test. The significant level was considered at P value <0.05.
Results
Replication of influenza A/H1N1/PR8 virus in pancreas cell line
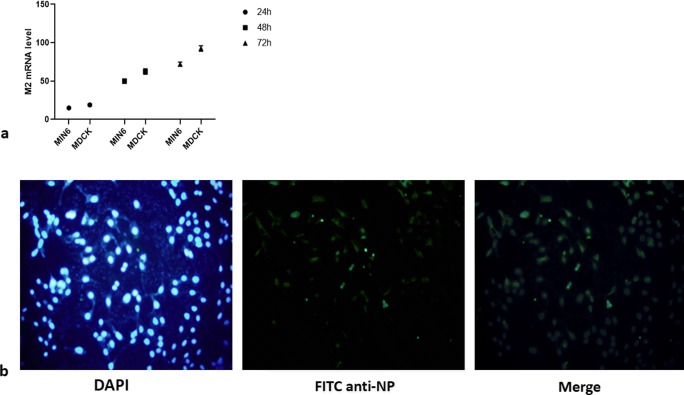
To evaluate the potential replication of influenza virus in MIN6, mouse pancreas cell line, (in-vitro), releasing the progeny infectious influenza virus test, real-time quantitative PCR, and immunocytochemistry assay were carried out. We expected the M2 mRNA gene expression after infection up to 72 h, thereby the expression of M2 mRNA in MIN6 was investigated at 24, 42, and 72 h after infection. As shown in Fig. 1a, expression of M2 mRNA increased at 72 h post-infection time as well as MDCK cell line was control for replication of influenza virus. The signal strength of NP of infected MIN6 cell line can be seen in Fig. 1b (using immunofluorescence cell staining) in which NP was produced at 72 h after infection. The production of NP indicates the MIN6 infection caused by influenza virus and influenza protein.
Fig. 1.
a Time interval of influenza A (H1N1) virus, Matrix 2 (M2), mRNA synthesis was analysed with Real-time PCR. Data are shown as mean ± S.D. ***P < 0.001, indicating that M2 mRNA expression at 72 h post infection time is significantly pronounced compared with its expression at 24 h post infection times. b Immunocytochemistry analysis of influenza A (H1N1) virus nucleoprotein synthesis in MIN6 at 72 h post infection time with immunofluorescence
Non-fasting glycaemia in mice infected by influenza virus
To investigate that whether the infection caused by influenza virus (H1N1/PR8) is capable to impair glucose tolerance in mouse, two sets of experiments were performed as follows: first, the mice were infected by IN method that can simulate the routine influenza infections, and second, the mice were infected by IV method that can indicate the entrance of influenza virus into bloodstream for any reason. In both treated groups, the significate weight loss was observed, which was notable compared with control. The weight loss in both groups indicates the presence of active infection in the mouse body. The weight loss in mice infected by IN method was more compared with mice infected by IV method despite no significant difference in weight loss.
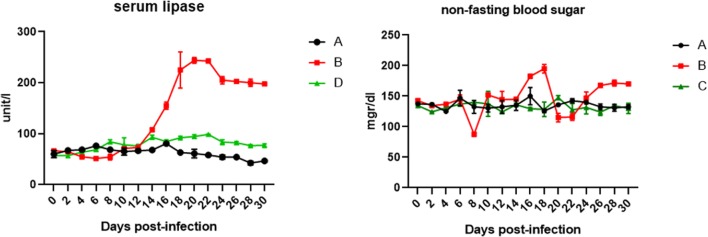
In this study, to determine the effect of influenza virus on the pancreas, non-fasting blood sugar and lipase enzyme as two important parameters were investigated. As shown in the Fig. 2, the rate of glucose in mice infected by IV method significantly increased compared with mice infected by IN method group. In addition, irregular changes in blood glucose level were observed in the mice infected by IV method, which sometimes reduced below the threshold level. These irregular changes in blood sugar indicate the pancreatic involvement. To investigation the pancreas damage, the serum lipase enzyme level is very important. As shown in Fig. 2, the serum lipase in mice infected by IV method was increased in duration of infection period, which the lipase level was higher compared with first group.
Fig. 2.
THE serum analysis of lipase an non fasting blood sugar in mice at during time post infection. A = Group control, B = mice were infected intranasally (IN), C = mice were infected intravenously (IV)
Cytokine response
To evaluate the secretion of serum cytokines in mouse, IL-6, IL-17a, IL-10, IL-33 and G-CSF samples were selected, in which the protein level at duration of infection was investigated using an ELISA Fig. 3.
Fig. 3.
Quantification of cytokines and chemokines using ELISA. IL-6, IL-17a, IL-10, IL-33, and G-CSF were selected in protein level at 1,3,5 and 7 post infection times in mice. C = Group control, A = mice were infected intranasally (IN), B = mice were infected intravenously (IV)
Accordingly, the G-CSF was increased over time, in which chemokine significantly increased the level of G-CSF in mice infected by IV and IN methods compared to control group. Notably, there was no significant difference in G-CSF levels in both infected groups until 5 days after infection. Whereas, at 7 days after infection, G-CSF level in mice infected by IV method was significantly increased compared with mice infected by IN method.
In addition, an increase in IL-33 was observed in both of infected groups. Initially, the serum IL-33 level was significantly higher in mice infected by IN method, but higher IL-33 level in 7 days’ post-infection was significantly in IV method. The increase in IL-6 level was started at the beginning of first day after infection in both infected groups, which continuously maintained until 7 days after infection. The IL-6 was reached its maximum level at 5 days after infections. Furthermore, IL-6 level was not significantly different between both infected groups. As such, the IL-10 was immediately increased at first day after infection in both infected groups. Notably, cytokine in mice infected by IV method was significantly decreased at 7 days after infection compared with that of mice infected by IN method. IL-17a increased significantly at the first day of infection in both of infected groups, in which the cytokine levels irregularly increased and decreased during infection despite no significant changes were observed.
Tissue processing
As mentioned earlier, to evaluate the replication of influenza virus, real-time PCR was carried out to detect influenza virus genome. As such, pancreas samples obtained from both infected groups were tested for detection of influenza RNA genome. The influenza genome was detected in five samples among 10 mice infected by IV method, in which the pancreas samples were obtained at seven days after infection. Whereas, the pancreas samples obtained from mice infected by IN method showed negative results for influenza RNA genome test.
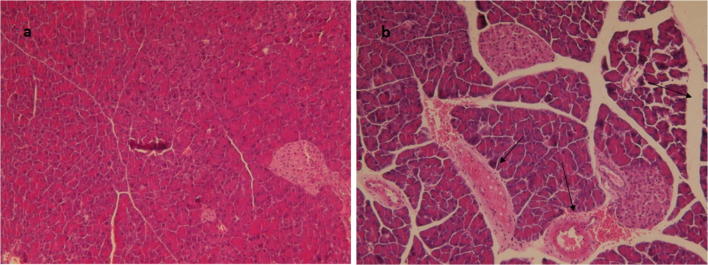
Further, the pancreas tissues were collected and analysed by histopathological assay. As shown in Fig. 4, pathological damage was not observed in control group (PBS treated) and mice infected by IN method. Conversely, tissue damage was found in mice infected by IV method including dilatation and exudate in pancreatic duct as well as necrosis of pancreatic cells and trace of immune cells in pancreas. These results indicate that H1N1 influenza virus could productively infect pancreatic cells.
Fig. 4.
Pancreatic tissue samples sectioned, stained with haematoxylin and eosin (H&E), a = control tissue, b = infectious pancreas
Discussion
The aim of this study was to evaluate H1N1 influenza virus replication in pancreas and investigate the impacts of infection on the serum cytokine and tissue using an in-vivo analysis. Previous studies reported that pancreatic injury was induced by H1N1 influenza virus infection in animals and humans cell lines [18]. Our study has served the novel in-vitro and in-vivo investigations, by which it was reported that H1N1 influenza virus can replicate in mouse pancreas cell line and pancreas tissue as well as the new suggest pro-inflammatory cytokine (IL-33) response.
H1N1 influenza virus, as an indoor and outdoor pathogen in air, causing flu-like illness can be recovered within 5–6 days. While, influenza virus is prone to cause other complications such as pneumonia that may cause a small bleeding in the respiratory tract. Such a damage in respiratory tract can facilitate the transmission of influenza virus from respiratory tract into blood vessel, thereby infecting the new target of internal tissue such as pancreas and liver that can support the replication of the influenza virus. To simulate this process in this study, the influenza virus was injected into the bloodstream. The attachment of hemagglutinin protein as the surface protein of influenza virus to sialic acid (SA) receptor on the cell surface is necessary for initial infection. Previous study reported that the both SA receptors including α-2,3- and α-2,6-linked are presented at the surface of pancreas cell [14]. Therefore, this study as an in-vivo investigation demonstrated that the H1N1 influenza virus as an airborne pathogen can infect the bloodstream through various pathways, thereby causing the positive pathogenicity and infectivity in the pancreas. As shown in the tissue pathology and RT-PCR tests, the influenza virus was actively presented in the pancreas and led to a tissue destruction. Further, in this study, pancreatitis was evaluated by measuring the serum lipase concentration and histopathologic examination of pancreas tissue. As it reported in mammals, pancreatic injury results in an increase in the serum lipase level. The increasing trend in serum lipase level was observed in mice infected by IV method in duration of infection, which was significantly higher compared with mice infected by IN method. This result was followed by an increase in the blood sugar at subsequent days, which can confirm the pancreas damage. In addition, the histological investigations apparently demonstrated the pancreas damage including dilatation and exudate in pancreatic duct as well as the disruption of the architecture of pancreatic cells and present of immune cells. Notably, the RT-PCR examination for M2 mRNA of influenza virus indicated the replication of influenza in pancreas. Therefore, our outputs prove that the pancreas tissue can be targeted by influenza virus as an air bore pathogen.
Flu as an immunopathogenic disease and immune system is double-edged sword that it can control the spread of the virus and also increase the severity of the disease, thus it can be concluded that cytokines are the key factor in the influenza disease. The IL- 33 as one of the members of IL-1 super family- can be considered as an alarming indicator because of releasing during cell damage [19]. It has been reported that after pancreas infection caused by Coxsackie virus B, exogenous IL-33 and IL-33 were elevated while viral load was not reduced. It can be implied that apoptosis and necrosis are exited in pancreas cells [20]. Results in this study exhibited that serum IL-33 in mice infected by IV method significantly increased at 7 days after infection, which was consistent with previous results that indicated the pancreatic injury and the long-term increase in serum IL-33 as the indicatives findings of pancreatic injury. Long-term high levels of IL-33 after infected by influenza virus in susceptible population can alert that pancreatic tissue can be infected with influenza virus, and this virus can be replicated in this organ. Furthermore, G-CSF is a critical cytokine for promotion of organ inflammation through immune cells trafficking [21]. In this study, the G-CSF levels were significantly elevated in both of infected groups, indicating present of immune cells in infected organ. In addition, in the pathologic slides of the pancreatic tissue, the presence of immune cells was also confirmed. The presence of immune cells for reducing the virus replication can increase the tissue damage.
Significant roles of IL-6 are secreted during viral infection and response to tissue damage [22, 23] as well as IL-17a is an important mediator related with lung injure in influenza infection [24] the high level of IL-6 and IL-17a in mice infected by IN method was expected because influenza virus induces the lunge injury. Notably, high level of IL-6 and IL-17a in mice infected by IV method is the important factor snice the high level of cytokines indicates the single or multiple organ damage, which was reported as pancreas damage in this study. In addition, IL-10 is described as the immunomodulator mediator, which particularly inhibits the action of T cells [25]. The increment in IL-10 level following infected by influenza virus causes weaken the activity of immune system for balancing the immunity and preventing the tissue damage. In this study, the IL-10 level in mice infected by IV method was reduced at 7 days after infection, indicating that the immune system of pancreas had high activity in long-term after infection. Therefore, high activity of immune system increased the inflammation and tissue damage. That is, we came up with the idea that the immune cells- a carrier of the Influenza virus- could transmit the influenza virus into target internal tissues, which still maintain open as our future study.
Conclusion
In this study, results proved that H1N1 influenza virus can infect the pancreas and impact the pancreas tissue, which is prone to increase the severity of disease. The results reported the replication of influenza virus in pancreas and a severe immune reaction against influenza virus as the main cause of the pancreatic disease. Also we concluded the health effects of airborne pathogens are not limited to the respiratory tract, as well as the process of treating patients with respiratory infections with the influenza virus requires an internal review.
Acknowledgements
The authors gratefully acknowledge the School of Public Health, Tehran university of Medical Science for providing financial support. This study was extracted from Ph.D. Dissertation. This study was funded by School of Public Health, Tehran university of Medical Science (grant number: 97-02-27-38327).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.López HC, Roca R, Daunis J. Pneumonia and the acute respiratory distress syndrome due to influenza a (H1N1) virus. Med Int. 2009;33(9):455–458. doi: 10.1016/j.medin.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Blum A, Podvitzky O, Shalabi R, Simsolo C. Acute pancreatitis may be caused by H1N1 influenza a virus infection. Isr Med Assoc J: IMAJ. 2010;12(10):640–641. [PubMed] [Google Scholar]
- 3.Calore EE, Uip DE, Perez NM. Pathology of the swine-origin influenza A (H1N1) flu. Pathol Res Pract. 2011;207(2):86–90. doi: 10.1016/j.prp.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Oikarinen S, Tauriainen S, Hober D, Lucas B, Vazeou A, Sioofy-Khojine A, Bozas E, Muir P, Honkanen H, Ilonen J, Knip M, Keskinen P, Saha MT, Huhtala H, Stanway G, Bartsocas C, Ludvigsson J, Taylor K, Hyöty H, VirDiab Study Group Virus antibody survey in different European populations indicates risk association between coxsackievirus B1 and type 1 diabetes. Diabetes. 2014;63(2):655–662. doi: 10.2337/db13-0620. [DOI] [PubMed] [Google Scholar]
- 5.Gale E. Congenital rubella: citation virus or viral cause of type 1 diabetes? Berlin: Springer; 2008. [DOI] [PubMed] [Google Scholar]
- 6.Aarnisalo J, Veijola R, Vainionpää R, Simell O, Knip M, Ilonen J. Cytomegalovirus infection in early infancy: risk of induction and progression of autoimmunity associated with type 1 diabetes. Diabetologia. 2008;51(5):769–772. doi: 10.1007/s00125-008-0945-8. [DOI] [PubMed] [Google Scholar]
- 7.Habib A, Jain A, Singh B, Jamshed N. H1N1 influenza presenting as severe acute pancreatitis and multiorgan dysfunction. Am J Emerg Med. 2016;34(9):1911.e1–1911.e2. doi: 10.1016/j.ajem.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez DS, Martínez A, Guzmán M, Robledo H, Capocasa P, Martinez L, et al. Severe acute pancreatitis and infection by influenza A (H1N1) virus in a child: case report. Arch Argent Pediatr. 2015;113(4):e215–e218. doi: 10.5546/aap.2015.e215. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez BA, Alcalá MP, Segura SS, Mira-Perceval JG. 2009 pandemic influenza A (H1N1) virus infection treated with oseltamivir and possible association with acute pancreatitis in a 12 years old patient. Enferm Infecc Microbiol Clin. 2015;33(2):139. doi: 10.1016/j.eimc.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz PL, Tapia G, Bakken IJ, Håberg SE, Hungnes O, Gulseth HL, Stene LC. Pandemic influenza and subsequent risk of type 1 diabetes: a nationwide cohort study. Diabetologia. 2018;61(9):1996–2004. doi: 10.1007/s00125-018-4662-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavicchioli L, Zappulli V, Beffagna G, Caliari D, Zanetti R, Nordio L, Mainenti M, Frezza F, Bonfante F, Patrono LV, Capua I, Terregino C. Histopathological and immunohistochemical study of exocrine and endocrine pancreatic lesions in avian influenza A experimentally infected turkeys showing evidence of pancreatic regeneration. Avian Pathol. 2015;44(6):498–508. doi: 10.1080/03079457.2015.1087640. [DOI] [PubMed] [Google Scholar]
- 12.Van Poucke SG, Nicholls JM, Nauwynck HJ, Van Reeth K. Replication of avian, human and swine influenza viruses in porcine respiratory explants and association with sialic acid distribution. Virol J. 2010;7(1):38. doi: 10.1186/1743-422X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raman R, Tharakaraman K, Shriver Z, Jayaraman A, Sasisekharan V, Sasisekharan R. Glycan receptor specificity as a useful tool for characterization and surveillance of influenza A virus. Trends Microbiol. 2014;22(11):632–641. doi: 10.1016/j.tim.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huo C, Xiao K, Zhang S, Tang Y, Wang M, Qi P, et al. H5N1 influenza A virus replicates productively in pancreatic cells and induces apoptosis and pro-inflammatory cytokine response. Front Cell Infect Microbiol. 2018;8:386. doi: 10.3389/fcimb.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gukovskaya AS, Vaquero E, Zaninovic V, Gorelick FS, Lusis AJ, Brennan ML, et al. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122(4):974–984. doi: 10.1053/gast.2002.32409. [DOI] [PubMed] [Google Scholar]
- 16.Abdulla A, Awla D, Thorlacius H, Regnér S. Role of neutrophils in the activation of trypsinogen in severe acute pancreatitis. J Leukoc Biol. 2011;90(5):975–982. doi: 10.1189/jlb.0411195. [DOI] [PubMed] [Google Scholar]
- 17.Eisfeld AJ, Neumann G, Kawaoka Y. Influenza A virus isolation, culture and identification. Nat Protoc. 2014;9(11):2663–2681. doi: 10.1038/nprot.2014.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capua I, Mercalli A, Pizzuto MS, Romero-Tejeda A, Kasloff S, De Battisti C, et al. Influenza A viruses grow in human pancreatic cells and cause pancreatitis and diabetes in an animal model. J Virol. 2013;87(1):597–610. doi: 10.1128/JVI.00714-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller AM. Role of IL-33 in inflammation and disease. J Inflamm. 2011;8(1):22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sesti-Costa R, Silva GK, Proença-Módena JL, Carlos D, Silva ML, Alves-Filho JC, et al. The IL-33/ST2 pathway controls Coxsackievirus B5–induced experimental pancreatitis. J Immunol. 2013;191(1):283–292. doi: 10.4049/jimmunol.1202806. [DOI] [PubMed] [Google Scholar]
- 21.Eyles JL, Hickey MJ, Norman MU, Croker BA, Roberts AW, Drake SF, et al. A key role for G-CSF–induced neutrophil production and trafficking during inflammatory arthritis. Blood, The Journal of the American Society of Hematology. 2008;112(13):5193–5201. doi: 10.1182/blood-2008-02-139535. [DOI] [PubMed] [Google Scholar]
- 22.Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV. The role of interleukin 6 during viral infections. Front Microbiol. 2019;10:1057. doi: 10.3389/fmicb.2019.01057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka T, Narazaki M, Kishimoto T. IL-6 en la inflamación, la inmunidad, y la enfermedad. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi: 10.1101/cshperspect.a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, et al. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol. 2009;183(8):5301–5310. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-β, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9(4):447–453. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]