Abstract
Because of the presence of tannin in the molecular structure of oak extract, this substance is used as a natural coagulant to remove turbidity from water. The aim of this study was to determine the efficiency of this coagulant alone and in combination with polyaluminium chloride (PACl) in turbidity removal from water under optimal conditions. In this experimental study, Iranian oak extract was prepared by maceration method using ethanol 96% as an extractor. Kaolin was used to prepare synthetic turbid water samples. Using the jar test, the optimum concentrations of oak extract and PACl were determined in various concentrations of initial turbidity and pH. Moreover, the central composite design (CCD) method was utilized to design experiments and RSM was applied for analyzing the obtained results. Optimum concentrations of oak extract and PACl were 62.6 mg/L and 52.6 mg/L, respectively. An increase in initial turbidity and pH led to an increase in turbidity removal by the two coagulants. The efficiency of turbidity removal by oak extract and PACl was 63.5% and 66.5%, respectively. The simultaneous application of oak extract and polyaluminium chloride increased removal efficiency (85%) and reduced the total organic carbon concentration (TOC) in water (42.3%). The results showed that the simultaneous application of Iranian oak extract and polyaluminium chloride had an acceptable performance in removing turbidity from water.
Keywords: Iranian oak, Water treatment, Natural coagulant, Polyaluminium chloride, Turbidity, Central composite design
Introduction
Water is a substance vital for all human and natural activities. However, due to its ability to dissolve other matters, pure water is not usually found in nature. The impurities contained in water include minerals, compounds, and organic gases that make changes in physical (e.g., turbidity, color, temperature, and electrical conductivity), chemical (e.g., chemical oxygen demand (COD), biochemical oxygen demand (BOD), pH, alkalinity, and total organic carbon, and biological features of water. The severity of the harmful effects of impurities in water depends on the concentration of compounds and chemical reactions between them [1–3]. Hence, to use unhealthy water resources for humans and other environmental organisms, it is necessary to utilize sequential purification processes. These processes have several steps including physical pre-purification, chemical purification via coagulation, deposition, and filtration, which are taken to achieve transparent disinfected water [4–6].
Coagulation is used as an essential step in the water purification process to eliminate suspended solid and very fine particles. These particles, called colloidal particles, have undesirable effects such as turbidity in water and negatively affect different steps of water purification processes such as filtration and disinfection. Stable colloids in water usually have a negative electrical charge at all levels; however, coagulant compounds make them unstable by neutralizing their negative electrical charge and eventually precipitating them in water via gravity [7, 8]. Coagulant compounds are also able to eliminate colors caused by humic acids and Fulvic acid, organic compounds, and even bacteria present in water resources. Thus, they increase the efficiency of subsequent steps of purification processes such as filtration and disinfection processes [9, 10].
The most common artificial coagulants used in water purification are aluminum sulfate, ferric chloride, lime, and artificial polymers. The mentioned types of coagulants have some disadvantages such as inefficiency at low temperatures, relatively high purchase costs, negative health effects, high volumes of sludge production, and a significant effect on the pH of purified water [11–14]. In recent years, there has been an increasing tendency toward the use of natural coagulant compounds obtained from the plant, animal tissues, or microorganisms in order to overcome potential problems [15]. The use of natural coagulants has some advantages including rapid biodegradability, a wide range of effective doses of their coagulation power for various colloid suspended substances, lack of negative health effects, and a low level of sludge production (20–30%) as compared with their chemical counterparts [16, 17].
Tannin is a general name used to refer to a large group of polyphenylene compounds obtained from natural materials such as organic extract of wood and shell of acacia or oak trees. The results of studies on the coagulation potential of the tannin extract obtained from wood show that tannin can be used as a very good alternative to chemical coagulants. The presence of phenolic groups in tannin structure results in the emergence of anionic features. Subsequently, its hydrogen-bearing feature increases, leading to an increase in the efficiency of tannin in the coagulation of suspended compounds in water. In this regard, the efficiency of tannin, as a natural coagulant used for water purification, depends on the chemical structure of the tannin extract and the level of treatment done on its extract [15, 18, 19].
Quercus Branti, as a member of the Fagaceae family, is a large tree with a height of 10–50 m and a large spherical crown that grows in the forests of Kurdistan, Lorestan, and Kohgiluyeh and Boyer Ahmad Provinces of Iran. Its leaves are often uniform and oval-shaped with toothed margins. Oak fruit has three sections including the hard shell, the middle layer, and the edible part. The middle layer, called “Jaft”, contains high amounts of tannin compounds that can be used in the coagulation process for water purification [20].
Therefore, the aim of this study was to assess the efficiency of Iranian oak extract as a natural herbal coagulant and polyaluminium chloride as a chemical coagulant in eliminating water turbidity. The study also aimed to assess the simultaneous application of these two coagulants to compare their efficiency with each other. The central composite design (CCD) method was used to design the experiments and the response surface methodology (RSM) method was applied to analyze the results and optimize the parameters. RSM is one of the most common statistical and mathematical techniques. RSM can be useful for developing and optimizing industrial, chemical, and biochemical processes, wherein the desired response is influenced by several independent variables and the objective is to optimize and control this response [21].
This method has been widely used to optimize reacting parameters by reducing the number of designed experiments to analyze the interaction between parameters, reducing chemical consumption, and decreasing the number of experimental trials. CCD is the most commonly used response surface designed experiment. This design consists of axial points, cube points, and center points. Using the CCD method, researchers can provide information on the experimental independent variable effects and the overall experimental error in a minimum number of runs [22, 23].
Materials and methods
This pilot-scale experimental study aimed to optimize the turbidity removal process using oak extract and polyaluminium chloride and to assess the simultaneous application of these two materials using the CCD and RSM. In this study, independent variables were pH, initial turbidity, and the amount of coagulant (oak extract). Also, the dependent variable (response) was the percentage of water turbidity removal. Chloride acid and sodium hydroxide were used to adjust pH.
Oak extract preparation
Oak fruit was collected from the forests around Yasuj city (Iran) and the middle layer of the fruit was separated and dried under shade. The obtained Jaft was ground to obtain powder and after weighing 100 g, it was extracted by maceration or soaking using 96% ethanol in two rounds each lasting 24 h. The extract was condensed as much as possible using the Whatman paper and Rotary machine and dried in an incubator at 50 °C. The dried extract was stored in a freezer at −20 °C until use [24, 25].
Preparing water samples with artificial turbidity
The white powder of kaolin (manufactured by Merck Company) was used to create artificial turbidity in water. It was weighed to collect 10 g and then put in a 105 °C oven for 3–4 h to dry. Then, it was placed in a desiccator for 30 min to dehydrate and cool the kaolin substances. Afterward, 50 ml of distilled water was added to the kaolin substance and it was left for 12 h to become wet enough. In the next step, the volume of the solution was increased to 1.5 L using distilled water and it was blended for 20 min. The obtained mixture was left for 4 h to deposit large substances. Next, 1 L of the liquid was transferred to the Arlene Mayer to provide a stock solution. This suspension was used in experimental studies to provide artificial water samples with diverse turbidity ranging from 20 to 250 NTU.
Coagulation experiments
All the experiments were carried out in a 6-cell Jar test unit (Phipps & Bird model manufactured in the United States), in accordance with the ASTM2001 standard. First, the pH of the artificial water samples was adjusted in five levels (3, 4.5, 6, 7.5, and 9) using sodium hydroxide 0.1 M or chloride acid 0.1 M. The pH values were measured by a pH meter machine (Metrohm-827lab model made in Switzerland). Then, 300 mL of the artificial water samples with different turbidities were added to the jars and they were centrifuged at 200 rpm to blend rapidly. The blending process was performed for 1 min by adding a specific amount of coagulant to the dishes. The slow-blending process was then performed at 70 rpm for 30 min. Then, the contents of the containers were stored for 60 min without moving in order to perform the deposition process. All the mentioned steps were performed on three separate groups of samples containing oak extract coagulant (5–75 mg/L), polyaluminium chloride (5–75 mg/L), and a combination of the two coagulants (10–90 and 5–65 mg/L) at room temperature. To measure the final turbidity of the solution, each sample was collected from a 3-cm depth of the dish and its turbidity was read by a turbidity meter device (HACH-2100 model) with a maximum sensitivity of 0.01 NTU. The efficiency of turbidity removal from artificial water was calculated using Eq. (1).
| 1 |
where T0 and T are the initial and final turbidity, respectively, and Re is the percentage of turbidity removal from artificial water. Total Organic Carbon (TOC) was measured in a HACH DR-2500 analyzer.
Experimental design and statistical analysis using RSM software
Design Expert 7 software was used for the statistical design of the experiments and data analysis. The CCD and the RSM methods were used in order to optimize three important variables including pH (3–9), initial turbidity (NTU 20–260), oak extract concentration (5–75 mg/L), polyaluminium chloride concentration (5–75 mg/L), and the concentration of combined coagulants (10–90 and 5–65 mg/L). Levels of three variables considered for the CCD in actual terms are presented in Table 1. Moreover, levels of four variables considered in the simultaneous effect of oak extract and polyaluminium chloride design are given in Table 2. First, the three independent variables were encoded into five levels (i.e., −2, −1, 0, +1, and + 2). The encoding of the variables into different levels was carried out using Eq. (2).
| 2 |
where X is factor code, x is the actual value of the factor, and xmin and xmax are the minimum and maximum values of the factor, respectively.
Table 1.
Uncoded levels of the three independent variables (A: Oak extract as coagulant, B: Polyaluminium chloride as coagulant)
| Independent Variables | Unit | Symbol | Levels | |||||
|---|---|---|---|---|---|---|---|---|
| -α | −1 | 0 | +1 | +α | ||||
| Turbidity | NTU | X1 | 20 | 68.64 | 140 | 211.35 | 260 | |
| pH | X2 | 3 | 4.21 | 6 | 7.78 | 9 | ||
| Coagulant Dose | A: oak extract | mg/L | X3 | 10 | 26.21 | 50 | 73.78 | 90 |
| B:polyaluminium chloride | mg/L | X3 | 5 | 17.16 | 35 | 52.83 | 65 | |
Table 2.
levels of four variables that considered in simultaneous effect of oak extract and polyaluminium chloride
| Independent Variables | Unit | Symbol | Levels | ||||
|---|---|---|---|---|---|---|---|
| -α | −1 | 0 | +1 | +α | |||
| Turbidity | NTU | X1 | 20 | 80 | 140 | 200 | 260 |
| pH | X2 | 3 | 4.5 | 6 | 7.5 | 9 | |
| Oak Extract | mg/L | X3 | 10 | 30 | 50 | 70 | 90 |
| Polyaluminium Chloride | mg/L | X4 | 5 | 20 | 35 | 50 | 65 |
The number of experiments was determined using Eq. (3). A total of 24 experiments were conducted using oak extract, 24 experiments using polyaluminium chloride, and 34 experiments using a combination of the two coagulants. The order of the experiments is presented in Tables 1, 2, and 3.
| 3 |
where n is the total number of tests, K is the number of variables, and Cp is the number of central points.
Table 3.
Number of coagulation tests performed using oak extract, PACl and oak extract+PACl
| Row | Oak extract | PACl | Oak extract+ PACl | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Turbidity (NTU) | pH | Oak extract (mg/L) | Turbidity (NTU) | pH | PACl (mg/L) | Turbidity (NTU) | pH | Oak extract (mg/L) | PACl (mg/L) | |
| 1 | 68.64 | 7.78 | 73.78 | 68.64 | 7.78 | 52.83 | 80 | 4.5 | 30 | 50 |
| 2 | 211.35 | 7.78 | 73.78 | 140 | 6 | 35 | 80 | 7.5 | 30 | 20 |
| 3 | 68.64 | 4.21 | 26.21 | 68.64 | 4.21 | 52.83 | 140 | 6 | 50 | 35 |
| 4 | 140 | 6 | 50 | 211.35 | 6 | 35 | 200 | 7.5 | 70 | 50 |
| 5 | 211.35 | 4.21 | 73.78 | 140 | 7.78 | 17.16 | 200 | 7.5 | 30 | 20 |
| 6 | 140 | 6 | 50 | 140 | 7.78 | 52.83 | 140 | 6 | 50 | 35 |
| 7 | 140 | 6 | 50 | 211.35 | 6 | 35 | 200 | 4.5 | 70 | 50 |
| 8 | 140 | 6 | 50 | 211.35 | 6 | 35 | 80 | 7.5 | 30 | 50 |
| 9 | 68.64 | 4.21 | 73.78 | 211.35 | 7.78 | 17.16 | 200 | 7.5 | 30 | 50 |
| 10 | 211.35 | 4.21 | 26.21 | 68.64 | 4.21 | 17.16 | 140 | 6 | 50 | 35 |
| 11 | 211.35 | 7.78 | 26.21 | 140 | 4.21 | 52.83 | 200 | 4.5 | 70 | 20 |
| 12 | 140 | 6 | 50 | 140 | 4.21 | 17.16 | 80 | 4.5 | 30 | 20 |
| 13 | 68.64 | 7.78 | 26.21 | 140 | 6 | 35 | 200 | 4.5 | 30 | 50 |
| 14 | 140 | 6 | 50 | 260 | 6 | 65 | 140 | 6 | 50 | 35 |
| 15 | 20 | 6 | 50 | 20 | 6 | 35 | 140 | 6 | 50 | 35 |
| 16 | 140 | 6 | 50 | 140 | 6 | 35 | 200 | 7.5 | 70 | 20 |
| 17 | 140 | 6 | 90 | 140 | 6 | 35 | 200 | 4.5 | 30 | 20 |
| 18 | 140 | 6 | 50 | 140 | 6 | 5 | 80 | 7.5 | 70 | 50 |
| 19 | 140 | 6 | 50 | 140 | 9 | 35 | 80 | 4.5 | 70 | 50 |
| 20 | 140 | 6 | 50 | 140 | 6 | 35 | 80 | 4.5 | 70 | 20 |
| 21 | 140 | 6 | 10 | 140 | 3 | 35 | 80 | 7.5 | 70 | 20 |
| 22 | 260 | 6 | 50 | 140 | 6 | 35 | 140 | 6 | 50 | 35 |
| 23 | 140 | 3 | 50 | 140 | 6 | 35 | 140 | 6 | 50 | 5 |
| 24 | 140 | 9 | 50 | 140 | 6 | 35 | 140 | 6 | 50 | 35 |
| 25 | 140 | 3 | 50 | 35 | ||||||
| 26 | 140 | 6 | 50 | 35 | ||||||
| 27 | 140 | 6 | 90 | 35 | ||||||
| 28 | 140 | 6 | 10 | 35 | ||||||
| 29 | 140 | 6 | 50 | 35 | ||||||
| 30 | 20 | 6 | 50 | 35 | ||||||
| 31 | 140 | 6 | 50 | 35 | ||||||
| 32 | 140 | 6 | 50 | 65 | ||||||
| 33 | 260 | 6 | 50 | 35 | ||||||
| 34 | 80 | 4.5 | 30 | 50 | ||||||
The behavior of the system was studied using the following equation, which is a second-degree polynomial model.
| 4 |
where η is the predicted response, Xi and Xj are variables, β is the constant coefficient, jß, jjß, and ijß are linear, square, and second-degree mutual coefficients, respectively, and ei is the error [21].
The analysis of variance (ANOVA) was used to test the relationships between the three independent variables and the response variable based on the results of experiments. The quality of fit of the statistical second-degree polynomial model was expressed by the coefficient of determination (R2) and coefficient of variations. Statistical significance was determined by the Fisher test (F-test). From a statistical point of view, a suitable model does not show a significant lack of fit and has the highest values of R2, R2-adjusted, R2-predicted (> 0.95), and a low CV (around 10%). The factors in the model were assessed by p value set at a confidence interval of 95%. The balance graphs were plotted to determine the efficiency of turbidity removal using oak extract based on the effects of three factors (i.e., pH, initial turbidity, and oak extract concentration).
Results
To determine the maximum turbidity removal efficiency using oak extract, polyaluminium chloride, and a combination of them, the Design-Expert software was used to specify the best levels of the main process variables, including oak extract concentration, polyaluminium chloride concentration, and combination of the two coagulants, initial turbidity, and pH.
Characterization of oak extract
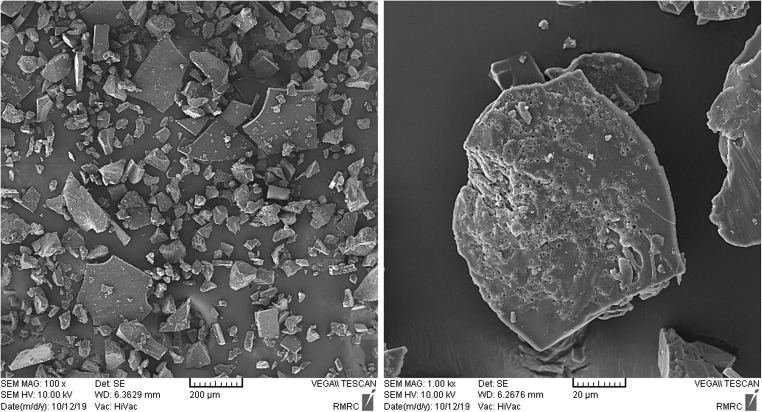
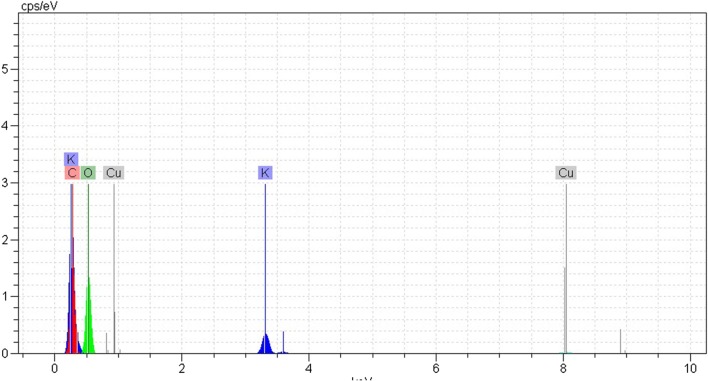
Scanning Electron Microscope (SEM) was used to characterize the structure of the oak extract. The SEM image of the powdered oak extract is shown in Fig. 1. The figure shows small pores in the structure of oak extract that are more visible at 1 k magnifications. The energy-dispersive spectroscopy (EDS) results of the oak extract are shown in Fig. 2. The EDS spectra approve the Carbon, Oxygen, Potassium, and Copper with weight percentage (wt.%) of 39.72, 59.46, 0.65, and 0.17 respectively.
Fig. 1.
SEM image of the powdered oak extract
Fig. 2.
EDS spectrum of oak extract
Analysis of variance (ANOVA)
The ANOVA results were used to validate the predicted model. Tables 4, 5 and 6 presented the results of the ANOVA and the regression equation analysis of the turbidity removal efficiency by the oak extract, PACl and oak extract+PACl.
Table 4.
ANOVA results for Turbidity removal efficiency by using oak extract and PACl
| Parameter | Coagulant type | Coefficient | Standard error | t value | P value |
|---|---|---|---|---|---|
| Intercept | Oak Extract | 73.527 | 0.781 | 94.065 | <0.001 |
| PACl | 60.226 | 0.735 | 81.88 | <0.001 | |
| X1 | Oak Extract | 8.05 | 1.125 | 7.152 | <0.001 |
| PACl | 9.872 | 1.059 | 9.32 | <0.001 | |
| X2 | Oak Extract | −1.556 | 1.125 | −1.382 | 0.18 |
| PACl | 32.756 | 1.059 | 30.926 | <0.001 | |
| X3 | Oak Extract | 1.951 | 1.125 | 1.734 | 0.1 |
| PACl | 4.304 | 1.059 | 4.063 | 0.001 | |
| X1X2 | Oak Extract | 11.939 | 2.473 | 4.827 | <0.001 |
| PACl | −12.57 | 2.327 | −5.401 | <0.001 | |
| X1X3 | Oak Extract | 12.107 | 2.473 | 4.895 | <0.001 |
| PACl | 9.523 | 2.327 | −3.059 | 0.001 | |
| X2X3 | Oak Extract | −16.866 | 2.473 | −6.819 | <0.001 |
| PACl | −7.12 | 2.327 | −3.059 | 0.008 | |
| X12 | Oak Extract | −12.122 | 1.736 | −6.979 | <0.001 |
| PACl | 0.555 | 1.634 | 0.34 | 0.738 | |
| X22 | Oak Extract | −24.153 | 1.736 | −13.906 | <0.001 |
| PACl | 9.483 | 1.634 | 5.802 | <0.001 | |
| X32 | Oak Extract | −13.332 | 1.736 | −7.676 | <0.001 |
| PACl | 20.951 | 1.634 | 12.819 | <0.001 | |
| Model p value | Oak Extract | <0.001 | |||
| PACl | <0.001 | ||||
| Lack of Fit | Oak Extract | 0.06 | |||
| PACl | 0.076 | ||||
Table 5.
ANOVA results for Turbidity removal efficiency by simultaneous using of oak extract and PACl
| Parameter | Coefficient | Standard error | t value | P value |
|---|---|---|---|---|
| Intercept | 96.092 | 1.099 | 87.416 | <0.001 |
| X1 | 17.874 | 1.419 | 12.595 | <0.001 |
| X2 | 11.447 | 1.419 | 8.066 | <0.001 |
| X3 | 6.449 | 1.419 | 8.544 | <0.001 |
| X4 | 2.182 | 1.419 | 1.538 | 0.14 |
| X1X2 | −2.136 | 3.476 | −0.614 | 0.546 |
| X1X3 | −3.739 | 3.476 | −1.075 | 0.29 |
| X1X4 | −17.668 | 3.476 | −5.082 | <0.001 |
| X2X3 | −18.334 | 3.476 | −5.274 | <0.001 |
| X2X4 | 35.926 | 3.476 | 10.335 | <0.001 |
| X3X4 | 0.084 | 3.476 | 0.024 | 0.98 |
| X12 | −13.563 | 2.498 | −5.428 | <0.001 |
| X22 | −6.692 | 2.489 | −2.678 | 0.01 |
| X32 | −3.817 | 2.498 | −1.528 | 0.14 |
| X42 | −42.239 | 2.498 | −16.905 | <0.001 |
| Model p value | <0.001 | |||
| Lack of Fit | 0.073 | |||
Table 6.
The results of regression equation analysis of turbidity removal by oak extract, PACl and Oak extract+PACl
| Final equation | R2 | Adjusted R2 | Predicted R2 | Enough Accuracy | Standard Deviation | Variable Coefficient | Prediction Residual |
|---|---|---|---|---|---|---|---|
| Oak Extract | 0.964 | 0.953 | 0.937 | 34.612 | 1.432 | 2.36 | 136.535 |
| PACl | 0.983 | 0.973 | 0.957 | 38.612 | 1.432 | 2.415 | 137.521 |
| Oak Extract + PACl | 0.964 | 0.956 | 0.957 | 32.322 | 3.592 | 2.36 | 125.834 |
Effect of operational factors on turbidity removal by using oak extract
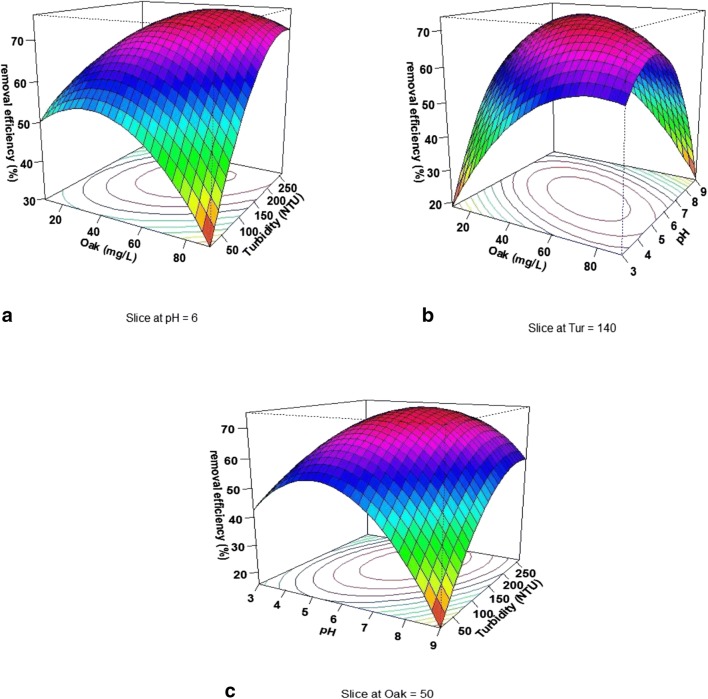
Figure 3a presents the simultaneous effect of oak extract concentration and initial turbidity on the efficiency of the turbidity removal at pH = 6. The results of this study indicated that with increasing the oak extract concentration in a range of 40–60 mg/L, the removal efficiency first increases and then decreases. The maximum removal efficiency (i.e., 76.57%) was obtained at a concentration of 62 mg/L. As shown in Fig. 3a, with increasing the initial turbidity in a range of 50 to 240 mg/L, the turbidity removal efficiency increased as well. Figure 3b presents the simultaneous effect of the sample pH and oak extract concentration on the removal efficiency in the initial turbidity of NTU = 140. As can be seen, with increasing pH from 3 to 6, there was an increase in turbidity removal efficiency. At higher pH values, the turbidity removal efficiency by the oak extract was reduced. Besides, with increasing the concentration of oak extract in the initial turbidity of NTU = 140, the removal efficiency increased as well. Figure 3c shows that the effect of the initial turbidity concentration and pH in the oak extract concentration is constant and equal to 50 mg/L on turbidity removal efficiency. According to the results obtained at low concentrations of initial turbidity, with increasing pH from 3 to 6, there was an increase in the removal efficiency; however, a further increase in pH reduced the turbidity removal efficiency. At higher initial turbidity, this trend was almost the same, but the intensity of the changes declined. With increasing the initial turbidity, the turbidity removal efficiency increased by oak extract coagulant and in initial turbidity of 196 NTU and reached its maximum efficiency (i.e., 75.66%).
Fig. 3.
3D plots of the effect of variable on the turbidity removal efficiency by oak extrac: (a) oak extract and initial turbidity concentration, (b) pH and oak extraxt, and (c) pH and initial turbidity concentration
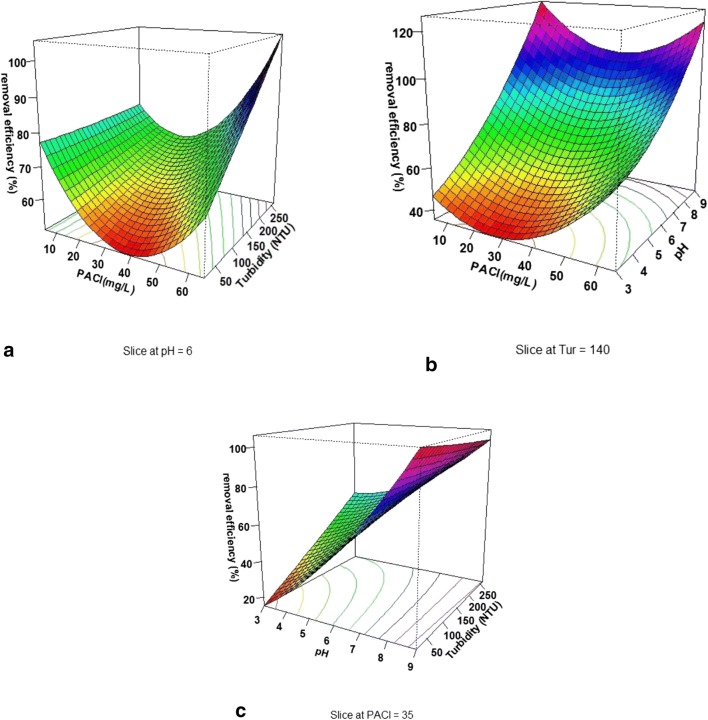
Effect of operational factors on turbidity removal by using polyaluminium chloride
Figure 4a presents the simultaneous effect of the initial turbidity and polyaluminium chloride concentration at pH = 6. As shown in this figure, with increasing the concentration of polyaluminium chloride up to 40 mg/L, the removal efficiency was first decreased and then increased. Figure 4b illustrates the simultaneous effect of the sample pH and polyaluminium chloride concentration on turbidity removal efficiency in the initial turbidity of NTU = 140. The results of this study indicated that turbidity removal efficiency increased with increasing pH, and the highest and the lowest turbidity removal efficiency were observed at pH = 8 and pH = 3, respectively. With increasing the concentration of polyaluminium chloride, turbidity removal efficiency decreased slowly and increased gradually at a concentration of 30 mg/L. In general, the effect of the initial concentration of polyaluminium chloride was not significant compared with the effect of pH. Figure 4c shows the simultaneous effect of initial turbidity and pH on turbidity removal efficiency. According to the results of this experiment, with increasing pH, the turbidity removal efficiency increased. It is of note that in lower initial turbidity values, this increase in removal efficiency is of gradient greater than the higher values of initial turbidity. At a low pH, the increase in turbidity increased the turbidity removal efficiency, but at higher pH values, the increase in turbidity reduced the turbidity removal efficiency.
Fig. 4.
3D plots of the effect of variable on the turbidity removal efficiency by polyaluminium chloride: (a) PACl and initial turbidity concentration, (b) PACl and pH, and (c) pH and initial turbidity concentration
Effect of operational factors on turbidity removal by simultaneous using oak extract and polyaluminium chloride
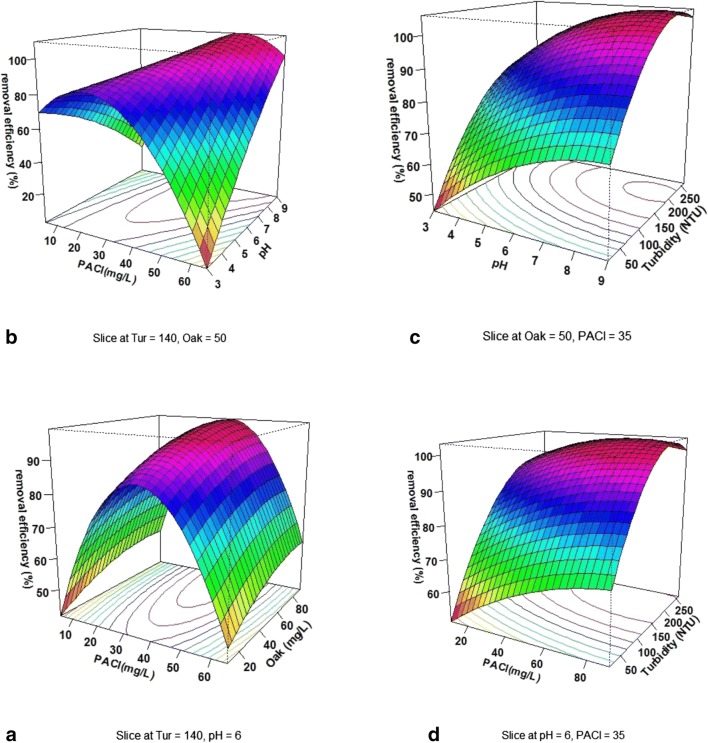
Figure 5a depicts the simultaneous effect of oak extract concentration and polyaluminium chloride concentration on turbidity removal efficiency at pH = 6 and at turbidity of NTU = 140. According to the results of this study, with increasing polyaluminium chloride concentration up to 32.6 mg/L, the turbidity removal efficiency increased. However, with a further increase in polyaluminium chloride concentration, turbidity removal efficiency decreased. The concentration of oak extract had no significant effect on the turbidity removal efficiency, but with increasing the oak extract concentration, in general, there was an increase in the turbidity removal efficiency. Figure 5b displays the simultaneous effect of polyaluminium chloride concentration and pH on the turbidity removal efficiency at the initial turbidity of NTU = 140 and oak extract concentration of 50 mg/L, respectively. According to the results of this study, the changes process of turbidity removal efficiency of pH changes is dependent on the concentration of polyaluminium chloride. At low concentrations of polyaluminium chloride coagulant, an increase in pH result in a decrease in the removal efficiency. In comparison, the changes process in the higher concentrations of polyaluminium chloride is reversed and the pH increase results in a significant increase in the turbidity removal efficiency. Figure 5c presents the simultaneous effect of initial turbidity concentration and pH on turbidity removal efficiency at the oak extract concentration = 140 mg/L and polyaluminium chloride concentration of 35 mg/L. At low pH values, with increasing the concentration of turbidity, there was an increase in turbidity removal efficiency; however, the trend was reversed at higher pH levels such that the increase in initial turbidity concentration decreased the turbidity removal efficiency. Figure 5d shows the simultaneous effect of polyaluminium chloride concentration and the initial turbidity on the turbidity removal efficiency at an oak extract concentration of 50 mg/L and pH of 6. As can be seen, the increase in polyaluminium chloride concentration resulted in an increase in turbidity removal efficiency. According to the results of this study, at lower initial turbidity, the trend of changes is steeper. On the other hand, with increasing the initial turbidity up to 210 mg/L, there was an increase in turbidity removal efficiency; however, with increasing initial turbidity, the turbidity removal efficiency decreased thereafter.
Fig. 5.
3D plots of the effect of variable on the turbidity removal efficiency by the simultaneous application of polyaluminium chloride: (a) PACl and oak extract, (b) PACl and pH, (c) pH and initial turbidity concentration, and (d) PACl and initial turbidity concentration
Discussion
Tables 4, 5 and 6 presents the ANOVA results of experiments designed by Design Expert software for natural and chemical coagulants (i.e., Iranian oak extract concentration and polyaluminium chloride concentration). As shown in the tables, F-value and P value were calculated for the model to evaluate the turbidity removal efficiency and the results indicated the validity of the model. The same values of F-Value and P Value obtained for the unfitness test in turbidity shows the validity of the model to predict the effect of variables on the response (percentage of turbidity removal efficiency) [26]. The results of this study show that with increasing initial turbidity, the turbidity removal efficiency of Iranian oak extract increases, the turbidity values decrease to less than NTU = 70, and turbidity removal efficiency decreases to less than 40%. With increasing initial turbidity, the turbidity removal efficiency of the natural coagulant increases as well. Such an increase can be attributed to an increase in the amount of colloidal and suspended particles in the water with higher turbidities, the increased probability of their collision with coagulant compounds, and the subsequent formation of larger particles. In this study, it was found that the highest turbidity removal efficiency in drinking water was obtained by means of oak extract at a pH of 6.5–8.5. Moreover, in a study by Šćiban et al., the use of European and Turkish oak extract was investigated. The results showed that after increasing the pH of the sample water, the turbidity removal efficiency was increased [27]. According to their results, turbidity removal efficiency by natural coagulants depends on the pH value of the sample water, which is consistent with the results of the present study. It should be noted that the effect of the sample pH on the performance of tannin-containing coagulants in removing water turbidity is attributed to the effect of pHzpc on the protein excretion potential in the phenolic groups present in the tannin molecular structure [28, 29]. The determination of pHzpc is very important in investigating the mechanism and efficiency of the coagulation process in water and wastewater treatment. As the pH changes, the dominant electric charge on the coagulant surface inversely changes with pHzpc. Exceeding the pHzpc, the surface charge of oak extract as a natural coagulant is negative, and below pHzpc, the surface charge of coagulant is positive. The pHzpc for the oak extract was determined to be 7.5. Therefore, at values less than 7.5, the surface of the oak extract was positive. Because the surface charge of the kaolin-induced turbidity was negative, electrical charge neutralization can take place more easily by positive surface charge coagulant. As the pH decreases, it was expected that the negatively surface charge turbidity removal would increase because of the increase in the positive surface charge of the oak extract [30]. It subsequently increases the deposition of suspended substances and finally results in water transparency. The efficiency of water turbidity removal by Iranian oak extract (63.5%) is similar to that of Turkish and European oaks (70% and 80%, respectively) [27]. The results of this study indicated that Iranian oak extract could reduce the total organic carbon present in the sample water by 10.3%, which indicates a higher efficiency in comparison with other natural coagulants such as Chitosan and Moringa oleifera [31]. Furthermore, the results indicated that concentration of protein in the oak extract was negligible (0.32%); it is less than the protein content of common extracts such as Turkey and European oak extract (ranging from 5.1 to 6.1%), and horseradish and Prosopis (40–50%) as natural coagulants. It should be noted that with reducing the amount of protein in the natural coagulant extracts, turbidity removal efficiency or their coagulation activity increases [27, 32–34]. However, the optimum conditions for using Iranian oak extract to remove turbidity from water are as follows: oak extract concentration of 40–60 mg/L, water sample with initial turbidity of NTU = 150–250, and pH = 5.6–7.5.
To date, several studies have used polyaluminium chloride as a coagulant to remove suspended substances in water. The results of previous studies indicated the higher efficiency of this coagulant compared with iron sulfate, iron chloride, and aluminum sulfate [35–38]. In the present study, it was found that after increasing the initial turbidity of the sample, turbidity removal efficiency by this chemical coagulant was increased, which is consistent with the results of previous studies [26]. It can be stated that with increasing the initial turbidity of the sample water, the amount of colloidal and suspended substances in the water increases. Consequently, the probability of their collisions with the coagulant compounds increases and helps form larger particles. Hence, with increasing initial turbidity, there is an increase in the efficiency of water turbidity removal by coagulants. In this study, it was found that turbidity removal efficiency by polyaluminium chloride coagulant depends on the sample pH. With increasing the sample pH, turbidity removal efficiency increases such that the highest efficiency of turbidity removal is observed at pH = 8 (69.7%), which is consistent with the results of other studies [38, 39]. It is worth noting that the results of previous studies have shown that the use of polyaluminium chloride as a coagulant reduces the pH of the water, which leads to a reduction in the efficiency of the water turbidity removal by the coagulant [26, 35]. Hence, it is recommended using polyaluminium chloride coagulant to achieve the maximum efficiency of turbidity removal in optimal pH conditions (range 7.5–8.5). The results of this study indicated that polyaluminium chloride does not play an effective role in decreasing the total organic carbon content in the sample water and cannot prevent the improper effects of these compounds in water (a 2.5% decrease in initial value). However, optimal conditions recommended using polyaluminium chloride to remove turbidity from water are using of 50–60 mg/L of polyaluminium chloride for water sample with initial turbidity of 120-250NTU at pH = 7–8.
The results of this study indicated that the simultaneous use of Iranian oak extract and polyaluminium chloride extract coagulants, after increasing initial turbidity and pH of the water sample, increased turbidity removal efficiency, which is consistent with the results obtained from the separate use of each coagulant. In this study, it was found that the optimum efficiency of water turbidity removal by simultaneous application of Iranian oak extract and polyaluminium chloride (85%) was significantly higher than that of the separate use of Iranian oak and polyaluminium chloride extract (63.5% and 66.5%, respectively). To achieve the same efficiency, there is a need for a lower amount of coagulant, which is consistent with the results of a study by Orooji et al. [38]. These authors investigated the simultaneous use of natural coagulant of chitosan and the chemical coagulant of polyaluminium chloride in the removal of water turbidity. It is of note that when using a chemical compound of polyaluminium chloride, it is likely to have aluminum metal ions remained in the water after the coagulation process, which could have a negative effect on the users’ health [40]. Nevertheless, the results of previous studies have shown that the simultaneous use of a natural coagulant with polyaluminium chloride decreases the amount of residual alumina in water compared with the separate use of polyaluminium chloride alone. As a result, the negative health effects of this toxic metal decrease in consumers. The results of the present research indicated that the simultaneous use of Iranian oak extract and polyaluminium chloride significantly decreased the total amount of organic carbon present in the sample (i.e., 42.3%) compared with the separate use of each of these coagulants. This can decrease the amount of coagulants, the growth of bacteria, and the probability of formation of trihalomethane compounds and subsequently prevent cancer induced by these compounds in humans [41]. In comparison with other natural coagulants, such as Horse chestnut with 80% turbidity efficiency, peanut seeds with 80% efficiency and chitosan with 84% efficiency in turbidity removal, the combination of oak extract with chloride was more effective [27, 31, 42]. By applying multiple regression analysis, it was found that a quadratic model is effective in explaining the relationship of independent variables and response variables in the coagulation process using oak extract and PACl, and simultaneous use of oak extract and PACl. The models are shown in Eqs. 5–7.
| 5 |
| 6 |
| 7 |
The desirability functions as a numerical tool of the software was used to evaluate the optimum level of parameters for maximum turbidity removal. Based on response surface and desirability functions, the optimum levels for turbidity removal were obtained. The predicted optimum levels of variables is shown in Table 7. In order to evaluate the accuracy of the predicted turbidity removal efficiency and the reliability of the optimum condition of coagulation process, an additional experiment was carried out at optimum level of variables. Table 7 presents the experimental results under the optimum levels of independent variables compared with the predicted values from the proposed models by Design Expert software. The results showed that there is a good agreement between predicted turbidity removal from proposed model of RSM and experimental data.
Table 7.
Optimum conditions found by design expert and verification for turbidity removal
| Condition | Turbidity (NTU) | pH | Oak extract (mg/L) | PACl (mg/L) | Predicted Removal Efficiency (%) | Experimental Removal Efficiency (%) |
|---|---|---|---|---|---|---|
| Oak Extract | 196 | 6 | 62 | – | 63.5 | 62.3 |
| PACl | 208 | 8 | – | 52 | 66.5 | 62.6 |
| Oak extract+ PACl | 218 | 6.2 | 63.5 | 32.6 | 85 | 86.1 |
The use of oak extract in water and wastewater treatment as a natural coagulant and coagulant aids has more benefits than chemicals, including biodegradability, far less effect on human health and environment, and non-existing residual metal problems in water and wastewater. For these reasons, oak extract may be of great interest since they are environment-friendly products, natural matter and low-cost coagulant, which increases the efficiency of the turbidity removal from water and wastewater in coagulation process.
Conclusion
In this study, the efficiency of Iranian oak extract as the main coagulant and polyaluminium chloride as an auxiliary coagulant was studied. With increasing the initial turbidity and pH of the sample water, the efficiency of coagulants in turbidity removal increased. The simultaneous use of the two coagulants, including Iranian oak extract and polyaluminium chloride, increased the efficiency of water turbidity removal and decreased the coagulant use rate compared with the use of each coagulant separately. In comparison to the non-Iranian oaks, Iranian oak extract showed the same or even higher efficiency in turbidity removal although having lower protein content. The simultaneous use of Iranian oak extract and polyaluminium chloride decreased the total amount of organic carbon present in the sample water.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Carr G, Potter RB, Nortcliff S. Water reuse for irrigation in Jordan: perceptions of water quality among farmers. Agric Water Manag. 2011;98(5):847–854. [Google Scholar]
- 2.Renault F, Sancey B, Badot PM, Crini G. Chitosan for coagulation/flocculation processes – an eco-friendly approach. Eur Polym J. 2009;45(5):1337–1348. [Google Scholar]
- 3.Wang LK, Hung Y-T, Shammas NK. Physicochemical treatment processes. Totowa: Humana Press; 2005.
- 4.Ferreira R, Napoleão TH, Santos AF, Sá R, Carneiro-da-Cunha M, Morais M, Silva-Lucca RA, Oliva ML, Coelho LC, Paiva PM. Coagulant and antibacterial activities of the water-soluble seed lectin from Moringa oleifera. Lett Appl Microbiol. 2011;53(2):186–192. doi: 10.1111/j.1472-765X.2011.03089.x. [DOI] [PubMed] [Google Scholar]
- 5.Gupta VK, Ali I, Saleh TA, Nayak A, Agarwal S. Chemical treatment technologies for waste-water recycling—an overview. RSC Adv. 2012;2(16):6380–6388. [Google Scholar]
- 6.Godini H, Hashemi F, Mansuri L, Sardar M, Hassani G, Mohseni S et al. Water polishing of phenol by walnut green hull as adsorbent: an insight of adsorption isotherm and kinetic. J Water Reuse Desal. 2016;6(4):544–52.
- 7.Wang J-P, Chen Y-Z, Ge X-W, Yu H-Q. Optimization of coagulation–flocculation process for a paper-recycling wastewater treatment using response surface methodology. Colloids Surf A Physicochem Eng Asp. 2007;302(1):204–210. [Google Scholar]
- 8.Birjandi N, Younesi H, Bahramifar N, Ghafari S, Zinatizadeh AA, Sethupathi S. Optimization of coagulation-flocculation treatment on paper-recycling wastewater: application of response surface methodology. J Environ Sci Health A. 2013;48(12):1573–1582. doi: 10.1080/10934529.2013.797307. [DOI] [PubMed] [Google Scholar]
- 9.Nougbodé YAEI, Agbangnan CP, Koudoro AY, Dèdjiho CA, Aïna MP, Mama D, et al. Evaluation of the Opuntia dillenii as natural coagulant in water clarification: case of treatment of highly turbid surface water. J Water Resour Prot. 2013;5(12):1242. [Google Scholar]
- 10.Al-Sameraiy M. A novel water pretreatment approach for turbidity removal using date seeds and pollen sheath. J Water Resour Prot. 2012;4(02):79. [Google Scholar]
- 11.Hassani G, Nasseri S, Gharibi H. Removal of cyanide by electrocoagulation process. Anal Bioanal Electrochem. 2011;3:625–634. [Google Scholar]
- 12.Kim M, Kim S, Kim J, Kang S, Lee S. Factors affecting flocculation performance of synthetic polymer for turbidity control. J Agric Chem Environ. 2013;2(01):16–21. [Google Scholar]
- 13.Flaten TP. Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull. 2001;55(2):187–196. doi: 10.1016/s0361-9230(01)00459-2. [DOI] [PubMed] [Google Scholar]
- 14.Hassani G, Alinejad A, Sadat A, Esmaeili A, Ziaei M, Bazrafshan AA, et al. Optimization of landfill leachate treatment process by electrocoagulation, Electroflotation and sedimentation sequential method. Int J Electrochem Sci. 2016;11:6705–6718. [Google Scholar]
- 15.Vijayaraghavan G, Sivakumar T, Kumar AV. Application of plant based coagulants for waste water treatment. Int J Adv Eng Res Stud. 2011;1(1):88–92. [Google Scholar]
- 16.Yin C-Y. Emerging usage of plant-based coagulants for water and wastewater treatment. Process Biochem. 2010;45(9):1437–1444. [Google Scholar]
- 17.Saranya P, Ramesh ST, Gandhimathi R. Effectiveness of natural coagulants from non-plant-based sources for water and wastewater treatment—a review. Desalin Water Treat. 2014;52(31–33):6030–6039. [Google Scholar]
- 18.Tondi G, Pizzi A. Tannin-based rigid foams: characterization and modification. Ind Crop Prod. 2009;29(2):356–363. [Google Scholar]
- 19.Antov MG, Šćiban MB, Petrović NJ. Proteins from common bean (Phaseolus vulgaris) seed as a natural coagulant for potential application in water turbidity removal. Bioresour Technol. 2010;101(7):2167–2172. doi: 10.1016/j.biortech.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Talebi M, Sagheb TK, Jahanbazi GH. Site demands and some quantitative and qualitative characteristics of Persian oak (Quercus brantii Lindl.) in Chaharmahal & Bakhtiari Province (western Iran) Iran J For Pop Res. 2006;14(1):67–79. [Google Scholar]
- 21.Hassani G, Takdastan A, Ghaedi M, Goudarzi G, Neisi A, Babaei AA. Optimization of 4-chlorophenol oxidation by manganese ferrite Nanocatalyst with response surface methodology. Int J Electrochem Sci. 2016;11:8471–8485. [Google Scholar]
- 22.Bhatti MS, Reddy AS, Thukral AK. Electrocoagulation removal of Cr (VI) from simulated wastewater using response surface methodology. J Hazard Mater. 2009;172(2–3):839–846. doi: 10.1016/j.jhazmat.2009.07.072. [DOI] [PubMed] [Google Scholar]
- 23.Ölmez T. The optimization of Cr (VI) reduction and removal by electrocoagulation using response surface methodology. J Hazard Mater. 2009;162(2–3):1371–1378. doi: 10.1016/j.jhazmat.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Hashemi S, Abdollahi M, Charehgani H. Inhibitory effect of Quercus brantii L. extract on Meloidogyne javanica, the causal agent of root-knot disease, in tomato plants. Iran J Med Aromat Plants. 2017;33(1).
- 25.Barmak MJ. Evaluation of the effect of the internal layer of oak fruit (jaft) extract on the prevention of gastric ulcers caused by stress in male rats. J Med Life. 2018;11(3):225. doi: 10.25122/jml-2017-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uosofi R, Moazed H, Karimi H, Nourmoradi H, Radmanesh F. Comparing the efficacy of cationic biopolymer chitosan and inorganic coagulant poly aluminum chloride in the removal of water turbidity. J Ilam Univ Med Sci. 2013;21(4):263–272. [Google Scholar]
- 27.Šćiban M, Klašnja M, Antov M, Škrbić B. Removal of water turbidity by natural coagulants obtained from chestnut and acorn. Bioresour Technol. 2009;100(24):6639–6643. doi: 10.1016/j.biortech.2009.06.047. [DOI] [PubMed] [Google Scholar]
- 28.Okuda T, Baes AU, Nishijima W, Okada M. Coagulation mechanism of salt solution-extracted active component in Moringa oleifera seeds. Water Res. 2001;35(3):830–834. doi: 10.1016/s0043-1354(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 29.Okuda T, Baes AU, Nishijima W, Okada M. Isolation and characterization of coagulant extracted from moringa oleifera seed by salt solution. Water Res. 2001;35(2):405–410. doi: 10.1016/s0043-1354(00)00290-6. [DOI] [PubMed] [Google Scholar]
- 30.Kim D-S. Mesurement of point of zero charge of bentonite by solubilization technique and its dependence of surface potential on pH. Environ Eng Res. 2003;8(4):222–227. [Google Scholar]
- 31.Bina B, Mehdinejad M, Nikaeen M, Attar HM. Effectiveness of chitosan as natural coagulant aid in treating turbid waters. J Environ Health Sci Eng. 2009;6(4):247–252. [Google Scholar]
- 32.Graham N, Gang F, Fowler G, Watts M. Characterisation and coagulation performance of a tannin-based cationic polymer: a preliminary assessment. Colloids Surf A Physicochem Eng Asp. 2008;327(1):9–16. [Google Scholar]
- 33.Narasiah K, Vogel A, Kramadhati N. Coagulation of turbid waters using Moringa oleifera seeds from two distinct sources. Water Sci Technol Water Supply. 2002;2(5–6):83–88. [Google Scholar]
- 34.Diaz A, Rincon N, Escorihuela A, Fernandez N, Chacin E, Forster CF. A preliminary evaluation of turbidity removal by natural coagulants indigenous to Venezuela. Process Biochem. 1999;35(3):391–395. [Google Scholar]
- 35.Pirsaheb M, Zinatizadeh AA, Dargahi A. Performance evaluation of coagulation process in removal of low turbidity and color from water using different inorganic coagulants. J Water Wastewater. 2011;23(1(81)):111–8.
- 36.Mahvi AH. Ahmadi Moghadam Mahdi, Naseri S, Kazem N. technical, economical and healthy evaluation of paci (poly aluminum chloride) application in water treatment. Iran J Public Health. 2003;32(2):6–8. [Google Scholar]
- 37.Sánchez-Martín J, González-Velasco M, Beltrán-Heredia J. Surface water treatment with tannin-based coagulants from Quebracho (Schinopsis balansae) Chem Eng J. 2010;165(3):851–858. [Google Scholar]
- 38.Orooji N, Takdastan A, Kargari A, Raeesi G. Efficiency of chitosan with polyaluminum chloride in turbidity removal from Ahwaz water treatment plant influent. J Water Wastewater. 2012;35:70–7.
- 39.Bina B, Shahsavani A, Asghari G, Hasanzadeh A. Comparison of water turbidity removal efficiencies of Moringa oleifera seed extract and poly-aluminum chloride. J Water Wastewater. 2007;18(61):24–33.
- 40.Mahdinezhad MH, Bina B, Nikaein M, Movahedian AH. Effectiveness of Moringa Oleifera coagulant protein and chitosan as natural coagulant aids in removal of colloidal particles and bacteria from turbid waters. J Gorgan Univ Med Sci. 2009;11(3):60–69. [Google Scholar]
- 41.Khan Ahmadi M, Borqei SM, Hasani AH. Comparison of “morning olifera seed extract and magnafloc lt25” in removal of water turbidity (case study: Tehran’s jalalieh (1) water treatment plant). J Water Wastewater. 2013;24(86):31–7.
- 42.Birima AH, Hammad H, Desa M, Muda ZC, editors. Extraction of natural coagulant from peanut seeds for treatment of turbid water. IOP Conference Series: Earth and Environmental Science; 2013: IOP Publishing.