Abstract
Shiga toxin-producing Escherichia coli (STEC) are important foodborne pathogens responsible for a wide spectrum of diseases including diarrhea, bloody diarrhea, and hemolytic uremic syndrome (HUS). A considerable number of outbreaks and sporadic cases of HUS have been associated with ingestion of fresh ready-to-eat products. Maintenance and persistence of STEC in the environment and foods can be related to its ability to form biofilm. A non-O157 STEC strain isolated from bovine feces was distinguished by its great ability to form biofilm in abiotic surfaces. In the present study, we aimed to investigate the ability of this strain to adhere to rocket leaves (Eruca sativa). Adherence assays were carried out for 3 h at 28 °C and analyzed by scanning electron microscopy. The non-O157 STEC strain adhered to leaf surface and inside the stomata forming several bacterial aggregates. The number of adherent bacteria per square millimeter of leaf was eightfold higher compared with an O157 STEC strain. Deletion of the STEC autotransporter protein contributing to biofilm (Sab) reduced the adherence ability of the non-O157 strain in almost 50%, and deletion of antigen 43 (Ag43) almost abolished this interaction. Very few bacteria were seen on the leaf surface, and these differences were statistically significant, suggesting the role of both proteins and especially Ag43 in the interaction of the non-O157 STEC strain with leaves. The risk posed by non-O157 STEC adherence to leaves on fresh produce contamination should not be neglected, and measures that effectively control adherence should be included in strategies to control non-O157 STEC.
Keywords: Shiga toxin-producing E. coli, Adherence, Leafy greens, Non-O157
Introduction
Shiga toxin-producing Escherichia coli (STEC) are considered important foodborne pathogens associated with diarrhea, bloody diarrhea, and hemolytic uremic syndrome (HUS) in humans [1]. Fresh leafy green vegetables contaminated with Escherichia coli have been associated to numerous outbreaks worldwide [2, 3]. It is suggested that maintenance and persistence of these organisms in the environment and food is related to its ability to form biofilm [4, 5]. Biofilm formation by STEC has been associated with the presence of different autotransporter (AT) proteins, such as “STEC autotransporter contributing to biofilm formation” (Sab) and antigen 43 (Ag43). Sab was identified in a virulent LEE-negative O113:H21 STEC and characterized as an AT protein that confers the strain the ability to adhere to human epithelial cells and mediate biofilm formation [6]. The Ag43 surface protein encoded by the chromosomal flu gene is not only involved in bacterial biofilm formation but also in its autoaggregation and characteristic frizzy colony morphology on the surface of host cells [7, 8].
In a previous study, one O105:H18 STEC strain isolated from bovine feces drew attention for its great ability to form biofilm in abiotic surfaces, and expression of Ag43 was suggested as having a role in this process [9].
Considering the number of outbreaks and sporadic HUS cases associated with ingestion of ready-to-eat fresh products, we aimed to investigate the ability of this non-O157 STEC strain to adhere to the surface of rocket leaves and search for the bacterial components involved in this process.
Our results show that wild-type O105:H8 strain extensively adhered to the rocket leaf, emphasizing the importance of the evaluation of STEC samples of different serotypes other than O157, as well as LEE-negative samples, in health and food industry.
Material and methods
Bacterial strains
STEC strain 473/01 was isolated from bovine feces and belonged to O105:H18 serotype [10]. Its ability to form biofilm in abiotic surfaces has been previously reported [9]. All strains and plasmids presently used are listed in Table 1. Strains were statically grown overnight at 37 °C on T medium (triptose, 10 g; beef extract, 3 g; NaCl, 5 g/L) broth, and bacterial cultures were standardized as previously described to an O.D.600 nm of 0.4 (approximately 1 × 109 CFU/mL) [12].
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristicsa | Reference |
|---|---|---|
| Strains | ||
| 473/1 (wt) | STEC O105:H18 biofilm forming in abiotic surfaces | [9] |
| 473/1 ∆sab | sab::zeo (Zeor) | This study |
| 473/1 ∆flu | flu::zeo (Zeor) | This study |
| 1711-4 ∆fliC | Source of zeocin cassette | [11] |
| 230/2 | STEC O157:H7 biofilm-forming in abiotic surfaces | [12] |
| Plasmids | ||
| pKOBEG-Para | Red recombinase system plasmid (Aprar) | [11] |
| pBAD/Myc-His A | Cloning vector | Invitrogen |
aZeor, zeocin resistant; Aprar, apramycin resistant
Adherence to rocket leaves
Prior to the adherence assays, rocket leaves (Eruca sativa) were immersed for 15 min at room temperature and with gentle agitation in 100 mL of H20 with four drops of Hidrosteril® (2.5% NaClO and 1% NaCl – commercial product for food sanitation) in order to minimize the interference of the leaf microbiota. The efficiency of this procedure was checked out growing suspension of macerated leaves in nutrient agar plates and MacConkey sorbitol agar plates. After the washing procedure, a piece of 1.5 cm2 was cut with a filter paper mold. The leaf piece was placed in a Petri dish containing 2.7 mL of T medium broth, in which 300 μL of standard bacterial cultures were added and incubation was carried out for 3 h at 28 °C as previously described [12]. Leaves were then washed in phosphate-buffered saline (PBS), prepared, and fixed for scanning electron microscopy (SEM).
Scanning electron microscopy (SEM)
After adherence assays using rocket leaves, the preparations were gently washed with 1x PBS for three times and subsequently fixed with Karnovsky fixative solution for at least 24 h at 4 °C. Preparations were then washed three times with 0.1 M cacodylate buffer (10 min) and post-fixed with 1% osmium tetroxide (prepared in the same buffer) for 30 min. After further three washes with distilled water, preparations were dehydrated through a graded series of ethanol (50%, 75%, 85%, 95%, and 100%) and, subsequently, critical point dried (CPD030, Leica EM, Germany), mounted on stubs, and sputter coated with gold (SCD050, Leica EM). Specimens were then examined under SEM (QUANTA 250, FEI Company, Netherlands) at 12.5 kV. The average number of adherent bacteria per square millimeter of leaf was counted in 10 different and representative fields (photos taken using spot size 2.5, average working distance of 5.5 mm, and magnification of ×2500).
Construction of mutant strains defective in Sab or Ag43 proteins
Derivative mutants were constructed using the lambda red system for recombination [13]. Primers containing a region homologous to the 5′ and 3′ extremities of the target genes (sab and flu) and sequence for the zeocin (Zeo) resistance-encoding gene were used to amplify the Zeo cassette. Amplicons were purified from the agarose gel with the QIAquick Gel Extraction Kit (Qiagen) and quantified (BioPhotometer; Eppendorf). The fragments were electroporated into competent STEC 473/1 cells containing the pKOBEG-Apra plasmid. Selection of recombinant bacteria on Zeo-containing LB agar plates (60 mg mL−1) was performed. Primers employed for mutagenesis are described in Table 2. PCR was used to confirm the loss of the target gene in the isogenic mutants, and mutants were checked for susceptibility to apramycin, indicative of the loss of pKOBEG-Apra. A growth curve of all mutant strains was obtained and evaluated by growing them as described above and measuring their O.D. every 30 min.
Table 2.
Primers used for PCR amplification
| Purpose and designation | Primer sequence (5′–3′) | Reference |
|---|---|---|
| Allelic exchange | ||
| sab-zeo F | ATG AAA TAT AAA AAA ACA CTG TCA TCG CTT GCA TTA GAA AGG | This study |
| sab-zeo R | TTA CCA CTG CCA GCC CAC ACC GGA ATG ATG CAG AGA TGT AAG | This study |
| flu-zeo F | ATG AAA CGA CAT CTG AAT ACG TCA TCG CTT GCA TTA GAA AGG | This study |
| flu-zeo R | TCA GAA GGT CAC ATT CAG CGT GGA ATG ATG CAG AGA TGT AAG | This study |
| Gene amplification by PCR | ||
| sab-F | GGT GGA TAC AGC AGG TAA TG | [6] |
| sab-R | TAT CTC ACC ACC TGC TAT CG | [6] |
| flu-F | CCG GCG GGC AAT GGG TAC A | [14] |
| flu-R | CAG CTC TCA CAA TCT GGC GAC | [14] |
Statistical analysis
Results of interactions of mutant strains with leaf surfaces were compared with the wt strain used as control by Student’s t test and considered significant when p < 0.05.
Results
Adherence of non-O157 STEC strain to rocket leaves
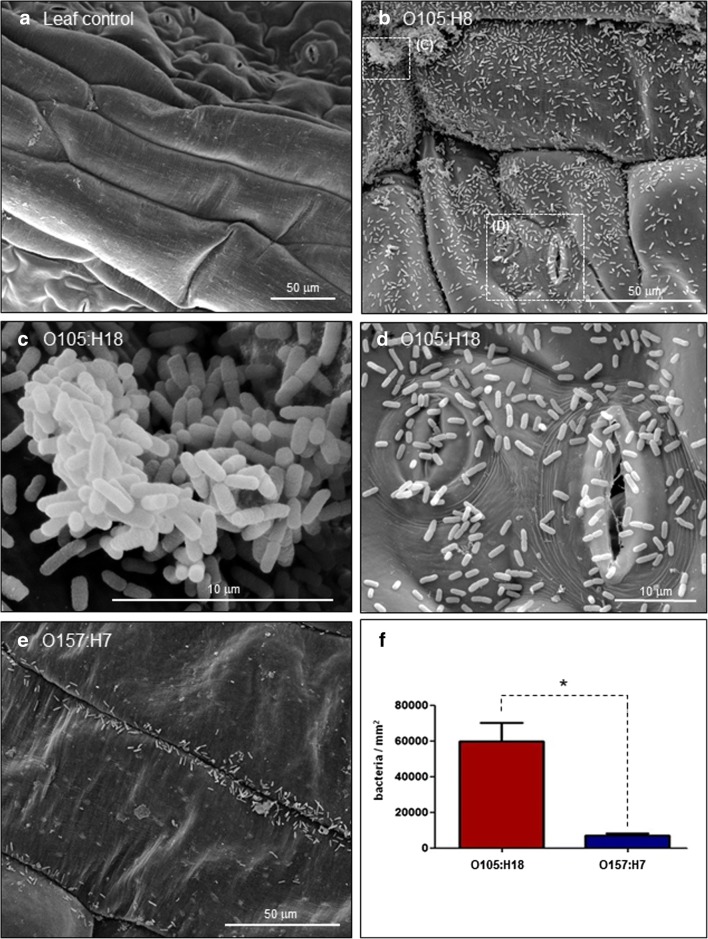
Prior to the adherence assays, rocket leaves were treated with a chlorine-based solution. This process significantly reduced the number of bacteria grown in nutrient agar plates and eliminated bacterial growth in MacConkey sorbitol agar plates. As a result of this washing process, bacteria could not be seen in the control leaf analyzed by SEM (Fig. 1a).
Fig. 1.
Adherence of O105:H18 and O157:H7 STEC strains to rocket leaves. Leaf squares were previously treated with Hidrosteril® in order to minimize the interference of the leaf microbiota. Uninfected leaf was used as the assay control (a). After 3-h infection at 28 °C, the O105:H18 strain presented a very pronounced ability to adhere to the rocket leaves (b). In some areas, O105:H18 could be observed forming several bacterial aggregates (c) and also adhered to and within the leaf stomata (d). O157:H7 strain adhered very weakly to the rocket leaves (e). The number of adherent bacteria per square millimeter of leaf observed for O105:H18 strain was eightfold higher than the number observed for O157:H7 strain (f). Differences observed were statistically significant (p < 0.05)
SEM analysis also showed that O105:H18 strain presented a pronounced ability to adhere (Fig. 1b) forming several bacterial aggregates on the leaf surface (Fig. 1c) and adhered to and within the leaf stomata (Fig. 1d). The O157:H7 STEC strain used as a control adhered weakly to the rocket leaves (Fig. 1e). Bacterial counting revealed that the number of O105:H18 adherent bacteria per square millimeter of leaf was eightfold higher compared with the O157:H7 strain (Fig. 1f). Differences observed were statistically significant (p < 0.05).
Mutagenesis
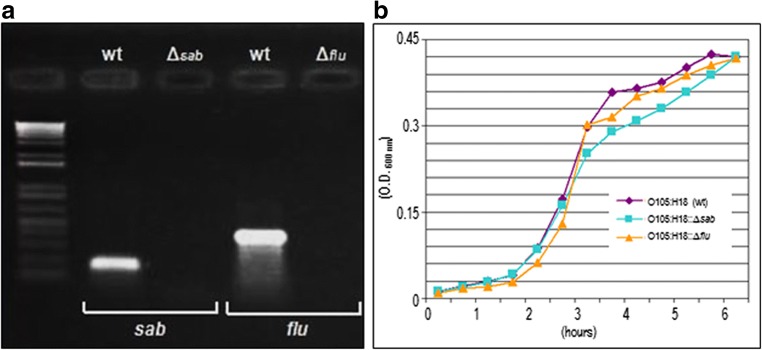
Loss of target genes in the isogenic mutants were confirmed by PCR (Fig. 2a), and all mutants were confirmed to grow at the same rate as the wild-type (wt) strain under the conditions tested (Fig. 2b).
Fig. 2.
PCR and growth curve of O105:H18 wt strain and mutants. PCR showed that target genes (sab and flu) were lost in the isogenic mutants (a). Bacterial growth curve (37 °C on T medium) showed that all mutants grew at the same rate as the wt strain (b)
Adherence of isogenic mutants to rocket leaves
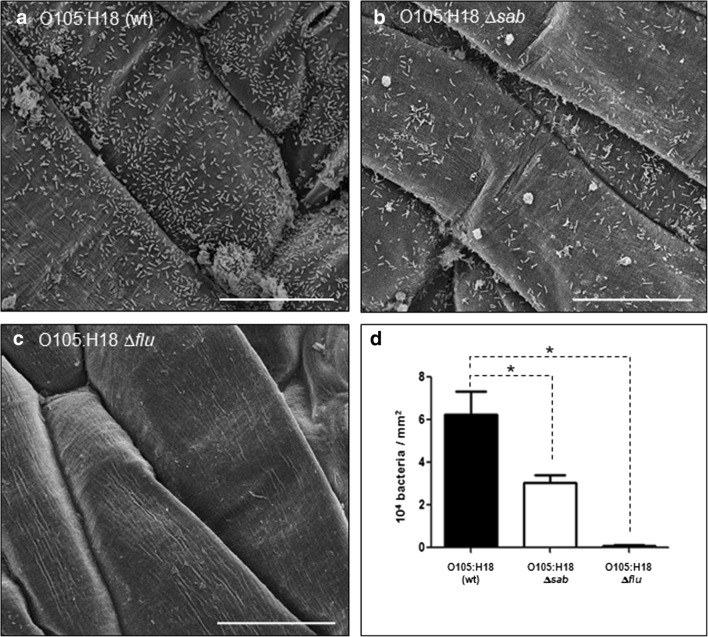
Deletion of sab reduced the ability of O105:H18 to adhere to leaves in almost 50% compared with the wt strain (Fig. 3a, b). Moreover, deletion of flu (Ag43) almost completely abolished this interaction (Fig. 3c), with the number of adherent bacteria being reduced in more than 50-fold compared with wt strain (Fig. 3d). Very few bacteria were seen on the leaf surface, and these differences were statistically significant (Fig. 3d).
Fig. 3.
Adherence of O105:H18 STEC and mutant strains to rocket leaves. After 3-h incubation at 28 °C, wt strain presented a strong adherence to the rocket leaves (a), while deletion of sab reduced in almost 50% the ability to adhere to leaves when compared with the wt strain (b and d). Deletion of flu almost abolished the interaction (c), as the number of adherent bacteria was reduced in more than 50-fold compared with wt strain (d). Differences observed were statistically significant (p < 0.05). Bars, 50 μm
Discussion
A large proportion of STEC-related illnesses have been associated to non-O157 strains [15], and estimates from the US Centers for Disease Control and Prevention indicate that non-O157 STEC causes more illnesses than STEC O157:H7 [16]. The growing importance of non-O157 STEC as agents of diarrhea and HUS stimulates studies on their virulence profile and pathogenic attributes [17, 18].
In more recent years, foodborne outbreaks linked to contaminated fresh produce is being recognized worldwide [19] leading to major concerns related to STEC widespread in agricultural environment. The occurrence of an outbreak caused by multiple non-O157 STEC strains was reported in Finland, and epidemiological investigations suggested rocket salad as the cause of the outbreak [20].
Indeed the ability of STEC to adhere and form biofilms on food and several other surfaces serves not only as an important source of contamination [21] but can also contribute to maintenance and persistence of bacteria protecting them against adverse environment conditions.
In the present study, the O105:H18 STEC strain showed a pronounced ability to adhere to rocket leaves. Several bacterial aggregates were seen on the leaf surface and bacteria adhered to and within the leaf stomata. One can suggest that such a behavior probably may aid bacterial maintenance even after leaves washing. Moreover, previous studies showed that this O105:H18 STEC strain was also able to produce a dense biofilm in abiotic surfaces [9]. Thus, the great ability of the O105:H18 STEC strain to adhere and form biofilm in abiotic and in biotic surfaces, such as fresh produce, can be considered as a serious problem for microbial safety of foods. Albeit, O105 serogroup is not among the non-O157 STEC serogroups most commonly found causing human diseases, known as the big six (O26, O45, O103, O111, O121, and O145), STEC strains belonging to serotype O105:H18 have been associated to HUS and diarrheal infections [22, 23]. Moreover, the pathogenic potential of a STEC isolate goes far beyond an association with a particular serogroup or serotype, and this became more evident from the German and Finland outbreaks [24, 20].
Different adhesive structures account for the capacity of STEC to bind to several surfaces, and autotransporters are important factors related to adherence and biofilm formation of non-O157 STEC [25]. A high occurrence of flu genes has been described among non-O157 STEC strains, but its presence was not statistically related to biofilm formation [26, 27]. In this study, it was shown that the adherence of O105:H18 to rocket leaves significantly decreased when genes related to Sab were deleted, confirming previous observations on the participation of this AT in the adherence ability of non-O157 isolates [6]. Moreover, deletion of Ag43 AT gene (flu) almost completely abolished the interaction of O105:H18 to leaf surfaces, showing its important role on adherence of this particular STEC isolate as previously suggested [9].
Conclusions
The data presently described highlight the important involvement of Sab and Ag43 on the adhesion of non-O157 STEC to fresh produce. Understanding STEC mechanisms involved on adhesion will certainly help the development of new approaches for control and prevention of bacterial contamination and persistence on food matrices and contact surfaces, therefore contributing to the microbiological safety of food.
Funding information
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to BEC Guth (Process No. 303403/2015-2).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaper JB, O’Brien AD (2014) Overview and historical perspectives. Microbiol Spectr 2. 10.1128/microbiolspec.EHEC-0028-2014 [DOI] [PMC free article] [PubMed]
- 2.Martínez-Vaz BM, Fink RC, Diez-Gonzalez F, Sadowsky MJ. Enteric pathogen-plant interactions: molecular connections leading to colonization and growth and implications for food safety. Microbes Environ. 2014;29:123–135. doi: 10.1264/jsme2.ME13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger CN, Sodha SV, Shaw RK, Griffin PM, Pink D, Hand P, Frankel G. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ Microbiol. 2010;12:2385–2397. doi: 10.1111/j.1462-2920.2010.02297.x. [DOI] [PubMed] [Google Scholar]
- 4.Segura A, Auffret P, Bibbal D, Bertoni M, Durand A, Jubelin G, Kérourédan M, Brugère H, Bertin Y, Forano E. Factors involved in the persistence of a Shiga toxin-producing Escherichia coli O157:H7 strain in bovine feces and gastro-intestinal content. Front Microbiol. 2018;9:375. doi: 10.3389/fmicb.2018.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kusumaningrum HD, Riboldi G, Hazeleger WC, Beumer RR. Survival of foodborne pathogens on stainless steel surfaces and cross-contamination to foods. Int J Food Microbiol. 2003;85:227–236. doi: 10.1016/s0168-1605(02)00540-8. [DOI] [PubMed] [Google Scholar]
- 6.Herold S, Paton JC, Paton AW. Sab, a novel autotransporter of locus of enterocyte effacement-negative Shiga-toxigenic Escherichia coli O113:H21, contributes to adherence and biofilm formation. Infect Immun. 2009;77:3234–3243. doi: 10.1128/IAI.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klemm P, Hjerrild L, Gjermansen M, Schembri MA. Structure–function analysis of the self-recognizing antigen 43 autotransporter protein from Escherichia coli. Mol Microbiol. 2004;51:283–296. doi: 10.1046/j.1365-2958.2003.03833.x. [DOI] [PubMed] [Google Scholar]
- 8.Hasman H, Schembri MA, Klemm P. Antigen 43 and type 1 fimbriae determine colony morphology of Escherichia coli K-12. J Bacteriol. 2000;182:1089–1095. doi: 10.1128/jb.182.4.1089-1095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biscola FT, Abe CM, Guth BEC. Determination of adhesin gene sequences in, and biofilm formation by, O157 and non-O157 Shiga toxin-producing Escherichia coli strains isolated from different sources. Appl Environ Microbiol. 2011;77:2201–2208. doi: 10.1128/AEM.01920-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irino K, Kato MAMF, Vaz TMI, Ramos II, Souza MAC, Cruz AS, Gomes TAT, Vieira MAM, Guth BEC. Serotypes end virulence markers of Shiga toxin-producing Escherichia coli (STEC) isolated from dairy cattle in São Paulo State, Brazil. Vet Microbiol. 2005;105:29–36. doi: 10.1016/j.vetmic.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Sampaio SC, Gomes TA, Pichon C, du Merle L, Guadagnini S, Abe CM, Sampaio JL, Le Bouguénec C. The flagella of an atypical enteropathogenic Escherichia coli strain are required for efficient interaction with and stimulation of interleukin-8 production by enterocytes in vitro. Infect Immun. 2009;77:4406–4413. doi: 10.1128/IAI.00177-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matheus-Guimarães C, Gonçalves EM, Cabilio Guth BE. Interactions of O157 and non-O157 Shiga toxin-producing Escherichia coli (STEC) recovered from bovine hide and carcass with human cells and abiotic surfaces. Foodborne Pathog Dis. 2014;11:248–255. doi: 10.1089/fpd.2013.1653. [DOI] [PubMed] [Google Scholar]
- 13.Chaveroche MK, Ghigo JM, d’Enfert C. A rapid method for efficient gene replacement in the filamentous fungus Aspergillus nidulans. Nucleic Acids Res. 2000;28:E97. doi: 10.1093/nar/28.22.e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Restieri C, Garriss G, Locas MC, Dozois CM. Autotransporter-encoding sequences are phylogenetically distributed among Escherichia coli clinical isolates and reference strains. Appl Environ Microbiol. 2007;73:1553–1562. doi: 10.1128/AEM.01542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith JL, Fratamico PM, Gunther NW. Shiga toxin-producing Escherichia coli. Adv Appl Microbiol. 2014;86:145–197. doi: 10.1016/B978-0-12-800262-9.00003-2. [DOI] [PubMed] [Google Scholar]
- 16.Valilis E, Ramsey A, Sidiq S, DuPont DL. Non-O157 Shiga toxin-producing Escherichia coli - a poorly appreciated enteric pathogen: systematic review. Int J Infect Dis. 2018;76:82–87. doi: 10.1016/j.ijid.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Feng PCH, Sabine Delannoy S, Lacher DW, Santos LF, Beutin L, Fach P, Rivas M, Hartland EL, Adrienne W, Paton AW, Guth BEC. Genetic diversity and virulence potential of Shiga toxin-producing Escherichia coli O113:H21 strains isolated from clinical, environmental, and food sources. Appl Environ Microbiol. 2014;80:4757–4763. doi: 10.1128/AEM.01182-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montero DA, Del Canto F, Velasco J, Colello R, Padola NL, Salazar JC, San Martin C, Oñate A, Blanco J, Rasko DA, Contreras C, Puente JL, Scheutz F, Franzi E, Vidal RM. Cumulative acquisition of pathogenicity islands has shaped virulence potential and contributed to the emergence of LEE-negative Shiga toxin-producing Escherichia coli strains. Emerg Microb Infect. 2019;8:486–502. doi: 10.1080/22221751.2019.1595985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olaimat AN, Holley RA. Factors influencing the microbial safety of fresh produce: a review. Food Microbiol. 2012;32:1–19. doi: 10.1016/j.fm.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Kinnula S, Hemminki K, Kotilainen H, Ruotsalainen E, Tarkka E, Salmenlinna S, Hallanvuo S, Leinonen E, Jukka O, Rimhanen-Finne R (2018) Outbreak of multiple strains of non-O157 Shiga toxin-producing and enteropathogenic Escherichia coli associated with rocket salad, Finland, autumn 2016. Euro Surveill 23(35). 10.2807/1560-7917.ES.2018.23.35.1700666 [DOI] [PMC free article] [PubMed]
- 21.Frank JF. Microbial attachment to food and food contact surfaces. Adv Food Nutr Res. 2001;43:319–370. doi: 10.1016/S1043-4526(01)43008-7. [DOI] [PubMed] [Google Scholar]
- 22.WHO. (1998) World Health Organization. Zoonotic non-O157 Shiga toxin-producing E coli (STEC) Report of a WHO Scientific Working group Meeting Berlin, Germany, June 1998. http://apps.who.int/iris/bitstream/10665/68880/1/WHO_CSR_APH_98.8.pdf
- 23.Girardeau JP, Dalmasso A, Bertin Y, Ducrot C, Bord S, Livrelli V, Christine Vernozy-Rozand C, Martin C. Association of virulence genotype with phylogenetic background in comparison to different seropathotypes of Shiga toxin-producing Escherichia coli isolates. J Clin Microbiol. 2005;43:6098–6107. doi: 10.1128/JCM.43.12.6098-6107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jandhyala DM, Vanguri V, Boll EJ, Laic Y, McCormick BA, Leong JM. Shiga toxin–producing Escherichia coli O104:H4: an emerging pathogen with enhanced virulence. Infect Dis Clin N Am. 2013;27(3):631–649. doi: 10.1016/j.idc.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaglic Z, Desvaux M, Weiss A, Nesse LL, Meyer RL, Demnerova K, Schmidt H, Giaouris E, Sipailiene A, Teixeira P, Kacániova M, Riedel CU, Susanne Knøchel S. Surface adhesins and exopolymers of selected foodborne pathogens. Microbiology. 2014;160:2561–2582. doi: 10.1099/mic.0.075887-0. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Stanford K, McAllister TA, Johnson RP, Chen J, Hou H, Zhang G, Yan D, Niu YD. Biofilm formation, virulence gene profiles, and antimicrobial resistance of nine serogroups of non-O157 Shiga toxin–producing Escherichia coli. Foodborne Pathog Dis. 2016;13:1–9. doi: 10.1089/fpd.2015.2099. [DOI] [PubMed] [Google Scholar]
- 27.Bumunang EW, McAllister TA, Zaheer R, Polo RO, Stanford K, King R, Niu YD, Ateba CN. Characterization of non-O157 Escherichia coli from cattle faecal samples in the North-West Province of South Africa. Microorganisms. 2019;7:272. doi: 10.3390/microorganisms7080272. [DOI] [PMC free article] [PubMed] [Google Scholar]