Abstract
Introduction
The objective of this systematic review is to reflect on assumptions in relation to codeine use in combination with other analgesics.
Methods
MEDLINE was searched according to the predetermined keywords and criteria. Only English language studies were taken into consideration and the outcome data of the final studies were extracted by two reviewers independently from each other and were checked by the third reviewer. Additionally, the available codeine-related Individual Case Safety Reports (ICSRs) retrieved from EudraVigilance were reviewed.
Results
Sixteen placebo-controlled studies that involved 3378 subjects suffering from acute pain were analyzed for the efficacy of low-dose codeine (≤ 30 mg) combination products. Twelve of them found low-dose codeine combinations more efficient in relieving pain than the assigned comparator. According to 20 randomized clinical trials which included at least one dose of codeine (from 30 to 240 mg daily), the vast majority of reported side-effects were mild or moderate in severity. A total of 20 ICSRs for dependence were identified in the EudraVigilance database with codeine as a suspect drug for the 10-year time period for the European region.
Conclusions
Low-dose codeine combinations are effective after a single application in treating acute pain. Codeine in doses ≤ 30 mg and higher was considered safe since only mild to moderate side-effects were observed. There is no indication in the available sources which clearly links low doses of codeine to substance use disorder in non-dependent subjects.
Electronic Supplementary Material
The online version of this article (10.1007/s40122-020-00162-8) contains supplementary material, which is available to authorized users.
Keywords: Acute pain, Clinical studies, Combination analgesic drugs, Efficacy, Low-dose codeine, Review, Safety, Substance-use disorder
Key Summary Points
| Sixteen placebo-controlled studies that involved 3378 subjects suffering from acute pain were analyzed for the efficacy of low-dose codeine (≤ 30 mg) combination products. |
| Twenty randomized clinical trials which included at least one dose of codeine (from 30 to 240 mg daily), were analyzed for the safety and adverse-effects profile. |
| In EudraVigilance database for the 10-year time period, a total of 20 individual case safety reports for dependence were identified with codeine as a suspect drug. |
| Low-dose codeine in fixed combinations with other drugs is effective and safe when used as recommended. |
| There is no exact proof in the available literature that clearly links low doses of codeine to substance use disorder issues in non-dependent subjects. |
Introduction
Codeine or 3-methylmorphine is a mild opioid with analgesic and antitussive effect [1]. Its analgesic activity is mostly due to the conversion to morphine by the cytochrome P450 enzyme CYP2D6, although codeine also has some (low) affinity for the μ-opioid receptor displayed in the central nervous system (CNS) and at peripheral tissues, like the gastrointestinal tract [2]. In the context of analgesic action, codeine can be considered as a prodrug. Only around 10% of codeine is converted to morphine. Other metabolic enzymes (CYP3A4, UDP-glucuronyltransferase) catalyse the conversion of codeine to other mostly inactive metabolites norcodeine (10–15%) and codeine-6-glucuronide (50–70%). Different rates of metabolism correlate with genotypes of the CYP2D6 [3]. Poor metabolizers, with one or two non-functional alleles are presented in 7–10% of the white population and may have decreased metabolism of codeine to morphine, and lower possibility for the analgesic effect in comparison to normal (extensive metabolizers). On the contrary, ultrarapid metabolism is considered to occur in 1–7% of the white population, whereas the incidence is 5–10% and 3% for Southern and Northern Europeans, respectively. As a result, in patients with more than two copies of the CYP2D6 functional allele, there is increased formation of morphine and a higher potential for experiencing adverse effects (AEs), such as sleepiness, confusion, and shallow breathing, even at recommended doses of codeine. Because of the unpredictable metabolism rate, codeine use as an analgesic drug is often replaced by other opioids, although its combination with non-opioid analgesic drugs is still largely available and used for pain treatment [4–8].
It has been found repeatedly that a combination of different analgesic drugs at fixed doses, rather than their single use, leads to a faster and better acute pain relief. The main purpose of such combination is the synergistic analgesic effect due to the multimodal approach to pain processing, alongside the better safety profile with no increase of the adverse effect incidence due to the initial lower doses of individual analgesics [9]. For example, the combination of non-opioid analgesic/antipyretic drugs, like paracetamol, ibuprofen, or acetylsalicylic acid with codeine was found to be rational since these substances have different mechanisms of action on pain with greater analgesic potential being possibly achieved, without reaching drugs individual toxic limits [10–12].
It is worth mentioning that doses of codeine in combinations with other drugs vary significantly, from 8 up to 60 mg, where a vast majority of studies investigated the effects of higher dose codeine combinations (codeine doses ≥ 30 mg).
A recent systematic review and meta-analysis by Abdel Shaheed et al. of ten randomized clinical trials investigated efficacy and safety of combination analgesic products with codeine in doses up to 30 mg and found low to moderate level of evidence for relief of acute pain. The authors found limited data about adverse effects outcomes [13].
At adult standard daily dose (30–60 mg every 4 h orally up to a maximum of 240 mg daily) codeine was found to cause no euphoria or respiratory depression. Also, despite the many speculations, existing proofs from clinical practice show it to be rarely addictive if applied as recommended [10, 14].
In this systematic review, using a comprehensive literature search strategy, we evaluated efficacy and safety of the low-dose codeine in combinations with other analgesics, to elaborate their efficacy and safety in treating acute pain. In contrast to a recent systematic review and meta-analysis [13] which evaluated the same ten studies for efficacy and safety and included single but also the multiple combination dosing regimens, here we evaluated just single-dose studies when assessing the efficacy of low-codeine combination medicinal products. Furthermore, here we focused on codeine safety in general and have included both, codeine in doses ≤ 30 mg and above, alone or in combination with other analgesics after single and multiple dosing regimens. Moreover, in addition to the scientific literature, the available individual safety reports data as retrieved from the European Medicines Agency’s (EMA) service—EudraVigilance were reviewed and the topic of substance use disorder is specially addressed.
Methods
General Search Strategy
A bibliographic database MEDLINE was searched according to the predetermined keywords (codeine, analgesia, pain, efficacy, safety, adverse event, side effect, addiction, dependence, overdose, misuse, abuse) and criteria (randomized clinical trial/study and range from inception to end of January 2019).
Search strategy was made concise enough to make sure that the exact data on efficacy and safety, along with potential for codeine use disorders is found, while remaining wide enough to include and extract the potential valuable data which could remain hidden if searching just for the codeine combination. Nevertheless, for the efficacy part, only placebo-controlled and combination studies were included in the final review.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Study Selection
Only English language studies were included in this review. Additional filters combining human species and studies published up to January 31, 2019 were taken into consideration for obtaining relevant literature findings. Following initial screening of the titles and abstracts found for codeine, two reviewers set the keywords and criteria in place for efficacy and safety studies on codeine. One of the exclusion criteria was paediatric population due to recommended restrictions on the use of codeine for cough and cold in children. Based on the abstracts, initial judgment call was made on the potentially valuable studies. After retrieving the full texts, final decision was made on to which studies shall be included in the review. Efficacy and safety differed in final decision on importance of the found studies.
Efficacy Study Search and Selection
Since codeine’s efficacy in combination was proven time and again, especially for higher doses (> 30 mg), the main focus was to find the randomized, placebo-controlled studies which included codeine in lower doses (≤ 30 mg) in combination with another non-opioid analgesic substance. Only single dose studies were deemed acceptable. Despite the wider spectrum of codeine use, only studies focusing on analgesia were searched for (regardless of the exact indication). The selection was not limited to the duration of treatment or to the analgesic effect duration. Also, different doses of the substances in the codeine combinations were not a restriction factor.
Safety Study Search and Selection
For the purpose of safety evaluation, randomized controlled clinical studies involving medicines containing codeine either alone or in combinations, irrespective of doses, were taken into consideration. Both single and multiple dose studies, where codeine-treated subjects received at least one dose of codeine (ranging from 15 to 240 mg daily dose), for any pain condition were reviewed in order to meet the eligibility criteria. The safety profile of codeine was additionally determined involving keywords such as dependence, addiction, misuse, abuse and overdose throughout MEDLINE and EudraVigilance.
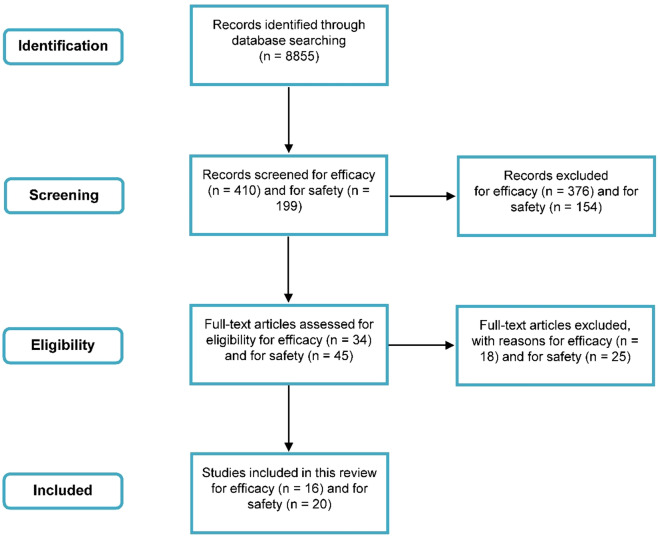
The summary on search strategy can be seen on Fig. 1.
Fig. 1.
Search strategy summary for efficacy and safety as per the predetermined inclusion and exclusion criteria
Data Extraction and Quality Assessment
Outcome data of the final studies were extracted by two reviewers independently from each other, and were checked by the third reviewer. Recommendations from the Cochrane Handbook were applied when and where applicable.
The quality of reports of randomised controlled trials was measured using a scale indexed on PEDro (provided as supplementary material), the Physiotherapy Evidence Database (https://www.pedro.org.au/).
Results
Overview of Efficacy
Efficacy of low-dose codeine combination medicinal products was analysed through review of 16 placebo-controlled studies which involved 3378 subjects. Most of the studies were focused on codeine combination efficacy regarding surgery pain with most of them relating to the dental surgery pain (9 out of the total 16 studies) [15–23]; two were focused on tension headache [20, 24]; one on acute migraine attack [25]; one on post-episiotomy pain [26]; one on post-orthopaedic surgery pain [27]; one on post-photorefractive keratectomy pain [28]; and one on post-operative pain [29].
Medicinal products as found in the aforementioned studies contained the following 9 substances alongside codeine: paracetamol—nine studies [10, 16–18, 20, 25, 27–29]; acetylsalicylic acid—seven studies [15, 17–19, 22–25]; and ibuprofen—six studies [16, 19, 22, 23 26, 27]; followed by butalbital—three studies [16, 17, 24]; caffeine—three studies [16, 17, 24]; zomepirac—one study [24]; meclofenam—one study [21]; pentazocine—one study [29]; and propoxyphene napsylate—one study [29].
The lowest and highest doses of the most commonly used substances mentioned above were as follows: for paracetamol—300 mg and 650 mg; for acetylsalicylic acid—325 mg and 1000 mg; and for ibuprofen—400 mg and 800 mg.
None of the studies were focused on the long-term effects of codeine combination use, with the analgesic efficacy period being evaluated for all of them: 2 h was the shortest time noted [25], while 72 h being the longest time observed following single doses of combination products [28].
Twelve studies based on statistically significant results provided the final conclusion that low-dose codeine combinations are more efficient in relieving pain compared to placebo [10, 15, 17–21, 24–26, 28, 29]. Complete or almost complete pain relief was noted after: 0.5 h [24]; 1 h [15, 26, 28, 29], 2 h [25], 5 h [19], 6 h [17, 18, 20, 21], and 12-24 h [10]. Cooper and Beaver [15] discussed the minor role of codeine in analgesia, while concluding that the statistically significant efficacy of the combination is due to acetylsalicylic acid.
In contrast, two studies found codeine in combinations to be effective, but less than the comparator [16, 27]. Daniels et al. [16] compared the efficacy of paracetamol and ibuprofen combinations (ibuprofen 200 mg + paracetamol 500 mg; ibuprofen 200 mg + codeine 12.8 mg; paracetamol 500 mg + codeine 15 mg) with placebo. They found that even though low-dose codeine combinations were statistically significantly superior to placebo, ibuprofen-paracetamol combinations were more effective in pain management. Heidrich et al. [27] compared the single dose ibuprofen 400 mg with a combination of paracetamol 300 mg and codeine 30 mg. They found the single dose of ibuprofen to be more effective in pain reduction than the combination containing codeine in terms of sensory descriptors of pain. However, combination with codeine was more effective when regarding the affective descriptors of pain.
Two studies found low-dose codeine in combination to be ineffective, or modestly effective in pain treatment [22, 23]. Giles et al. [22] argued in their study the efficacy of low-dose codeine, concluding that 15 mg codeine, alone or in combination, has little to none analgesic efficacy. A dose of 15 mg was discussed to be a subtherapeutic dose with questionable possibility to show the additive effect in combination. Squires et al. [23] found no statistically significant difference between 30 mg codeine (in combination with 375 mg acetylsalicylic acid and 30 mg caffeine) and placebo in their study.
There are four studies which found low-dose codeine in combination to be effective, but less than the comparator [16, 21, 25, 27]. This potentially suggests that low-dose codeine combinations are more efficient when compared to placebo, but not when compared to other non-opioid agents.
Most of the studies which were included are from more than 30 years ago, with the oldest study found dated 1976. Conclusions from four newer studies (time period from 2011–2017) are in line with conclusions from the older ones—stating that low-dose codeine combination is more efficient in pain management when compared to placebo [10, 16, 20, 28]. Still, one of those studies found that low-dose codeine combination is as effective as the comparator [20], while one study found that such combination is less efficient than the comparator [16].
The characteristics and conclusions of the included efficacy studies are available in the Table 1.
Table 1.
Characteristic and conclusions of the included efficacy studies
| Authors (reference) | Study design | Number of participants | Population characteristics/Mean age of the participants (years) | Indication | Intervention (compared to placebo) |
Outcome measures | Analgesic efficacy period measurement (h) | Study conclusion on codeine/codeine combinations compared to comparator/placebo |
|---|---|---|---|---|---|---|---|---|
| Cooper and Beaver [15] | A randomized, double-blind, single-dose study | 128 | 64 Female and 64 male patients/aged between 16 and 35 | Oral surgery pain |
1. Ibuprofen 400 mg + codeine phosphate 30 mg 2. zomepirac 100 mg 3. codeine 30 mg 4. acetylsalicylic acid 650 mg 5. codeine 30 mg + acetylsalicylic acid 650 mg |
Pain relief Pain intensity difference |
3 | Low-dose codeine combination is more efficient in relieving pain compared to placebo, acetylsalicylic acid 650 mg and codeine 30 mg alone |
| Cater et al. [26] | A double-blind, single-dose study | 127 | All female patients/22.8 | Moderate or severe postepisiotomy pain |
1. ibuprofen 400 mg + codeine phosphate 30 mg 2. zomepirac 100 mg 3. codeine 30 mg 4. acetylsalicylic acid 650 mg 5. codeine 30 mg + acetylsalicylic acid 650 mg |
Degree of pain Pain relief Pain half gone |
8 | Low-dose codeine combination is more efficient in relieving pain compared to placebo and zomepirac 100 mg |
| Heidrich et al. [27] | A double-blind, single-dose, parallel-group | 120 | 48 Female and 72 male patients/31 | Post-orthopaedic surgery pain |
1. ibuprofen 400 mg 2. paracetamol 300 mg + codeine 30 mg |
Pain relief Pain intensity Mood |
6 | Codeine in combination is effective, but less than the comparator |
| Frame et al. [19] | A double-blind, single dose study | 165 | Both genders were included/24.4 | Removal of an impacted mandibular third molar tooth |
1. acetylsalicylic acid 600 mg 2. ibuprofen 200 mg + codeine phosphate 15 mg 3. ibuprofen 400 mg + codeine phosphate 30 mg 4. ibuprofen 800 mg + codeine phosphate 60 mg |
Pain relief (degree of pain) | 5 | Low-dose codeine combinations are more efficient in relieving pain compared to placebo and acetylsalicylic acid |
| Desjardins et al. [17] | A double-blind, randomized, single-dose study | 123 | 77 Female and 46 male patients/23.8 | Oral surgery pain |
1. paracetamol 300 mg + codeine 30 mg 2. acetylsalicylic acid 325 mg + butalbital 50 mg + caffeine 40 mg + codeine 30 mg |
Sum of pain intensity difference (SPID) Peak pain intensity difference Total pain relief (TOTPAR) Peak pain relief Sum of observations with pain half gone Total anxiety Peak anxiety Total relaxation Peak relaxation Overall evaluation Time to remedication with an alternate analgesic |
6 | Low-dose codeine combinations are more efficient in relieving pain than placebo |
| Forbes et al. [18] | A double-blind, parallel group, randomized study | 122 | 77 Female and 45 male patients/21.63 | Moderate or severe pain after the surgical removal of impacted third molars |
1. acetylsalicylic acid 325 mg + caffeine 40 mg + codeine 30 mg + butalbital 50 mg 2. paracetamol 300 mg + codeine 30 mg |
Pain intensity difference score (PIO) Sum of the pain intensity difference scores (SPIO) Peak PIO score Pain relief score Total pain relief score Peak pain relief score Total hours of 50% relief Overall evaluation Anxiety difference score Sum of the anxiety difference scores Peak anxiety difference score Relaxation difference score Sum of the relaxation difference scores Peak relaxation difference score Time until taking the backup medication |
6 | Low-dose codeine combinations are more efficient in relieving pain than placebo |
| Giles et al. [22] | A double-blind, single dose study | 202 | Both genders were included/24.8 | Surgical removal of third molars pain |
1. acetylsalicylic acid 600 mg 2. ibuprofen 200 mg 3. codeine 15 mg 4. ibuprofen 200 mg + codeine 15 mg |
Degree of pain Pain relief If the pain was half gone |
5 | Codeine, alone or in combination, has little to none analgesic efficacy |
| Hwang et al. [24] | A randomized, double-blind, placebo-controlled, multicenter trial | 208 | Both genders were included/aged between 18 and 65 | Tension headache |
1. butalbital 50 mg + caffeine 40 mg + acetylsalicylic acid 200 mg + phenacetin 130 mg 2. codeine phosphate 30 mg 3. butalbital 50 mg + caffeine 40 mg + acetylsalicylic acid 200 mg + phenacetin 130 mg + codeine 30 mg |
Patients’ self-evaluation: Pain severity Pain relief Tension and uptight feeling Relaxation and contentment Muscle stiffness Daily activities Average tension composite Average pathology Physician’s global evaluation: Headache pain Psychic tension Muscle contraction |
4 | Low-dose codeine combinations are more efficient in relieving pain compared to placebo, butalbital 50 mg + caffeine 40 mg + acetylsalicylic acid 200 mg + phenacetin 130 mg and codeine phosphate 30 mg |
| Petti et al. [29] | A single-blind, parallel-group study | 129 | Both genders were included/aged between 18 and 80 | Moderate postoperative pain |
1. paracetamol 650 mg + pentazocine 25 mg 2. paracetamol 300 mg + codeine 30 mg 3. paracetamol 650 mg + propoxyphene napsylate 100 mg |
Severity of pain Degree of pain relief |
6 | Low-dose codeine combination is more efficient in relieving pain compared to placebo, paracetamol 650 mg + pentazocine 25 mg and paracetamol 650 mg + propoxyphene napsylate 100 mg |
| Giglio and Laskin [21] | A single-dose, randomized, double-blind, parallel-treatment study | 200 | 165 Female and 35 male patients/22.7 | Surgical removal of third molars pain |
1. meclofenamate 100 mg + codeine 60 mg 2. meclofenamate 50 mg + codeine 30 mg 3. meclofenamate 100 mg 4. codeine 60 mg |
Pain intensity difference (PID) Sum of pain intensity differences (SPID) Sum of pain relief scores (TOTPAR) Peak pain relief Number of observations at which pain was half relieved Overall evaluation of study medication effectiveness Time to remedication with a backup analgesic |
6 | Low-dose codeine combination is more efficient in relieving pain compared to placebo and codeine 60 mg, but slightly less efficient than meclofenamate 100 mg and meclofenamate 100 mg + codeine 60 mg |
| Boureau et al. [25] | A randomized, multicentre, double-blind study | 247 | 190 Female and 57 male patients/40.1 ± 11.6 | Acute migraine attack |
1. paracetamol 400 mg + codeine 25 mg 2. acetylsalicylic acid 1000 mg |
Complete or near complete relief of pain | 2 | Low-dose codeine combination is more efficient in relieving pain compared to placebo and slightly less efficient than acetylsalicylic acid 1000 mg |
| Daniels et al. [16] | A double-blind, 5-arm, parallel-group, placebo-controlled, randomised, single-dose study | 678 | 105 Female and 68 male patients/20.2 | Postoperative dental pain | 1. ibuprofen 200 mg + paracetamol 500 mg |
Pain relief Pain intensity difference |
12 | Codeine in combinations is effective, but less than the comparator ibuprofen + paracetamol combination |
| Gatoulis et al. [20] | A randomized, double-blind, placebo-controlled, single-dose clinical trial | 302 | 185 Female and 115 male patients/23.13 | Dental pain |
1. acetylsalicylic acid 1000 mg 2. paracetamol 300 mg + codeine 30 mg |
Sum of pain intensity differences from baseline (SPID) The time-interval–weighted sum of pain relief scores (TOTPAR) Pain intensity difference (PID) Peak PID Pain relief score (PAR) Peak PAR Time to onset of meaningful and complete relief Time to use of rescue medication |
6 | Low-dose codeine combination is more efficient in relieving pain compared to placebo and comparably efficient compared to acetylsalicylic acid 1000 mg |
| 676 | 304 Female and 183 male patients/37.12 | Tension-type headache | 4 | |||||
| Cristalli et al. [10] | A randomized, placebo-controlled, double-blind | 32 | Both genders were included/aged between 20 and 29 | Mandibular third molar surgery pain | Paracetamol 500 mg + codeine 30 mg |
Postoperative pain The number of patients using rescue therapy, Time to the first use of rescue analgesia Total number of postoperative-supplement paracetamol + codeine tablets |
48 | Low-dose codeine combination is more efficient in relieving pain than placebo |
| Pereira et al. [28] | A randomized, double-blind, placebo-controlled add-on trial | 40 | 27 Female and 13 male patients/30 | Pain after photorefractive keratectomy | Codeine 30 mg + paracetamol 500 mg |
Difference in pain intensity Mean pain scores |
72 | Low-dose codeine combination is more efficient in relieving pain compared to placebo |
Overview of Safety
Analgesics containing codeine are in general well-tolerated and usually exhibit favourable safety profile with most AEs classified as mild to moderate in severity if used for short period of time and in recommended doses. Based on the literature data discussed further in the text, the incidence of treatment discontinuation due to severe AEs is low and comparable to other treatment options for pain management.
Most of the analysed studies for safety overview included combination of non-steroidal antiinflammatory drugs (NSAIDs) with codeine in higher single dose (> 30 mg).
The overall safety of codeine alone or in combinations was assessed through 20 randomized clinical trials where codeine-treated subjects received at least one dose of codeine (ranging from 30 to 240 mg daily dose). The most represented dose within the studies was 60 mg single dose and was used in 75% of all evaluated clinical trials. Safety profiles of study medications were determined in adult patients by self-reporting of undesirable effects, including suggestive questioning regarding adverse symptoms, dependently on a study design.
Most of the performed single and multiple dose studies included codeine in combinations with paracetamol (65%), acetylsalicylic acid (25%) and ibuprofen (15%) usually for acute pain management including pre- and post-operative analgesia, headache and low-back pain. Only 3 from total of 20 evaluated studies (15%) were dealing with codeine containing analgesics being administered for up to 10 days, and these were the studies regarding chronic cancer pain [30, 31], along with outpatient breast surgery study [32]. Comparators that were used throughout the studies were standard pain killers such as paracetamol, nefopam, NSAIDs (ibuprofen, acetylsalicylic acid, ketorolac, flurbiprofen, etoricoxib, ketoprofen, piroxicam, and naproxen), opioids (tramadol) and placebo.
The list of all of the studies included in the safety analysis is provided in Table 2.
Table 2.
Studies included in the safety analysis with number of patients from randomized clinical trials reporting adverse effects
| Studies | Treatment | Comparator | Study conclusion | Number of patients reporting adverse drug reactions (n/N) | ||
|---|---|---|---|---|---|---|
| Treatment (n/N) | Comparator (n/N) | Treatment vs. comparator | ||||
| Cooper et al. [33] |
I. codeine 60 mg, II. acetylsalicylic acid 650 mg + codeine 60 mg, III. ibuprofen 400 mg + codeine 60 mg |
A. acetylsalicylic acid 650 mg, B. ibuprofen 400 mg, C. placebo |
There were no significant differences regarding AEs within treatment groups. No withdrawal from the study was recorded |
I. 26.8% (11/41) II. 26.7% (12/45) III. 43.9% (18/41) |
A. 23.7% (9/38) B. 28.9% (11/38) C. 10.9% (5/46) |
32.3% vs. 20.5% |
| Forbes et al. [34] | Paracetamol 600 mg + codeine 60 mg |
A. diflunisal 500 mg, B. diflunisal 1000 mg, C. paracetamol 600 mg, D. placebo |
There were no significant differences regarding AEs within treatment groups | 42.3% (11/26) |
A. 46.2% (12/26) B. 46.4% (13/28) C. 42.3% (11/26) D. 15.4% (4/26) |
42.3% vs. 37.7% |
| Desjardins et al. [17] |
I. paracetamol 300 mg + codeine 30 mg, II. acetylsalicylic acid 325 mg + butalbital 50 mg + caffeine 40 mg + codeine 30 mg |
Placebo | There were no significant differences regarding AEs within treatment groups |
I. 5.1% (2/39) II. 11.6% (5/43) |
9.8% (4/41) | 8.5% vs 9.8% |
| Forbes et al. [18] |
I. codeine sulfate 60 mg, II. naproxen sodium 550 mg + codeine 60 mg |
A. naproxen sodium 550 mg, B. acetylsalicylic acid 650 mg, C. placebo |
Report includes AEs occurred within 12 h of taking study medication. Incidence of AEs was higher in codeine treatment groups |
I. 25.5% (12/47) II. 35.6% (16/45) |
A. 16.3% (7/43) B. 7.3% (3/41) C. 15.2% (7/46) |
30.4% vs. 13.1% |
| Sagne et al. [35] | Paracetamol 650 mg + codeine 60 mg | Paracetamol 650 mg + dextropropoxyphene 65 mg | Adverse events were more frequent in women from paracetamol + codeine group | 38.1% (37/97) | 26.6% (25/94) | 38.1% vs. 26.6% |
| Stambaugh and Drew [36] | Acetylsalicylic acid 650 mg + codeine 60 mg |
A. ketoprofen 100 mg, B. ketoprofen 300 mg, C. placebo |
There were no significant differences in relation with safety data within treatment groups | 22.5% (9/40) |
A. 17.5% (7/40) B. 17.5% (7/40) C. 22.5% (9/40) |
22.5% vs. 19.2% |
| Sunshine et al. [37] | Codeine 60 mg |
A. piroxicam 20 mg, B. placebo |
The highest incidence of reported AEs was observed in the placebo group | 19.6% (10/51) |
A. 14.0% (7/50) B. 50.0% (25/50) |
19.6% vs 32.0% |
| Turek et al. [38] | Paracetamol 650 mg + codeine 60 mg |
A. ketoprofen 50 mg, B. ketoprofen 150 mg, C. placebo |
There was a significantly greater incidence of central nervous system AEs in the paracetamol plus codeine group | 28.2% (11/39) |
A. 34.1% (14/41) B. 20.5% (8/39) C. 9.8% (4/41) |
28.2% vs. 21.5% |
| Forbes et al. [39] | Paracetamol 600 mg + codeine 60 mg |
A. flurbiprofen 100 mg, B. paracetamol 600 mg, C. placebo |
Report includes AEs occurred within 12 h of taking study medication | 5.9% (1/17) |
A. 3.4% (1/29) B. 11.5% (3/26) C. 7.7% (2/26) |
5.9% vs. 7.4% |
| Minotti et al. [31] | Acetylsalicylic acid 640 mg + codeine 40 mg |
A. nefopam 60 mg, B. diclofenac sodium 50 mg |
AEs were slightly more frequent within acetylsalicylic acid + codeine and nefopam treatment groups | 36.4% (12/33) |
A. 39.4% (13/33) B. 6.1% (2/33) |
36.4% vs. 27.3% |
| Carlson et al. [30] | Paracetamol 600 mg + codeine 60 mg | Ketorolac 10 mg | AEs were acceptable for both treatment groups | 47.5% (19/40) | 61.8% (21/34) | 47.5% vs. 61.8% |
| Forbes et al. [40] | Paracetamol 600 mg + codeine 60 mg |
A. ketorolac 10 mg, B. ketorolac 20 mg, C. ibuprofen 400 mg, D. paracetamol 600 mg, E. placebo |
All AEs were transient, and none of them required additional treatment | 20.0% (8/40) |
A. 12.8% (5/39) B. 18.6% (8/43) C. 18.6% (8/43) D. 12.2% (5/41) E. 0% |
20.0% vs. 15.7% |
| Hellman et al. [41] |
I. codeine 30 mg, II. ibuprofen 200 mg + codeine 30 mg, III.acetylsalicylic acid 500 mg + codeine 30 mg |
NA | Codeine alone caused a higher rate of AEs than in combinations (17% vs. 11%) |
I. 16.7% (8/48) II. 10.6% (5/47) III. 10.3% (4/39) |
NA | 12.7% vs. 0% |
| Petersen et al. [42] | Ibuprofen 400 mg + codeine 60 mg | Ibuprofen 400 mg | Incidence of AEs were higher in ibuprofen + codeine group | 48.3% (14/29) | 19.4% (6/31) | 48.3% vs. 19.4% |
| Stubhaug et al. [43] | Paracetamol 1000 mg + codeine 60 mg |
A. tramadol 50 mg, B. tramadol 100 mg, C. placebo |
AEs were more common for tramadol treatment groups | 27.0% (10/37) |
A. 54.3% (19/35) B. 50.0% (18/36) C. 41.7% (15/36) |
27.0% vs. 49.1% |
| Soulier et al. [44] | Paracetamol 300 mg + codeine phosphate 30 mg | Flurbiprofen 50 mg | Differences between 2 groups were not statistically significant | 57.5% (23/40) | 46.3% (19/41) | 57.4% vs. 46.3% |
| Innes et al. [45] | Paracetamol 600 mg + 60 mg codeine | Ketorolac 10 mg | Patients in the paracetamol + codeine group reported significantly more AEs; 7 out of 60 patients withdrew from the study because of AEs after 7-day treatment | 64.4% (38/59) | 33.9% (21/62) | 64.4% vs. 33.9% |
| Daniels et al. [16] | Paracetamol 2400 mg + codeine 240 mg |
A. ibuprofen 2400 mg, B. etoricoxib 90 mg, C. etoricoxib 120 mg, D. placebo |
Significantly more discontinuations due to AEs were observed in paracetamol + codeine group | 48.4% (30/62) |
A. 9.4% (18/192) B. 11.0% (21/191) C. 12.4% (12/97) D. 13.0% (6/46) |
48% vs. 10.8% |
| Gatoulis et al. (2 studies included; 1. and 2.) [20] | Paracetamol 600 mg + codeine phosphate 60 mg |
A. acetylsalicylic acid 1000 mg, B. placebo |
There were no statistically significant differences regarding AEs and no serious AEs were reported | 1. 31.4% (38/121) |
A. 28.3% (34/120) B. 39.3% (24/61) |
31.4% vs. 32.0% |
| 2. 24.5% (57/233) |
A. 17.0% (38/223) B. 18.4% (19/103) |
24% vs. 17.5% | ||||
| Mitchell et al. [32] | Paracetamol 600 mg + caffeine 30 mg + codeine 60 mg | Paracetamol 650 mg + ibuprofen 400 mg | There were no significant differences regarding AEs within treatment groups, however, discontinuations due to AEs were higher in the codeine group | 41.4% (29/70) | 42.3% (30/71) | 41.4% vs. 42.3% |
Different comparators were differentiated with capital alphabetical letters (A., B., etc.). “Treatment” denotes combination therapy with codeine or codeine alone
n number of patients experiencing an adverse effect, N total number of patients in the respective treatment group, AEs adverse effects
The most commonly reported AEs, regardless of study drug-relatedness, were: nausea, gastrointestinal pain, constipation, dyspepsia, vomiting, dizziness, tiredness, headache, photophobia, somnolence, dry mouth, euphoria, and faintness. The vast majority of them were mild or moderate in severity. There was no observed difference regarding the incidence of undesirable effects within the treatment groups, irrespective of acute vs. chronic pain treatment. Incidence of AEs reporting was shown to be consistent, regardless of the patient’s age, race and gender. However, study from Sagne et al. [35] indicates that AEs are more frequently experienced by women taking codeine containing pain killers and that the frequency of unwanted effects is probably weight-dependent.
Discontinuation due to AEs in both treatment and comparator group was observed in 10 from total of 20 clinical trials (50%) and was more attributed to the treatment group when comparing with the comparator group (10% vs. 3%). It is worth mentioning that majority of the serious AEs were due to toxicity of the other drug in combination product, not to codeine (ibuprofen, acetylsalicylic acid, paracetamol).
In general, the safety profile of codeine containing analgesics in doses of 30 mg and higher has been well characterised combining the results from clinical studies and extensive post-marketing surveillance. Most of the reported AEs were transient and mild to moderate in severity. There were no deaths nor serious, drug-related AEs observed following treatment with codeine.
Codeine Use Disorders
Codeine, acting as opioid receptor agonist, is a substance with a well-known risk for substance use disorder (mild, moderate or severe) due to its conversion to morphine, usually when used at higher than recommended doses for chronic pain treatment. Besides dose and duration of codeine use, a patient psychosocial characteristics (substance use disorder, previous experience with drugs, mental illnesses, etc.) are relevant for the risk of codeine use disorder. Although the liability for substance use disorder is lower than with stronger opioids, neuro-adaption and the development of such disorder may appear following inappropriate use of codeine. The misuse of codeine is considered in case of taking higher doses and/or for longer period than advisable. The potential for codeine use disorder cannot be excluded when used for recreational purposes and in excessive doses [1, 46].
Due to the fact that codeine is the very often used either alone or in combo-preparation for pain relief, there is a growing concern regarding both intentional and unintentional misuse of codeine-containing products. There are available reports claiming both, low potential for substance use disorder and common issues in relation with addictive-related disorders linked to codeine use. Following search strategy within this systematic review, there were no randomised controlled clinical studies related to codeine use disorder found. The prevalence of codeine use disorder is not likely to be determined as most of the available evidence is addressed within case study reports. Moreover, there is limitation regarding data for doses < 30 mg [1, 7, 46, 47].
Consumption of higher doses was evident in Canadian survey which involved 339 subjects who had used codeine for 3 days/week for at least 6 months and where was found that 37% of subjects who have used approximately 180 mg per day have met DSM-IV criteria for dependence [48]. Another big survey involving total of 800 subjects compared characteristics of dependent vs. non-dependent codeine users. Similar to findings from the study from Sproule et al. [49], most of the dependent patients reported family history of substance use disorders and long-term treatment for chronic pain.
Following review of EudraVigilance database, a total of 20 ICSRs (Individual Case Safety Reports) reporting dependence as a reaction were identified with codeine as a suspect drug (as a single substance, not in combination) for the time period since the January 2009 up until January 2019, for the European region. Additionally, there were 3 cases reporting medication overuse headache, while withdrawal syndrome was recorded in 25 ICSRs where codeine was defined as a suspect drug [50].
For medicinal product containing codeine phosphate sesquihydrate or hemihydrate in dose of 10 mg and in combination with paracetamol, propyphenazone and caffeine for acute pain treatment, a total of 66 ICSRs were reported for the Republic of Croatia (as found in EudraVigilance). A substance use disorder was recorded in 4 ICSRs and in most of the cases there were missing information related to other possible suspect drugs [50]. It is worth mentioning that during this 10 year period, in total, 29,520,348 packages of such combined products with low-dose codeine phosphate were sold in the territory of Croatia [51].
Discussion
Acute pain is a common condition which needs adequate therapy. Non-steroidal anti-inflammatory drugs, along with paracetamol and acetylsalicylic acid are the first line in combating the pain of low to moderate severity. Additionally, concomitant use of drugs from different classes has proved to be rational. Codeine, as a weak opioid agonist is a common ingredient of combination analgesic drugs. However, due to its opioid-like features, the codeine-containing products are often tagged with an increased risk of AEs, and with a risk of substance use disorder development. It should be noted that a vast majority of published studies regarding codeine combinations are related to the higher doses of codeine (mostly for doses ≥ 30 mg) with non-opiate analgesic, leaving a very large gap of evidence for the codeine doses below 30 mg, which are often available as over-the-counter products.
Therefore, with this systematic review, we wanted to evaluate available data related to low-dose codeine efficacy and safety along with the potential for codeine substance use disorder using a comprehensive search strategy. Efficacy and safety differed in final decision on importance of the founded studies, however with limitations of only English studies and adult population that were taken into consideration. In this systematic review, efficacy of low-dose codeine (≤ 30 mg) combination medicinal products was analysed by reviewing the 16 placebo-controlled, single-dose studies which involved 3378 subjects suffering from different types of acute pain. Out of 16 studies, 12 of them were based on statistically significant results leading to the final conclusion that low-dose codeine combinations are more efficient in relieving pain than the assigned comparator (as shown and referenced in Table 1). Four studies out of 16 found codeine in combinations to be either less effective than the comparator (two studies) or to be ineffective altogether in pain treatment (also two studies).
Similar findings in terms of efficacy for low-dose codeine combinations (15–30 mg) have been presented in recently published systematic review and meta-analysis which included 10 randomized placebo controlled clinical trials. For the purpose of codeine safety analysis, and in contrast to Shaheed et al. [13], we have extended our research and included respective data from EudraVigilance database along with available data from RTC, regardless of the codeine doses.
This review assessed the overall safety of codeine, alone or in combinations, by reviewing the 20 randomized clinical trials (as shown and referenced in Table 2). These trials included codeine-treated subjects that received at least one dose of codeine, ranging from 30 to 240 mg daily dose (with 60 mg single dose being the most represented one). Codeine containing analgesics have shown good tolerability with no statistically significant differences in adverse reactions reporting among the treatment and the comparator group (Table 2) It was also demonstrated that the most AEs are in relationship with higher dose combinations (30 or 60 mg of codeine per tablet).
In general, the safety profile of codeine containing analgesics in doses of 30 mg and higher has been well characterised combining the results from clinical studies and extensive post-marketing surveillance. When used as prescribed, for short periods of time and in recommended doses (up to 240 mg daily), most of the AEs are classified as mild to moderate in severity.
The most common challenge faced with codeine use is its well-known potential for substance use disorder. This is attributed to its conversion to morphine, usually when used at higher doses than advised and for longer periods of time (e.g. for chronic pain treatment). Although observed substance use disorder is lower than with stronger opioids, the development of such disorder may appear following the inappropriate use, usually higher than recommended daily doses either recreationally or continuously for chronic pain treatment in vulnerable patient population [1, 46].
Currently available reports can be found which claim both, low potential for substance use disorder (mild, moderate, severe) and common issues in relation with addictive-related disorders linked to codeine use. Following search strategy within this systematic review, no randomised controlled clinical studies related to codeine use disorders were found. The prevalence of codeine use disorder is not likely to be determined as most of the available evidence is addressed within case study reports. Moreover, there is limitation regarding data for doses < 30 mg. Only findings that were found for codeine use disorder pertained to the higher doses, with no exact and statistically significant data. Most of the patients with codeine use disorder have reported family history of substance use disorders and long-term treatment for chronic pain.
Conclusion
This comprehensive systematic review has found low-dose codeine combinations to be effective and safe when applied in recommended daily doses and for short periods of time. There is no indication in the available literature which clearly links low doses of codeine (alone or in combination) to substance use disorder (low, moderate, severe) issues in non-dependent subjects. In fact, we have found low-dose codeine combinations to be safe, with most of the adverse reactions reported as mild to moderate in severity. Publicly accessible data from European Medicines Agency also show a very small number of codeine use disorder adverse reactions as reported. However, to finally eliminate issues related to low-dose codeine in combination analgesic products, further well-designed clinical studies are warranted to provide more data relevant for efficacy, safety profile, and risk for substance use disorder.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Croatian Chamber of Pharmacists for all of their support.
Funding
This project and the journal’s Rapid Service Fee was funded by a project by the Croatian Chamber of Pharmacists funded by PLIVA Hrvatska and Alkaloid AD Skopje.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Lidija Bach-Rojecky is a member of the Advisory Board at Alkaloid AD Skopje. Lidija Bach-Rojecky reports personal fees from Alkaloid AD Skopje, PLIVA Hrvatska, Sandoz Hrvatska, and Medis Adria for giving lectures, outside the submitted work. Ivan Ćelić reports speaking fees for the following companies: Alkaloid AD Skopje and Sandoz. Iveta Merćep, Anja Kos Petrak, Ana Soldo and Ana Bučan have nothing to disclose. Anja Kos Petrak is now affiliated with Makpharm Ltd., Pharmacovigilance Department, Zagreb, Croatia.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Footnotes
Enhanced Digital Features
To view enhanced digital features for this article go to 10.6084/m9.figshare.11925480.
References
- 1.Nielsen S, Van Hout MC. Over-the-counter codeine-from therapeutic use to dependence, and the grey areas in between. Curr Top Behav Neurosci. 2017;34:59–75. doi: 10.1007/7854_2015_422. [DOI] [PubMed] [Google Scholar]
- 2.Yaksh TL, Wallace MS. Opiods, analgesia, and pain management. In: Brunton LL, Hilal-Dundan R, Knollmann BC, editors. Goodman & Gilman’s: the pharmacological basis of therapeutics. New York: McGraw Hill Education; 2018. pp. 355–385. [Google Scholar]
- 3.Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE, Caudle KE, Haidar CE, Shen DD, Callaghan JT, Sadhasivam S, Prows CA, Kharasch ED, Skaar TC, Clinical Pharmacogenetics Implementation Consortium Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther. 2014;95(4):376–382. doi: 10.1038/clpt.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekhri NK, Cooney MF. Opioid metabolism and pharmacogenetics: clinical implications. J Perianesth Nurs. 2017;32(5):497–505. doi: 10.1016/j.jopan.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Dean L. Codeine therapy and CYP2D6 genotype. In: Pratt V, McLeod H, Dean L, Malheiro A, Rubinstein W (eds) Medical genetics summaries. Bethesda: National Center for Biotechnology Information; 2012. http://www.ncbi.nlm.nih.gov/books/NBK100662/. Acessed 31 May 2019. [PubMed]
- 6.He YJ, Brockmöller J, Schmidt H, Roots I, Kirchheiner J. CYP2D6 ultrarapid metabolism and morphine/codeine ratios in blood: was it codeine or heroin? J Anal Toxicol. 2008;32(2):178–182. doi: 10.1093/jat/32.2.178. [DOI] [PubMed] [Google Scholar]
- 7.Gasche Y, Daali Y, Fathi M, Chiappe A, Cottini S, Dayer P, Desmeules J. Codeine intoxication associated with ultrarapid CYP2D6 metabolism. N Engl J Med. 2004;351(27):2827–2831. doi: 10.1056/NEJMoa041888. [DOI] [PubMed] [Google Scholar]
- 8.Bradley CM, Nicholson AN. Effects of a mu-opioid receptor agonist (codeine phosphate) on visuo-motor coordination and dynamic visual acuity in man. Br J Clin Pharmacol. 1986;22(5):507–512. doi: 10.1111/j.1365-2125.1986.tb02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardhan R, Chelly J. Recent advances in acute pain management: understanding the mechanisms of acute pain, the prescription of opioids, and the role of multimodal pain therapy. F1000Res. 2017;6:2065. doi: 10.12688/f1000research.12286.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cristalli MP, La Monaca G, De Angelis C, Pranno N, Annibali S. Efficacy of preoperative administration of paracetamol-codeine on pain following impacted mandibular third molar surgery: a randomized, split-mouth, placebo-controlled, double-blind clinical trial. Pain Res Manag. 2017;2017:9246352. doi: 10.1155/2017/9246352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Craen AJ, Di Giulio G, Lampe-Schoenmaeckers JE, Kessels AG, Kleijnen J. Analgesic efficacy and safety of paracetamol-codeine combinations versus paracetamol alone: a systematic review. BMJ. 1996;313(7053):321–325. doi: 10.1136/bmj.313.7053.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehlisch DR. The efficacy of combination analgesic therapy in relieving dental pain. J Am Dent Assoc. 2002;133(7):861–871. doi: 10.14219/jada.archive.2002.0300. [DOI] [PubMed] [Google Scholar]
- 13.Abdel Shaheed C, Maher CG, McLachlan AJ. Efficacy and safety of low-dose codeine-containing combination analgesics for pain: systematic review and meta-analysis. Clin J Pain. 2019;35(10):836–843. doi: 10.1097/AJP.0000000000000746. [DOI] [PubMed] [Google Scholar]
- 14.Kim I, Barnes AJ, Oyler JM, Schepers R, Joseph RE, Jr, Cone EJ, Lafko D, Moolchan ET, Huestis MA. Plasma and oral fluid pharmacokinetics and pharmacodynamics after oral codeine administration. Clin Chem. 2002;48(9):1486–1496. doi: 10.1093/clinchem/48.9.1486. [DOI] [PubMed] [Google Scholar]
- 15.Cooper SA, Beaver WT. A model to evaluate mild analgesics in oral surgery outpatients. Clin Pharmacol Ther. 1976;20(2):241–250. doi: 10.1002/cpt1976202241. [DOI] [PubMed] [Google Scholar]
- 16.Daniels SE, Bandy DP, Christensen SE, Boice J, Losada MC, Liu H, Mehta A, Peloso PM. Evaluation of the dose range of etoricoxib in an acute pain setting using the postoperative dental pain model. Clin J Pain. 2011;27(1):1–8. doi: 10.1097/AJP.0b013e3181ed0639. [DOI] [PubMed] [Google Scholar]
- 17.Desjardins PJ, Cooper SA, Finizio T. Efficacy of low dose combination analgesics: acetaminophen/codeine, aspirin/butalbital/caffeine/codeine, and placebo in oral surgery pain. Anesth Prog. 1986;33(3):143–146. [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes JA, Keller CK, Smith JW, Zeleznock JR, Sevelius H, Beaver WT. Analgesic effect of naproxen sodium, codeine, a naproxen-codeine combination and aspirin on the postoperative pain of oral surgery. Pharmacotherapy. 1986;6(5):211–218. doi: 10.1002/j.1875-9114.1986.tb03479.x. [DOI] [PubMed] [Google Scholar]
- 19.Frame JW, Fisher SE, Pickvance NJ, Skene AM. A double-blind placebo-controlled comparison of three ibuprofen/codeine combinations and aspirin. Br J Oral Maxillofac Surg. 1986;24(2):122–129. doi: 10.1016/0266-4356(86)90007-0. [DOI] [PubMed] [Google Scholar]
- 20.Gatoulis SC, Voelker M, Fisher M. Assessment of the efficacy and safety profiles of aspirin and acetaminophen with codeine: results from 2 randomized, controlled trials in individuals with tension-type headache and postoperative dental pain. Clin Ther. 2012;34(1):138–148. doi: 10.1016/j.clinthera.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 21.Giglio JA, Laskin DM. Double-blind comparison of meclofenamate sodium plus codeine, meclofenamate sodium, codeine, and placebo for relief of pain following surgical removal of third molars. J Oral Maxillofac Surg. 1990;48(8):785–790. doi: 10.1016/0278-2391(90)90332-V. [DOI] [PubMed] [Google Scholar]
- 22.Giles AD, Hill CM, Shepherd JP, Stewart DJ, Pickvance NJ. A single dose assessment of an ibuprofen/codeine combination in postoperative dental pain. Int J Oral Maxillofac Surg. 1986;15(6):727–732. doi: 10.1016/S0300-9785(86)80114-4. [DOI] [PubMed] [Google Scholar]
- 23.Squires DJ, Masson EL. A double-blind comparison of ibuprofen, ASA-codeine-caffeine compound and placebo in the treatment of dental surgery pain. J Int Med Res. 1981;9(4):257–260. doi: 10.1177/030006058100900404. [DOI] [PubMed] [Google Scholar]
- 24.Hwang DS, Mietlowski MJ, Friedman AP. Fiorinal with Codeine in the management of tension headache: impact of placebo response. Clin Ther. 1987;9(2):201–222. [PubMed] [Google Scholar]
- 25.Boureau F, Joubert JM, Lasserre V, Prum B, Delecoeuillerie G. Double-blind comparison of an acetaminophen 400 mg-codeine 25 mg combination versus aspirin 1000 mg and placebo in acute migraine attack. Cephalalgia. 1994;14(2):156–161. doi: 10.1046/j.1468-2982.1994.1402156.x. [DOI] [PubMed] [Google Scholar]
- 26.Cater M, O’Brien PM, Pickvance NJ. A double-blind comparison of the new ibuprofen-codeine phosphate combination, zomepirac, and placebo in the relief of postepisiotomy pain. Clin Ther. 1985;7(4):442–447. [PubMed] [Google Scholar]
- 27.Heidrich G, Slavic-Svircev V, Kaiko RF. Efficacy and quality of ibuprofen and acetaminophen plus codeine analgesia. Pain. 1985;22(4):385–397. doi: 10.1016/0304-3959(85)90044-2. [DOI] [PubMed] [Google Scholar]
- 28.Pereira VBP, Garcia R, Torricelli AAM, Mukai A, Bechara SJ. Codeine plus acetaminophen for pain after photorefractive keratectomy: a randomized, double-blind, placebo-controlled add-on trial. Cornea. 2017;36(10):1206–1212. doi: 10.1097/ICO.0000000000001328. [DOI] [PubMed] [Google Scholar]
- 29.Petti A. Postoperative pain relief with pentazocine and acetaminophen: comparison with other analgesic combinations and placebo. Clin Ther. 1985;8(1):126–133. [PubMed] [Google Scholar]
- 30.Carlson RW, Borrison RA, Sher HB, Eisenberg PD, Mowry PA, Wolin EM. A multiinstitutional evaluation of the analgesic efficacy and safety of ketorolac tromethamine, acetaminophen plus codeine, and placebo in cancer pain. Pharmacotherapy. 1990;10(3):211–216. doi: 10.1002/j.1875-9114.1990.tb02577.x. [DOI] [PubMed] [Google Scholar]
- 31.Minotti V, Patoia L, Roila F, Basurto C, Tonato M, Pasqualucci V, Maresca V, Del Favero A. Double-blind evaluation of analgesic efficacy of orally administered diclofenac, nefopam, and acetylsalicylic acid (ASA) plus codeine in chronic cancer pain. Pain. 1989;36(2):177–183. doi: 10.1016/0304-3959(89)90021-3. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell A, McCrea P, Inglis K, Porter G. A randomized, controlled trial comparing acetaminophen plus ibuprofen versus acetaminophen plus codeine plus caffeine (Tylenol 3) after outpatient breast surgery. Ann Surg Oncol. 2012;19(12):3792–3800. doi: 10.1245/s10434-012-2447-7. [DOI] [PubMed] [Google Scholar]
- 33.Cooper SA, Engel J, Ladov M, Precheur H, Rosenheck A, Rauch D. Analgesic efficacy of an ibuprofen-codeine combination. Pharmacotherapy. 1982;2(3):162–167. doi: 10.1002/j.1875-9114.1982.tb04528.x. [DOI] [PubMed] [Google Scholar]
- 34.Forbes JA, Kolodny AL, Beaver WT, Shackleford RW, Scarlett VR. A 12-hour evaluation of the analgesic efficacy of diflunisal, acetaminophen, and acetaminophen-codeine combination, and placebo in postoperative pain. Pharmacotherapy. 1983;3(2 Pt 2):47S–54S. [PubMed] [Google Scholar]
- 35.Sagne S, Henrikson PA, Kahnberg KE, Thilander H, Bertilson SO. Analgesic efficacy and side-effect profile of paracetamol/codeine and paracetamol/dextropropoxyphene after surgical removal of a lower wisdom tooth. J Int Med Res. 1987;15(2):83–88. doi: 10.1177/030006058701500204. [DOI] [PubMed] [Google Scholar]
- 36.Stambaugh J, Drew J. A double-blind parallel evaluation of the efficacy and safety of a single dose of ketoprofen in cancer pain. J Clin Pharmacol. 1988;28(s1):S34–S39. doi: 10.1002/j.1552-4604.1988.tb05975.x. [DOI] [PubMed] [Google Scholar]
- 37.Sunshine A, Roure C, Colon A, Olson NZ, Gonzalez L, Siegel C, Laska E. Analgesic efficacy of piroxicam in the treatment of postoperative pain. Am J Med. 1988;84(5A):16–22. doi: 10.1016/0002-9343(88)90472-X. [DOI] [PubMed] [Google Scholar]
- 38.Turek MD, Baird WM. Double-blind parallel comparison of ketoprofen (Orudis), acetaminophen plus codeine, and placebo in postoperative pain. J Clin Pharmacol. 1988;28(s1):S23–S28. doi: 10.1002/j.1552-4604.1988.tb05973.x. [DOI] [PubMed] [Google Scholar]
- 39.Forbes JA, Butterworth GA, Burchfield WH, Yorio CC, Selinger LR, Rosenmertz SK, Beaver WT. Evaluation of flurbiprofen, acetaminophen, an acetaminophen-codeine combination, and placebo in postoperative oral surgery pain. Pharmacotherapy. 1989;9(5):322–330. doi: 10.1002/j.1875-9114.1989.tb04144.x. [DOI] [PubMed] [Google Scholar]
- 40.Forbes JA, Kehm CJ, Grodin CD, Beaver WT. Evaluation of ketorolac, ibuprofen, acetaminophen, and an acetaminophen-codeine combination in postoperative oral surgery pain. Pharmacotherapy. 1990;10(6):94S–105S. doi: 10.1002/j.1875-9114.1990.tb03568.x. [DOI] [PubMed] [Google Scholar]
- 41.Hellman M, Ahlström U, Andersson L, Strid S. Analgesic efficacy of an ibuprofen-codeine combination in patients with pain after removal of lower third molars. Eur J Clin Pharmacol. 1992;43(4):347–350. doi: 10.1007/BF02220607. [DOI] [PubMed] [Google Scholar]
- 42.Petersen JK, Hansson F, Strid S. The effect of an ibuprofen-codeine combination for the treatment of patients with pain after removal of lower third molars. J Oral Maxillofac Surg. 1993;51(6):637–640. doi: 10.1016/S0278-2391(10)80262-9. [DOI] [PubMed] [Google Scholar]
- 43.Stubhaug A, Grimstad J, Breivik H. Lack of analgesic effect of 50 and 100 mg oral tramadol after orthopaedic surgery: a randomized, double-blind, placebo and standard active drug comparison. Pain. 1995;62(1):111–118. doi: 10.1016/0304-3959(95)00056-X. [DOI] [PubMed] [Google Scholar]
- 44.Soulier SM, Page JC, Larsen LC, Grose BC. The efficacy of ANSAID (flurbiprofen) as an analgesic in foot surgery. J Foot Ankle Surg. 1997;36(6):414–417. doi: 10.1016/S1067-2516(97)80091-9. [DOI] [PubMed] [Google Scholar]
- 45.Innes GD, Croskerry P, Worthington J, Beveridge R, Jones D. Ketorolac versus acetaminophen-codeine in the emergency department treatment of acute low back pain. J Emerg Med. 1998;16(4):549–556. doi: 10.1016/S0736-4679(98)00044-4. [DOI] [PubMed] [Google Scholar]
- 46.Nielsen S, MacDonald T, Johnson JL. Identifying and treating codeine dependence: a systematic review. Med J Aust. 2018;208(10):451–461. doi: 10.5694/mja17.00749. [DOI] [PubMed] [Google Scholar]
- 47.Shaheed CA, Maher CG, McLachlan A. Investigating the efficacy and safety of over-the-counter codeine containing combination analgesics for pain and codeine-based antitussives. Woden: Government of Australia, Department of Health, Therapeutic Goods Administration; 2016.
- 48.Sproule BA, Busto UE, Somer G, Romach MK, Sellers EM. Characteristics of dependent and nondependent regular users of codeine. J Clin Psychopharmacol. 1999;19(4):367–372. doi: 10.1097/00004714-199908000-00014. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen S, Cameron J, Lee N. Characteristics of a nontreatment-seeking sample of over-the-counter codeine users: implications for intervention and prevention. J Opioid Manag. 2011;7(5):363–370. doi: 10.5055/jom.2011.0077. [DOI] [PubMed] [Google Scholar]
- 50.EudraVigilance (2019) Online access to suspected side-effect reports https://bi.ema.europa.eu/analyticsSOAP/saw.dll?PortalPages. Accessed 24 Sept 2019.
- 51.MIDAS® (IQVIA) (2019). Sales of codeine phosphate for the territory of Croatia. Accessed Aug 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.