Abstract
In our daily life, we are surrounded by harmful pollutants, including heavy metals that are not visible in the macroscopic view easily. Heavy metals can disrupt different aspects of human health, such as the immune system which has gained a lot of attention in recent decades. This had led to its rapid progression and new insights into its alterations in different diseases especially cancer. Heavy metals are non-biodegradable materials that exist in different parts of the food cycle, such as fruits and vegetables as commonly consumed foods and also unexpected sources such as street dust, that exists in the streets that we pass every day, soil, air, and water. These heavy metals can enter the human body through respiratory, cutaneous, and gastrointestinal pathways and then accumulate in different organs, leading to their encountering with various parts of the body. These sources and natural characteristics of heavy metals facilitate their interaction with the immune system. In this review, we investigated the effect of lead and cadmium, as pollutants that exist in many different parts of the human environment, on the immune system which is known to have a key role in the pathophysiology of cancer.
Keywords: Heavy metals, Lead, Cadmium, Immune system, Cancer
Introduction
Environmental pollution of soil, water, and air by heavy metal pollutants is becoming a global problem with rapid industrial development and modernization [1–3]. Heavy metals are defined as naturally occurring elements that have a high atomic weight and high density [4]. Unlike organic contaminants, heavy metals are not biodegradable [5, 6]. Most of the heavy metals are toxic and dangerous because they can enter the human body through the uptake of food, water, air, and skin contact and easily accumulate in body organs and living organisms [7–11]. The presence of some heavy metals such as Iron (Fe), Zinc (Zn), and Selenium (Se) in the diet is essential since they are known to have a protective role against many diseases [12, 13]. However, some heavy metals that are non-essential such as Cadmium (Cd), Lead (Pb), Arsenic (As), and Nickel (Ni) may have negative impacts on human health, including susceptibility to infection and increased risk of autoimmune diseases and various cancers, even in minor quantity [14–18]. Lead and cadmium are two toxic, non-essential heavy metals that are soft, malleable, and bluish-gray and bluish-white respectively [19, 20].
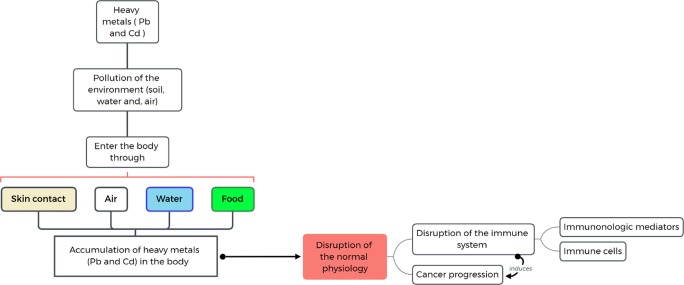
Lead and cadmium have various uses; for example, lead is used in gasoline, paints, pesticides, batteries and plumbing fixtures [20, 21] and cadmium is used in zinc or lead smelting, steel galvanizing, manufacturing television screens, paint pigments, batteries, lasers, phosphate fertilizers, and the aircraft industry [9, 10]. Lead accumulation in various organs induces adverse effects that may lead to anemia, nervous system disorders, kidney and liver damage, auditory impairment, gastrointestinal damage, decreased IQ and behavioral and learning disorders in children, Alzheimer’s disease, cancer and progression of cancers such as breast cancer [11, 22–26]. Similarly, cadmium can damage various organs such as the lungs, liver, and kidneys and can cause liver, prostate, breast, lung, kidney, skin, and pancreatic cancer [9, 17, 27–31]. According to the International Agency for Cancer Research (IACR), cadmium and its compounds have been classified as group 1 carcinogens, while lead and its compounds have been classified as ‘probably’ human carcinogens (group 2A) [32]. In addition, lead and cadmium have adverse effects on the immune system [33, 34]. They upregulate expression of some inflammatory mediators and markers and modify the immune responses, lymphocyte function, cytokine and immunoglobulin production [11, 33–39] (Fig. 1). The half-life of cadmium is estimated to be 12 to 30 years [40]. Cadmium exposure causes alterations in the number, maturation, and function of T-cells through complex effects on innate cells, such as macrophages, natural killer (NK) cells, splenic cells, and thymus cells [41]. Acute exposure to cadmium affects the immune system and cell structures via enhancing reactive oxygen species (ROS) generation, which in turn induces oxidative stress [42–44]. By induction of oxidative stress and downregulation of T cell-specific cytokines, cadmium can cause T cell apoptosis [45]. Lead also can induce oxidative stress via upregulation of ROS and is capable of altering the T cell half-life and function [45, 46]. The present review aims to summarize the studies investigating the environmental pollution of lead and cadmium and their effects on the immune system and cancer progression.
Fig. 1.
Pathway of heavy metals sources and exposure to humans, and their effects on the immune system
Environmental pollution caused by lead and cadmium
Heavy metals first pollute the environment and then enter the human body via the gastrointestinal tract and airways [8]. We are surrounded by a large spectrum of heavy metal sources. Heavy metals may be found in unexpected sources such as street dust. A study in China showed the high level of heavy metals such as cadmium in street dust, which is primarily derived from anthropogenic sources, especially industrial activities, and also lead which is derived from the traffic emission. Heavy metals, especially cadmium, had a moderate to very high potential ecological risk. According to this study, young children were under threat of non-carcinogenic and carcinogenic risk due to their low toxic tolerance [47]. Another study showed that anthropogenic sources are the major origins of lead and cadmium; however, the results of this study indicated that cadmium does not have a local anthropogenic source [48]. Street dust was found to be greatly enriched in lead, the only element posing a possible risk to human health by exposure to street dust and surface soil [49]. Epidemiologic studies imply the relationship between economic changes and aerial level of heavy metals. In this regard, a study in China that evaluated the atmospheric heavy metals pollution during a century confirmed this idea [50]. The results obtained from a study on the elements of lead and cadmium in PM2.5 of Xi’an, Northwestern China in summer and winter indicated the high concentrations of elements of lead and cadmium exceeded AAQS (Ambient Air Quality Standard) in China. Additionally, they found that mass concentrations of the elements of lead and cadmium in winter were much higher than those in summer. The increase of elements such as lead can be due to extra coal and biomass burning in winter for domestic heating. It should be noted that in similar situations, children are prone to more exposure than adults [51]. In another study, Qari et al. evaluated the air quality in Albania. They found that heavy metals including lead and cadmium, disrupt the ecological system and this disruption increases in areas with high metal content [52].
The rapid increase in water pollution is mainly due to the growth of the human population, the generation of hazardous waste, and the release of untreated wastewater from various industries and domestic purposes. The presence of heavy metals in the wastewater is a serious threat to human health, as well as to animals and plants, making it a global health dilemma in recent years [53–56]. The provisional maximum tolerable daily intake (PMTDI) of lead and cadmium is 0.015 and 0.003 ppm, respectively, as proposed by the WHO (World Health Organization) [57, 58]. The results obtained from one study indicated that the concentration of lead and cadmium in the wastewater and tube well water irrigated food crops in some cities of Pakistan were higher than permissible limits. Also, the concentration of these two metals in samples of soil and food crops irrigated with wastewater were higher than samples irrigated with tube well water [59].
Heavy metals also may affect human health by entering the human food chain. For example, studies have shown that milk and dairy products as a major source of nutrition, especially in young children, human milk as the best source of nutrition for infants, vegetables, fruits, fish, canned products, especially canned fish, chicken eggs, candies, cocoa powder, and chocolate contain varying amounts of heavy metals [14, 60–64].
Contamination of soil with heavy metals may be the result of bedrock mineralization, precipitation, dust settlement, or human activities [65]. The soil is the main source of cadmium in vegetables and cereals [66]. In a study examining soil quality in a particular area in China, lead and cadmium were two of the heavy metals found in the sampled soil. The mean concentration of all heavy metal elements did not exceed China’s national secondary standards. Among heavy metals, cadmium was more concentrated than the others. In addition, cadmium, along with Hg (mercury) was the most hazardous heavy metal pollutant in the study area [65]. The results obtained from another study indicated that there is a positive correlation between backyard soil lead levels and lead concentrations in chicken eggs [62].
Plants are contaminated by heavy metals through different routes. First of all, these metals can be absorbed via contaminated soil. Secondly, plants’ exposure to contaminated air leads to deposition of heavy metals on the plants. Plants also can be contaminated after irrigation with heavy metal polluted water [67]. Environmental pollutants can enter into the food cycle, including vegetables leading to our exposure to heavy metals such as cadmium and lead. The result obtained from one study showed that cadmium is one of the main trace metal elements causing greenhouse vegetable production (GVP) soil pollution. Based on this study, more than half of cadmium and lead were related to non-residual fractions. The pollution of heavy metals such as cadmium has resulted in an increase of their concentrations in mobile fractions [68]. In a study designed to determine the level of heavy metals in edible vegetables, sweet basil and garden cress had the highest level of lead and cadmium, respectively. The results of this study showed that the high amount of heavy metals in the vegetables is hazardous to human health when taken on a daily basis [67].
Rice, one of the main fundamental food for humanity, is an important cereal that can be contaminated with heavy metals [69]. Cadmium accumulates in rice easier than other cereal crops [70]. The level of some heavy metals such as lead and cadmium in different types of rice (domestic cultivated and imported) was investigated by researchers. In this study, the mean values for lead and cadmium were considerably higher than the permissible limits set by FAO (Food and Agriculture Organization) /WHO. Additionally, the estimated weekly intake (EWI) of lead was considerably higher than that of other toxic metals [71].
Canned food, considered as widely consumed sources of carbohydrates, proteins, vitamins, minerals, and trace elements can be contaminated with heavy metals [72]. In a study, the level of lead and cadmium was analyzed in four popular canned tuna fish in the market of Tehran, Iran. The results of this analysis showed that lead and cadmium concentrations were generally higher than the recommended limits for fish, set by different health authorities. This study also suggested that long-term consumption of canned fish in children and pregnant women must be avoided [73]. In another study in Irbid city, the concentration of toxic heavy metals including lead and cadmium was examined in canned vegetable and fruit samples of the Jordanian market. Results revealed that the levels of these heavy metals surpassed the permissible limits set by health organizations [72].
Other unexpected sources of heavy metals such as lead and cadmium are candies, cocoa powder, and chocolate [63, 64]. A study carried out in Nigeria indicated that the level of heavy metals in candies collected from different stores was above the standard limits, constituting significant health risks [64]. Lo Dico et al. have stated that daily consumption of cocoa powder could be harmful to health, particularly in children who are great consumers of chocolate [63].
Inadequate nutrition during infancy has both short-term and long-term consequences on human health [74]. Maternal milk, as the major source of nutrition in infancy, may be contaminated with toxic metals that can be transferred to the infant through milk ingestion [75]. Importantly, the results of a study indicated that the lead level in 94% of breast milk samples was above the suggested WHO limit for lead contamination of breast milk, which can be a potential health risk for infants. It is also worth mentioning that the lead content in the breast milk of mothers consuming lipsticks was significantly higher compared to those who did not use cosmetics [76]. One should not overlook the fact that lead can pass through the placenta during pregnancy, leading to fetal exposure to toxic metals [77]. In a similar study in Lebanon, random smoke exposure was found to be an independent predictor of cadmium level in the breast milk of lactating women [78]. Other studies have also stated that apart from occupational exposure, food, and water, cigarette smoking is an important source of cadmium contamination [40].
It is estimated that about 5% of ingested cadmium is absorbed through the gastrointestinal tract. However, several factors influence the rate of its intestinal absorption; studies have shown that the nutritional status of zinc, iron, and calcium of an individual is conversely related to cadmium absorption [66].
Effects of lead and cadmium on the immune system
Lead and cadmium can damage the immune system in humans and animals [79]. These two toxic elements affect innate and adaptive (cellular and humoral) immune responses, and cytokine production [35]. Cadmium induces oxidative stress by generating ROS, through which it exerts its toxicity [42]. Lead also can induce oxidative stress via overproduction of ROS [38, 80]. High levels of ROS induced by environmental lead and cadmium can elicit harm to immunity and cell structures, including lipids, proteins, and DNA due to their reactivity with ROS [38, 42].
Heavy metals are considered to be occupational pollutants. Some studies have revealed increases in interleukin (IL)-10 and tumor necrosis factor-α (TNF-α) and decreases in serum IgA levels in lead-exposed workers in comparison to non-exposed workers [81, 82]. A study by Yucesoy et al. confirmed that chronic exposure to lead and cadmium led to alterations in cytokine levels. According to this study, serum IL-1β level decreased, while IL-2 and TNF-α levels did not differ compared to those of the control group. They also showed that Interferon-gamma (IFN-γ) level decreased in the lead-exposed workers while, an increase was observed in the cadmium-exposed group [83, 84]. A study exploring the link between cadmium and psoriasis found that the accumulation of cadmium may give rise to the worsening of psoriasis, possibly through mechanisms such as inducing oxidative stress, changes in immune response, and upregulation of inflammation markers, including IL-6, TNF-α, IL-8, and IL-1β [33, 85]. Results of a study in rats exposed to CdCl2-polluted drinking water showed a significant elevation in TNF-α and IL-6 plasma levels, resulting in systematic oxidative stress [86]. Similar results have also been observed in reptiles. Intraperitoneal injection of cadmium caused oxidative stress and damage to the freshwater turtles, Chinemys reevesii [87].
Lead, an environmental pollutant, increases the percentage of immune cells, such as peripheral CD4+ and CD8+ central memory T cells. Cao et al. have recently demonstrated a positive correlation between blood lead levels and CD4+ central memory T cells in preschool children living in e-waste recycling areas [45]. Pathak and colleagues demonstrated a significant cadmium-induced decline in CD4+/ CD8+ ratio accompanied by T-cell apoptosis (CD4+> CD8+) in BALB/c mice, which was thought to be due to higher depletion of intracellular glutathione. As a consequence, Th1-derived cytokines (IL-2 and IFN-γ) declined more than those derived from Th-2 cells (IL-4) [37]. Also, the results obtained from one study on lead-exposed workers indicated that despite no alterations in the number of NK cells, CD4+ T-cells, IgG, IgM, C3, and C4 complement levels decreased significantly compared to healthy controls [88]. Several studies have reported the relationship between e-waste heavy metal toxicity and erythrocytes. In a cross-sectional study by Huo et al., e-waste-exposed preschool children had higher blood and erythrocyte lead levels compared to the control group. Interestingly, elevated erythrocyte lead levels were associated with a decrease in expression of erythrocyte immune adhesion molecules (CD44 and CD58). In addition, lead exposure stimulated the secretion of cytokines, including IL-1β, IL-12p70, and IFN-γ [89]. In a previous study by his colleagues, exposed children had higher blood lead and cadmium levels. The results of this study showed that absolute counts of monocytes, eosinophils, neutrophils, and basophils were significantly higher in the Guiya group. However, NK cell percentages were lower in this group compared to the reference group. According to the authors, these findings provided evidence on the impact of heavy metal toxicity on innate and adaptive immunity [35].
As mentioned earlier, cadmium exposure via maternal milk during infancy can cause both transient and continuous effects on immune functions. In a study by Pillet et al., cadmium exposure in the neonatal period caused a significant delay in the early development of females but not males. Also, exposure to cadmium altered the cytotoxic activity of NK cells in adult and juvenile male rats [90]. In a recent study, Nygaard and colleagues showed that prenatal exposure to cadmium resulted in the reduction of T helper memory cells in cord blood samples, increasing the host’s susceptibility to infectious diseases [91]. Hanson et al. have also reported that prenatal cadmium exposure causes detrimental effects on the immune system of mice offspring. The results of this study showed a decrease in IL-2 and IFN-γ of both male and female cadmium-exposed offspring, whereas IL-4 only reduced in the female offspring. In addition, a marked decrease in the number of splenic CD8+CD223+ cells was seen in both sexes. These findings, according to the authors, were suggestive of increased susceptibility of the offspring to tumor growth and infections [92].
Effects of lead and cadmium on cancer immunology and progression
Several studies have demonstrated that exposure to lead can affect the production of T and B lymphocytes and NK cells performing the crucial anti-cancer role [84]. Lead triggers oxidative stress and increases the sensitivity of genes to oxidative stress leading to higher estrogen levels as an important risk factor of breast cancer. Elevated levels of estrogen can damage normal tissue via different pathways. For example, many cellular populations of our body, including immune cells are provoked by estrogen via estrogen receptors. Kasten –Jolly et al. have suggested that the higher lead-induced ROS in females might be the result of higher estrogen levels in the respective sex [80].
Regarding lead carcinogenesis, a study investigating the association between urinary lead and cancer mortality showed that urinary lead levels were predictive of cancer-related death [93]. In contrast, a previous case-control study found no evidence of an association between urinary lead concentrations and breast cancer risk [94]. Also, cadmium may induce some other malignancies such as pancreatic cancer. Different studies in different levels including human, animal and cell culture imply a connection between cadmium and pancreatic cancer induced by damage to epigenetic part of cells, as a pathway cooperating with cellular nucleic acids. Also, cadmium ameliorates the survival of healthy cells and induces oxidative stress during induction of pancreatic cancer [30, 95, 96]. Another cancer that is thought to be induced by cadmium is renal cancer. Cadmium can lead to a large spectrum of pathologies in renal tissue from renal dysfunction to renal cancer. One of the worst aspects about the relation between cadmium and kidney is its accumulation in renal tissue through binding to metallothionein leading to accumulation without deletion. This accumulated cadmium can induce the development of cancer through some mechanisms such as the suppression of genes that suppress tumors and inhibiting some mechanisms that correct DNA repairs derived from mutations [97, 98]. Besides, cadmium induces melanoma, a kind of skin cancer, via stimulation of cell growth and suppression of apoptotic pathways in tumor cells [31]. A recent meta-analysis showed that cadmium concentration has a positive association with breast cancer risk [99]. Results of another study showed that heavy metals including lead and cadmium accumulate in the breast cancer tissue. A positive correlation was found between DNA methylation level and heavy metal concentration in the neoplastic tissue. Also, increasing levels of heavy metals was associated with an increase in HER2/neu, p53, Ki-67, MGMT expression and a decrease in ER and PR expression. Conclusively, heavy metals stimulated breast cancer progression while suppressing the response to therapy [25]. Grioni et al. have also reported a significant association between dietary cadmium intake and the risk of developing breast cancer, regardless of ER, PR, and HER2 status [66]. Likewise, a meta-analysis conducted by Cho et al. demonstrated a positive association between dietary cadmium intake and hormone-related cancers, including breast, prostate, and endometrial tumors in Western countries [100]. Consistent with the previous findings, White et al. recently demonstrated a higher risk of postmenopausal breast cancer in women exposed to increasing levels of lead and cadmium [101]. Other studies, however, have reported conflicting results. Wu et al., Maele-Fabry et al., and Adams et al. found no significant association between dietary cadmium intake/urinary cadmium and breast cancer risk [102–104].
The underlying mechanisms of cancer promotion due to heavy metal exposure have not been clearly defined yet. Some of the most recent studies have suggested that cadmium can mimic steroid hormones such as androgen and estrogen supporting its possible role in the development of hormone-related cancers [105–108]. Other potential mechanisms include enhancement of cell proliferation, immune dysregulation, induction of oxidative stress, inhibition of DNA damage repair, and inhibition of apoptosis [93, 109, 110]. In a study by Ju et al., cadmium stimulated the gene expression of stem-cell markers (CD44, CD24, CD133, and ALDH1) in the breast and liver cancer lineage and promoted the generation of cancer stem cells (CSCs) [17].
Conclusion
Since lead and cadmium are non-biodegradable toxicants, improper disposal of such heavy metals is a growing concern. The major sources of contamination with heavy metals are air, water, soil, food, and smoke. Many studies have investigated the impact of heavy metal pollutants on human health. These toxic elements are known to disrupt various systems of the body, including respiratory, neurologic, digestive, cardiovascular, urinary, and most importantly the immune system. Recent studies have demonstrated that cadmium and lead may have a carcinogenic and estrogenic function, inducing alterations in the immune cells and inflammatory markers. Some studies have found a significant association between cadmium/lead levels and various cancers; however, no conclusive results could be extracted since other studies have not found any relationship. Further large-scale epidemiologic and experimental studies are warranted to provide insight on heavy metal toxicity in human.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Behbahani M, Ghareh Hassanlou P, Amini MM, Omidi F, Esrafili A, Farzadkia M, et al. Application of solvent-assisted dispersive solid phase extraction as a new, fast, simple and reliable preconcentration and trace detection of lead and cadmium ions in fruit and water samples. Food Chem. 2015;187:82–88. doi: 10.1016/j.foodchem.2015.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Feng W, Dong T, Li K, Wang T, Chen Z, Wang R. Characterization of binding behaviors of Cd2+ to rice proteins. Food Chem. 2019;275:186–192. doi: 10.1016/j.foodchem.2018.09.123. [DOI] [PubMed] [Google Scholar]
- 3.Yao Y, Wu H, Ping J. Simultaneous determination of cd (II) and Pb (II) ions in honey and milk samples using a single-walled carbon nanohorns modified screen-printed electrochemical sensor. Food Chem. 2019;274:8–15. doi: 10.1016/j.foodchem.2018.08.110. [DOI] [PubMed] [Google Scholar]
- 4.Masindi V, Muedi KL. Environmental contamination by heavy metals. In: Saleh HE-DM, Fekry Eid Sayed Aglan R, editors. Heavy Metals: IntechOpen; 2018.
- 5.Pugazhendhi A, Boovaragamoorthy GM, Ranganathan K, Naushad M, Kaliannan T. New insight into effective biosorption of lead from aqueous solution using Ralstonia solanacearum: characterization and mechanism studies. J Clean Prod. 2018;174:1234–1239. doi: 10.1016/j.jclepro.2017.11.061. [DOI] [Google Scholar]
- 6.Abbas A, Al-Amer AM, Laoui T, Al-Marri MJ, Nasser MS, Khraisheh M, et al. Heavy metal removal from aqueous solution by advanced carbon nanotubes: critical review of adsorption applications. Sep Purif Technol. 2016;157:141–161. doi: 10.1016/j.seppur.2015.11.039. [DOI] [Google Scholar]
- 7.Kaplan Ince O, Ince M, Yonten V, Goksu A. A food waste utilization study for removing lead(II) from drinks. Food Chem. 2017;214:637–643. doi: 10.1016/j.foodchem.2016.07.117. [DOI] [PubMed] [Google Scholar]
- 8.Kampa M, Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151(2):362–367. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Lee C-Y, Su C-H, Tsai P-K, Yang M-L, Ho Y-C, Lee S-S, Chen CH, Chen WY, Lin ML, Chen CJ, Chian CY, Huang-Liu R, Chang YL, Kuan YH. Cadmium nitrate-induced neuronal apoptosis is protected by N-acetyl-l-cysteine via reducing reactive oxygen species generation and mitochondria dysfunction. Biomed Pharmacother. 2018;108:448–456. doi: 10.1016/j.biopha.2018.09.054. [DOI] [PubMed] [Google Scholar]
- 10.Caini S, Bendinelli B, Masala G, Saieva C, Lundh T, Kyrtopoulos SA, Palli D. Predictors of erythrocyte cadmium levels in 454 adults in Florence. Italy Science of the Total Environment. 2018;644:37–44. doi: 10.1016/j.scitotenv.2018.06.347. [DOI] [PubMed] [Google Scholar]
- 11.Boskabady M, Marefati N, Farkhondeh T, Shakeri F, Farshbaf A, Boskabady MH. The effect of environmental lead exposure on human health and the contribution of inflammatory mechanisms, a review. Environ Int. 2018;120:404–420. doi: 10.1016/j.envint.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Saghazadeh A, Ahangari N, Hendi K, Saleh F, Rezaei N. Status of essential elements in autism spectrum disorder: systematic review and meta-analysis. Rev Neurosci. 2017;28(7):783–809. doi: 10.1515/revneuro-2017-0015. [DOI] [PubMed] [Google Scholar]
- 13.Abolhassani H, Honarvar NM, Mosby TT, Mahmoudi M. Nutrition, immunity, and cancers. In: Rezaei N, editor. Cancer immunology. Berlin, Heidelberg: Springer; 2015. [Google Scholar]
- 14.Esposito F, Nardone A, Fasano E, Scognamiglio G, Esposito D, Agrelli D, et al. A systematic risk characterization related to the dietary exposure of the population to potentially toxic elements through the ingestion of fruit and vegetables from a potentially contaminated area. A case study: the issue of the "land of fires" area in Campania region, Italy. Environ Pollut. 2018;243:1781–1790. doi: 10.1016/j.envpol.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 15.Dai J, Zhang L, Du X, Zhang P, Li W, Guo X, et al. Effect of Lead on antioxidant ability and immune responses of Crucian carp. Biol Trace Elem Res. 2018:1–8. [DOI] [PubMed]
- 16.Krueger WS, Wade TJ. Elevated blood lead and cadmium levels associated with chronic infections among non-smokers in a cross-sectional analysis of NHANES data. Environ Health. 2016;15(1):16. doi: 10.1186/s12940-016-0113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ju H, Arumugam P, Lee J, Song JM. Impact of environmental pollutant cadmium on the establishment of a Cancer stem cell population in breast and hepatic Cancer. ACS Omega. 2017;2(2):563–572. doi: 10.1021/acsomega.6b00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadeghi F, Nasseri S, Mosaferi M, Nabizadeh R, Yunesian M, Mesdaghinia A. Statistical analysis of arsenic contamination in drinking water in a city of Iran and its modeling using GIS. Environ Monit Assess. 2017;189(5):230–212. doi: 10.1007/s10661-017-5912-8. [DOI] [PubMed] [Google Scholar]
- 19.Pan C, Liu H-D, Gong Z, Yu X, Hou X-B, Xie D-D, et al. Cadmium is a potent inhibitor of PPM phosphatases and targets the M1 binding site. Sci Rep. 2013;3:2333. doi: 10.1038/srep02333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Acharya J, Sahu JN, Mohanty CR, Meikap BC. Removal of lead(II) from wastewater by activated carbon developed from tamarind wood by zinc chloride activation. Chem Eng J. 2009;149(1):249–262. doi: 10.1016/j.cej.2008.10.029. [DOI] [Google Scholar]
- 21.Rosen MB, Pokhrel LR, Weir MH. A discussion about public health, lead and Legionella pneumophila in drinking water supplies in the United States. Sci Total Environ. 2017;590–591:843–852. doi: 10.1016/j.scitotenv.2017.02.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Naggar NE-A, Hamouda RA, Mousa IE, Abdel-Hamid MS, Rabei NH. Biosorption optimization, characterization, immobilization and application of Gelidium amansii biomass for complete Pb2+ removal from aqueous solutions. Sci Rep. 2018;8(1):13456. doi: 10.1038/s41598-018-31660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silver MK, Li X, Liu Y, Li M, Mai X, Kaciroti N, Kileny P, Tardif T, Meeker JD, Lozoff B. Low-level prenatal lead exposure and infant sensory function. Environ Health. 2016;15(1):65. doi: 10.1186/s12940-016-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chibowska K, Baranowska-Bosiacka I, Falkowska A, Gutowska I, Goschorska M, Chlubek D. Effect of Lead (Pb) on inflammatory processes in the brain. Int J Mol Sci. 2016;17(12):2140. doi: 10.3390/ijms17122140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romaniuk А, Lyndin M, Sikora V, Lyndina Y, Romaniuk S, Sikora K. Heavy metals effect on breast cancer progression. J Occupat Med Toxicol. 2017;12(1):32. doi: 10.1186/s12995-017-0178-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saghazadeh A, Rezaei N. Systematic review and meta-analysis links autism and toxic metals and highlights the impact of country development status: Higher blood and erythrocyte levels for mercury and lead, and higher hair antimony, cadmium, lead, and mercury. Progress in neuro-psychopharmacology & biological psychiatry. 2017;79(Pt B):340–368. doi: 10.1016/j.pnpbp.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Shan Z, Wei Z, Shaikh ZA. Suppression of ferroportin expression by cadmium stimulates proliferation, EMT, and migration in triple-negative breast cancer cells. Toxicol Appl Pharmacol. 2018;356:36–43. doi: 10.1016/j.taap.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson JB, Dancy BC, Horton CL, Lee YS, Madejczyk MS, Xu ZZ, et al. Exposure to toxic metals triggers unique responses from the rat gut microbiota. Sci Rep. 2018;8. [DOI] [PMC free article] [PubMed]
- 29.Waalkes MP. Cadmium carcinogenesis. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis. 2003;533(1–2):107–120. doi: 10.1016/j.mrfmmm.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Buha A, Wallace D, Matovic V, Schweitzer A, Oluic B, Micic D, et al. Cadmium exposure as a putative risk factor for the development of pancreatic cancer: three different lines of evidence. Biomed Res Int. 2017;2017. [DOI] [PMC free article] [PubMed]
- 31.Venza M, Visalli M, Biondo C, Oteri R, Agliano F, Morabito S, Teti D, Venza I. Epigenetic marks responsible for cadmium-induced melanoma cell overgrowth. Toxicol in Vitro. 2015;29(1):242–250. doi: 10.1016/j.tiv.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 32.IARC . Working group on the evaluation of carcinogenic risks to humans: inorganic and organic lead compounds. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon (FR): International Agency for Research on Cancer; 2006. [PMC free article] [PubMed] [Google Scholar]
- 33.Liaw F-Y, Chen W-L, Kao T-W, Chang Y-W, Huang C-F. Exploring the link between cadmium and psoriasis in a nationally representative sample. Sci Rep. 2017;7(1):1723. doi: 10.1038/s41598-017-01827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.García-Lestón J, Roma-Torres J, Mayan O, Schroecksnadel S, Fuchs D, Moreira AO, Pásaro E, Méndez J, Teixeira JP, Laffon B. Assessment of immunotoxicity parameters in individuals occupationally exposed to lead. J Toxic Environ Health A. 2012;75(13–15):807–818. doi: 10.1080/15287394.2012.690327. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Xu X, Sun D, Cao J, Zhang Y, Huo X. Alteration of the number and percentage of innate immune cells in preschool children from an e-waste recycling area. Ecotoxicol Environ Saf. 2017;145:615–622. doi: 10.1016/j.ecoenv.2017.07.059. [DOI] [PubMed] [Google Scholar]
- 36.Olszowski T, Gutowska I, Baranowska-Bosiacka I, Piotrowska K, Korbecki J, Kurzawski M, Chlubek D. The effect of cadmium on COX-1 and COX-2 gene, protein expression, and enzymatic activity in THP-1 macrophages. Biol Trace Elem Res. 2015;165(2):135–144. doi: 10.1007/s12011-015-0234-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pathak N, Khandelwal S. Impact of cadmium in T lymphocyte subsets and cytokine expression: differential regulation by oxidative stress and apoptosis. Biometals. 2008;21(2):179–187. doi: 10.1007/s10534-007-9106-7. [DOI] [PubMed] [Google Scholar]
- 38.Yin Y, Zhang P, Yue X, Du X, Li W, Yin Y, et al. Effect of sub-chronic exposure to lead (Pb) and Bacillus subtilis on Carassius auratus gibelio: bioaccumulation, antioxidant responses and immune responses. Ecotoxicol Environ Saf. 2018;161:755–762. doi: 10.1016/j.ecoenv.2018.06.056. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q, Huang Y, Zhang K, Yan Y, Wu J, Wang F, Zhao Y, Xu H, Jiang W, Yu D, Chen Y, Ye D. Progesterone attenuates hypertension and autoantibody levels to the angiotensin II type 1 receptor in response to elevated cadmium during pregnancy. Placenta. 2018;62:16–24. doi: 10.1016/j.placenta.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Fittipaldi S, Bimonte V, Soricelli A, Aversa A, Lenzi A, Greco E, et al. Cadmium exposure alters steroid receptors and proinflammatory cytokine levels in endothelial cells in vitro: a potential mechanism of endocrine disruptor atherogenic effect. J Endocrinol Investig. 2018:1–13. [DOI] [PubMed]
- 41.Holásková I, Elliott M, Hanson ML, Schafer R, Barnett JB. Prenatal cadmium exposure produces persistent changes to thymus and spleen cell phenotypic repertoire as well as the acquired immune response. Toxicol Appl Pharmacol. 2012;265(2):181–189. doi: 10.1016/j.taap.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng J-L, Yuan S-S, Wu C-W, Lv Z-M. Acute exposure to waterborne cadmium induced oxidative stress and immunotoxicity in the brain, ovary and liver of zebrafish (Danio rerio) Aquat Toxicol. 2016;180:36–44. doi: 10.1016/j.aquatox.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Almeer RS, Alarifi S, Alkahtani S, Ibrahim SR, Ali D, Moneim A. The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression. Biomed Pharmacother. 2018;106:1490–1498. doi: 10.1016/j.biopha.2018.07.089. [DOI] [PubMed] [Google Scholar]
- 44.Sun M, Li YT, Liu Y, Lee SC, Wang L. Transcriptome assembly and expression profiling of molecular responses to cadmium toxicity in hepatopancreas of the freshwater crab Sinopotamon henanense. Sci Rep. 2016;6:19405. doi: 10.1038/srep19405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao J, Xu X, Zhang Y, Zeng Z, Hylkema MN, Huo X. Increased memory T cell populations in Pb-exposed children from an e-waste-recycling area. Sci Total Environ. 2018;616:988–995. doi: 10.1016/j.scitotenv.2017.10.220. [DOI] [PubMed] [Google Scholar]
- 46.Chwalba A, Maksym B, Dobrakowski M, Kasperczyk S, Pawlas N, Birkner E, et al. The effect of occupational chronic lead exposure on the complete blood count and the levels of selected hematopoietic cytokines. Toxicol Appl Pharmacol. 2018. [DOI] [PubMed]
- 47.Xiao Q, Zong Y, Malik Z, Lu S. Source identification and risk assessment of heavy metals in road dust of steel industrial city (Anshan), Liaoning, Northeast China. Hum Ecol Risk Assess: Int J. 2019:1–20. 10.1080/10807039.2019.1578946.
- 48.Saeedi M, Li LY, Salmanzadeh M. Heavy metals and polycyclic aromatic hydrocarbons: pollution and ecological risk assessment in street dust of Tehran. J Hazard Mater. 2012;227:9–17. doi: 10.1016/j.jhazmat.2012.04.047. [DOI] [PubMed] [Google Scholar]
- 49.Dehghani S, Moore F, Keshavarzi B, Beverley AH. Health risk implications of potentially toxic metals in street dust and surface soil of Tehran, Iran. Ecotoxicol Environ Saf. 2017;136:92–103. doi: 10.1016/j.ecoenv.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 50.Wan D, Song L, Mao X, Yang J, Jin Z, Yang H. One-century sediment records of heavy metal pollution on the southeast Mongolian plateau: implications for air pollution trend in China. Chemosphere. 2019;220:539–545. doi: 10.1016/j.chemosphere.2018.12.151. [DOI] [PubMed] [Google Scholar]
- 51.Liu P, Lei Y, Ren H, Gao J, Xu H, Shen Z, et al. Seasonal variation and health risk assessment of heavy metals in PM2. 5 during winter and summer over Xi’an, China. Atmosphere. 2017;8(5):91. [Google Scholar]
- 52.Qarri F, Lazo P, Allajbeu S, Bekteshi L, Kane S, Stafilov T. The evaluation of air quality in Albania by Moss biomonitoring and metals atmospheric deposition. Arch Environ Contam Toxicol. 2019:1–18. [DOI] [PubMed]
- 53.Carolin CF, Kumar PS, Saravanan A, Joshiba GJ, Naushad M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: a review. J Environ Chem Eng. 2017;5(3):2782–2799. doi: 10.1016/j.jece.2017.05.029. [DOI] [Google Scholar]
- 54.Matouq M, Jildeh N, Qtaishat M, Hindiyeh M, Al Syouf MQ. The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. Journal of Environmental Chemical Engineering. 2015;3(2):775–784. doi: 10.1016/j.jece.2015.03.027. [DOI] [Google Scholar]
- 55.Kobielska PA, Howarth AJ, Farha OK, Nayak S. Metal–organic frameworks for heavy metal removal from water. Coord Chem Rev. 2018;358:92–107. doi: 10.1016/j.ccr.2017.12.010. [DOI] [Google Scholar]
- 56.Bouabidi ZB, El-Naas MH, Cortes D, McKay G. Steel-making dust as a potential adsorbent for the removal of lead (II) from an aqueous solution. Chem Eng J. 2018;334:837–844. doi: 10.1016/j.cej.2017.10.073. [DOI] [Google Scholar]
- 57.Koushkbaghi S, Zakialamdari A, Pishnamazi M, Ramandi HF, Aliabadi M, Irani M. Aminated-Fe3O4 nanoparticles filled chitosan/PVA/PES dual layers nanofibrous membrane for the removal of Cr(VI) and Pb(II) ions from aqueous solutions in adsorption and membrane processes. Chem Eng J. 2018;337:169–182. doi: 10.1016/j.cej.2017.12.075. [DOI] [Google Scholar]
- 58.Xu L, Xu X, Wu D. Initial dissolved oxygen-adjusted electrochemical generation of sulfate green rust for cadmium removal using a closed-atmosphere Fe–electrocoagulation system. Chem Eng J. 2019;359:1411–1418. doi: 10.1016/j.cej.2018.11.032. [DOI] [Google Scholar]
- 59.Khan MU, Malik RN, Muhammad S. Human health risk from heavy metal via food crops consumption with wastewater irrigation practices in Pakistan. Chemosphere. 2013;93(10):2230–2238. doi: 10.1016/j.chemosphere.2013.07.067. [DOI] [PubMed] [Google Scholar]
- 60.Rahimi E. Lead and cadmium concentrations in goat, cow, sheep, and buffalo milks from different regions of Iran. Food Chem. 2013;136(2):389–391. doi: 10.1016/j.foodchem.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 61.Okyere H, Voegborlo RB, Agorku SE. Human exposure to mercury, lead and cadmium through consumption of canned mackerel, tuna, pilchard and sardine. Food Chem. 2015;179:331–335. doi: 10.1016/j.foodchem.2015.01.038. [DOI] [PubMed] [Google Scholar]
- 62.Leibler JH, Basra K, Ireland T, McDonagh A, Ressijac C, Heiger-Bernays W, et al. Lead exposure to children from consumption of backyard chicken eggs. Environ Res. 2018;167:445–452. doi: 10.1016/j.envres.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lo Dico GM, Galvano F, Dugo G, D'Ascenzi C, Macaluso A, Vella A, et al. Toxic metal levels in cocoa powder and chocolate by ICP-MS method after microwave-assisted digestion. Food Chem. 2018;245:1163–1168. doi: 10.1016/j.foodchem.2017.11.052. [DOI] [PubMed] [Google Scholar]
- 64.Orisakwe OE, Igweze ZN, Udowelle NA. Candy consumption may add to the body burden of lead and cadmium of children in Nigeria. Environ Sci Pollut Res. 2018:1–11. [DOI] [PubMed]
- 65.Zhang P, Qin C, Hong X, Kang G, Qin M, Yang D, Pang B, Li Y, He J, Dick RP. Risk assessment and source analysis of soil heavy metal pollution from lower reaches of Yellow River irrigation in China. Sci Total Environ. 2018;633:1136–1147. doi: 10.1016/j.scitotenv.2018.03.228. [DOI] [PubMed] [Google Scholar]
- 66.Grioni S, Agnoli C, Krogh V, Pala V, Rinaldi S, Vinceti M, et al. Dietary cadmium and risk of breast cancer subtypes defined by hormone receptor status: a prospective cohort study. Int J Cancer. 2018. [DOI] [PubMed]
- 67.Maleki A, Zarasvand MA. Heavy metals in selected edible vegetables and estimation of their daily intake in Sanandaj, Iran. Southeast Asian J Trop Med Public Health. 2008;39(2):335–340. [PubMed] [Google Scholar]
- 68.Yang L, Liu G, Di L, Wu X, You W, Huang B. Occurrence, speciation, and risks of trace metals in soils of greenhouse vegetable production from the vicinity of industrial areas in the Yangtze River Delta. China Environmental Science and Pollution Research. 2019:1–13. [DOI] [PubMed]
- 69.Yang P, Zhou R, Zhang W, Yi R, Tang S, Guo L, Hao Z, Li X, Lu Y, Zeng X. High-sensitivity determination of cadmium and lead in rice using laser-induced breakdown spectroscopy. Food Chem. 2019;272:323–328. doi: 10.1016/j.foodchem.2018.07.214. [DOI] [PubMed] [Google Scholar]
- 70.Huang Y, Feng F, Chen Z-G, Wu T, Wang Z-H. Green and efficient removal of cadmium from rice flour using natural deep eutectic solvents. Food Chem. 2018;244:260–265. doi: 10.1016/j.foodchem.2017.10.060. [DOI] [PubMed] [Google Scholar]
- 71.Naseri M, Vazirzadeh A, Kazemi R, Zaheri F. Concentration of some heavy metals in rice types available in shiraz market and human health risk assessment. Food Chem. 2015;175:243–248. doi: 10.1016/j.foodchem.2014.11.109. [DOI] [PubMed] [Google Scholar]
- 72.Massadeh AM, Allah A, Al-Massaedh T. Determination of heavy metals in canned fruits and vegetables sold in Jordan market. Environ Sci Pollut Res. 2018;25(2):1914–1920. doi: 10.1007/s11356-017-0611-0. [DOI] [PubMed] [Google Scholar]
- 73.Hosseini SV, Sobhanardakani S, Miandare HK, Harsij M, Mac RJ. Determination of toxic (Pb, cd) and essential (Zn, Mn) metals in canned tuna fish produced in Iran. J Environ Health Sci Eng. 2015;13(1):59. doi: 10.1186/s40201-015-0215-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ozdemir S, Kilinc E, Oner ET. Preconcentrations and determinations of copper, nickel and lead in baby food samples employing Coprinus silvaticus immobilized multi-walled carbon nanotube as solid phase sorbent. Food Chem. 2019;276:174–179. doi: 10.1016/j.foodchem.2018.07.123. [DOI] [PubMed] [Google Scholar]
- 75.Klein LD, Breakey AA, Scelza B, Valeggia C, Jasienska G, Hinde K. Concentrations of trace elements in human milk: comparisons among women in Argentina, Namibia, Poland, and the United States. PLoS One. 2017;12(8):e0183367. doi: 10.1371/journal.pone.0183367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Samiee F, Vahidinia A, Taravati Javad M, Leili M. Exposure to heavy metals released to the environment through breastfeeding: a probabilistic risk estimation. Sci Total Environ. 2019;650:3075–3083. doi: 10.1016/j.scitotenv.2018.10.059. [DOI] [PubMed] [Google Scholar]
- 77.Al-Saleh I, Al-Enazi S, Shinwari N. Assessment of lead in cosmetic products. Regul Toxicol Pharmacol. 2009;54(2):105–113. doi: 10.1016/j.yrtph.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 78.Bassil M, Daou F, Hassan H, Yamani O, Kharma JA, Attieh Z, Elaridi J. Lead, cadmium and arsenic in human milk and their socio-demographic and lifestyle determinants in Lebanon. Chemosphere. 2018;191:911–921. doi: 10.1016/j.chemosphere.2017.10.111. [DOI] [PubMed] [Google Scholar]
- 79.Xing M, Jin X, Wang J, Shi Q, Cai J, Xu S. The antagonistic effect of selenium on lead-induced immune dysfunction via recovery of cytokine and heat shock protein expression in chicken neutrophils. Biol Trace Elem Res. 2018;185(1):162–169. doi: 10.1007/s12011-017-1200-2. [DOI] [PubMed] [Google Scholar]
- 80.Kasten-Jolly J, Lawrence DA. Sex-specific effects of developmental lead exposure on the immune-neuroendocrine network. Toxicol Appl Pharmacol. 2017;334:142–157. doi: 10.1016/j.taap.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 81.Valentino M, Rapisarda V, Santarelli L, Bracci M, Scorcelletti M, Di Lorenzo L, et al. Effect of lead on the levels of some immunoregulatory cytokines in occupationally exposed workers. Hum Exp Toxicol. 2007;26(7):551–556. doi: 10.1177/0960327107073817. [DOI] [PubMed] [Google Scholar]
- 82.Mishra K, Chauhan U, Naik S. Effect of lead exposure on serum immunoglobulins and reactive nitrogen and oxygen intermediate. Hum Exp Toxicol. 2006;25(11):661–665. doi: 10.1177/0960327106070453. [DOI] [PubMed] [Google Scholar]
- 83.Yücesoy B, Turhan A, Üre M, Imir T, Karakaya A. Effects of occupational lead and cadmium exposure on some immunoregulatory cytokine levels in man. Toxicology. 1997;123(1–2):143–147. doi: 10.1016/S0300-483X(97)00107-8. [DOI] [PubMed] [Google Scholar]
- 84.Metryka E, Chibowska K, Gutowska I, Falkowska A, Kupnicka P, Barczak K, et al. Lead (Pb) exposure enhances expression of factors associated with inflammation. Int J Mol Sci. 2018;19(6):1813. doi: 10.3390/ijms19061813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Olszowski T, Baranowska-Bosiacka I, Gutowska I, Chlubek D. Pro-inflammatory properties of cadmium. Acta Biochim Pol. 2012;59(4). [PubMed]
- 86.Alghasham A, Salem TA, Meki A-RM. Effect of cadmium-polluted water on plasma levels of tumor necrosis factor-α, interleukin-6 and oxidative status biomarkers in rats: protective effect of curcumin. Food Chem Toxicol. 2013;59:160–164. doi: 10.1016/j.fct.2013.05.059. [DOI] [PubMed] [Google Scholar]
- 87.Huo J, Dong A, Niu X, Dong A, Lee S, Ma C, Wang L. Effects of cadmium on oxidative stress activities in plasma of freshwater turtle Chinemys reevesii. Environ Sci Pollut Res. 2018;25(8):8027–8034. doi: 10.1007/s11356-017-1139-z. [DOI] [PubMed] [Google Scholar]
- 88.Ündeg̃er Ü, Başaran N, Canpmar H, Kansu E. Immune alterations in lead-exposed workers. Toxicology. 1996;109(2):167–172. doi: 10.1016/0300-483X(96)03333-1. [DOI] [PubMed] [Google Scholar]
- 89.Huo X, Dai Y, Yang T, Zhang Y, Li M, Xu X. Decreased erythrocyte CD44 and CD58 expression link e-waste Pb toxicity to changes in erythrocyte immunity in preschool children. Sci Total Environ. 2019;664:690–697. doi: 10.1016/j.scitotenv.2019.02.040. [DOI] [PubMed] [Google Scholar]
- 90.Pillet S, Rooney AA, Bouquegneau J-M, Cyr DG, Fournier M. Sex-specific effects of neonatal exposures to low levels of cadmium through maternal milk on development and immune functions of juvenile and adult rats. Toxicology. 2005;209(3):289–301. doi: 10.1016/j.tox.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 91.Nygaard UC, Li Z, Palys T, Jackson B, Subbiah M, Malipatlolla M, Sampath V, Maecker H, Karagas MR, Nadeau KC. Cord blood T cell subpopulations and associations with maternal cadmium and arsenic exposures. PLoS One. 2017;12(6):e0179606. doi: 10.1371/journal.pone.0179606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hanson ML, Holásková I, Elliott M, Brundage KM, Schafer R, Barnett JB. Prenatal cadmium exposure alters postnatal immune cell development and function. Toxicol Appl Pharmacol. 2012;261(2):196–203. doi: 10.1016/j.taap.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li S, Wang J, Zhang B, Liu Y, Lu T, Shi Y, et al. Urinary lead concentration is an independent predictor of cancer mortality in the US general population. Front Oncol. 2018;8:242. doi: 10.3389/fonc.2018.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.McElroy JA, Shafer MM, Gangnon RE, Crouch LA, Newcomb PA. Urinary lead exposure and breast cancer risk in a population-based case-control study. Cancer Epidemiol Prev Biomark. 2008;17(9):2311–2317. doi: 10.1158/1055-9965.EPI-08-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen C, Xun P, Nishijo M, Sekikawa A, He K. Cadmium exposure and risk of pancreatic cancer: a meta-analysis of prospective cohort studies and case–control studies among individuals without occupational exposure history. Environ Sci Pollut Res. 2015;22(22):17465–17474. doi: 10.1007/s11356-015-5464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Djordjevic VR, Wallace DR, Schweitzer A, Boricic N, Knezevic D, Matic S, et al. Environmental cadmium exposure and pancreatic cancer: evidence from case control, animal and in vitro studies. Environ Int. 2019;128:353–361. doi: 10.1016/j.envint.2019.04.048. [DOI] [PubMed] [Google Scholar]
- 97.Kun Song J, Luo H, Hai Yin X, Lei Huang G, Yang Luo S, Yuan DB, et al. Association between cadmium exposure and renal cancer risk: a meta-analysis of observational studies. Sci Rep. 2015;5:17976. doi: 10.1038/srep17976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu H, Liao Q, Chillrud SN, Yang Q, Huang L, Bi J, et al. Environmental exposure to cadmium: health risk assessment and its associations with hypertension and impaired kidney function. Sci Rep. 2016;6:29989. doi: 10.1038/srep29989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jouybari L, Naz MSG, Sanagoo A, Kiani F, Sayehmiri F, Sayehmiri K, et al. Toxic elements as biomarkers for breast cancer: a meta-analysis study. Cancer Manag Res. 2018;10:69. doi: 10.2147/CMAR.S151324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cho YA, Kim J, Woo HD, Kang M. Dietary cadmium intake and the risk of cancer: a meta-analysis. PLoS One. 2013;8(9):e75087. doi: 10.1371/journal.pone.0075087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.White AJ, O’brien KM, Niehoff NM, Carroll R, Sandler DP. Metallic air pollutants and breast Cancer risk in a Nationwide cohort study. Epidemiology. 2019;30(1):20–28. doi: 10.1097/EDE.0000000000000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu X, Zhu X, Xie M. Association between dietary cadmium exposure and breast cancer risk: an updated meta-analysis of observational studies. Medical science monitor: international medical journal of experimental and clinical research. 2015;21:769. doi: 10.12659/MSM.892743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Maele-Fabry G, Lombaert N, Lison D. Dietary exposure to cadmium and risk of breast cancer in postmenopausal women: a systematic review and meta-analysis. Environ Int. 2016;86:1–13. doi: 10.1016/j.envint.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 104.Adams SV, Shafer MM, Bonner MR, LaCroix AZ, Manson JE, Meliker JR, Neuhouser ML, Newcomb PA. Urinary cadmium and risk of invasive breast cancer in the women's health initiative. Am J Epidemiol. 2016;183(9):815–823. doi: 10.1093/aje/kwv285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Byrne C, Divekar SD, Storchan GB, Parodi DA, Martin MB. Cadmium—a metallohormone? Toxicol Appl Pharmacol. 2009;238(3):266–271. doi: 10.1016/j.taap.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sawada N, Iwasaki M, Inoue M, Takachi R, Sasazuki S, Yamaji T, et al. Long-term dietary cadmium intake and cancer incidence. Epidemiology. 2012:368–76. [DOI] [PubMed]
- 107.Johnson MD, Kenney N, Stoica A, Hilakivi-Clarke L, Singh B, Chepko G, Clarke R, Sholler PF, Lirio AA, Foss C, Reiter R, Trock B, Paik S, Martin MB. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med. 2003;9(8):1081–1084. doi: 10.1038/nm902. [DOI] [PubMed] [Google Scholar]
- 108.Martin MB, Voeller HJ, Gelmann EP, Lu J, Stoica E-G, Hebert EJ, Reiter R, Singh B, Danielsen M, Pentecost E, Stoica A. Role of cadmium in the regulation of AR gene expression and activity. Endocrinology. 2002;143(1):263–275. doi: 10.1210/endo.143.1.8581. [DOI] [PubMed] [Google Scholar]
- 109.Joseph P. Mechanisms of cadmium carcinogenesis. Toxicol Appl Pharmacol. 2009;238(3):272–279. doi: 10.1016/j.taap.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 110.Luevano J, Damodaran C. A review of molecular events of cadmium-induced carcinogenesis. J Environ Pathol Toxicol Oncol. 2014;33(3). [DOI] [PMC free article] [PubMed]