Abstract
Eosinophils play roles in the pathogenesis of various diseases. In order to accumulate within sites of inflammation, eosinophils must adhere to, and migrate across the microvasculature. These processes are largely controlled by type 2-immune responses; interleukin (IL)-4 and IL-13 induce the expression of endothelial adhesion molecule vascular cell adhesion molecule-1 (VCAM-1), a representative adhesive ligand for eosinophils, while also stimulating generations of CC chemokines from structural cells, including epithelial cells. VCAM-1 and CC chemokines synergistically induce transmigration of eosinophils to the tissue inflammation site. Another type 2 cytokine, IL-5, prolongs survival, and enhances the effector functions of eosinophils. Recently, accumulating evidence has established that corticosteroid-resistant group 2 innate lymphoid cells are cellular sources for IL-5. Another immunological mechanism that may be contributing to eosinophilic inflammation involves type 1 immune system-associated molecules such as interferons and IP-10. In addition to these immune pathways, lipid mediators, such as cysteinyl leukotrienes, directly provoke the infiltration and activation of eosinophils. Extracellular matrix proteins including periostin also induce the adhesion and activation of eosinophils. Finally, activated neutrophils can also induce eosinophil transmigration. In summary, various mechanisms are involved within eosinophilic inflammation, and effective therapeutic strategies targeting these pathways should be established.
Keywords: Eosinophils, Allergy, Allergic inflammation
INTRODUCTION
Eosinophils are generally believed to play important roles in the pathogenesis of certain allergic or inflammatory disorders. For example, anti-interleukin (IL)-5 treatments that selectively attenuate the number and functional status of eosinophils dramatically improve disease control of severe bronchial asthma with eosinophilia or eosinophilic granulomatosis with polyangiitis [1,2]. In a murine model of asthma, a lack of eosinophils is sufficient to abolish airway remodeling [3]. Eosinophils play roles within various diseases via the release of inflammatory mediators into tissue sites such as specific granule proteins, cysteinyl leukotrienes, radical oxygen species, and a variety of cytokines or chemokines [4,5]. Moreover, cytolysis of eosinophils generates nuclear-derived DNA traps that are major extracellular structural components in eosinophil-rich secretion and can contribute to viscosity [6]. More recently, Charcot-Leyden crystals, which are formed from the eosinophil granule protein galectin-10, were found to act as an adjuvant to augment the type 2 -immune response [7]. For eosinophils to accumulate within the site of allergic inflammation, they must first adhere to and then migrate across the microvasculature [4,8]. In this review article, the current understanding of the control mechanisms of eosinophilic inflammation within pathological conditions will be discussed.
“CLASSICAL” TYPE 2-IMMUNITY
It is historically well established that eosinophil adhesion to and their transmigration across endothelial cells are largely controlled by type 2-immune pathways. The representative type 2 cytokines IL-4 and IL-13 induce the expression of adhesion molecule vascular cell adhesion molecule-1 (VCAM-1), but not intercellular adhesion molecule-1 (ICAM-1), on endothelial cells [9]. VCAM-1 is representative of a powerful adhesive ligand for peripheral blood eosinophils, but not neutrophils [9].
VCAM-1 induces a higher degree of adhesion of human peripheral blood eosinophils via interaction with alpha 4 integrins, such as alpha 4-beta 1 (CD49d/CD29, very late antigen [VLA]-4) and alpha 4-beta 7, expressed on eosinophil surfaces as compared to ICAM-1, which is constitutively expressed on endothelial cells [10]. Therefore, IL-4 and IL-13 play a role in capturing eosinophils at the level of the vasculature within inflammation sites [10]. The counter-ligands for ICAM-1 expressed on eosinophils are beta2-integirns including alpha L beta2 (CD11a/CD18, lymphocyte function-associated antigen-1) and alpha M beta2 (CD11b/CD18, Mac-1). ICAM-1 is essentially involved in the induction of transendothelial migration of eosinophils with its counter ligand beta2 integrins expressed on eosinophils [11]. In the presence of type-2 inflammation, another endothelial adhesion protein, such as P-selectin, may also play a role in capturing and interacting with eosinophils [12].
The process of interaction with VCAM-1 augment the effector functions of eosinophils. For example, we have observed that adhesion to recombinant human (rh)-VCAM-1 or VCAM-1-expressing endothelial cells upregulate superoxide generation of eosinophils [13,14]. In terms of the immunological significance of such a respiratory burst of eosinophils, we confirmed that hydrogen peroxide is able to upregulate the function of beta 2 integrins on these cells [15], suggesting that the process of cell adhesion to VCAM-1 facilitates eosinophil interactions with ICAM-1.
IL-4 and IL-13 also result in the generation of CC chemokines from epithelial cells, airway smooth muscle cells, and even airway fibroblasts [16,17]. CC chemokines and VCAM-1, but not ICAM-1, synergistically and effectively induce eosinophil migration into tissue. We observed that culture supernatants of specific allergen-stimulated peripheral blood mononuclear cells (PBMCs) obtained from atopic asthmatics enhanced eosinophil transmigration across VCAM-1-expressing endothelial cells, and this migration was blocked by anti-α4-integrin mAb [18]. Furthermore, the enhancement of eosinophil transmigration with the PBMC supernatant was blocked by mAb against CCR3, a major chemokine receptor present on eosinophils [18]. We then confirmed that eosinophil migrations induced by the CC chemokines RANTES (regulated on activation, normal T cell expressed and secreted), eotaxin, eotaxin-2, monocyte chemoattractant protein (MCP)-3, and MCP-4 were all augmented in the presence of rh-VCAM-1, but not in the presence of rh-ICAM-1 [19]. These observations suggest that the CCR3/CC-chemokine pathway plays an essential role in eosinophil trafficking in the presence of type 2 inflammation.
The type 2 cytokine IL-5 controls the development and maturation of eosinophils inthe bone marrow. Following migration into the tissue site, IL-5 prolongs survival and enhances effector functions of eosinophils [20]. It is noteworthy that although IL-5 at physiological concentrations is not a potent chemoattractant for human eosinophils, it does prime the chemotactic response of these cells. IL-5 is generated by Th2 cells, group 2 innate lymphoid cells (ILC2s), mast cells, and even natural killer T cells [21]. Among them, ILC2s secrete tremendous amounts of IL-5 and IL-13 as compared with other cell types [22]. At inflammation sites, the cytokines IL-33, IL-25, and thymic stromal lymphopoietin (TSLP) may be released from a variety of cells—including epithelial cells—and activate ILC2s [22]. There is evidence that IL-33 and TSLP are increased in the lower airways of severe asthmatics [23,24]. Eosinophils are IL-25-producing cells and hence could activate ILC2s [25]. ILC2s expressing IL-5 mRNA are increased in the sputum of severe asthmatics despite the use of high-dose inhaled corticosteroids (ICS) and thus could be important cellular sources of IL-5 in the airways of these patients [26]. Interestingly, anti-IL-5 treatment, which provides clinical effectiveness for severe “eosinophilic” asthma, is not sufficiently effective within mild to moderate asthma cases, especially in patients not treated with ICS [27]. A mechanism to explain this discrepancy may be contributed to other eosinophil growth factors, such as granulocyte-macrophage colony-stimulating factor (GM-CSF), which strongly prolongs the survival and upregulates the functional status of eosinophils. Eosinophil viability-enhancing activity in sputum from patients with acute asthma is mainly attributed to GM-CSF, not IL-5 [28]. Eosinophils by themselves are capable of spontaneously generating GM-CSF, but not IL-5, during the process of their transendothelial migration [29]. Following exposure to GM-CSF, the expression of the IL-5 receptor (R) is reduced in vitro [30]. Finally, airway eosinophils obtained following segmental allergen challenge express GM-CSF-R, but not IL-5-R [31], raising the possibility that GM-CSF plays a role within certain asthmatic conditions, especially in mild atopic situation. Such actions related to GM-CSF may not be seriously problematic in clinical settings, as ICS is usually sufficient to attenuate airway expression of GM-CSF [32].
POSSIBLE INVOLVEMENT OF TYPE 1 IMMUNITY
During viral infection, both eosinophilic and neutrophilic inflammation are increased in the airways [33]. Type 1 immunity involves production of interferons (IFNs) and CXC chemokines—such as CXCL10/IP-10—with both being related to antiviral immunity [34]. IFN-gamma is upregulated in the lower airways of severe persistent asthma [35,36]. Furthermore, the IFN-gamma-producing ability of PBMCs is higher in patients with corticosteroid-resistant asthma as compared to those with corticosteroid-sensitive asthma [37]. In the case of IP-10, concentrations of this specific chemokine are increased during viral-acute asthma [38].
We have confirmed that eosinophil adhesion-inducing activity of endothelial cells stimulated with IFN-beta is significantly augmented when TNF-alpha is present [39]. Furthermore, such augmented adhesion was inhibited by anti-alpha 4 integrin or anti-beta 2 integrin antibodies. Finally, IFN-beta enhanced the expression of both VCAM-1 and ICAM-1 on endothelial cells. These findings indicate that IFN-beta augments the adhesiveness of endothelial cells for eosinophils, primarily via expression of the aforementioned adhesion proteins. We also found that IP-10 significantly enhanced eosinophil adhesion to ICAM-1 and induced eosinophil superoxide anion generation in the presence of ICAM-1 [40]. Finally, we observed that IP-10 concentrations in sputum were higher in asthmatics that exhibited a mixed granulocyte subtype (eosinophils ≥ 2% and neutrophils ≥ 40%) as compared to healthy subjects [41]. Therefore, CXCR3 ligands such as IP-10 may serve as potent promoters for eosinophilic airway inflammation in asthma. Taken together, type 1 immune system-associated molecules such as IFNs and IP-10 could be involved in the development of eosinophilic inflammation within viral-associated or severe persistent asthmatic disease; however, the clinical relevance of these contributing factors should be further investigated.
ROLE OF LIPID MEDIATORS
When activated, inflammatory cells—including mast cells, basophils, neutrophils, and eosinophils—are capable of releasing lipids as newly generated mediators. Among them, platelet-activating factor (PAF) and leukotriene (LT) B4 were first described as inducing both eosinophil and neutrophil chemotaxis more than 30 years ago [42]. Subsequently, several lipid mediators—including prostaglandin (PG) D2, 5-Oxo-6, 8, 11, 14-eicosatetraenoic acid (5-oxo-ETE), and cysteinyl leukotrienes (CysLTs)—were found to be eosinophil chemoattractants [43,44,45]. Among these mediators, there is evidence that CysLTs clinically contribute to the accumulation of eosinophils within asthmatic airway tissues, such as inhalation of LTE4 [46]. Regarding this mechanism, we previously observed that the CysLT LTD4 directly upregulates the expression of β2 integrins on human eosinophils and augments eosinophil adhesion in vitro, mainly via CysLT 1 reeceptor, a receptor for CysLTs expressed on their surface [47]. Moreover, we confirmed that LTD4 induces transendothelial migration, respiratory burst, and degranulation of eosinophils through β2 integrin and the CysLT 1 receptor [48]. Such enhanced eosinophil functions provoked by LTD4 are blocked by montelukast, a CysLT 1 receptor antagonist, but not by beta-adrenergic agonist [49], Even in clinical settings, addition of a CysLT 1 receptor antagonist, but not long-acting beta-agonists, to ICS further attenuates airway eosinophilia in asthmatic patients [50]. Collectively, CysLTs partly contribute to eosinophilic infiltration and activation in asthmatic airways. Chemoattractant receptor-homologous molecule expressed on TH2 cells mediates prostaglandin D2 (PGD2)-dependent migration of eosinophils; however, the clinical significance of PGD2 in allergic disease remains to be elucidated.
ROLES OF EXTRACELLULAR MATRIX PROTEINS
Some extracellular matrix proteins exert promoting effects upon eosinophilic inflammation. For example, fibronectin, an adhesive ligand for VLA-4 that is constitutively expressed on eosinophils, prolongs survival and increases generation of leukotriene C4 from eosinophils [51]. Similar effects are also observed with laminin [52]. Periostin, an extracellular matrix protein that is highly expressed in the airways of asthmatics in response to Th2 cytokines such as IL-13, functions as a matricellular protein that binds to receptors and activates cells, including eosinophils. In this context, we confirmed that periostin directly induces eosinophil adhesion, which is comparable to the effect of VCAM-1. Furthermore, periostin induces eosinophil superoxide anion generation and degranulation through the αMβ2 integrin in vitro [53]. Collectively, such extracellular matrix proteins may contribute to the enhancement of eosinophilic inflammation in certain conditions.
INTERACTIONS WITH ACTIVATED NEUTROPHILS
Neutrophilic inflammation has been shown to be associated with eosinophilic inflammation in severe asthma. For example, the European Network Study for Understanding Mechanisms of Severe Asthma study showed that severe asthmatics have both a greater sputum neutrophil count and an increased release of eosinophil-derived mediators [54]. There is evidence that IL-8 plays an important role in the accumulation of neutrophils within inflammation sites. For example, we and others confirmed that IL-8 expression is upregulated in the airways of severe asthmatic patients [55,56]. Concerning the relationship between neutrophils and eosinophils within severely asthmatic airways, we observed that neutrophils that had migrated in response to IL-8 strikingly induced the transbasement membrane migration of eosinophils in vitro, even without the presence of eosinophil chemoattractant [57]. This neutrophil-induced eosinophil migration is inhibited by either leukotriene B4 (LTB4) or PAF antagonists. Therefore, IL-8-stimulated neutrophils are capable of enhancing eosinophil accumulation in asthmatic airways through release of LTB4 or PAF from neutrophils.
Lipopolysaccharide (LPS) may play a role in inducing IL-8 or neutrophilic inflammation in the airways of severe asthmatics. In the bronchoalveolar lavage (BAL) fluid of asthmatic children, LPS levels correlate with airway neutrophils or IL-8 [58]. Furthermore, concentrations of LPS in BAL fluid and genes associated with LPS signaling activation are higher in corticosteroid-resistant asthma. A positive correlation is observed between IL-8 mRNA expression in BAL cells and the amount of LPS in BAL fluid [59]. In a study investing house dust mite (HDM)-sensitive mild asthmatics treated with ICS, inhalation of a combination of LPS and mite allergen-induced activation of eosinophils in the lower airways, while mite allergen alone did not [60]. In this context, we confirmed that LPS-stimulated neutrophils can induce the transbasement membrane migration of eosinophils in vitro [61]. Taken together, activated neutrophils, either in the presence of IL-8 or endotoxin, may be involved in inducing eosinophil transmigration.
ENVIRONMENTAL FACTORS
Environmental factors may also facilitate eosinophilic inflammation. As noted above, LPS is a factor that can augment eosinophil migration via activation of neutrophils and is increased in certain living situations, including within the presence of household pets, cockroaches, or carpeted floors [62]. Some fungi existing within common living environments are also capable of inducing eosinophil activations. For example, aspartate protease activities secreted by Alternaria induce activation and degranulation of human eosinophils through protease-activated receptor-2 expressed on the cells [63]. Additionally, Aspergillus fumigatus can induce extracellular DNA traps of human eosinophils, which is dependent upon the Syl tyrosine kinase pathway [64]. We recently observed that Dermatophagoides farinae extract, a representative HDM, or its major allergen Derf 1 directly induced adhesion, respiratory burst, and release of specific granule protein of eosinophils obtained from normal subjects that were not sensitized against HDM, thereby suggesting that exposure to HDM in the environment might augment eosinophilic inflammation [65]. From this point of view, the clinical significance of cleanliness of the home and surrounding environment could be profound in terms of eosinophilic inflammation; however, more study would be required to determine if this is the case.
CONCLUSION
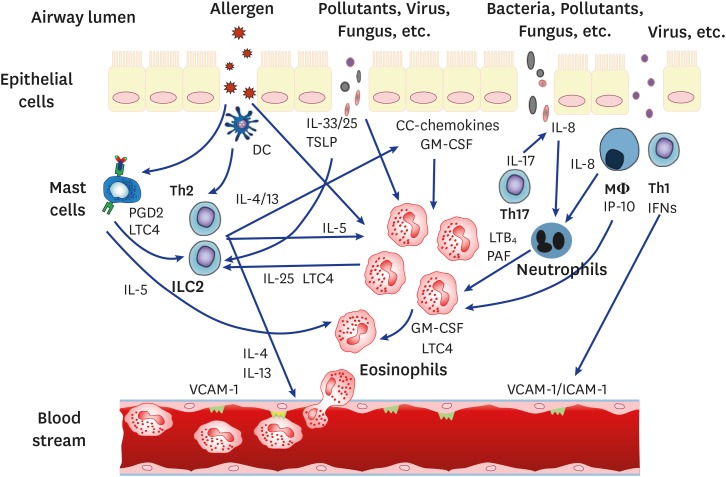
Both classical and novel mechanisms are involved in eosinophilic inflammation (Fig. 1). Further development of effective therapeutic strategies targeting effective control of eosinophilic inflammation is required.
Fig. 1. Summary of major contributing mechanisms involved in eosinophilic inflammation. PGD2, prostaglandin D2; LTC4, leukotriene C4; Th2, T-helper 2 cells; ILC2, innate lymphoid cell 2; IL, interleukin; DC, dendritic cells; TSLP, thymic stromal lymphopoietin; GM-CSF, granulocyte-macrophage colony-stimulating factor; LTB4, leukotriene B4; PAF, platelet-activating factor; VCAM-1, vascular cell adhesion molecule-1; ICAM-1, intercellular adhesion molecule-1.
Footnotes
Conflict of Interest: MN received honoraria from Astra Zeneca. Glaxo Smith Kline, Kyorin Pharma, Novartis Pharma, and Sanofi Aventis. KN received honoraria from Astra Zeneca.
- Conceptualization: Makoto Nagata.
- Data curation: Makoto Nagata, Kazuyuki Nakagome.
- Formal analysis: Makoto Nagata, Tomoyuki Soma.
- Funding acquisition: Makoto Nagata.
- Methodology: Makoto Nagata.
- Project administration: Makoto Nagata, Kazuyuki Nakagome.
- Visualization: Makoto Nagata, Tomoyuki Soma.
- Writing - original draft: Makoto Nagata.
- Writing - review & editing: Tomoyuki Soma, Kazuyuki Nakagome.
References
- 1.Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, Barker P, Sproule S, Ponnarambil S, Goldman M ZONDA Trial Investigators. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017;376:2448–2458. doi: 10.1056/NEJMoa1703501. [DOI] [PubMed] [Google Scholar]
- 2.Steinfeld J, Bradford ES, Brown J, Mallett S, Yancey SW, Akuthota P, Cid MC, Gleich GJ, Jayne D, Khoury P, Langford CA, Merkel PA, Moosig F, Specks U, Weller PF, Wechsler ME. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol. 2019;143:2170–2177. doi: 10.1016/j.jaci.2018.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 4.Sedgwick JB, Nagata M. Mechanism of eosinophil activation. In: Busse W, Holgate S, editors. Asthma and rhinitis. Boston (MA): Blackwell Scientific; 2000. pp. 373–393. [Google Scholar]
- 5.Melo RCN, Weller PF. Contemporary understanding of the secretory granules in human eosinophils. J Leukoc Biol. 2018;104:85–93. doi: 10.1002/JLB.3MR1217-476R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ueki S, Konno Y, Takeda M, Moritoki Y, Hirokawa M, Matsuwaki Y, Honda K, Ohta N, Yamamoto S, Takagi Y, Wada A, Weller PF. Eosinophil extracellular trap cell death-derived DNA traps: their presence in secretions and functional attributes. J Allergy Clin Immunol. 2016;137:258–267. doi: 10.1016/j.jaci.2015.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persson EK, Verstraete K, Heyndrickx I, Gevaert E, Aegerter H, Percier JM, Deswarte K, Verschueren KHG, Dansercoer A, Gras D, Chanez P, Bachert C, Gonçalves A, Van Gorp H, De Haard H, Blanchetot C, Saunders M, Hammad H, Savvides SN, Lambrecht BN. Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Science. 2019;364:eaaw4295. doi: 10.1126/science.aaw4295. [DOI] [PubMed] [Google Scholar]
- 8.Nakagome K, Nagata M. Pathogenesis of airway inflammation in bronchial asthma. Auris Nasus Larynx. 2011;38:555–563. doi: 10.1016/j.anl.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Bochner BS, Klunk DA, Sterbinsky SA, Coffman RL, Schleimer RP. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799–803. [PubMed] [Google Scholar]
- 10.Nagata M, Sedgwick JB, Kita H, Busse WW. Granulocyte macrophage colony-stimulating factor augments ICAM-1 and VCAM-1 activation of eosinophil function. Am J Respir Cell Mol Biol. 1998;19:158–166. doi: 10.1165/ajrcmb.19.1.3001. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto H, Nagata M. Regulatory mechanisms of eosinophil adhesion to and transmigration across endothelial cells by alpha4 and beta2 integrins. Int Arch Allergy Immunol. 1999;120(Suppl 1):24–26. doi: 10.1159/000053588. [DOI] [PubMed] [Google Scholar]
- 12.Woltmann G, McNulty CA, Dewson G, Symon FA, Wardlaw AJ. Interleukin-13 induces PSGL-1/P-selectin-dependent adhesion of eosinophils, but not neutrophils, to human umbilical vein endothelial cells under flow. Blood. 2000;95:3146–3152. [PubMed] [Google Scholar]
- 13.Nagata M, Sedgwick JB, Bates ME, Kita H, Busse WW. Eosinophil adhesion to vascular cell adhesion molecule-1 activates superoxide anion generation. J Immunol. 1995;155:2194–2202. [PubMed] [Google Scholar]
- 14.Nagata M, Sedgwick JB, Vrtis R, Busse WW. Endothelial cells upregulate eosinophil superoxide generation via VCAM-1 expression. Clin Exp Allergy. 1999;29:550–561. doi: 10.1046/j.1365-2222.1999.00506.x. [DOI] [PubMed] [Google Scholar]
- 15.Nagata M, Yamamoto H, Shibasaki M, Sakamoto Y, Matsuo H. Hydrogen peroxide augments eosinophil adhesion via beta2 integrin. Immunology. 2000;101:412–418. doi: 10.1046/j.1365-2567.2000.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stellato C, Beck LA, Gorgone GA, Proud D, Schall TJ, Ono SJ, Lichtenstein LM, Schleimer RP. Expression of the chemokine RANTES by a human bronchial epithelial cell line. Modulation by cytokines and glucocorticoids. J Immunol. 1995;155:410–418. [PubMed] [Google Scholar]
- 17.Nonaka M, Pawankar R, Fukumoto A, Ogihara N, Sakanushi A, Yagi T. Induction of eotaxin production by interleukin-4, interleukin-13 and lipopolysaccharide by nasal fibroblasts. Clin Exp Allergy. 2004;34:804–811. doi: 10.1111/j.1365-2222.2004.1954.x. [DOI] [PubMed] [Google Scholar]
- 18.Nagata M, Yamamoto H, Tabe K, Sakamoto Y. Eosinophil transmigration across VCAM-1-expressing endothelial cells is upregulated by antigen-stimulated mononuclear cells. Int Arch Allergy Immunol. 2001;125(Suppl 1):7–11. doi: 10.1159/000053844. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto H, Nagata M, Sakamoto Y. CC chemokines and transmigration of eosinophils in the presence of vascular cell adhesion molecule 1. Ann Allergy Asthma Immunol. 2005;94:292–300. doi: 10.1016/S1081-1206(10)61311-7. [DOI] [PubMed] [Google Scholar]
- 20.Takatsu K. Interleukin-5 and IL-5 receptor in health and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:463–485. doi: 10.2183/pjab.87.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakuishi K, Oki S, Araki M, Porcelli SA, Miyake S, Yamamura T. Invariant NKT cells biased for IL-5 production act as crucial regulators of inflammation. J Immunol. 2007;179:3452–3462. doi: 10.4049/jimmunol.179.6.3452. [DOI] [PubMed] [Google Scholar]
- 22.Kabata H, Moro K, Koyasu S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol Rev. 2018;286:37–52. doi: 10.1111/imr.12706. [DOI] [PubMed] [Google Scholar]
- 23.Préfontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125:752–754. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 24.Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, Shelley M, Abbas AR, Austin CD, Jackman J, Wu LC, Heaney LG, Arron JR, Bradding P. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012;129:104–111.e1-9. doi: 10.1016/j.jaci.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Terrier B, Bièche I, Maisonobe T, Laurendeau I, Rosenzwajg M, Kahn JE, Diemert MC, Musset L, Vidaud M, Sène D, Costedoat-Chalumeau N, Le Thi-Huong D, Amoura Z, Klatzmann D, Cacoub P, Saadoun D. Interleukin-25: a cytokine linking eosinophils and adaptive immunity in Churg-Strauss syndrome. Blood. 2010;116:4523–4531. doi: 10.1182/blood-2010-02-267542. [DOI] [PubMed] [Google Scholar]
- 26.Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O'Byrne PM, Gauvreau GM, Boulet LP, Lemiere C, Martin J, Nair P, Sehmi R. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86.e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 27.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, Hansel TT, Holgate ST, Sterk PJ, Barnes PJ. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356:2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 28.Adachi T, Motojima S, Hirata A, Fukuda T, Makino S. Eosinophil viability-enhancing activity in sputum from patients with bronchial asthma, contributions of interleukin-5 and granulocyte/macrophage colony-stimulating factor. Am J Respir Crit Care Med. 1995;151(3 Pt 1):618–623. doi: 10.1164/ajrccm.151.3.7881646. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto H, Sedgwick JB, Vrtis RF, Busse WW. The effect of transendothelial migration on eosinophil function. Am J Respir Cell Mol Biol. 2000;23:379–388. doi: 10.1165/ajrcmb.23.3.3707. [DOI] [PubMed] [Google Scholar]
- 30.Gregory B, Kirchem A, Phipps S, Gevaert P, Pridgeon C, Rankin SM, Robinson DS. Differential regulation of human eosinophil IL-3, IL-5, and GM-CSF receptor alpha-chain expression by cytokines: IL-3, IL-5, and GM-CSF down-regulate IL-5 receptor alpha expression with loss of IL-5 responsiveness, but up-regulate IL-3 receptor alpha expression. J Immunol. 2003;170:5359–5366. doi: 10.4049/jimmunol.170.11.5359. [DOI] [PubMed] [Google Scholar]
- 31.Liu LY, Sedgwick JB, Bates ME, Vrtis RF, Gern JE, Kita H, Jarjour NN, Busse WW, Kelly EA. Decreased expression of membrane IL-5 receptor alpha on human eosinophils: I. Loss of membrane IL-5 receptor alpha on airway eosinophils and increased soluble IL-5 receptor alpha in the airway after allergen challenge. J Immunol. 2002;169:6452–6458. doi: 10.4049/jimmunol.169.11.6452. [DOI] [PubMed] [Google Scholar]
- 32.Wilson SJ, Wallin A, Della-Cioppa G, Sandström T, Holgate ST. Effects of budesonide and formoterol on NF-kappaB, adhesion molecules, and cytokines in asthma. Am J Respir Crit Care Med. 2001;164:1047–1052. doi: 10.1164/ajrccm.164.6.2010045. [DOI] [PubMed] [Google Scholar]
- 33.Pizzichini MM, Pizzichini E, Efthimiadis A, Chauhan AJ, Johnston SL, Hussack P, Mahony J, Dolovich J, Hargreave FE. Sputum eosinophilia predicts benefit from prednisone in smokers with chronic obstructive bronchitis. Am J Respir Crit Care Med. 1998;158:1178–1184. doi: 10.1164/ajrccm.158.5.9804028. [DOI] [PubMed] [Google Scholar]
- 34.Nakagome K, Bochkov YA, Ashraf S, Brockman-Schneider RA, Evans MD, Pasic TR, Gern JE. Effects of rhinovirus species on viral replication and cytokine production. J Allergy Clin Immunol. 2014;134:332–341. doi: 10.1016/j.jaci.2014.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truyen E, Coteur L, Dilissen E, Overbergh L, Dupont LJ, Ceuppens JL, Bullens DM. Evaluation of airway inflammation by quantitative Th1/Th2 cytokine mRNA measurement in sputum of asthma patients. Thorax. 2006;61:202–208. doi: 10.1136/thx.2005.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, Huff R, Pilewski J, Holguin F, Kolls J, Wenzel S, Ray P, Ray A. High IFN-γ and low SLPI mark severe asthma in mice and humans. J Clin Invest. 2015;125:3037–3050. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chambers ES, Nanzer AM, Pfeffer PE, Richards DF, Timms PM, Martineau AR, Griffiths CJ, Corrigan CJ, Hawrylowicz CM. Distinct endotypes of steroid-resistant asthma characterized by IL-17A(high) and IFN-γ(high) immunophenotypes: potential benefits of calcitriol. J Allergy Clin Immunol. 2015;136:628–37.e4. doi: 10.1016/j.jaci.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wark PA, Bucchieri F, Johnston SL, Gibson PG, Hamilton L, Mimica J, Zummo G, Holgate ST, Attia J, Thakkinstian A, Davies DE. IFN-gamma-induced protein 10 is a novel biomarker of rhinovirus-induced asthma exacerbations. J Allergy Clin Immunol. 2007;120:586–593. doi: 10.1016/j.jaci.2007.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi T, Takaku Y, Yokote A, Miyazawa H, Soma T, Hagiwara K, Kanazawa M, Nagata M. Interferon-beta augments eosinophil adhesion-inducing activity of endothelial cells. Eur Respir J. 2008;32:1540–1547. doi: 10.1183/09031936.00059507. [DOI] [PubMed] [Google Scholar]
- 40.Takaku Y, Nakagome K, Kobayashi T, Hagiwara K, Kanazawa M, Nagata M. IFN-γ-inducible protein of 10 kDa upregulates the effector functions of eosinophils through β2 integrin and CXCR3. Respir Res. 2011;12:138. doi: 10.1186/1465-9921-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takaku Y, Soma T, Uchida Y, Kobayashi T, Nakagome K, Nagata M. CXC chemokine superfamily induced by Interferon-γ in asthma: a cross-sectional observational study. Asthma Res Pract. 2016;2:6. doi: 10.1186/s40733-016-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wardlaw AJ, Moqbel R, Cromwell O, Kay AB. Platelet-activating factor. A potent chemotactic and chemokinetic factor for human eosinophils. J Clin Invest. 1986;78:1701–1706. doi: 10.1172/JCI112765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Powell WS, Ahmed S, Gravel S, Rokach J. Eotaxin and RANTES enhance 5-oxo-6,8,11,14-eicosatetraenoic acid-induced eosinophil chemotaxis. J Allergy Clin Immunol. 2001;107:272–278. doi: 10.1067/mai.2001.112847. [DOI] [PubMed] [Google Scholar]
- 44.Monneret G, Gravel S, Diamond M, Rokach J, Powell WS. Prostaglandin D2 is a potent chemoattractant for human eosinophils that acts via a novel DP receptor. Blood. 2001;98:1942–1948. doi: 10.1182/blood.v98.6.1942. [DOI] [PubMed] [Google Scholar]
- 45.Domingo C, Palomares O, Sandham DA, Erpenbeck VJ, Altman P. The prostaglandin D2 receptor 2 pathway in asthma: a key player in airway inflammation. Respir Res. 2018;19:189. doi: 10.1186/s12931-018-0893-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laitinen LA, Laitinen A, Haahtela T, Vilkka V, Spur BW, Lee TH. Leukotriene E4 and granulocytic infiltration into asthmatic airways. Lancet. 1993;341:989–990. doi: 10.1016/0140-6736(93)91073-u. [DOI] [PubMed] [Google Scholar]
- 47.Nagata M, Saito K, Tsuchiya K, Sakamoto Y. Leukotriene D4 upregulates eosinophil adhesion via the cysteinyl leukotriene 1 receptor. J Allergy Clin Immunol. 2002;109:676–680. doi: 10.1067/mai.2002.122841. [DOI] [PubMed] [Google Scholar]
- 48.Saito K, Nagata M, Kikuchi I, Sakamoto Y. Leukotriene D4 and eosinophil transendothelial migration, superoxide generation, and degranulation via beta2 integrin. Ann Allergy Asthma Immunol. 2004;93:594–600. doi: 10.1016/S1081-1206(10)61269-0. [DOI] [PubMed] [Google Scholar]
- 49.Kushiya M, Saito K, Kikuchi I, Kobayashi T, Hagiwara K, Kanazawa M, Nagata M. Differential effects of salbutamol and montelukast on eosinophil adhesion and superoxide anion generation. Int Arch Allergy Immunol. 2006;140(Suppl 1):17–22. doi: 10.1159/000092706. [DOI] [PubMed] [Google Scholar]
- 50.Bjermer L, Bisgaard H, Bousquet J, Fabbri LM, Greening AP, Haahtela T, Holgate ST, Picado C, Menten J, Dass SB, Leff JA, Polos PG. Montelukast and fluticasone compared with salmeterol and fluticasone in protecting against asthma exacerbation in adults: one year, double blind, randomised, comparative trial. BMJ. 2003;327:891. doi: 10.1136/bmj.327.7420.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anwar AR, Moqbel R, Walsh GM, Kay AB, Wardlaw AJ. Adhesion to fibronectin prolongs eosinophil survival. J Exp Med. 1993;177:839–843. doi: 10.1084/jem.177.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walsh GM, Wardlaw AJ. Dexamethasone inhibits prolonged survival and autocrine granulocyte-macrophage colony-stimulating factor production by human eosinophils cultured on laminin or tissue fibronectin. J Allergy Clin Immunol. 1997;100:208–215. doi: 10.1016/s0091-6749(97)70226-4. [DOI] [PubMed] [Google Scholar]
- 53.Noguchi T, Nakagome K, Kobayashi T, Uchida Y, Soma T, Nakamoto H, Nagata M. Periostin upregulates the effector functions of eosinophils. J Allergy Clin Immunol. 2016;138:1449–52.e5. doi: 10.1016/j.jaci.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 54.The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J. 2003;22:470–477. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 55.Kikuchi S, Kikuchi I, Takaku Y, Kobayashi T, Hagiwara K, Kanazawa M, Nagata M. Neutrophilic inflammation and CXC chemokines in patients with refractory asthma. Int Arch Allergy Immunol. 2009;149(Suppl 1):87–93. doi: 10.1159/000211379. [DOI] [PubMed] [Google Scholar]
- 56.Shannon J, Ernst P, Yamauchi Y, Olivenstein R, Lemiere C, Foley S, Cicora L, Ludwig M, Hamid Q, Martin JG. Differences in airway cytokine profile in severe asthma compared to moderate asthma. Chest. 2008;133:420–426. doi: 10.1378/chest.07-1881. [DOI] [PubMed] [Google Scholar]
- 57.Kikuchi I, Kikuchi S, Kobayashi T, Hagiwara K, Sakamoto Y, Kanazawa M, Nagata M. Eosinophil trans-basement membrane migration induced by interleukin-8 and neutrophils. Am J Respir Cell Mol Biol. 2006;34:760–765. doi: 10.1165/rcmb.2005-0303OC. [DOI] [PubMed] [Google Scholar]
- 58.Hauk PJ, Krawiec M, Murphy J, Boguniewicz J, Schiltz A, Goleva E, Liu AH, Leung DY. Neutrophilic airway inflammation and association with bacterial lipopolysaccharide in children with asthma and wheezing. Pediatr Pulmonol. 2008;43:916–923. doi: 10.1002/ppul.20880. [DOI] [PubMed] [Google Scholar]
- 59.Goleva E, Hauk PJ, Hall CF, Liu AH, Riches DW, Martin RJ, Leung DY. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol. 2008;122:550–559.e3. doi: 10.1016/j.jaci.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berger M, de Boer JD, Bresser P, van der Poll T, Lutter R, Sterk PJ, van der Zee JS. Lipopolysaccharide amplifies eosinophilic inflammation after segmental challenge with house dust mite in asthmatics. Allergy. 2015;70:257–264. doi: 10.1111/all.12544. [DOI] [PubMed] [Google Scholar]
- 61.Nishihara F, Nakagome K, Kobayashi T, Noguchi T, Araki R, Uchida Y, Soma T, Nagata M. Trans-basement membrane migration of eosinophils induced by LPS-stimulated neutrophils from human peripheral blood in vitro . ERJ Open Res. 2015;1:00003-2015. doi: 10.1183/23120541.00003-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thorne PS, Mendy A, Metwali N, Salo P, Co C, Jaramillo R, Rose KM, Zeldin DC. Endotoxin exposure: predictors and prevalence of associated asthma outcomes in the United States. Am J Respir Crit Care Med. 2015;192:1287–1297. doi: 10.1164/rccm.201502-0251OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuwaki Y, Wada K, White TA, Benson LM, Charlesworth MC, Checkel JL, Inoue Y, Hotta K, Ponikau JU, Lawrence CB, Kita H. Recognition of fungal protease activities induces cellular activation and eosinophil-derived neurotoxin release in human eosinophils. J Immunol. 2009;183:6708–6716. doi: 10.4049/jimmunol.0901220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muniz VS, Silva JC, Braga YAV, Melo RCN, Ueki S, Takeda M, Hebisawa A, Asano K, Figueiredo RT, Neves JS. Eosinophils release extracellular DNA traps in response to Aspergillus fumigatus. J Allergy Clin Immunol. 2018;141:571–85.e7. doi: 10.1016/j.jaci.2017.07.048. [DOI] [PubMed] [Google Scholar]
- 65.Ueda Y, Nakagome K, Kobayashi T, Noguchi T, Soma T, Ohashi-Doi K, Tokuyama K, Nagata M. Dermatophagoides farinae upregulates the effector functions of eosinophils through αMβ2-integrin and protease-activated receptor-2. Int Arch Allergy Immunol. 2019;178:295–306. doi: 10.1159/000495008. [DOI] [PubMed] [Google Scholar]