Abstract
Background
The upregulation of the cyclooxygenase and lipoxygenase pathways of arachidonic acid is thought to be involved in the development of rheumatoid arthritis. Recently, the presence of specialized pro-resolving lipid mediators in synovial tissues from patients with osteoarthritis has been reported.
Objective
To clarify the quantitative and qualitative changes in lipid mediators in the synovium of severe rheumatoid arthritis patients, we compared the profiles of lipid mediators in synovial fluid obtained from patients with severe rheumatoid arthritis and from those with severe osteoarthritis.
Methods
We enrolled 18 patients with rheumatoid arthritis and 26 patients with osteoarthritis. All the patients had undergone total knee replacement surgery. Synovial fluid samples had been obtained during the surgery. Lipid profiling in the synovial fluid from these patients was performed using liquid chromatography-tandem mass spectrometry/mass spectrometry.
Results
Among the 150 oxidized fatty acids examined so far, 119 were substantially detected in synovial fluid from the patients. Not only the concentrations of pro-inflammatory lipid mediators such as prostaglandins and leukotrienes, but also those of specialized pro-resolving lipid mediators such as lipoxins, resolvins, and protectin D1 were significantly higher in synovial fluid obtained from rheumatoid arthritis patients than from synovial fluid obtained from osteoarthritis patients.
Conclusion
The activation of both inflammation and resolution pathways of lipid mediators might be a fatty acid signature in the synovial fluid of patients with severe rheumatoid arthritis. Inflammatory, anti-inflammatory and pro-resolving mediators in synovial fluid could be good biomarkers for differentiating between severe rheumatoid arthritis and severe osteoarthritis.
Keywords: Liquid chromatography-tandem mass spectrometry/mass spectrometry, Lipid mediator, Osteoarthritis, Oxidized fatty acid, Rheumatoid arthritis, Specialized pro-resolving lipid mediator
INTRODUCTION
Rheumatoid arthritis (RA) and osteoarthritis (OA) are representative inflammatory joint diseases, that are characterized by different pathophysiological mechanisms but display common clinical characteristics, such as pain and structural damage [1]. In both RA and OA, total knee replacement (total knee arthroplasty) is performed to restore function and to relieve pain in patients with severely damaged knees.
Eicosanoids, which are produced by the enzymatic oxygenation of arachidonic acid (AA; C20:4), an ω6 polyunsaturated fatty acid (PUFA), represent one of the most complex networks in the body controlling many physiological and pathophysiological processes, including inflammation, autoimmunity and cancer. AA constitutes a substrate for 2 cyclooxygenase (COX) isoforms (COX-1 and COX-2), several lipoxygenase (LO) isoforms (5-LO, 12-LO, and 15-LO), and cytochrome P-450 (P450). The produced intermediates are subsequently converted by specific downstream enzymes into various prostaglandins (PGs) via the COX pathways and leukotrienes (LTs) or lipoxins (LXs) via the LO pathways. The upregulation of these pathways of AA is thought to be involved in the development of rheumatic diseases, and targeting this pathway might enable improved treatment strategies [2]. Indeed, PGs, such as PGE2 and PGI2, and LTs, such as LTB4, have been considered to play important roles in the onset and development of arthritic diseases in animals and humans [1,2,3,4], although some PGs, such as the nonenzymatic PGD2 metabolite 15-deoxy-PGJ2, play anti-inflammatory roles according to the disease contexts [2]. Nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit COXs and thereby shut off the biosynthesis of PGs, are first-line drugs for the treatment of inflammation and pain in RA and OA.
Similar to ω6 AA, ω3 eicosapentaenoic acid (EPA; C20:5) and docosahexaenoic acid (DHA; C22:6) can be further metabolized by the oxidative enzymes COXs, LOs and P450. EPA can be metabolized by COXs and 5-LO to prostanoids series 3 (e.g., PGE3, PGI3, and TXA3) and LT series 5 (e.g., LTB5, LTC5 and LTD5), respectively; these compounds display anti-inflammatory properties through competing receptor occupation with AA-derived PGs and LTs [5]. Specialized pro-resolving lipid mediators (SPMs), including ω6 AA-derived LXs and ω3 EPA/DHA-derived resolvins, protectins and maresins, are each temporally produced in resolving exudates with distinct actions for their return to homeostasis. SPMs have the abilities to promote microbial defense, tissue regeneration and wound healing, resolve inflammation and pain, and prevent cancer [6]. Several SPMs, such as LXA4, resolvin (Rv) E1, RvD1, and maresin 1 (MaR1), are detected in both human RA and mouse RA models and can attenuate synovial inflammation, promote bone resolution, and correct the imbalance of regulatory T cells and Th17 cells through multiple mechanisms [7,8,9,10,11,12,13,14,15].
To date, a couple of studies have been conducted to gain insight into the profile of lipid mediators (LMs) in human synovial fluid (SF) from patients with RA or OA using liquid chromatography-tandem mass spectrometry/mass spectrometry (LC-MS/MS) [7,8,9,16]. However, no direct comparison of LM profiling of SF in patients with severe RA and those with severe OA has currently been performed. In this study, we compared, for the first time, the quantitative and qualitative changes in LMs that occur in the synovium of patients with severe RA or severe OA, who had undergone total knee replacement surgery.
MATERIALS AND METHODS
Ethical considerations
The study was approved by the Ethics Committees of Nihon University School of Medicine (approved number, RK-160112-2), and all the subjects provided written informed consent in accordance with the Helsinki Declaration of the World Medical Association.
Patient enrollment
We enrolled 18 patients with RA and 26 patients with OA. The diagnosis of each patient was established by the treating doctors. SF samples were obtained during total knee arthroplasty performed at the Department of Orthopedic Surgery, Nihon University, after receiving informed consent.
Processing of SF
Two milliliters of SF were treated with hyaluronidase, followed by centrifugation at 860 ×g for 10 minutes. The supernatants were collected, and the tubes were filled with N2 gas. The samples were then frozen at -80°C.
Lipidomics analysis
Electrospray ionization-tandem mass spectrometry (ESI-MS) was performed in accordance with our current protocol [10]. In brief, for the detection of phospholipids, SF obtained from the patients was diluted in 10 volumes of 20 mM Tris-HCl (pH 7.4). Lipids were extracted from the homogenates using the method described by Bligh and Dyer [11]. An MS analysis was performed using a 4000 Q-TRAP quadrupole-linear ion trap hybrid mass spectrometer (AB Sciex, Framingham, MA, USA) with LC (NexeraX2 system; Shimazu, Kyoto, Japan). The samples were applied to a Kinetex C18 column (1 × 150 mm i.d., 1.7-μm particle) (Phenomenex, Torrance, CA, USA) coupled to ESI-MS/MS. The samples injected by an autosampler (10 μL) were separated using a step gradient with mobile phase A (acetonitrile/methanol/water = 1:1:1 [v/v/v] containing 5 μM phosphoric acid and 1 mM ammonium formate) and mobile phase B (2-propanol containing 5 μM phosphoric acid and 1 mM ammonium formate) at a flow rate of 0.2 mL/min at 50°C. For the detection of fatty acids and their oxygenated metabolites, the plasma was diluted in 10 volumes of methanol. After overnight incubation at −20°C, water was added to the mixture to give a final methanol concentration of 10% (v/v). The samples in 10% methanol were applied to Oasis HLB cartridges (Waters, Milford, MA, USA), washed with 10 mL of hexane, eluted with 3 mL of methyl formate, dried under N2 gas, and dissolved in 60% methanol. The samples were then applied to a Kinetex C18 column (1 × 150 mm i.d., 1.7-μm particles) (Phenomenex) coupled to ESI-MS/MS as described above. The samples injected by an autosampler (10 μL) were separated using a step gradient with mobile phase C (water containing 0.1% acetic acid) and mobile phase D (acetonitrile/methanol = 4:1; v/v) at a flow rate of 0.2 mL/min at 45°C. Identification was conducted using multiple reaction monitoring (MRM) transition and retention times, and quantification was performed based on the peak area of the MRM transition and the calibration curve obtained with an authentic standard for each compound. As internal standards, d5-labeled EPA and d7-labeled 17:0 lysophosphatidylcholine (1 nmol; Cayman Chemicals, Ann Arbor, MI, USA) were added to each sample [12].
Statistical analysis
The Mann-Whitney U test was used to compare the 2 groups; to compare categorical variables, a 2-sided Fisher exact test was used. The receiver operator characteristic curve (ROC curve) was used to determine the optimal cutoff values that maximized the sum of the specificity and sensitivity. Spearman rank correlation coefficients were calculated to determine the strength of the correlations between continuous variables. A p value <0.05 was considered significant. The data analyses were performed using Prism ver. 7 (GraphPad software; San Diego, CA, USA).
RESULTS
Recruitment and baseline characteristics
We enrolled 18 patients with RA and 26 patients with OA. The baseline characteristics of the patients with RA or OA are shown in Tables 1 and 2, respectively. There was no significant difference in age or percentage of females between patients with RA and those with OA.
Table 1. Characteristics of the patients with rheumatoid arthritis (RA).
| Sex | Age (yr) | Duration of illness (yr) | Peripheral leukocyte counts (/mm3) | Serum CRP level (mg/dL) | Serum anti-CCP Ab level (U/mL) | Serum MMP3 level (ng/mL) | Serum RF level (mg/dL) | Medication | |
|---|---|---|---|---|---|---|---|---|---|
| RA1 | F | 64 | 22 | 10,500 | 2.3 | 88.4 | 396.8 | 330 | PSL+SASP |
| RA2 | F | 80 | 4 | 5,700 | 4.1 | >1,200 | 417.4 | 258 | MTX+PSL+SASP+TAC |
| RA3 | F | 77 | 5 | 7,400 | 0.1 | 0.6 | n.d. | 5.3 | NSAIDa) |
| RA4 | F | 69 | 27 | 5,400 | 1.4 | 135.6 | 166.6 | 53 | MTX+PSL |
| RA5 | F | 54 | 21 | 6,200 | 4.6 | 47.5 | 509.6 | 35 | IGU+PSL+SASP |
| RA6 | M | 80 | 1 | 7,100 | 2.51 | >500 | 711.6 | 164 | PSL+TAC |
| RA7 | F | 64 | 1 | 12,900 | 0.1 | 0.6 | 185.5 | 21.9 | MTX+NSAIDb)+PSL |
| RA8 | F | 74 | 10 | 5,300 | 0.31 | 860 | 47.6 | 240 | MTX |
| RA9 | F | 69 | 9 | 4,300 | 0.6 | 362.5 | 87.3 | 45.7 | MTX+NSAIDb)+PSL |
| RA10 | F | 77 | 14 | 5,700 | 0.1 | 85.9 | 73.2 | 28.3 | BUC+SASP |
| RA11 | F | 84 | 7 | 4,800 | 0.11 | 320 | 463.1 | 55.1 | ABT+MTX |
| RA12 | F | 74 | 3 | 7,500 | 0.1 | 32.1 | 415.4 | 3 | APAP+PSL |
| RA13 | F | 78 | 22 | 8,000 | 3.78 | 727 | 386.7 | ND | MTX+NSAIDa) |
| RA14 | F | 54 | 3 | 5,400 | 0.63 | 491 | 225.8 | 703.1 | MTX |
| RA15 | F | 63 | 5 | 6,600 | 0.54 | 166 | 105 | 115.2 | MTX |
| RA16 | F | 60 | 9 | 8,200 | 0.15 | 358.1 | 414.8 | 186.5 | MTX+PSL |
| RA17 | F | 60 | 2 | 8,000 | 0.84 | 0.6 | 132.6 | 6.7 | IGU |
| RA18 | F | 77 | 1 | 7,500 | 0.47 | 0.6 | 108.2 | 3.7 | NSAIDb) |
ABT, abatacept; anti-CCP Ab, anti-cyclic citrullinated peptide antibody; APAP, acetaminophen; BUC, bucillamine; CRP, C-reactive protein; IGU, iguratimod; MMP3, matrix metalloproteinase-3; MTX, methotrexate; NSAID, nonsteroidal anti-inflammatory drug; ND, not done; PSL, prednisolone; RA, rheumatoid arthritis; RF, rheumatoid factor; SASP, salazosulfapyridine; TAC, tacrolimus.
a)Loxoprofen 180 mg/day. b)Celecoxib 200 mg/day.
Table 2. Characteristics of the patients with osteoarthritis (OA).
| Sex | Age (yr) | Duration of illness (yr) | Peripheral leukocyte counts (/mm3) | Serum CRP level (mg/dL) | Serum anti-CCP Ab level (U/mL) | Serum MMP3 level (ng/mL) | Serum RF level (mg/dL) | Medication | |
|---|---|---|---|---|---|---|---|---|---|
| OA1 | F | 88 | 1 | 3,500 | 0.1 | ND | ND | ND | NSAIDb) |
| OA2 | F | 69 | 27 | 3,200 | 0.11 | ND | ND | ND | APAP |
| OA3 | M | 58 | 2 | 5,000 | 0.23 | ND | ND | ND | NSAIDa) |
| OA4 | M | 53 | 1 | 4,600 | 0.1 | ND | ND | ND | NSAIDa) |
| OA5 | F | 85 | 3 | 5,600 | 0.1 | ND | ND | ND | NSAIDa) |
| OA6 | F | 80 | 6 | 7,600 | 0.21 | ND | ND | ND | NSAIDb) |
| OA7 | M | 75 | 6 | 6,900 | 0.1 | ND | ND | ND | APAP |
| OA8 | M | 69 | 10 | 5,200 | 0.15 | ND | ND | ND | NSAIDa) |
| OA9 | M | 77 | 10 | 6,100 | 0.1 | ND | ND | ND | APAP |
| OA10 | M | 84 | 1 | 6,500 | 0.1 | ND | ND | ND | NSAIDb) |
| OA11 | F | 67 | 10 | 7,000 | 0.1 | ND | ND | ND | NSAIDa) |
| OA12 | M | 75 | 6 | 5,100 | 0.1 | ND | ND | ND | NSAIDb) |
| OA13 | M | 65 | 2 | 4,300 | 0.1 | ND | ND | ND | NSAIDa) |
| OA14 | F | 67 | 8 | 4,800 | 0.14 | ND | ND | ND | NSAIDb) |
| OA15 | F | 85 | 20 | 3,800 | 0.1 | ND | ND | ND | NSAIDb) |
| OA16 | F | 73 | 10 | 4,900 | 0.1 | ND | ND | ND | APAP |
| OA17 | F | 74 | 2 | 5,600 | 0.1 | ND | ND | ND | NSAIDb) |
| OA18 | M | 88 | 44 | 7,300 | 0.1 | ND | ND | ND | APAP |
| OA19 | F | 86 | 29 | 6,100 | 0.14 | ND | ND | ND | APAP |
| OA20 | M | 80 | 3 | 6,200 | 0.89 | ND | ND | ND | NSAIDb) |
| OA21 | F | 76 | 4 | 6,600 | 0.1 | ND | ND | ND | NSAIDb) |
| OA22 | M | 80 | 2 | 3,200 | 0.1 | ND | ND | ND | NSAIDb) |
| OA23 | F | 83 | 7 | 7,800 | 0.37 | ND | ND | ND | NSAIDb) |
| OA24 | F | 81 | 31 | 5,100 | 0.1 | ND | ND | ND | APAP |
| OA25 | M | 75 | 8 | 4,400 | 0.11 | ND | ND | ND | APAP |
| OA26 | F | 72 | 9 | 7,000 | 2.2 | ND | ND | ND | NSAIDa) |
APAP, acetaminophen; anti-CCP Ab, anti-cyclic citrullinated peptide antibody; CRP, C-reactive protein; MMP3, matrix metalloproteinase-3; ND, not done; NSAID, nonsteroidal anti-inflammatory drug; OA, osteoarthritis; RF, rheumatoid factor.
a)Loxoprofen 180 mg/day. b)Celecoxib 200 mg/day.
Profiles of LMs derived from COX-1/2 and 5-LO products of AA in SF from patients with RA or OA
Among the 150 oxidized fatty acids examined so far, 119 were substantially detected in SF from patients with RA or OA at levels based on the area under the curve/mL. Oxidized products (both enzymatic and nonenzymatic) of ω6 PUFAs included COX-1/2, 5-LO, 12/15-LO, and P450 products of AA (Supplementary Fig. 1A, B). The profiles of LMs derived from COX-1/2 and the 5-LO products of AA in SF from patients with RA were compared with those from patients with OA (Fig. 1A). The concentrations of the majority of COX-1/2 and five 5-LO products of AA appeared to be higher in SF from patients with RA than in SF from patients with OA (Fig. 1A, Supplementary Fig. 1A). The absolute amounts (pmol/mL) of representative eicosanoids, including 6-keto PGF1α (a stable metabolite of PGI2), PGF2α, PGE2, PGD2, 12-hydroxyheptadecatrienoic acid, 5-hydroxyeicosatetraenoic acid (HETE), 5-oxo-eicosatetraenoic acid (ETE), LTB4, LTC4, LTD4, and LTE4, in SF from patients with RA or OA were quantified (Fig. 1B). The concentrations of PGF2α, 5-HETE, and LTB4 were significantly higher in SF from patients with RA than in SF from patients with OA (Fig. 1B). The concentrations of LTC4, LTD4, and LTE4 via the LO pathways were under the detection limits (Fig. 1B). Thus, we confirmed the previous findings regarding the upregulation of the COX and 5-LO pathways in RA, relative to OA [2].
Fig. 1. Comparison of profiles of lipid mediators (LMs) derived from COX-1/2, 5-LO, 12/15-LO, and P450 products of arachidonic acid (AA) from patients with rheumatoid arthritis (RA) or osteoarthritis (OA). (A) Profiles of LMs derived from COX-1/2, 5-LO, 12/15-LO, and P450 products of AA in synovial fluid (SF) from patients with RA, compared with those in SF from patients with OA. The concentration (area under the curve/mL) ratios (RA/OA) are shown. (B) Comparison of concentrations of LMs derived from COX-1/2, 5-LO, 12/15-LO, and P450 products of AA. The data are shown as a box-plot. The concentrations of LMs are shown as pmol/mL. p values are shown. COX, cyclooxygenase; DHETE, dihydroxyeicosatrienoic acid; DiHETE, dihydroxyeicosatrienoic acid; EET, epoxyeicosatrienoic acids; ETE, eicosatetraenoic acid; HETE, hydroxyeicosatetraenoic acid; HHT, hydroxyheptadecatrienoic acid; HpETE, hydroperoxyeicosatetraenoic acid; LT, leukotriene; LO, lipoxygenase; LX, lipoxin; P450, cytochrome p450; PG, prostaglandin; Tx, thromboxane.
Profiles of LMs derived from 12/15-LO and P450 products of AA
The roles of 12/15-LO and P450 metabolites in the pathogenesis of RA are poorly understood. Among these metabolites, LXA4, an AA-derived SPM produced by the combined actions of 5-LO and 12/15-LO, was reportedly detected in SF from patients with RA [8]. The profiles of LMs derived from 12/15-LO and P450 products of AA showed that the concentrations of 12/15-LO products of AA, including 12-, 15- and 5-Hp-15-HETEs, 8, 15- and 5, 15-DiHETEs, LXA4 and 12-oxo-ETE, appeared to be higher in SF from patients with RA than in SF from patients with OA (Fig. 1A, Supplementary Fig. 1B). Also, the concentrations of P450 products of AA, including several epoxyeicosatrienoic acids (EETs) and their hydrolytic metabolites dihydroxyeicosatrienoic acids, which have anti-inflammatory and analgesic activities, appeared to be higher in SF from patients with RA than in SF from patients with OA (Fig. 1A, Supplementary Fig. 1B). The absolute amounts (pmol/mL) of representative 12/15-LO and P450 metabolites, including LXA4, LXB4,12-HETE, and 8,9-EET in SF from patients with RA or OA, were quantified (Fig. 1B). The concentrations of LXA4, LXB4, 12-HETE, and 8,9-EET were significantly higher in SF from patients with RA than in SF from patients with OA (Fig. 1B).
Profiles of LMs derived from EPA
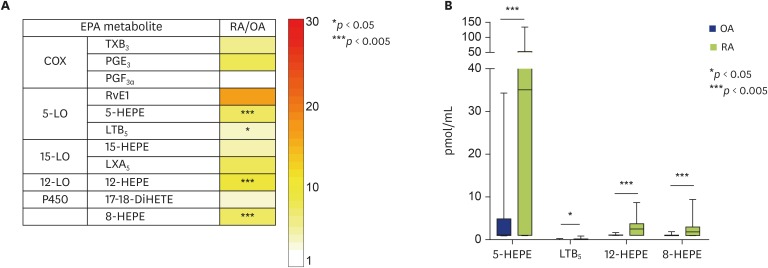
The profiling of LMs derived from EPA showed that the concentrations of EPA-derived COX metabolites, such as TXB3 and PGE3, and of SPMs, such as RvE1 and LXA5, appeared to be higher in SF from RA patients than in SF from OA patients (Fig. 2A, Supplementary Fig. 2). The quantified concentrations of 5-, 8-, and 12-HEPEs, and LTB5 were significantly higher in SF from patients with RA than in SF from patients with OA (Fig. 2B).
Fig. 2. Comparison of profiles of lipid mediators (LMs) derived from eicosapentaenoic acid (EPA) from patients with rheumatoid arthritis (RA) or osteoarthritis (OA). (A) Profiles of LMs derived from EPA in synovial fluid (SF) from patients with RA, compared with those in SF from patients with OA. The concentration (area under the curve/mL) ratios (RA/OA) are shown. (B) Comparison of concentrations of LMs derived from EPA. The data are shown as a box-plot. The concentrations of LMs are shown as pmol/mL. p values are shown. COX, cyclooxygenase; DiHETE, dihydroxyeicosatetraenoic acid; HEPE, hydroxyeicosapentaenoic acid; LT, leukotriene; LO, lipoxygenase; LX, lipoxin; PG, prostaglandin; Rv, resolvin; TX, thromboxane.
Profiles of LMs derived from DHA
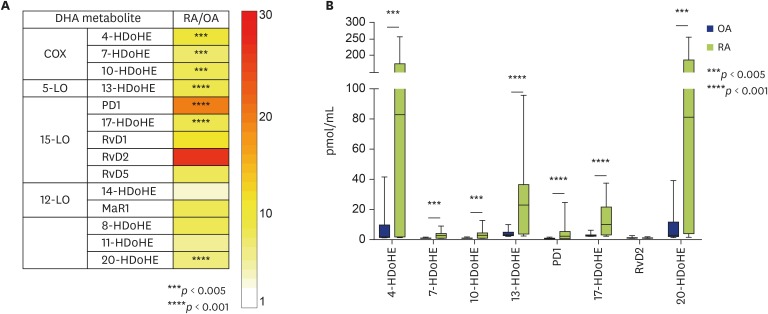
Although a previous report has shown that a few LMs derived from DHA were detected in SF obtained from patients with RA or OA, the levels did not differ significantly between patients with RA and those with OA [7]. In our study, the concentrations of DHA-derived LMs (both enzymatic and nonenzymatic) appeared to be higher in SF obtained from patients with RA than in SF from patients with OA (Fig. 3A, Supplementary Fig. 3). The absolute amounts (pmol/mL) of representative LMs derived from DHA, including 4-, 7-, 10-, 13-, 17-, and 20-hydroxydocosahexaenoic acids (HDoHEs) as well as SPMs such as protectin D1 (PD1) and RvD2, were quantified (Fig. 3B). The absolute amounts (pmol/mL) of 4-, 7-, 10-, 13-, 17-, and 20-HDoHEs and PD1 and RvD2 were significantly higher in SF from patients with RA than in SF from patients with OA (Fig. 3B).
Fig. 3. Comparison of profiles of lipid mediators (LMs) derived from docosahexaenoic acid (DHA) from patients with rheumatoid arthritis (RA) or osteoarthritis (OA). (A) Profiles of LMs derived from DHA in synovial fluid (SF) from patients with RA, compared with those in SF from patients with OA. The concentration (area under the curve/mL) ratios (RA/OA) are shown. (B) Comparison of concentrations of LMs derived from DHA. The data are shown as a box-plot. The concentrations of LMs are shown as pmol/mL. p values are shown. COX, cyclooxygenase; HDoHE, hydroxydocosahexaenoic acid; LO, lipoxygenase; MaR1, maresin 1; PD1, protectin D1; Rv, resolvin.
Overall, we concluded that most, if not all, AA, EPA, and DHA metabolites in the COX, 5-LO, 12/15-LO, and P450 pathways with both pro- and anti-inflammatory functions were concomitantly increased in the synovium of patients with RA, compared with the levels in patients with OA. Next, we analyzed which mediators in SF would be good biomarkers for differentiating between severe RA and severe OA. Among 19 mediators with SF concentrations that were significantly higher in patients with RA than in patients with OA, 17 mediators showed significant specificities to RA, compared with OA, using ROC curves (Table 3). Thus, inflammatory mediators such as 5-HETE, 12-HETE and 8,9-EET and anti-inflammatory and pro-resolving mediators such as LXA4, PD1, 12-HEPE, 4-HDoHE, and 17-HDoHE in SF might be good biomarkers for differentiating severe RA and severe OA, as positive likelihood ratios of these lipid mediators showed more than 10 (Table 3).
Table 3. Analysis of 19 lipid mediators, which concentrations in synovial fluids from patients with rheumatoid arthritis were significantly higher than the concentrations in synovial fluids from patients with osteoarthritis based on a receiver operator characteristic curve analysis.
| Acid | Major pathway | Mediator | Cutoff (pmol/mL) | Sensitivity (%) | Specificity (%) | p value | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|---|---|---|
| AA | COX | PGF2α | 1.124 | 55.56 | 92.31 | 0.0357 | 7.225 | 0.481 |
| 5-LO | 5-HETE | 63.61 | 66.67 | 96.15 | 0.0007 | 17.317 | 0.347 | |
| 5-oxo-ETE | 0.05 | 22.22 | 100 | 0.215 | Infinity | 0.778 | ||
| LTB4 | 9.065 | 61.11 | 80.77 | 0.015 | 3.178 | 0.481 | ||
| 15-LO | LXA4 | 2.474 | 61.11 | 100 | 0.0009 | Infinity | 0.389 | |
| LXB4 | 2.33 | 61.11 | 92.31 | 0.0033 | 7.947 | 0.421 | ||
| 12-LO | 12-HETE | 4.028 | 66.67 | 100 | <0.0001 | Infinity | 0.333 | |
| P450 | 8,9-EET | 2.912 | 61.11 | 100 | 0.0011 | Infinity | 0.389 | |
| EPA | 5-LO | 5-HEPE | 16.24 | 66.67 | 92.31 | 0.0021 | 8.670 | 0.361 |
| LTB5 | 0.065 | 37.5 | 96.15 | 0.12 | 9.740 | 0.650 | ||
| 12-LO | 12-HEPE | 1.982 | 61.11 | 100 | 0.0045 | Infinity | 0.389 | |
| 8-HEPE | 1.258 | 66.67 | 92.31 | 0.0019 | 8.670 | 0.361 | ||
| DHA | COX | 4-HDoHE | 53.38 | 66.67 | 100 | 0.0019 | Infinity | 0.333 |
| 7-HDoHE | 2.02 | 61.11 | 100 | 0.0017 | Infinity | 0.389 | ||
| 10-HDoHE | 1.369 | 66.67 | 96.15 | 0.0016 | 17.317 | 0.347 | ||
| 5-LO | 13-HDoHE | 7.669 | 66.67 | 96.15 | 0.0012 | 17.317 | 0.347 | |
| 15-LO | PD1 | 1.761 | 61.11 | 100 | 0.0005 | Infinity | 0.389 | |
| 17-HDoHE | 4.966 | 66.67 | 96.15 | 0.0002 | 17.317 | 0.347 | ||
| 20-HDoHE | 53.49 | 66.67 | 100 | 0.0016 | Infinity | 0.333 |
AA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; COX, cyclooxygenase; LO, lipoxygenase; PG, prostaglandin; HETE, hydroxyeicosatetraenoic acid; EET, epoxyeicosatrienoic acids; ETE, eicosatetraenoic acid; HDoHE, hydroxydocosahexaenoic acid; HEPE, hydroxyeicosapentaenoic acid; LT, leukotriene; LX, lipoxin; PD1, protectin D1; P450, cytochrome p450.
DISCUSSION
In this study, we demonstrated that the activation of both inflammation and resolution pathways of LMs might represent a fatty acid signature in the SF of patients with severe RA. The upregulation of the COX and 5-LO pathways of AA in patients with RA is well known. Using LC-MS/MS, Giera et al. [7] identified several SPMs, including MaR1, LXA4, and RvD5, in SF from patients with RA. Both LXA4 and 15-epi-LXA4 reportedly showed significantly higher levels in SF from patients with RA (10.34 ± 14.12 ng/mL for LXA4) than in SF from patients with OA (0.66 ± 0.77 ng/mL for LXA4) using an enzyme-linked immunosorbent assay [13]. On the other hand, Jónasdóttir et al. [8] reported that none of the SPMs were detected in any of the SF obtained from patients with RA or OA. Herein, as a result of comprehensive LM profiling using an LC-MS/MS-based lipidomics approach, we demonstrated, for the first time, the significantly increased productions of various SPMs including LXA4, LXB4, and PD1 in SF from patients with severe RA, compared with SF from patients with severe OA. We confirmed the findings by Hashimoto et al. [13], which showed that the level of LXA4 was significantly higher in RA SF than in OA SF. Thus, despite the continued inflammation in the synovium, the active process of resolving inflammation via anti-inflammatory and pro-resolving mediators was concomitantly upregulated, probably as a counter-regulatory event. These alterations in the oxidized fatty acid signature might contribute to the pathogenesis of severe RA. We also showed that inflammatory mediators such as 5-HETE, 12-HETE, and 8,9-EET and anti-inflammatory and pro-resolving mediators such as LXA4, PD1, 12-HEPE, 4-HDoHE, and 17-HDoHE in SF could be good biomarkers for differentiating severe RA and severe OA.
The concentrations of 19 lipid mediators in SF from patients with RA were significantly higher than the concentrations in SF from patients with OA (Fig. 1B, 2B, and 3B), and among 19 mediators, 17 lipid mediators showed significant specificities to RA, compared with OA, based on an ROC curve analysis (Table 3). Among these 17 mediators, 5-HETE, LTB4, and 12-HETE are neutrophil-chemoattractants and/or activators [17,18,19]. In addition, 8,9-EET enhances paramethoxyamphetamine-induced neutrophil adhesion to endothelial cells [20]. On the other hand, LXA4 and LXB4 inhibit neutrophil transmigration and adherence [21]. PD1 enhances phagocyte removal during acute inflammation by regulating leukocyte infiltration, increasing the macrophage ingestion of apoptotic neutrophils [22]. Neutrophils are one of the cell sources of 5-HETE, LTB4, 12-HETE, LXA4, and LXB4. Thus, these lipid mediators may partially reflect neutrophil activation levels in SF from patients with RA. PGF2α enhances chondrogenic differentiation and hyaline cartilage matrix deposition by expanded human articular chondrocytes [23]. LXA4 inhibits IL-1β-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances the synthesis of tissue inhibitors of metalloproteinases [24]. Therefore, these lipid mediators may reflect various inflammatory statuses during the pathogenesis of RA.
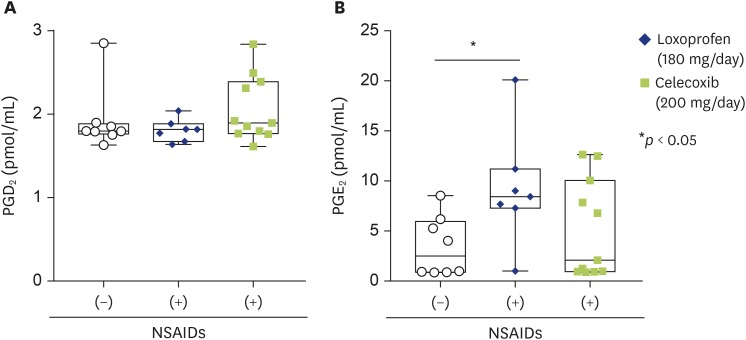
This study has some limitations. First, the samples used in this study were restricted to those obtained from hospitalized patients who had undergone total knee replacement surgery. Thus, the samples were limited to enrollees with severe clinical illnesses, hence limiting the external validity of the results to broader samples of patients with milder clinical illnesses. Second, this was a hypothesis-generating study, and the findings must be confirmed in a different population. Third, the majority of patients with OA, i.e., 18 out of 26 patients with OA, had been treated with NSAIDs. Thus, the significant difference in the COX-1/2 products of ω6 and ω3 PUFAs could be explained by the inhibitory effect of NSAIDs on the activity of COX-1 and COX-2 in patients with OA. However, we found that the concentrations of PGE2, PGD2, and PGE2 in SF from OA patients who had been treated with loxoprofen (180 mg/day, n = 7) or celecoxib (200 mg/day, n = 11) were not significantly lower than those from patients with OA who had not been treated with these NSAIDs (n = 8; Fig. 4A, B). Therefore, the treatment of RA patients with NSAIDs might not profoundly affect the concentrations of COX products in SF. Despite the use of a wide range of antirheumatic drugs including methotrexate, oral glucocorticoids, anti-TNF therapy, and B-cell-depleting agents, which potently suppress specific inflammation, AA metabolites in the COX and 5-LO pathways reportedly remain overproduced in inflamed tissue in patients with RA [14,15,25]. Although the possibility that these antirheumatic drugs might affect the profile of LMs cannot be ruled out, among patients treated with multiple antirheumatics, the activation of both inflammatory and resolution pathways of LMs was observed in SF from patients with severe RA.
Fig. 4. Comparison of PGD2 (A) and PGE2 concentrations in synovial fluid from patients with OA who had been treated with (+) or without (-) nonsteroidal anti-inflammatory drugs (NSAIDs). ○ indicates patients treated without NSAIDs (n = 8). ◆ and ■ indicate patients treated with loxoprofen (180 mg/day, n = 7) and celecoxib (200 mg/day, n = 11), respectively. PG, prostaglandins. The data are shown as a box-plot. *p < 0.05.
In conclusion, the profiles of LMs in SF from patients with severe RA were quite different from those in SF from patients with severe OA. The hyperproduction of LMs involved in inflammatory pathways, which are not suppressed by the upregulated resolving pathways of LMs, might lead to disease relapse.
ACKNOWLEDGEMENTS
This work was supported in part by Grants-in-Aid for Scientific Research from the Nihon University Multidisciplinary Research Grant for 2018-2019 (Project No. So18-009, awarded to Y.O.), the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2015-2019 (Project No. S1511014, awarded to Y.O.), and the Practical Research Project for Allergic Diseases and Immunology 19ek0410052 and FORCE 19gm4010005 (to M.M.) from the Japan Agency for Medical Research and Development (AMED).
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Yoshimichi Okayama.
- Data curation: Yutaka Sano, Shota Toyoshima, Yoshimi Miki, Mana Ito, Hyunho Lee.
- Formal analysis: Yutaka Sano, Shota Toyoshima, Yoshimi Miki.
- Funding acquisition: Yoshimichi Okayama, Makoto Murakami.
- Methodology: Yoshimi Miki, Yoshitaka Taketomi, Makoto Murakami.
- Project administration: Yoshimichi Okayama, Makoto Murakami, Shu Saito.
- Visualization: Yutaka Sano, Shota Toyoshima, Yoshimi Miki.
- Writing - original draft: Yoshimichi Okayama, Makoto Murakami.
- Writing - review & editing: Yoshimichi Okayama, Makoto Murakami.
SUPPLEMENTARY MATERIALS
Supplementary Figures 1–3 can be found via https://www.apallergy.org/src/sm/apallergy-10-e21-s001.pdf.
Concentrations (AUC/mL) of lipid mediators derived from COX-1/2 and 5-LO (A) and 12/15-LO and P450 products (B) of AA from patients with rheumatoid arthritis (RA) or osteoarthritis (OA). Each dot represents one patient. The median values are shown. Groups were compared using the Mann-Whitney U test. p values are shown. AA, arachidonic acid; AUC, area under the curve; COX, cyclooxygenase; DHETE, dihydroxyeicosatrienoic acid; DiHETE, dihydroxyeicosatetraenoic acid; EET, epoxyeicosatrienoic acids; ETE, eicosatetraenoic acid; HETE, hydroxyeicosatetraenoic acid; HHT, hydroxyheptadecatrienoic acid; HpETE, hydroperoxyeicosatetraenoic acid; LO, lipoxygenase; LT, leukotriene; LX, lipoxin; P450, cytochrome p450; PG, prostaglandin; Tx, thromboxane.
Concentrations (AUC/mL) of lipid mediators derived from EPA from patients with rheumatoid arthritis (RA) or osteoarthritis (OA). Each dot represents one patient. The median values are shown. Groups were compared using the Mann-Whitney U test. p values are shown. AUC, area under the curve; COX, cyclooxygenase; EPA, eicosapentaenoic acid; DiHETE, dihydroxyeicosatetraenoic acid; HEPE, hydroxyeicosapentaenoic acid; HpEPE, hydroperoxyeicosapentaenoic acid, LO, lipoxygenase; LT, leukotriene; LX, lipoxin; P450, cytochrome p450; PG, prostaglandin; Rv, resolvin; Tx, thromboxane.
Concentrations (AUC/mL) of lipid mediators derived from DHA from patients with RA or OA. Each dot represents one patient. The median values are shown. Groups were compared using the Mann-Whitney U test. p values are shown. AUC, area under the curve; DHA, docosahexaenoic acid; HDoHE, hydroxydocosahexaenoic acid; HpDHA, hydroperoxydocosahexaenoic acid; LO, lipoxygenase; MaR1, maresin 1; PD1, protectin D1; Rv, resolvin.
References
- 1.Brouwers H, von Hegedus J, Toes R, Kloppenburg M, Ioan-Facsinay A. Lipid mediators of inflammation in rheumatoid arthritis and osteoarthritis. Best Pract Res Clin Rheumatol. 2015;29:741–755. doi: 10.1016/j.berh.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Korotkova M, Jakobsson PJ. Persisting eicosanoid pathways in rheumatic diseases. Nat Rev Rheumatol. 2014;10:229–241. doi: 10.1038/nrrheum.2014.1. [DOI] [PubMed] [Google Scholar]
- 3.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, Lee DM. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med. 2006;203:837–842. doi: 10.1084/jem.20052371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiktorowska-Owczarek A, Berezińska M, Nowak JZ. PUFAs: structures, metabolism and functions. Adv Clin Exp Med. 2015;24:931–941. doi: 10.17219/acem/31243. [DOI] [PubMed] [Google Scholar]
- 6.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giera M, Ioan-Facsinay A, Toes R, Gao F, Dalli J, Deelder AM, Serhan CN, Mayboroda OA. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC-MS/MS. Biochim Biophys Acta. 2012;1821:1415–1424. doi: 10.1016/j.bbalip.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jónasdóttir HS, Brouwers H, Kwekkeboom JC, van der Linden HM, Huizinga T, Kloppenburg M, Toes RE, Giera M, Ioan-Facsinay A. Targeted lipidomics reveals activation of resolution pathways in knee osteoarthritis in humans. Osteoarthritis Cartilage. 2017;25:1150–1160. doi: 10.1016/j.joca.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Mustonen AM, Käkelä R, Lehenkari P, Huhtakangas J, Turunen S, Joukainen A, Kääriäinen T, Paakkonen T, Kröger H, Nieminen P. Distinct fatty acid signatures in infrapatellar fat pad and synovial fluid of patients with osteoarthritis versus rheumatoid arthritis. Arthritis Res Ther. 2019;21:124. doi: 10.1186/s13075-019-1914-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murase R, Taketomi Y, Miki Y, Nishito Y, Saito M, Fukami K, Yamamoto K, Murakami M. Group III phospholipase A2 promotes colitis and colorectal cancer. Sci Rep. 2017;7:12261. doi: 10.1038/s41598-017-12434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto K, Miki Y, Sato H, Murase R, Taketomi Y, Murakami M. Secreted phospholipase A2 specificity on natural membrane phospholipids. Methods Enzymol. 2017;583:101–117. doi: 10.1016/bs.mie.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto A, Hayashi I, Murakami Y, Sato Y, Kitasato H, Matsushita R, Iizuka N, Urabe K, Itoman M, Hirohata S, Endo H. Antiinflammatory mediator lipoxin A4 and its receptor in synovitis of patients with rheumatoid arthritis. J Rheumatol. 2007;34:2144–2153. [PubMed] [Google Scholar]
- 14.Korotkova M, Westman M, Gheorghe KR, af Klint E, Trollmo C, Ulfgren AK, Klareskog L, Jakobsson PJ. Effects of antirheumatic treatments on the prostaglandin E2 biosynthetic pathway. Arthritis Rheum. 2005;52:3439–3447. doi: 10.1002/art.21390. [DOI] [PubMed] [Google Scholar]
- 15.Korotkova M, Helmers SB, Loell I, Alexanderson H, Grundtman C, Dorph C, Lundberg IE, Jakobsson PJ. Effects of immunosuppressive treatment on microsomal prostaglandin E synthase 1 and cyclooxygenases expression in muscle tissue of patients with polymyositis or dermatomyositis. Ann Rheum Dis. 2008;67:1596–1602. doi: 10.1136/ard.2007.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jónasdóttir HS, Nicolardi S, Jonker W, Derks R, Palmblad M, Ioan-Facsinay A, Toes R, van der Burgt YE, Deelder AM, Mayboroda OA, Giera M. Detection and structural elucidation of esterified oxylipids in human synovial fluid by electrospray ionization-fourier transform ion-cyclotron mass spectrometry and liquid chromatography-ion trap-MS(3): detection of esterified hydroxylated docosapentaenoic acid containing phospholipids. Anal Chem. 2013;85:6003–6010. doi: 10.1021/ac400826z. [DOI] [PubMed] [Google Scholar]
- 17.Goetzl EJ, Sun FF. Generation of unique mono-hydroxy-eicosatetraenoic acids from arachidonic acid by human neutrophils. J Exp Med. 1979;150:406–411. doi: 10.1084/jem.150.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harvath L. Neutrophil chemotactic factors. EXS. 1991;59:35–52. doi: 10.1007/978-3-0348-7494-6_3. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham FM, Woollard PM. 12(R)-hydroxy-5,8,10,14-eicosatetraenoic acid is a chemoattractant for human polymorphonuclear leucocytes in vitro. Prostaglandins. 1987;34:71–78. doi: 10.1016/0090-6980(87)90264-4. [DOI] [PubMed] [Google Scholar]
- 20.Pratt PF, Rosolowsky M, Campbell WB. Effects of epoxyeicosatrienoic acids on polymorphonuclear leukocyte function. Life Sci. 2002;70:2521–2533. doi: 10.1016/s0024-3205(02)01533-3. [DOI] [PubMed] [Google Scholar]
- 21.Lee TH, Horton CE, Kyan-Aung U, Haskard D, Crea AE, Spur BW. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin Sci (Lond) 1989;77:195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- 22.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakob M, Démarteau O, Suetterlin R, Heberer M, Martin I. Chondrogenesis of expanded adult human articular chondrocytes is enhanced by specific prostaglandins. Rheumatology (Oxford) 2004;43:852–857. doi: 10.1093/rheumatology/keh197. [DOI] [PubMed] [Google Scholar]
- 24.Sodin-Semrl S, Taddeo B, Tseng D, Varga J, Fiore S. Lipoxin A4 inhibits IL-1 beta-induced IL-6, IL-8, and matrix metalloproteinase-3 production in human synovial fibroblasts and enhances synthesis of tissue inhibitors of metalloproteinases. J Immunol. 2000;164:2660–2666. doi: 10.4049/jimmunol.164.5.2660. [DOI] [PubMed] [Google Scholar]
- 25.Gheorghe KR, Thurlings RM, Westman M, Boumans MJ, Malmström V, Trollmo C, Korotkova M, Jakobsson PJ, Tak PP. Prostaglandin E2 synthesizing enzymes in rheumatoid arthritis B cells and the effects of B cell depleting therapy on enzyme expression. PLoS One. 2011;6:e16378. doi: 10.1371/journal.pone.0016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Concentrations (AUC/mL) of lipid mediators derived from COX-1/2 and 5-LO (A) and 12/15-LO and P450 products (B) of AA from patients with rheumatoid arthritis (RA) or osteoarthritis (OA). Each dot represents one patient. The median values are shown. Groups were compared using the Mann-Whitney U test. p values are shown. AA, arachidonic acid; AUC, area under the curve; COX, cyclooxygenase; DHETE, dihydroxyeicosatrienoic acid; DiHETE, dihydroxyeicosatetraenoic acid; EET, epoxyeicosatrienoic acids; ETE, eicosatetraenoic acid; HETE, hydroxyeicosatetraenoic acid; HHT, hydroxyheptadecatrienoic acid; HpETE, hydroperoxyeicosatetraenoic acid; LO, lipoxygenase; LT, leukotriene; LX, lipoxin; P450, cytochrome p450; PG, prostaglandin; Tx, thromboxane.
Concentrations (AUC/mL) of lipid mediators derived from EPA from patients with rheumatoid arthritis (RA) or osteoarthritis (OA). Each dot represents one patient. The median values are shown. Groups were compared using the Mann-Whitney U test. p values are shown. AUC, area under the curve; COX, cyclooxygenase; EPA, eicosapentaenoic acid; DiHETE, dihydroxyeicosatetraenoic acid; HEPE, hydroxyeicosapentaenoic acid; HpEPE, hydroperoxyeicosapentaenoic acid, LO, lipoxygenase; LT, leukotriene; LX, lipoxin; P450, cytochrome p450; PG, prostaglandin; Rv, resolvin; Tx, thromboxane.
Concentrations (AUC/mL) of lipid mediators derived from DHA from patients with RA or OA. Each dot represents one patient. The median values are shown. Groups were compared using the Mann-Whitney U test. p values are shown. AUC, area under the curve; DHA, docosahexaenoic acid; HDoHE, hydroxydocosahexaenoic acid; HpDHA, hydroperoxydocosahexaenoic acid; LO, lipoxygenase; MaR1, maresin 1; PD1, protectin D1; Rv, resolvin.