Abstract
Real-world experience with mepolizumab for pediatric asthma is still limited. We report 3 patients who were treated with mepolizumab for severe adolescent asthma. Two patients, a 12-year-old boy and a 14-year-old girl, responded well to mepolizumab and showed apparent improvement in lung function from a downward trend over time before treatment. The third patient, a 16-year-old boy, whose treatment was switched from omalizumab to mepolizumab, did not have satisfactory response. The 2 successful cases had eosinophil counts of 440 and 371/μL and multiple comorbid allergic diseases including food allergies. The clinical benefit to them included elimination of both exacerbation and exercise-induced asthma. Interestingly, the boy's food-induced gastrointestinal symptoms disappeared following start of mepolizumab treatment.
Keywords: Severe asthma, Adolescent, Mepolizumab, Interleukin-5, Eosinophilic inflammation, Eosinophil count
INTRODUCTION
Mepolizumab, a humanized monoclonal antibody against interleukin-5, is approved for severe eosinophilic asthma. Although accumulating evidence indicates that mepolizumab reduces exacerbation and improves asthma control in adults [1,2], clinical experience in children remains limited [3]. Reduced lung function growth in children with asthma can increase the risk of chronic obstructive pulmonary disease in adulthood [4,5]. Thus, we report 3 adolescent cases who were treated with mepolizumab for uncontrolled asthma and then analyzed for lung function changes.
CASE REPORTS
Written informed consent for publication of clinical data was obtained from the patients.
Case 1
A 12-year-old boy had suffered from asthma and been treated with inhaled corticosteroid (ICS) since 1 year of age. He had multiple comorbid allergic diseases, including atopic dermatitis, allergy to multiple foods and allergic rhinitis. To alleviate the burden of his multiple-elimination diet, oral immunotherapy (OIT) for milk and egg allergies was performed, resulting in desensitization to a medium amount of milk and hard-boiled egg. However, he nevertheless sometimes complained of abdominal pain after eating hard-boiled eggs.
His asthma had been well-controlled with low-dose ICS until his 10th birthday. However, after he joined a baseball team, frequent exercise-induced asthma (EIA) badly impaired his quality of life and necessitated ICS dose escalation. At age 11, he was hospitalized due to severe exacerbation of asthma, and 500 μg of fluticasone plus long-acting ß2 agonist (LABA) were initiated. Despite the high-dose ICS, however, he continued to suffer from severe EIA, and he had to give up practice with his baseball team. Mepolizumab was then started to control his problems based on his laboratory test results (Table 1), such as high serum IgE, above the range for omalizumab, and high eosinophils.
Table 1. Laboratory data at initiation of mepolizumab.
| Variable | Case 1 | Case 2 | Case 3 | |
|---|---|---|---|---|
| WBC (/μL) | 5,860 | 7,400 | 5,260 | |
| Eos (%) | 7.5 | 5.8 | 4.8 | |
| Eos (/μL) | 440 | 371 | 252 | |
| Total IgE (IU/mL) | 1,770 | 948 | - | |
| Specific IgE (UA/mL) | ||||
| House dust mite | 211 | 57.8 | 29.4 | |
| Japanese cedar pollen | 325 | 12.3 | 7.47 | |
| Dog dander | - | 24.2 | 0.1 | |
| Cat dander | - | - | 0.1 | |
| Egg white | 0.87 | 5.56 | - | |
| Ovomucoid | 0.14 | 0.12 | - | |
| Milk | 0.16 | 5.01 | - | |
| Casein | 0.25 | 5.56 | - | |
| %FEV1 | 92.6 | 96.2 | 85.8 | |
| %FEF50 | 88.4 | 66.5 | 57 | |
| FeNO (ppb) | 31 | 64 | 10 | |
WBC, white blood cell count; Eos, eosinophils; %FEV1, % of predicted forced expiratory volume in 1 second; %FEF50, % of predicted forced expiratory flow at 50% of forced lung capacity; FeNO, fractional exhaled nitric oxide.
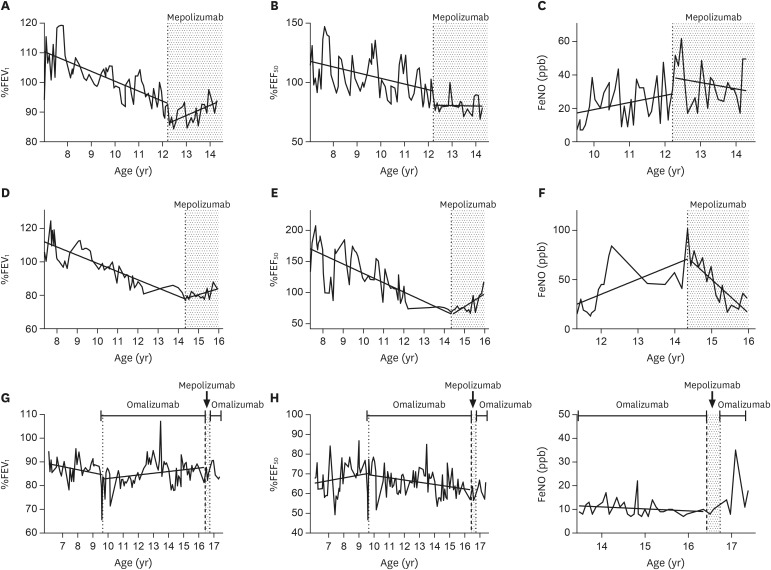
After the first dose of mepolizumab, his EIA was alleviated and the Childhood Asthma Control Test (C-ACT) score [6] improved from 12 to 19. After the second dose the EIA disappeared, and he was able to fully participate in baseball with C-ACT score of 27. He experienced no exacerbation of asthma for 2 years after starting mepolizumab. The abdominal pain that often followed egg ingestion also disappeared, and he became able to consume even uncooked eggs, which are more allergenic than boiled eggs. As expected, his eosinophil count decreased drastically, but serum IgE was unchanged after starting mepolizumab (Table 2). His lung function had been declining before mepolizumab treatment but improved after mepolizumab (Fig. 1A, B). Fractional exhaled nitric oxide (FeNO) had been increasing before mepolizumab, but tended to decrease after mepolizumab (Fig. 1C).
Table 2. Serum IgE and eosinophil count before and after 2 months of mepolizumab treatment.
| Case | IgE & Eos | Pre | 2 Months |
|---|---|---|---|
| 1 | IgE (IU/mL) | 1,770 | 1,815 |
| Eos (/μL) | 440 | 30 | |
| 2 | IgE (IU/mL) | 948 | 1,070 |
| Eos (/μL) | 371 | 50 | |
| 3 | IgE (IU/mL) | - | - |
| Eos (/μL) | 252 | 39 |
IgE was not measured in case 3 because of possible influence of omalizumab.
Eos, eosinophils.
Fig. 1. Linear regression analysis was performed for the measurements of %FEV1 (A, D), %FEF50 (B, E), and FeNO (C, F, I) before and during mepolizumab treatment. For Case 3, the analysis was performed for the measurements (G, H, I) before and during omalizumab treatment. Fitted lines by regression are depicted. %FEV1, % of predicted forced expiratory volume in 1 second; %FEF50, % of predicted forced expiratory flow at 50% of forced lung capacity; FeNO, fractional exhaled nitric oxide.
Case 2
A 14-year-old girl, the older sister of case 1, had suffered from asthma and been treated with ICS since 2 years of age. She had atopic dermatitis, allergic rhinitis, and food allergies to egg, milk, wheat, soy and shrimp. OIT was performed for egg and milk allergies, but unsuccessfully. Her asthma became uncontrolled at 12 years of age, and it was often exacerbated by upper respiratory infections despite high-dose ICS plus LABA. EIA was also a problem, so she tried to prevent attacks by reducing her activity.
After starting mepolizumab treatment at age 14, she experienced no exacerbation for 2 years and EIA disappeared, allowing her to be active with C-ACT changed from 24 to 27. Her eosinophil count decreased, while her serum IgE levels were unchanged (Table 2). Declining trends of lung function (Fig. 1D, E) turned to elevation after mepolizumab treatment, and FeNO decreased (Fig. 1F).
Case 3
A 16-year-old boy who was diagnosed with asthma during infancy and had been treated with ICS from 5 years old. Despite step-up to high-dose ICS/LABA at age 7, he frequently had to be hospitalized due to exacerbations, and omalizumab was started. Asthma control improved. After omalizumab, % of predicted forced expiratory volume in 1 second (%FEV1); %FEV1 appeared to improve (Fig. 1G), but % of predicted forced expiratory flow at 50% of forced lung capacity (%FEF50) decreased (Fig. 1H). At age 16, his asthma again became uncontrolled due to viral respiratory infections, and the biologic treatment was switched from omalizumab to mepolizumab. However, 3 doses of mepolizumab did not improve the patient perception of asthma control despite a significant decrease in the eosinophil count (Table 2). Actually, Asthma Control Test (ACT) score [7] was 18 before mepolizumab improved to 22 after 1st, 2nd, and 3rd dose but he still had exercise-induced symptom and the level of control for him was not as good as he expected to the “new” medicine and he wanted to go back on omalizumab treatment. Changes in lung function and FeNO after mepolizumab were not calculated because of the short period of treatment (Fig. 1G, H, I).
DISCUSSION
We reported 3 adolescent cases of mepolizumab treatment, 2 with favorable responses and 1 who did not respond. Since we followed up their lung function for more than 7 years, especially for more than 2 years after mepolizumab in the successful cases, we were able to depict the lung function trajectories. We found that the %FEV1 had declined before use of any biologic in all 3 cases of severe asthma, and that it increased after mepolizumab in the 2 successful cases. In the unsuccessful case, omalizumab treatment changed the trend to elevation FEV1 although the %FEF50 continued to decline; however, we could not evaluate the trend because of the short period of mepolizumab treatment.
The effect of biologics on lung function in children and adolescents with severe asthma has not been well established. In 2 randomized controlled studies of omalizumab, FEV1 in the omalizumab arm was no better than with the placebo at 24 weeks [8] or 48 weeks [9]. Real-world studies of omalizumab reported that it improved FEV1 from the baseline at 24 weeks [10] and at 1 year [11], but not at 2 years [12]. For mepolizumab, because of the low prevalence of severe eosinophilic asthma in adolescents, a subgroup analysis of 4 phase II/III mepolizumab trials was recently reported [13]. In 34 adolescents recruited, there was a reduction in the annual rate of clinically significant exacerbations with no statistical difference due to the low number of the subjects. The effect of mepolizumab on lung function in adolescents has not been reported. Our observations suggest the potential of mepolizumab to improve lung function and warrant further study.
In addition to reduction in asthma exacerbations, we observed that mepolizumab significantly alleviated EIA in our 2 successful cases. The pathogenesis of exercise-induced bronchoconstriction [14] is complex, but eosinophilic airway inflammation is one of the important mechanisms underlying EIA [15]. In this context, antieosinophilic treatment may have been beneficial for controlling EIA. This effect is especially important for children who lead a physically active life and in whom EIA can be a significant burden compared with in adults.
Further, in case 1, food-induced abdominal pain was also alleviated by mepolizumab. Since mepolizumab reduced esophageal eosinophilic inflammation in patients with eosinophilic esophagitis [16], it may be a potential remedy for the disease [17]. Although we did not confirm that his gastrointestinal symptoms were due to eosinophilic inflammation, the efficacy of mepolizumab may support that hypothesis.
In case 3, mepolizumab actually improved asthma control with ACT score of 18 to 22, however, in spite of 3 doses of mepolizumab ACT score remained at 22 and the patient was not satisfied with the effect. Similar cases were reported, in which 3 adult patients with severe asthma attained favorable, but not perfect, responses to mepolizumab after significant reduction of peripheral blood eosinophils [18]. Then, they attained full control after switching to benralizumab that made eosinophil counts almost zero. In a segmental allergen challenge model in mild human asthma, mepolizumab inhibited allergen-induced eosinophilia in peripheral blood and bronchoalveolar lavage fluid, but activation markers of eosinophils were maintained [19]. These observations suggest that mepolizumab treatment may not be sufficient for patients who need complete control of eosinophil activation.
In conclusion, our findings suggest the importance of observation of the lung function trajectory and the potential efficacy of mepolizumab in treatment of adolescent asthma.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Takao Fujisawa, Mizuho Nagao.
- Data curation: Miyuki Hoshi, Mizuho Nagao.
- Formal analysis: Mizuho Nagao.
- Funding acquisition: Takao Fujisawa.
- Methodology: Takao Fujisawa, Mizuho Nagao.
- Project administration: Takao Fujisawa.
- Visualization: Miyuki Hoshi, Mizuho Nagao.
- Writing - original draft: Miyuki Hoshi.
- Writing - review & editing: Miyuki Hoshi, Mayumi Matsunaga, Kazutaka Nogami, Kana Hamada, Taiga Kobori, Keigo Kainuma, Mizuho Nagao, and Takao Fujisawa.
References
- 1.Yancey SW, Ortega HG, Keene ON, Mayer B, Gunsoy NB, Brightling CE, Bleecker ER, Haldar P, Pavord ID. Meta-analysis of asthma-related hospitalization in mepolizumab studies of severe eosinophilic asthma. J Allergy Clin Immunol. 2017;139:1167–1175.e2. doi: 10.1016/j.jaci.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Pavord ID. Mepolizumab, quality of life, and severe eosinophilic asthma. Lancet Respir Med. 2017;5:362–363. doi: 10.1016/S2213-2600(17)30132-7. [DOI] [PubMed] [Google Scholar]
- 3.Comberiati P, McCormack K, Malka-Rais J, Spahn JD. Proportion of severe asthma patients eligible for mepolizumab therapy by age and age of onset of asthma. J Allergy Clin Immunol Pract. 2019;7:2689–2696.e2. doi: 10.1016/j.jaip.2019.05.053. [DOI] [PubMed] [Google Scholar]
- 4.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, Wise RA, Szefler SJ, Sharma S, Kho AT, Cho MH, Croteau-Chonka DC, Castaldi PJ, Jain G, Sanyal A, Zhan Y, Lajoie BR, Dekker J, Stamatoyannopoulos J, Covar RA, Zeiger RS, Adkinson NF, Williams PV, Kelly HW, Grasemann H, Vonk JM, Koppelman GH, Postma DS, Raby BA, Houston I, Lu Q, Fuhlbrigge AL, Tantisira KG, Silverman EK, Tonascia J, Weiss ST, Strunk RC. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374:1842–1852. doi: 10.1056/NEJMoa1513737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, Bowatte G, Gurrin L, Johns DP, Thompson BR, Hamilton GS, Frith PA, James AL, Thomas PS, Jarvis D, Svanes C, Russell M, Morrison SC, Feather I, Allen KJ, Wood-Baker R, Hopper J, Giles GG, Abramson MJ, Walters EH, Matheson MC, Dharmage SC. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6:535–544. doi: 10.1016/S2213-2600(18)30100-0. [DOI] [PubMed] [Google Scholar]
- 6.Liu AH, Zeiger R, Sorkness C, Mahr T, Ostrom N, Burgess S, Rosenzweig JC, Manjunath R. Development and cross-sectional validation of the Childhood Asthma Control Test. J Allergy Clin Immunol. 2007;119:817–825. doi: 10.1016/j.jaci.2006.12.662. [DOI] [PubMed] [Google Scholar]
- 7.Thomas M, Kay S, Pike J, Williams A, Rosenzweig JR, Hillyer EV, Price D. The Asthma Control Test (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Prim Care Respir J. 2009;18:41–49. doi: 10.4104/pcrj.2009.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, Taylor AF, Rohane P. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab) Pediatrics. 2001;108:E36. doi: 10.1542/peds.108.2.e36. [DOI] [PubMed] [Google Scholar]
- 9.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic JA, Chmiel JF, Steinbach SF, Calatroni A, Togias A, Thompson KM, Szefler SJ, Sorkness CA. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–1015. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Odajima H, Ebisawa M, Nagakura T, Fujisawa T, Akasawa A, Ito K, Doi S, Yamaguchi K, Katsunuma T, Kurihara K, Kondo N, Sugai K, Nambu M, Hoshioka A, Yoshihara S, Sato N, Seko N, Nishima S. Omalizumab in Japanese children with severe allergic asthma uncontrolled with standard therapy. Allergol Int. 2015;64:364–370. doi: 10.1016/j.alit.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Deschildre A, Marguet C, Salleron J, Pin I, Rittié JL, Derelle J, Taam RA, Fayon M, Brouard J, Dubus JC, Siret D, Weiss L, Pouessel G, Beghin L, Just J. Add-on omalizumab in children with severe allergic asthma: a 1-year real life survey. Eur Respir J. 2013;42:1224–1233. doi: 10.1183/09031936.00149812. [DOI] [PubMed] [Google Scholar]
- 12.Deschildre A, Marguet C, Langlois C, Pin I, Rittié JL, Derelle J, Abou Taam R, Fayon M, Brouard J, Dubus JC, Siret D, Weiss L, Pouessel G, Beghin L, Just J. Real-life long-term omalizumab therapy in children with severe allergic asthma. Eur Respir J. 2015;46:856–859. doi: 10.1183/09031936.00008115. [DOI] [PubMed] [Google Scholar]
- 13.Yancey SW, Ortega HG, Keene ON, Bradford ES. Efficacy of add-on mepolizumab in adolescents with severe eosinophilic asthma. Allergy Asthma Clin Immunol. 2019;15:53. doi: 10.1186/s13223-019-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kippelen P, Anderson SD, Hallstrand TS. Mechanisms and Biomarkers of Exercise-Induced Bronchoconstriction. Immunol Allergy Clin North Am. 2018;38:165–182. doi: 10.1016/j.iac.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Duong M, Subbarao P, Adelroth E, Obminski G, Strinich T, Inman M, Pedersen S, O'Byrne PM. Sputum eosinophils and the response of exercise-induced bronchoconstriction to corticosteroid in asthma. Chest. 2008;133:404–411. doi: 10.1378/chest.07-2048. [DOI] [PubMed] [Google Scholar]
- 16.Assa'ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, Perschy TL, Jurgensen CH, Ortega HG, Aceves SS. An antibody against IL-5 reduces numbers of esophageal intraepithelial eosinophils in children with eosinophilic esophagitis. Gastroenterology. 2011;141:1593–1604. doi: 10.1053/j.gastro.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 17.Eskian M, Khorasanizadeh M, Assa'ad AH, Rezaei N. Monoclonal Antibodies for Treatment of Eosinophilic Esophagitis. Clin Rev Allergy Immunol. 2018;55:88–98. doi: 10.1007/s12016-017-8659-7. [DOI] [PubMed] [Google Scholar]
- 18.Matsuno O, Minamoto S. Eosinophils depletion therapy for severe asthma management following favorable response to mepolizumab. Respir Med Case Rep. 2019;28:100899. doi: 10.1016/j.rmcr.2019.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly EA, Esnault S, Liu LY, Evans MD, Johansson MW, Mathur S, Mosher DF, Denlinger LC, Jarjour NN. Mepolizumab attenuates airway eosinophil numbers, but not their functional phenotype, in asthma. Am J Respir Crit Care Med. 2017;196:1385–1395. doi: 10.1164/rccm.201611-2234OC. [DOI] [PMC free article] [PubMed] [Google Scholar]