Abstract
Background
Even in subjects who are not sensitized to house dust mite (HDM), allergic symptoms can be clinically aggravated by exposure to dust. We previously reported that Dermatophagoides farinae (Df), an important HDM, or Der f 1, a major allergen of Df, induced the effector functions of eosinophils, which may be an important mechanism for HDM-induced symptoms in nonsensitized patients. In a clinical setting, β2-adrenergic agonists, such as salbutamol and formoterol, are used for the treatment of asthma attacks or exacerbation to release the airway obstruction. Several reports have suggested that some β2-adrenergic agonists have an anti-inflammatory capacity.
Objective
In this study, we investigated whether β2-adrenergic agonist could modify the Df- or Der f 1-induced activation of eosinophils.
Methods
Blood eosinophils obtained from healthy donors were preincubated with either formoterol (1 μM), salbutamol (1 μM), or buffer control and then stimulated with Df extract (1 μg/mL) or Der f 1 (100 pg/mL). Eosinophil adhesion to intercellular adhesion molecule (ICAM)-1 was measured using eosinophil peroxidase assays. Generation of superoxide anion (O2−) was examined based on the superoxide dismutase-inhibitable reduction of cytochrome C. Eosinophil-derived neurotoxin (EDN) concentrations in cell media were measured by enzyme-linked immunosorbent assay.
Results
Formoterol, but not salbutamol, suppressed the Df- or Der f 1-induced eosinophil adhesion to ICAM-1. Furthermore, formoterol, but not salbutamol, suppressed Df-induced O2− generation or EDN release. Neither formoterol nor salbutamol suppressed spontaneous eosinophil adhesion, O2− generation, or EDN release.
Conclusion
These findings suggested that formoterol, but not salbutamol, suppressed Df- or Der f 1-induced eosinophil activation when used at the same concentration. Therefore, formoterol could potentially be used for the treatment of bronchial asthma via both bronchodilation and anti-inflammatory effect.
Keywords: Beta2-adrenergic agonists, Der f 1, Eosinophils, Formoterol, House dust mite
INTRODUCTION
Bronchial asthma is characterized by airway inflammation, reversible airway obstruction and airway hyperresponsiveness [1]. Although numerous cell types are involved in airway inflammation associate with asthma, eosinophils play an important role in asthma pathogenesis through the release of inflammatory mediators, such as specific granule proteins, reactive oxygen species, cysteinyl leukotrienes and cytokines [2].
Adaptive immune responses are involved in the development of allergies or allergic diseases such as bronchial asthma [3,4]. When patients are exposed to a specific allergen, dendritic cells present it to naïve T cells, and activating them as they polarize into T helper type 2 (Th2) cells [3]. Activated Th2 cells produce cytokines, such as interleukin (IL)-4 and IL-13, which promote the production of the allergen-specific immunoglobulin (Ig) E from B cells, and IL-5 that activates eosinophils. Among the allegens, house dust mite (HDM) is a significant cause of allergic disease including bronchial asthma [5]. Dermatophagoides farina (Df) and Dermatophagoides pteronyssinus (Dp) are important HDMs, and Der f 1 or Der p 1 is a major allegen of Df or Dp [5,6]. HDM play important role in the development of allergic sensitization and disease [7,8]. Furthermore, HDM exposure exacerbated allergic symptoms including asthma attacks in the case of sensitized patients.
However, innate immune responses also contribute to the development of allergic disease [4,5,9,10,11]. For example, the role of type 2 innate lymphoid cells (ILC2) in severe asthma has recently been established [9]. Furthermore, even in subjects who are not sensitized to HDM or pollen, allergic symptoms can be clinically induced or aggravated by exposure to HDM or pollen [10,11]. In this context, we reported that Df or Der f 1 directly activate the effector functions of eosinophils, such as adhesion, superoxide anion (O2-) generation and degranulation [12], which may be the important mechanisms for induction or aggravation of allergic symptoms in nonsensitized patients. Protease activated receptor-2 on the eosinophils is involved in Df or Der f 1-induced eosinophil activation [12].
In a clinical setting, β2-adrenergic agonists, especially short-acting β2 agonists (SABA) such as salbutamol, are used in the treatment of asthma attacks to release airway obstruction [13]. In some cases, formoterol, a long-acting β2 agonist (LABA), is used with budesonide, an inhaled glucocorticoid (ICS), as a maintenance and reliever therapy for preventing or treating asthma exacerbation [14]. In addition to their bronchodilation effect, some β2-adrenergic agonists exert anti-inflammatory effects [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. In this study, we investigated whether the β2-adrenergic agonist could modify Df- and Der f 1-induced eosinophilic activation.
MATERIALS AND METHODS
Preparation of eosinophils and Df
Eosinophils were isolated from peripheral blood from nonallergic healthy volunteers with a blood differential eosinophil count of <5%. Healthy volunteers were defined as subjects with no history of allergic disease, such as asthma or rhinitis, with no symptoms. The number of males and females was comparable among the donors. This study was approved by the Ethical Committee of Saitama Medical University (IRB number: 781-III), and written informed consent was obtained before collection of each blood sample. Eosinophils were isolated by negative selection using anti-CD16 Ab-coated magnetic beads (Miltenyi Biotec, Auburn, CA, USA), as previously described [12,27,28,29,30]. Over 98% of the cells were eosinophils, as determined by morphological criteria using May-Grünwald-Giemsa staining. Eosinophils were resuspended in Hanks' balanced salt solution (HBSS) supplemented with gelatin to a final concentration of 0.1% (HBSS/gel). Df was collected and a dried extract prepared as previously described [31]. Der f 1, purified from mite culture by affinity chromatography, was obtained from Indoor Biotechnologies Inc. (Charlottesville, VA, USA).
Eosinophil adhesion
The effect of formoterol or salbutamol on Df extract- or Der f 1-induced eosinophil adhesion to recombinant human (rh) intercellular adhesion molecule (ICAM) 1-coated plates was assessed based on the residual eosinophil peroxidase activity of adherent eosinophils, as previously described [12,27,28,29,30]. Briefly, eosinophils (100 µL of 1 × 105 cells/mL in HBSS/gel) from nonallergic volunteers were preincubated with formoterol (1 μM) or salbutamol (1 μM) at 37oC for 20 minutes. Next, the cells were incubated in rh-ICAM-1-coated plates (10 µg/mL; R&D Systems, Minneapolis, MN, USA) in the presence or absence of Df extract (1 µg/mL) or Der f 1 (100 pg/mL) at 37 oC for 20 minutes. Corresponding control wells were coated with HBSS/gel. The plates were washed with HBSS and 100 µL of HBSS/gel was then added to the wells. Standards comprised of 100 µL of serially diluted cell suspensions (1 × 103, 3 × 103, 1 × 104, 3 × 104, and 1 × 105 cells/mL) were added to the empty wells. The eosinophil peroxidase substrate (1 mM o-phenylenediamine, 1 mM H2O2, and 0.1% Triton X-100 in Tris buffer, pH 8.0) was then added to all wells, and the plates were incubated for 30 minutes at room temperature. The reaction was stopped by adding 20 µL of 4 M H2SO4, and absorbance was measured at 490 nm. Each experiment was performed in quadruplicate using eosinophils from a single donor, and the percentage eosinophil adhesion was determined from mean values that were calculated from log dose-response curves. Eosinophil viability after incubation was >98%, as determined by trypan blue dye exclusion.
Eosinophil O2− generation
Eosinophil O2− generation was measured in 96-well enzyme-linked immunosorbent assay (ELISA) plates by superoxide dismutase (SOD)-inhibitable reduction of cytochrome C [12,27,28,29,30]. We initially added SOD (0.2 mg/mL in HBSS/gel; 20 µL) to SOD control wells and then HBSS/gel to all of the wells of rh-ICAM-1-coated plates (10 µg/mL) to bring the final volume to 100 µL. Eosinophils were pretreated with formoterol (1 μM) or salbutamol (1 μM) for 20 minutes. The density of eosinophils was adjusted to 1.25 × 106 cells/mL of HBSS/gel mixed in a 4:1 ratio with cytochrome C (12 mg/mL of HBSS/gel), and 100 µL of eosinophil suspension was then added to all wells. Immediately following the addition of Df extract (1 µg/mL) to the eosinophils, the absorbance of the cell suspensions in the wells was measured at 550 nm (Immuno-Mini NJ-2300; Japan Intermed Co., Tokyo, Japan), followed by repeated measurements over the next 240 minutes (0, 10, 20, 30, 40, 50, 60, 90, 120, 150, 180, 210, 240 minutes). Each reaction was measured in duplicate and compared to control reactions in wells containing 20 µg/mL of SOD. The results were adjusted for a 1-mL reaction volume, and O2− generation was calculated using an extinction coefficient of 21.1/mM/cm, as nanomoles of cytochrome C reduced per 1.0 × 106 cells/mL minus the SOD control. The maximum value observed over the incubation period was determined to evaluate the effects of various factors on eosinophil O2− generation. Cell viability, as determined by trypan blue exclusion at the end of each experiment, remained at 95% after 240 minutes of incubation.
Eosinophil degranulation
Eosinophils (1 × 106 cells/mL) in 96-well plates were incubated for the 240 minutes that were required for the measurement of O2− generation before being immediately centrifuged (1,500 rpm) at 4oC for 10 minutes. Recovered cell-free supernatants were measured for eosinophil-derived neurotoxin (EDN), as described previously [12,27,28,29,30]. Concentrations of EDN were quantified by ELISA (Medical and Biological Laboratory Co. Ltd., Nagoya, Japan).
Statistical analysis
Values are expressed as means ± standard error of the mean. Results were compared using a 1-way analysis of variance followed by the Tukey-Kramer test when differences were significant, or a paired t test for analysis of the differences between the 2 groups. Values of p < 0.05 were considered statistically significant.
RESULTS
Effect of formoterol or salbutamol on Df extract-induced eosinophil adhesion
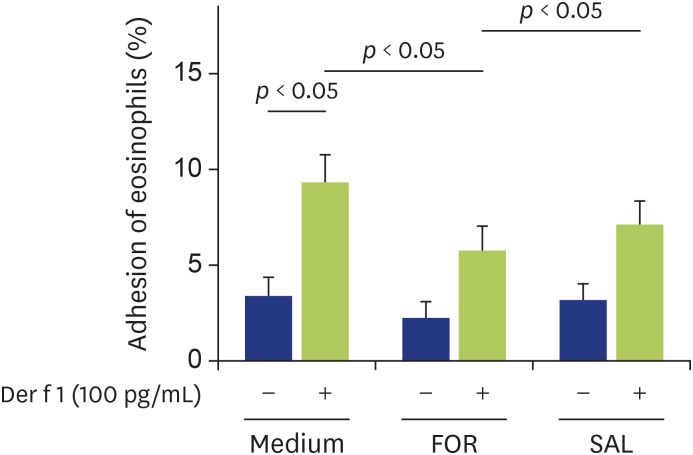
First, we examined whether formoterol or salbutamol could modify Df extract-induced eosinophil adhesion. Eosinophils were preincubated with formoterol (1 μM) or salbutamol (1 μM) for 20 minutes, stimulated with or without Df extract (1 µg/mL), and adhesion to rh-ICAM-1 was evaluated. We previously reported that Df extract at a concentration of 1 µg/mL enhanced the adhesion of eosinophils to ICAM-1 [12], which was also confirmed in this study (Fig. 1). We selected the concentration (1 μM) of formoterol or salbutamol in this study based on our previous findings [27,28]. Formoterol significantly suppressed Df extract-induced eosinophil adhesion to ICAM-1 (Fig. 1; medium [without formoterol or salbutamol] 9.7% ± 1.9%, formoterol 5.6% ± 2.1%) (p < 0.05). On the other hand, salbutamol did not modify the Df extract-induced eosinophil adhesion (Fig. 1; salbutamol 9.9% ± 4.1%; not significant). In the absence of Df extract, neither formoterol nor salbutamol suppressed eosinophil adhesion to ICAM-1 (Fig. 1).
Fig. 1. Effect of formoterol or salbutamol on Dermatophagoides farinae (Df) extract-induced eosinophil adhesion. Eosinophils from nonallergic volunteers were preincubated with or without formoterol (1 µM) or salbutamol (1 µM) for 20 min prior to analysis of adhesion to intercellular adhesion molecule-1 in the presence or absence of Df extract (1 µg/mL) (n = 7). FOR, formoterol; SAL, salbutamol.
Effect of formoterol or salbutamol on Der f 1-induced eosinophil adhesion
Next, we examined whether formoterol or salbutamol could modify Der f 1-induced eosinophil adhesion. Eosinophils were preincubated with formoterol (1 μM) or salbutamol (1 μM) for 20 minutes, stimulated with or without Der f 1 (100 pg/mL), and adhesion to rh-ICAM-1 was evaluated. We previously reported that Der f 1 at a concentration of 100 pg/mL enhanced the eosinophil adhesion to ICAM-1 [12]. Formoterol significantly suppressed Der f 1-induced eosinophil adhesion to ICAM-1 (Fig. 2; medium 8.8% ± 2.8%, formoterol 5.6% ± 2.4%) (p < 0.05), while salbutamol did not modify the Der f 1-induced eosinophil adhesion (salbutamol 7.1% ± 2.1%; not significant). In the absence of Der f 1, neither formoterol nor salbutamol suppressed eosinophil adhesion to ICAM-1 (Fig. 2).
Fig. 2. Effect of formoterol or salbutamol on Der f 1-induced eosinophil adhesion. Eosinophils from nonallergic volunteers were preincubated with or without formoterol (1 µM) or salbutamol (1 µM) for 20 minutes prior to analysis of adhesion to intercellular adhesion molecule-1 in the presence or absence of Der f 1 (100 pg/mL) (n = 7). FOR, formoterol; SAL, salbutamol.
Effect of formoterol or salbutamol on Df extract-induced O2− generation of eosinophils
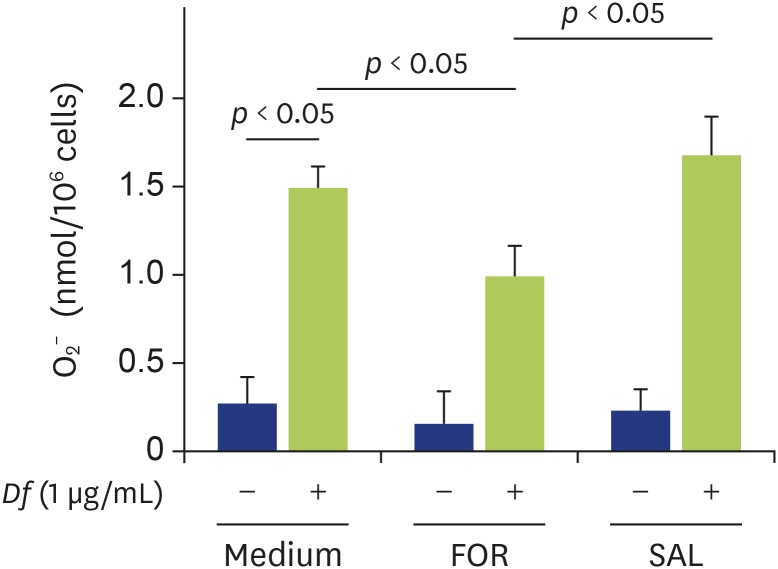
Next, we examined whether formoterol or salbutamol could modify Df extract-induced eosinophil O2− generation. We previously reported that Df extract at a concentration of 1 μg/mL induced eosinophil O2− generation in ICAM-1-coated plates [12], which we also confirmed in this study (Fig. 3). Formoterol significantly suppressed Df extract-induced eosinophil O2− generation (Fig. 3; medium 1.50 ± 0.17 nmol/million cells, formoterol 1.04 ± 0.26 nmol/million cells) (p < 0.05). On the other hand, salbutamol did not suppress the Df extract-induced eosinophil O2− generation (Fig. 3; salbutamol 1.70 ± 0.57 nmol/million cells; not significant).
Fig. 3. Effect of formoterol or salbutamol on Dermatophagoides farinae (Df) extract-induced eosinophil O2− generation. Eosinophils were preincubated with or without formoterol (1 µM) or salbutamol (1 µM) for 20 min prior to analysis of O2− generation of eosinophils in the presence or absence of Df extract (1 µg/mL) (n = 6). FOR, formoterol; SAL, salbutamol.
Effect of formoterol or salbutamol on Df extract-induced EDN release from eosinophils
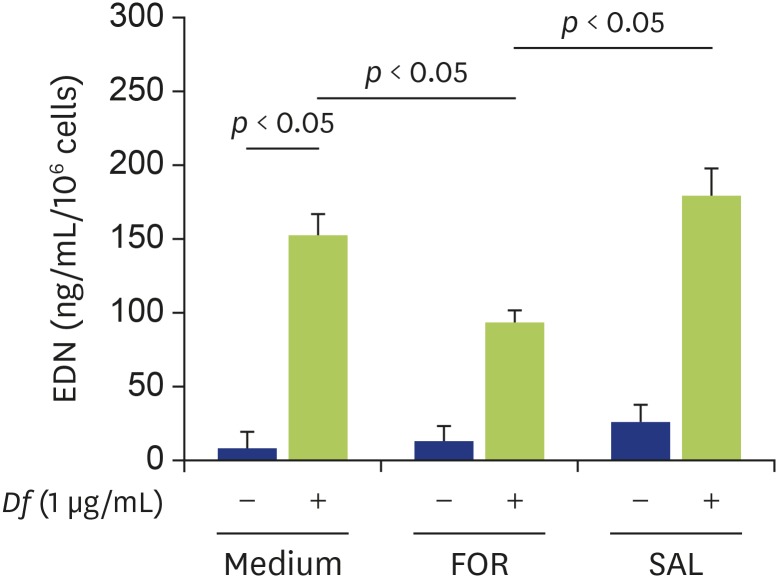
Finally, we examined whether formoterol or salbutamol could modify Df extract-induced eosinophil degranulation. We previously reported that Df extract at a concentration of 1 μg/mL induced EDN release from eosinophils in ICAM-1-coated plates [12], which was also confirmed in this study (Fig. 4). Formoterol, but not salbutamol, significantly suppressed the Df extract-induced EDN release from eosinophils (Fig. 4; medium 154.3 ± 24.0 ng/mL, formoterol 93.0 ± 11.1 ng/mL; p < 0.05, salbutamol 175.5 ± 34.3 ng/mL; not significant).
Fig. 4. Effect of formoterol or salbutamol on Dermatophagoides farinae (Df) extract-induced eosinophil eosinophil-derived neurotoxin (EDN) release. Eosinophils were preincubated with or without formoterol (1 µM) or salbutamol (1 µM) for 20 minutes prior to analysis of EDN release of eosinophils in the presence or absence of Df extract (1 µg/mL) (n = 6). FOR, formoterol; SAL, salbutamol.
DISCUSSION
In this study, we found that formoterol, but not salbutamol, suppressed Df- or Der f 1-induced eosinophil adhesion to ICAM-1 when used at the same concentration. We also found that formoterol suppressed Df-induced eosinophil O2− generation or EDN release in ICAM-1 coated plates. Since HDM exposure can induce or aggravate asthma symptoms, not only in HDM-sensitized patients, but also clinically in nonsensitized patients, the bronchodilation and such anti-inflammatory actions associate formoterol administration could be an effective strategy for asthma treatment.
In patients who, despite treatment with ICS, have persistent asthma symptoms, the addition of LABA to ICS is more useful than a higher dose of ICS alone [32]. The combination of ICS and LABA therapy (ICS/LABA) is now widely used for the treatment of moderate to severe asthma. Formoterol is a LABA that it is suited to the treatment of asthma or its exacerbation; for example, budesonide/formoterol (Symbicort, AstraZeneca, London, UK) maintenance and reliever therapy can be an important option for preventing or treating asthma exacerbation [14,33]. Furthermore, a recent study suggested that budesonide-formoterol used as needed for symptom relief is more effective at preventing severe exacerbations than maintenance low-dose budesonide plus as-needed terbutaline [34]. The Global Initiative for Asthma (2019) also recommends that ICS-formoterol reliever therapy be used as an alternative regimen to daily low-dose ICS in patients with mild asthma [13]. Thus, in addition to the bronchodilation effect associate with formoterol administration, it may have other benefits for the treatment of bronchial asthma.
Several studies have suggested that formoterol has anti-inflammatory properties in vivo [15,16,17,18], although some β2-adrenergic agonists, especially SABA, can increase eosinophilic airway inflammation and airway hyperresponsiveness [35,36,37]. For example, compared to budesonide alone, the addition of formoterol to budesonide suppressed the sputum eosinophil counts [15]. Formoterol suppressed neutrophilic airway inflammation in asthma [16], and formoterol, but not salbutamol, inhibited platelet activating factor (PAF)-induced eosinophil accumulation in the guinea-pig lung [17].
The presence of β2-adrenoreceptor receptors on inflammatory cells, such as mast cells, monocytes, neutrophils, eosinophils, T cells, and ILC2 [19,23,38], implies that formoterol may affect these cells through their β2-adrenergic receptors in vitro. As for eosinophils, Eda et al. [24] reported that formoterol concentrations greater than 1 μM inhibited PAF-induced eosinophil chemotaxis and degranulation. We previously reported that 1 µM formoterol, but not salbutamol, inhibited IL-5, leukotriene (LT) D4, or interferon-γ inducible protein of 10 kDa-induced eosinophil adhesiveness, O2− generation and EDN release [27]. Furthermore, 1 µM formoterol, but not salbutamol, suppressed the periostin-induced eosinophil adhesion, O2− generation and EDN release [28]. In this study, we also found that 1 µM formoterol, but not salbutamol, inhibited Df- or Der f 1-induced eosinophil adhesion to ICAM-1, Df-induced O2− generation or EDN release. Df has components that have exhibit serine protease activity, whereas Der f 1 has cysteine protease activity [5,6]. Therefore, formoterol may suppress both serine protease and cysteine protease induced eosinophil adhesion. Furthermore, 1 μM formoterol appears to down-modulate the overall effector functions of eosinophils.
The involvement of the β2-adrenergic receptor in β2 agonist-mediated suppression of eosinophil functioning has not been fully clarified. For example, Kainuma et al. [25] reported that procaterol suppressed integrin expression on human eosinophils (CD11b, CD18, CD49d, CD29) when cocultured with epithelial cells, as well as the eosinophil-induced epithelial-to-mesenchymal transition of bronchial epithelial cells. Butoxamine, a specific β2-adrenergic antagonist, restored the suppression of eosinophil functions by procaterol [25], suggesting the involvement of β2-adrenergic receptor dependent mechanisms in the case of procaterol. Tachibana et al. [26] reported that fenoterol suppressed phorbol myristate acetate (PMA)-induced eosinophil O2− generation. However, inhibition of PMA-induced O2− generation by fenoterol was not reversed by ICI-118551 [26], a selective β2-adrenoreceptor antagonist, suggesting the involvement of mechanism independent from β2-adrenergic receptor in the case of fenoterol. As for formoterol, Bowden et al. [17] reported that ICI-118551 restored the formoterol-induced suppression of eosinophil or neutrophil adhesion to rat trachea. Therefore, although we did not examine the effect of β2-adrenoreceptor antagonists in this study, we consider that formoterol suppresses the function of eosinophils through β2-adrenergic receptors.
The exact mechanisms by which formoterol, but not salbutamol, suppressed Df-induced eosinophil effector functions remain unknown. One possibility is that levels of cyclic 3',5'-adenosine monophosphate (cAMP) in eosinophils, which are increased after β2-adrenoreceptor stimulation, differed between formoterol and salbutamol, and that increased cAMP in eosinophils or neutrophils suppressed the functions of these cells. For example, Kita et al. [39] reported that a cAMP analogue and cAMP phosphodiesterase inhibitors suppressed immunoglobulin-induced human eosinophil degranulation. They also reported previously that cAMP levels were inversely correlated with eosinophil degranulation [39]. Kainuma et al. [25] reported that forskolin, a part of adenylate cyclase, synthesis cAMP from adenosine triphosphate and thus suppresses integrin expression on human eosinophils, or eosinophil-induced epithelial-to-mesenchymal transition of bronchial epithelial cells. As for neutrophils, formoterol increased cAMP levels more than salbutamol [21,22], and inhibited neutrophil functions, including O2− generation, LTB4 generation, and elastase release [21,22]. In fact, increased cAMP levels in neutrophils inhibited their adhesion [40]. Furthermore, in cultured rat cardiomyocytes, formoterol increased cAMP levels more than salbutamol [41]. Thus, formoterol is likely to increase cAMP levels in eosinophils more than salbutamol, and in so doing, can suppress eosinophil functions. Another possibility is that β2-adrenoreceptor-independent mechanisms may be involved in the suppression of Df-induced eosinophil effector functions by formoterol, as described above. Inhibition by β2-adrenergic agonists may include a component not mediated via the β2-adrenoreceptor.
A major limitation of this study is that all of the experiments were performed in vitro study. These agonists could have different concentration-response curve and receptor binding capacities. We did in vitro study with same concentration of these agonists. Therefore, it is difficult to conclude the general salbutamol invalidity, especially in the clinical setting. Furthermore, there is currently little information about formoterol concentrations in plasma or airways. For example, Campestrini et al. [42] reported that the plasma concentration of formoterol reached approximately about 300 pM after inhalation of 90 μg of formoterol. Gravett et al. [20] speculated that the local concentration of formoterol is approximately 10 nM if a maximal single dose of 24 μg, lung deposition of 18.6%, and a tidal volume of 500 mL is assumed. Although the actual concentration in the airway is difficult to estimate, concentrations greater than 1 μM at site of inflammation appear to be difficult to reach after β2-adrenoreceptor agonists treatment even during asthma attack. Another limitation is that we did not investigate the role of β2-adrenoreceptors in the suppression of eosinophil functions by formoterol. For example, we did not use selective β2-adrenoreceptor antagonists such as ICI-11855110 in this study, so whether the β2-adrenoreceptor-dependent or -independent pathway is involved in the suppression of Df-induced eosinophil activation by formoterol remains unknown.
In conclusion, we found that formoterol, but not salbutamol, suppressed Df- and Der f1-induced eosinophil adhesion to ICAM-1 when used at the same concentration. Furthermore, formoterol, but not salbutamol, suppressed Df-induced O2− generation or EDN release. Therefore, formoterol may have both bronchodilation and anti-inflammatory effects, which can contribute to the treatment of bronchial asthma.
ACKNOWLEDGEMENTS
We would like to thank Ms. Akemi Yokote for her excellent technical assistance. This study was funded by a grant from the Ministry of Education, Culture, Sports, Science and Technology (15K09228).
Footnotes
Conflict of Interest: KN received honoraria from AstraZeneca. KOD is employee of Torii Pharmaceutical Co., Ltd. MN received honoraria from AstraZeneca and KYORIN Pharmaceutical Co., Ltd. The other authors have no conflicts of interest.
- Conceptualization: Kazuyuki Nakagome, Makoto Nagata.
- Data curation: Yutaka Ueda, Kazuyuki Nakagome.
- Formal analysis: Yutaka Ueda, Kazuyuki Nakagome.
- Funding acquisition: Kazuyuki Nakagome, Makoto Nagata.
- Methodology: Takehito Kobayashi, Toru Noguchi, Katsuyo Ohashi-Doi.
- Project administration: Kazuyuki Nakagome, Makoto Nagata.
- Visualization: Tomoyuki Soma, Kenichi Tokuyama.
- Writing - original draft: Yutaka Ueda, Kazuyuki Nakagome.
- Writing - review & editing: Kazuyuki Nakagome, Makoto Nagata.
References
- 1.Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Annu Rev Immunol. 1994;12:295–335. doi: 10.1146/annurev.iy.12.040194.001455. [DOI] [PubMed] [Google Scholar]
- 2.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–663. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 3.Lambrecht BN. Dendritic cells and the regulation of the allergic immune response. Allergy. 2005;60:271–282. doi: 10.1111/j.1398-9995.2005.00708.x. [DOI] [PubMed] [Google Scholar]
- 4.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–683. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 5.Jacquet A. The role of innate immunity activation in house dust mite allergy. Trends Mol Med. 2011;17:604–611. doi: 10.1016/j.molmed.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Holck A, Dale S, Sletten K. Purification and characterization of a major allergen from the house dust mite Dermatophagoides farinae. Allergy. 1986;41:408–417. doi: 10.1111/j.1398-9995.1986.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 7.Calderón MA, Linneberg A, Kleine-Tebbe J, De Blay F, Hernandez Fernandez de Rojas D, Virchow JC, Demoly P. Respiratory allergy caused by house dust mites: what do we really know? J Allergy Clin Immunol. 2015;136:38–48. doi: 10.1016/j.jaci.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Platts-Mills TA, Vervloet D, Thomas WR, Aalberse RC, Chapman MD. Indoor allergens and asthma: report of the Third International Workshop. J Allergy Clin Immunol. 1997;100(6 Pt 1):S2–24. doi: 10.1016/s0091-6749(97)70292-6. [DOI] [PubMed] [Google Scholar]
- 9.Smith SG, Chen R, Kjarsgaard M, Huang C, Oliveria JP, O'Byrne PM, Gauvreau GM, Boulet LP, Lemiere C, Martin J, Nair P, Sehmi R. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75–86.e8. doi: 10.1016/j.jaci.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 10.Andersen I, Mølhave L, Proctor DF. Human response to controlled levels of combinations of sulfur dioxide and inert dust. Scand J Work Environ Health. 1981;7:1–7. doi: 10.5271/sjweh.2570. [DOI] [PubMed] [Google Scholar]
- 11.Andersen I, Lundqvist GR, Proctor DF, Swift DL. Human response to controlled levels of inert dust. Am Rev Respir Dis. 1979;119:619–627. doi: 10.1164/arrd.1979.119.4.619. [DOI] [PubMed] [Google Scholar]
- 12.Ueda Y, Nakagome K, Kobayashi T, Noguchi T, Soma T, Ohashi-Doi K, Tokuyama K, Nagata M. Dermatophagoides farinae upregulates the effector functions of eosinophils through αMβ2-integrin and protease-activated receptor-2. Int Arch Allergy Immunol. 2019;178:295–306. doi: 10.1159/000495008. [DOI] [PubMed] [Google Scholar]
- 13.Global Initiative for Asthma (GINA) The global initiative for asthma. GINA report, global strategy for asthma management and prevention. Global Initiative for Asthma; 2019. [Google Scholar]
- 14.O'Byrne PM, Bisgaard H, Godard PP, Pistolesi M, Palmqvist M, Zhu Y, Ekström T, Bateman ED. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171:129–136. doi: 10.1164/rccm.200407-884OC. [DOI] [PubMed] [Google Scholar]
- 15.Kelly MM, O'Connor TM, Leigh R, Otis J, Gwozd C, Gauvreau GM, Gauldie J, O'Byrne PM. Effects of budesonide and formoterol on allergen-induced airway responses, inflammation, and airway remodeling in asthma. J Allergy Clin Immunol. 2010;125:349–56.e13. doi: 10.1016/j.jaci.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Maneechotesuwan K, Essilfie-Quaye S, Meah S, Kelly C, Kharitonov SA, Adcock IM, Barnes PJ. Formoterol attenuates neutrophilic airway inflammation in asthma. Chest. 2005;128:1936–1942. doi: 10.1378/chest.128.4.1936. [DOI] [PubMed] [Google Scholar]
- 17.Bowden JJ, Sulakvelidze I, McDonald DM. Inhibition of neutrophil and eosinophil adhesion to venules of rat trachea by beta 2-adrenergic agonist formoterol. J Appl Physiol (1985) 1994;77:397–405. doi: 10.1152/jappl.1994.77.1.397. [DOI] [PubMed] [Google Scholar]
- 18.Whelan CJ, Johnson M, Vardey CJ. Comparison of the anti-inflammatory properties of formoterol, salbutamol and salmeterol in guinea-pig skin and lung. Br J Pharmacol. 1993;110:613–618. doi: 10.1111/j.1476-5381.1993.tb13855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson M. Effects of beta2-agonists on resident and infiltrating inflammatory cells. J Allergy Clin Immunol. 2002;110(6 Suppl):S282–S290. doi: 10.1067/mai.2002.129430. [DOI] [PubMed] [Google Scholar]
- 20.Gravett CM, Theron AJ, Steel HC, Tintinger GR, Cockeran R, Feldman C, Anderson R. Interactive inhibitory effects of formoterol and montelukast on activated human neutrophils. Eur Respir J. 2010;36:1417–1424. doi: 10.1183/09031936.00157409. [DOI] [PubMed] [Google Scholar]
- 21.Anderson R, Theron AJ, Steel HC, Durandt C, Tintinger GR, Feldman C. The beta-2-adrenoreceptor agonists, formoterol and indacaterol, but not salbutamol, effectively suppress the reactivity of human neutrophils in vitro. Mediators Inflamm. 2014;2014:105420. doi: 10.1155/2014/105420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tintinger GR, Anderson R, Theron AJ, Ramafi G, Ker JA. Comparison of the effects of selective and non-selective beta-adrenoreceptor agonists on the pro-inflammatory activities of human neutrophils in vitro. Inflammation. 2000;24:239–249. [Google Scholar]
- 23.Moriyama S, Brestoff JR, Flamar AL, Moeller JB, Klose CSN, Rankin LC, Yudanin NA, Monticelli LA, Putzel GG, Rodewald HR, Artis D. β2-adrenergic receptor-mediated negative regulation of group 2 innate lymphoid cell responses. Science. 2018;359:1056–1061. doi: 10.1126/science.aan4829. [DOI] [PubMed] [Google Scholar]
- 24.Eda R, Sugiyama H, Hopp RJ, Okada C, Bewtra AK, Townley RG. Inhibitory effects of formoterol on platelet-activating factor induced eosinophil chemotaxis and degranulation. Int Arch Allergy Immunol. 1993;102:391–398. doi: 10.1159/000236588. [DOI] [PubMed] [Google Scholar]
- 25.Kainuma K, Kobayashi T, D'Alessandro-Gabazza CN, Toda M, Yasuma T, Nishihama K, Fujimoto H, Kuwabara Y, Hosoki K, Nagao M, Fujisawa T, Gabazza EC. β2 adrenergic agonist suppresses eosinophil-induced epithelial-to-mesenchymal transition of bronchial epithelial cells. Respir Res. 2017;18:79. doi: 10.1186/s12931-017-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tachibana A, Kato M, Kimura H, Fujiu T, Suzuki M, Morikawa A. Inhibition by fenoterol of human eosinophil functions including beta2-adrenoceptor-independent actions. Clin Exp Immunol. 2002;130:415–423. doi: 10.1046/j.1365-2249.2002.01997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noguchi T, Nakagome K, Kobayashi T, Ueda Y, Soma T, Nakamoto H, Nagata M. Effect of beta2-adrenergic agonists on eosinophil adhesion, superoxide anion generation, and degranulation. Allergol Int. 2015;64(Suppl):S46–S53. doi: 10.1016/j.alit.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Noguchi T, Nakagome K, Kobayashi T, Ueda Y, Soma T, Nakamoto H, Nagata M. Effects of β2-adrenergic agonists on periostin-induced adhesion, superoxide anion generation, and degranulation of human eosinophils. Allergol Int. 2018;67S:S48–S50. doi: 10.1016/j.alit.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Takaku Y, Nakagome K, Kobayashi T, Hagiwara K, Kanazawa M, Nagata M. IFN-γ-inducible protein of 10 kDa upregulates the effector functions of eosinophils through β2 integrin and CXCR3. Respir Res. 2011;12:138. doi: 10.1186/1465-9921-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noguchi T, Nakagome K, Kobayashi T, Uchida Y, Soma T, Nakamoto H, Nagata M. Periostin upregulates the effector functions of eosinophils. J Allergy Clin Immunol. 2016;138:1449–1452.e5. doi: 10.1016/j.jaci.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 31.Shima K, Koya T, Tsukioka K, Sakagami T, Hasegawa T, Fukano C, Ohashi-Doi K, Watanabe S, Suzuki E, Kikuchi T. Effects of sublingual immunotherapy in a murine asthma model sensitized by intranasal administration of house dust mite extracts. Allergol Int. 2017;66:89–96. doi: 10.1016/j.alit.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Pauwels RA, Löfdahl CG, Postma DS, Tattersfield AE, O'Byrne P, Barnes PJ, Ullman A. Effect of inhaled formoterol and budesonide on exacerbations of asthma. Formoterol and Corticosteroids Establishing Therapy (FACET) International Study Group. N Engl J Med. 1997;337:1405–1411. doi: 10.1056/NEJM199711133372001. [DOI] [PubMed] [Google Scholar]
- 33.Patel M, Pilcher J, Pritchard A, Perrin K, Travers J, Shaw D, Holt S, Harwood M, Black P, Weatherall M, Beasley R SMART Study Group. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerbations: a randomised controlled trial. Lancet Respir Med. 2013;1:32–42. doi: 10.1016/S2213-2600(13)70007-9. [DOI] [PubMed] [Google Scholar]
- 34.Hardy J, Baggott C, Fingleton J, Reddel HK, Hancox RJ, Harwood M, Corin A, Sparks J, Hall D, Sabbagh D, Mane S, Vohlidkova A, Martindale J, Williams M, Shirtcliffe P, Holliday M, Weatherall M, Beasley R PRACTICAL study team. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394:919–928. doi: 10.1016/S0140-6736(19)31948-8. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi T, Soma T, Takaku Y, Nakagome K, Hagiwara K, Kanazawa M, Nagata M. Salbutamol modulates the balance of Th1 and Th2 cytokines by mononuclear cells from allergic asthmatics. Int Arch Allergy Immunol. 2010;152(Suppl 1):32–40. doi: 10.1159/000312123. [DOI] [PubMed] [Google Scholar]
- 36.Mcivor RA, Pizzichini E, Turner MO, Hussack P, Hargreave FE, Sears MR. Potential masking effects of salmeterol on airway inflammation in asthma. Am J Respir Crit Care Med. 1998;158:924–930. doi: 10.1164/ajrccm.158.3.9802069. [DOI] [PubMed] [Google Scholar]
- 37.Lindqvist A, Karjalainen EM, Laitinen LA, Kava T, Altraja A, Pulkkinen M, Halme M, Laitinen A. Salmeterol resolves airway obstruction but does not possess anti-eosinophil efficacy in newly diagnosed asthma: a randomized, double-blind, parallel group biopsy study comparing the effects of salmeterol, fluticasone propionate, and disodium cromoglycate. J Allergy Clin Immunol. 2003;112:23–28. doi: 10.1067/mai.2003.1500. [DOI] [PubMed] [Google Scholar]
- 38.Barnes PJ. Beta-adrenoceptors on smooth muscle, nerves and inflammatory cells. Life Sci. 1993;52:2101–2109. doi: 10.1016/0024-3205(93)90725-i. [DOI] [PubMed] [Google Scholar]
- 39.Kita H, Abu-Ghazaleh RI, Gleich GJ, Abraham RT. Regulation of Ig-induced eosinophil degranulation by adenosine 3′,5′-cyclic monophosphate. J Immunol. 1991;146:2712–2718. [PubMed] [Google Scholar]
- 40.Bloemen PG, van den Tweel MC, Henricks PA, Engels F, Kester MH, van de Loo PG, Blomjous FJ, Nijkamp FP. Increased cAMP levels in stimulated neutrophils inhibit their adhesion to human bronchial epithelial cells. Am J Physiol. 1997;272(4 Pt 1):L580–L587. doi: 10.1152/ajplung.1997.272.4.L580. [DOI] [PubMed] [Google Scholar]
- 41.Freyss-Beguin M, Griffaton G, Lechat P, Picken D, Quennedey MC, Rouot B, Schwartz J. Comparison of the chronotropic effect and the cyclic AMP accumulation induced by beta 2-agonists in rat heart cell culture. Br J Pharmacol. 1983;78:717–723. doi: 10.1111/j.1476-5381.1983.tb09425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campestrini J, Lecaillon JB, Godbillon J. Automated and sensitive method for the determination of formoterol in human plasma by high-performance liquid chromatography and electrochemical detection. J Chromatogr B Biomed Sci Appl. 1997;704:221–229. doi: 10.1016/s0378-4347(97)00425-8. [DOI] [PubMed] [Google Scholar]