Diet, especially with respect to consumption of dietary fibers, is well recognized as one of the most important factors shaping the colonic microbiota composition. Accordingly, many studies have been conducted to explore dietary fiber types that could predictably manipulate the colonic microbiota for improved health. However, the majority of these studies underappreciate the vastness of fiber structures in terms of their microbial utilization and omit detailed carbohydrate structural analysis. In some cases, this causes conflicting results to arise between studies using (theoretically) the same fibers. In this investigation, by performing in vitro fecal fermentation studies using bran arabinoxylans obtained from different classes of wheat, we showed that even subtle changes in the structure of a dietary fiber result in divergent microbial communities and metabolic outputs. This underscores the need for much higher structural resolution in studies investigating interactions of dietary fibers with gut microbiota, both in vitro and in vivo.
KEYWORDS: wheat, arabinoxylan, carbohydrate, linkage, monosaccharide, colonic microbiome short-chain fatty acids, 16S rRNA, Bacteroides, Prevotella, carbohydrate structure, dietary fiber
ABSTRACT
The chemical structures of soluble fiber carbohydrates vary from source to source due to numerous possible linkage configurations among monomers. However, it has not been elucidated whether subtle structural variations might impact soluble fiber fermentation by colonic microbiota. In this study, we tested the hypothesis that subtle structural variations in a soluble polysaccharide govern the community structure and metabolic output of fermenting microbiota. We performed in vitro fecal fermentation studies using arabinoxylans (AXs) from different classes of wheat (hard red spring [AXHRS], hard red winter [AXHRW], and spring red winter [AXSRW]) with identical initial microbiota. Carbohydrate analyses revealed that AXSRW was characterized by a significantly shorter backbone and increased branching compared with those of the hard varieties. Amplicon sequencing demonstrated that fermentation of AXSRW resulted in a distinct community structure of significantly higher richness and evenness than those of hard-AX-fermenting cultures. AXSRW favored OTUs within Bacteroides, whereas AXHRW and AXHRS favored Prevotella. Accordingly, metabolic output varied between hard and soft varieties; higher propionate production was observed with AXSRW and higher butyrate and acetate with AXHRW and AXHRS. This study showed that subtle changes in the structure of a dietary fiber may strongly influence the composition and function of colonic microbiota, further suggesting that physiological functions of dietary fibers are highly structure dependent. Thus, studies focusing on interactions among dietary fiber, gut microbiota, and health outcomes should better characterize the structures of the carbohydrates employed.
IMPORTANCE Diet, especially with respect to consumption of dietary fibers, is well recognized as one of the most important factors shaping the colonic microbiota composition. Accordingly, many studies have been conducted to explore dietary fiber types that could predictably manipulate the colonic microbiota for improved health. However, the majority of these studies underappreciate the vastness of fiber structures in terms of their microbial utilization and omit detailed carbohydrate structural analysis. In some cases, this causes conflicting results to arise between studies using (theoretically) the same fibers. In this investigation, by performing in vitro fecal fermentation studies using bran arabinoxylans obtained from different classes of wheat, we showed that even subtle changes in the structure of a dietary fiber result in divergent microbial communities and metabolic outputs. This underscores the need for much higher structural resolution in studies investigating interactions of dietary fibers with gut microbiota, both in vitro and in vivo.
OBSERVATION
Although plant carbohydrates are composed of a relatively limited set of different monosaccharides, astronomical structural diversity arises from the multiplicity of anomeric configurations, linkage types, backbone lengths, branching units, and reducing terminal attachments that occur in these often-branched polymers (1–5). Further, as biosynthetic enzymes generating plant cell wall polysaccharides are sometimes imprecise, these reactions can be viewed to generate a family of related structural features that can vary significantly among plant species or even different tissues within a plant (1). Conversely, microbial hydrolases are often quite structure specific in their degradative activities (6), requiring a large complement of distinct enzymes to degrade complex polysaccharides and limiting microbial access to such carbohydrates (7). However, this structural diversity is often overlooked when examining the effects of fiber carbohydrates on the community structure and metabolic function of human gut microbiota, raising the question of whether distinct structural variants might interact differently. Recently, the fine structure of insoluble starch molecules was found to influence gut microbiome structural and functional responses in a dose-dependent way in a human feeding study (8). This suggests tight interrelationships between physical and chemical structuring and the organisms most competitive in exploiting these differences (8). In this study, we tested the hypothesis that subtle variation in a soluble fiber carbohydrate’s chemical structure would govern the outcome of microbial competition, using arabinoxylan (AX) hemicelluloses extracted from brans of three different commonly consumed classes of wheat (Triticum aestivum)—hard red spring (AXHRS), hard red winter (AXHRW), and soft red winter (AXSRW)—as model fiber carbohydrates.
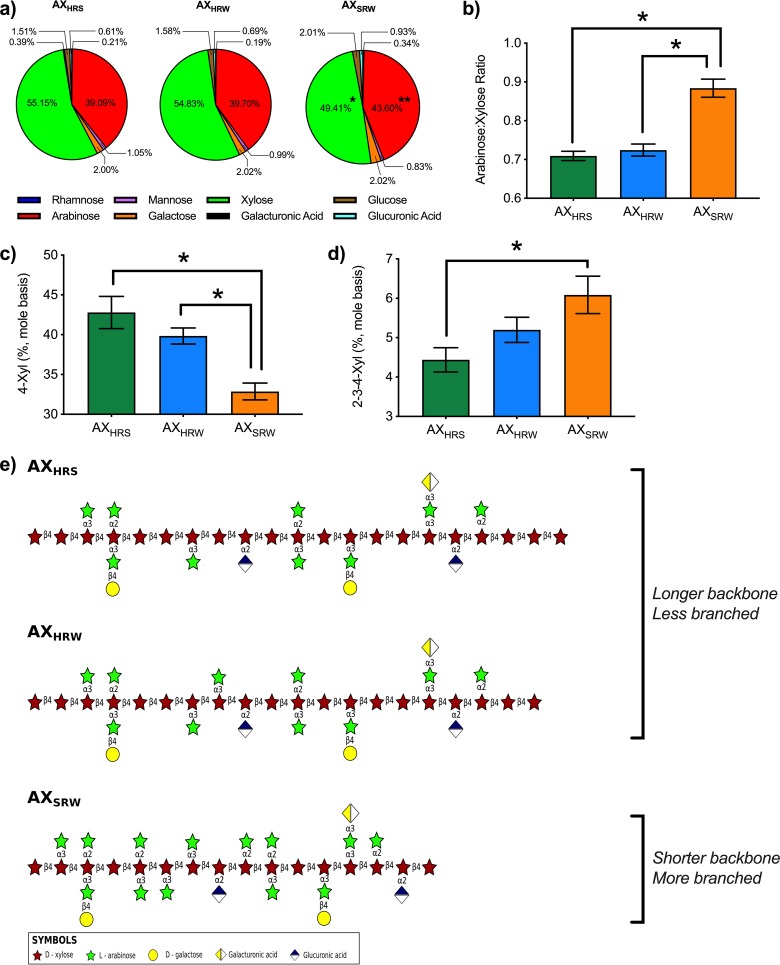
We observed no significant differences in the starch content, molecular weight, or dispersity values of the extracted AXs (P > 0.05) (see Fig. S1 in the supplemental material). AXHRS and AXHRW were almost identical in sugar composition, displaying no significant differences in any of their acidic and neutral monosaccharides (Fig. 1a). However, AXSRW exhibited a significantly higher abundance of arabinose and a lower abundance of xylose (P < 0.05) (Fig. 1a). Xylose and arabinose are the main building blocks of AX; the former composes the linear backbone and the latter the major branches of the AX. Thus, the arabinose-to-xylose ratio provides an estimate of an AX’s branching density. The arabinose-to-xylose ratios of AXHRS and AXHRW were not distinguishable, whereas that of AXSRW was significantly higher (Fig. 1b), indicating an increasingly branched structure compared to those of AXHRS and AXHRW. Examination of the glycosyl linkages (Table S1) further supports significant differences in branch structures. AXHRS and AXHRW exhibited a significantly higher proportion of 4-xylose linkages (Fig. 1c), which join the xylose units in the linear backbone of AXs. Conversely, a greater abundance of the branch-associated 2-3-4-linked xylose was observed in AXSRW (P < 0.05) (Fig. 1d). Collectively, these data indicate that AXSRW has a shorter backbone and increased branch density compared to those of AXHRS and AXHRW (Fig. 1e). It should be noted that we did not quantify the protein contents of the AXs, as extensive enzyme (proteases pepsin and pancreatin) treatments applied during the extraction and mimic upper gastrointestinal tract digestion are considered to be sufficient to remove proteins (Text S1). Similarly, ferulic acid contents of the AXs were not quantified, as alkali-extracted wheat bran AXs have been shown to possess no detectable ferulic acid (9). However, as the polysaccharides were extracted from intact wheat brans, it cannot be ruled out that retained traces of these or other components may have influenced the observed fermentation dynamics.
FIG 1.
Compositional and structural features of AXs used. (a) Monosaccharide compositions (mole basis) (*, significantly smaller amount of xylose; **, significantly larger amount of arabinose; two-tailed Student’s t test, P < 0.05). (b) Arabinose-to-xylose ratio (as an indicator of branching density). (c) Relative abundances of 4-Xyl linkage (typical linkage presented in the backbone of AX). (d) Relative abundances of 2-3-4-Xyl linkage (typical linkage presented in the backbone of AX that bear side chains) (other linkages detected are given in Table S1). (e) Schematic of proposed generalized structures of the AX samples drawn based on monosaccharide compositions and linkage profiles. Arabinoxylan extracted from hard red spring wheat (AXHRS) and arabinoxylan extracted from hard red winter wheat (AXHRW) have longer backbones and fewer branching points than arabinoxylan extracted from soft red winter wheat (AXSRW). Statistical analyses were done using two-tailed Student’s t test (P < 0.05). Error bars represent the standard errors of three separate replicates.
Materials and methods. Download Text S1, DOCX file, 0.04 MB (36.3KB, docx) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Starch contents, molecular sizes, and dispersity values of the AXs used. Statistical analyses were done using two-tailed Student’s t test. Error bars represent the standard errors of three separate replicates. ns, not significant. Download FIG S1, TIF file, 0.6 MB (564.8KB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Linkage compositions of AXs (percent, mole basis). Download Table S1, DOCX file, 0.08 MB (83.3KB, docx) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
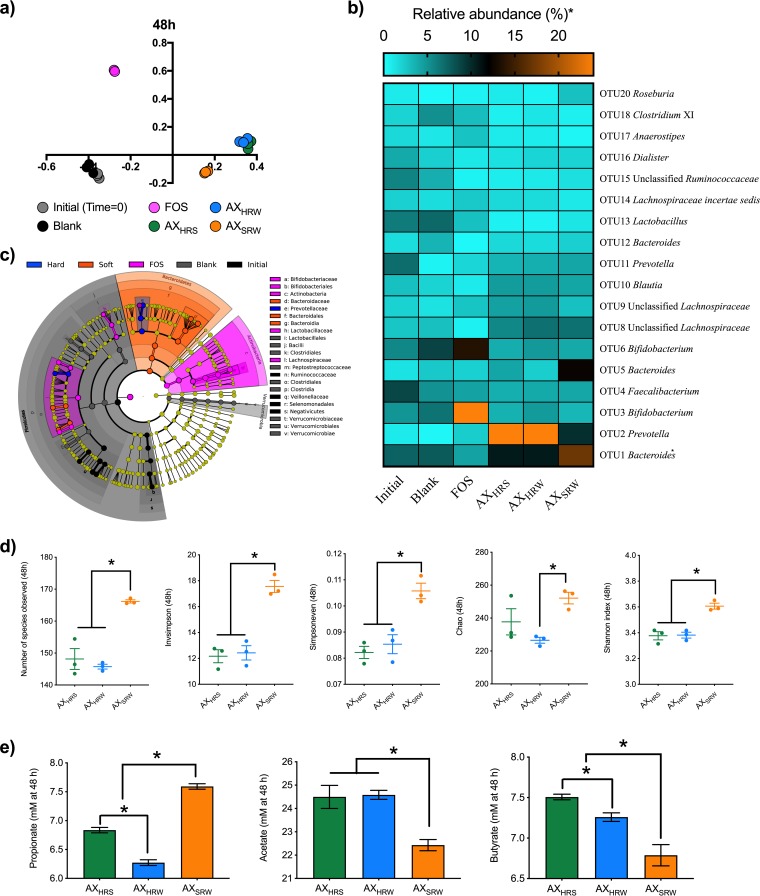
To investigate how these differences in branch densities of AXs impact the structure and metabolism of gut microbiota, we performed an in vitro fermentation study using fecal microbiota obtained from three healthy donors. Microbial composition was assayed with 16S rRNA amplicon sequencing and metabolism with respect to short-chain fatty acid (SCFA) production using gas chromatography. Communities fermenting AXSRW were significantly different in structure than those fermenting AXHRS or AXHRW based upon Bray-Curtis dissimilarity (analysis of molecular variance [AMOVA], P < 0.001), with AX cultures from the hard varieties not distinguishable from one another (Fig. 2a and Fig. S2). These data aligned with the significant structural differences between AXSRW and AXHRS/HRW, though not between the hard varieties. The structural variants also differentially affected community richness and evenness, with AXSRW resulting in greater α-diversity indices (number of species observed, Simpson evenness, and inverse Simpson, Chao, and Shannon indices); AXHRS and AXHRW treatments were not distinguishable (Fig. 2d and Fig. S3). We hypothesize that the higher α-diversity values observed in AXSRW-fermenting cultures arose from its more complex branching structure, requiring a greater number of glycoside hydrolases for complete consumption and potentially affording more microbial niches.
FIG 2.
Microbial community analyses after in vitro fermentation for 48 h, as determined by 16S rRNA gene amplicon sequencing. (a) Bray-Curtis dissimilarity of fecal microbial communities based on the relative abundances of OTUs at 97% similarity level (principal-component analysis [PCA] plots for the 12- and 24-h time points are given in Fig. S2). Dissimilarity was also calculated using ThetaYC; the result was not substantially different from that visualized by Bray-Curtis dissimilarity. (b) Relative abundances (percentage of sequences) based on the top 50 OTUs in each sample. The top 50 OTUs accounted for more than 90% of the total sequences of all AX treatment groups at all time points (relative abundances at the 12- and 24-h time points are given in Fig. S4, and relative abundances displayed in a bar graph are provided in Fig. S5). (c) Cladogram (obtained as a result of linear discriminant analysis) depicting taxa that are overrepresented in the AX samples obtained from hard and soft wheat classes compared with abundances in the initial inoculum and substrate-free blank incubations. (d) Changes in α-diversity of the fecal microbiota communities, as measured by number of species observed and inverse Simpson, Simpson, Chao, and Shannon’s index calculators (α-diversity of the fecal microbiota communities at the 12- and 24-h time points are given in Fig. S3). (e) Short-chain fatty acid (SCFA; namely, propionate, acetate, and butyrate) production by fecal microbiota at the end of the fermentation (the amounts of SCFAs produced after in vitro fermentation for 12 and 24 h are given in Fig. S6a; the proportions of all SCFAs produced after in vitro fermentation for 12, 24, and 48 h are given in Fig. S6b). Fructooligosaccharide (FOS) was used as a fast-fermenting, butyrate-producing positive control. The blank did not contain any substrate. Statistical analyses were done using two-tailed Student’s t test. Error bars represent the standard errors of three separate replicates.
Bray-Curtis dissimilarity of fecal microbial communities based on the relative abundances of OTUs at 97% similarity after in vitro fermentation for 12 and 24 h. Download FIG S2, TIF file, 0.5 MB (534.8KB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Changes in α-diversity of the fecal microbiota communities after in vitro fermentation for 12, 24, and 48 h, as measured by number of species observed and inverse Simpson, Simpson, Chao, and Shannon’s index calculators. Values with different letters in the same raw are statistically different (P < 0.05, Tukey’s test). Fructooligosaccharide (FOS) was used as a fast-fermenting, butyrate-producing positive control. The blank did not contain any substrate. Abbreviations: HRS, arabinoxylan extracted from hard red spring wheat; HRW, arabinoxylan extracted from hard red winter wheat; SRW, arabinoxylan extracted from soft red winter wheat. Download FIG S3, TIF file, 1.8 MB (1.8MB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative abundances (percentage of sequences) based on the top 50 OTUs in each sample after in vitro fermentation for 12 and 24 h. The top 50 OTUs accounted for more than 90% of the total sequences of all AX treatment groups at all time points. FOS was used as a fast-fermenting, butyrate-producing positive control. The blank did not contain any substrate. Download FIG S4, TIF file, 2.1 MB (2.1MB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative abundances (percentage of sequences) based on the top 50 OTUs in each sample after in vitro fermentation for 48 h. The top 50 OTUs accounted for more than 90% of the total sequences of all AX treatment groups at all time points. FOS was used as a fast-fermenting, butyrate-producing positive control. The blank did not contain any substrate. Download FIG S5, TIF file, 2.2 MB (2.2MB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative abundances (percentage of reads) of OTU20 (Roseburia) in each sample based on the top 50 OTUs after in vitro fermentation for 48 h. The top 50 OTUs accounted for more than 90% of the total sequences of all AX treatment groups at all time points. Error bars represent the standard errors of three separate replicates. SCFA, short-chain fatty acid. Download FIG S6, TIF file, 0.4 MB (431.1KB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
AX fine structure strongly governed the relative abundances of operational taxonomic units (OTUs), such that the less branched AXHRS and AXHRW promoted OTU2 (Prevotella) and the more branched AXSRW favored OTUs within genus Bacteroides (Fig. 2b and Fig. S4 and S5). Specifically, after 24 h of fermentation, AXSRW elicited a 9.8-fold increase in the relative abundance of OTU2 but 30- and 29-fold increases in AXHRS and AXHRW cultures, respectively. Conversely, although fermentation of AXHRS and AXHRW resulted in <2.4-fold increases in the relative abundance of OTU5 (Bacteroides), we observed a 12.3-fold increase with AXSRW. AXSRW also significantly increased the abundance of OTU1 (Bacteroides) compared with that obtained with AXHRS and AXHRW. Linear discriminant analysis effect size (LEfSe) identified the members of family Prevotellaceae and subordinate taxa as discriminators for hard wheat AXs, and Bacteroidales and subordinate taxa were discriminators of AXSRW (Fig. 1c). Both Bacteroides and Prevotella species are known to be very well equipped for the degradation of (arabino)xylans (10–14). Our findings further add to this that the structural complexity of xylans determines which of these taxa outcompete the other; Prevotella species seemed to better compete for less densely branched AXs but Bacteroides species, in general, for the more densely branched one. Moreover, in addition to Bacteroides and Prevotella species, AX consumption was shown to promote Roseburia species (10, 12). Our findings reveal that increases in Roseburia relative abundances on AX are structure dependent; neither AXHRS nor AXHRW significantly changed Roseburia relative abundances after 48 h of fermentation, whereas the fermentation of highly branched AXSRW resulted in 3.2-fold increases in the abundance of Roseburia spp. (OTU20) (Fig. 2b and Fig. S4, S5, and S6). Collectively, these findings support the idea that soluble polysaccharides can act as “discrete fiber structures,” such that unique fiber chemical structures specifically promote particular microbial taxa in the colon (2, 8, 15).
Accordingly, structure-specific SCFA formations were observed; compared to AXHRS and AXHRW, AXSRW treatment resulted in formation of significantly higher propionate but lower acetate and butyrate concentrations (P < 0.05) (Fig. 2e). As both the genera Bacteroides and Prevotella are regarded to increase community propiogenesis, differences in other SCFAs may stem from altered abundance or activity of acetate-consuming, butyrate-producing members of Lachnospiraceae. Moreover, total SCFA production occurred at a lower rate in AXSRW than in hard varieties (Fig. S7a), indicating that AXSRW was more slowly fermented. This was further supported by the slower decrease in pH in fermentations of AXSRW than for AXHRW/HRS (Fig. S8). We hypothesize that the increased branch density in AXSRW may limit enzyme access to the xylan backbone until branches are partially removed, potentially allowing coordinated activity of multiple species.
(a) Absolute amounts (mole basis) of total SCFAs, acetate, propionate, and butyrate produced after in vitro fermentation for 12, 24, 36, and 48 h. Total SCFA was calculated by summing up the amounts of acetate, propionate, and butyrate. (b) Proportions of acetate, propionate, and butyrate (relative to total SCFA; mole basis) produced after in vitro fermentation for 12, 24, 36, and 48 h. FOS was used as a fast-fermenting, butyrate-producing positive control. The blank did not contain any substrate. Error bars represent the standard errors of three separate replicates. Abbreviations: AXHRS, arabinoxylan extracted from hard red spring wheat; AXHRW, arabinoxylan extracted from hard red winter wheat; AXSRW, arabinoxylan extracted from soft red winter wheat. Download FIG S7, TIF file, 2.1 MB (2.1MB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Changes in pH of the treatments after in vitro fermentation for 0, 6, 12, 24, 36, and 48 h. Download FIG S8, TIF file, 0.5 MB (527.5KB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Overall, our results demonstrate that although the general polysaccharide chemical structures obtained from closely related plant sources may seem similar, subtle structural differences significantly alter their effects on the structure and metabolic function of colonic microbiota. We submit that studies focusing on interactions among dietary fiber carbohydrates, gut microbiota, and health outcomes should include much more extensive characterization of the carbohydrate structures employed. Further, we propose that use of fibers in general categories (e.g., high- or low-fiber diets or arabinoxylan of unspecified or poorly characterized origin) without such structural data may confuse the conflicting effects of subtle structural variants, which may elicit different microbial responses.
Data availability.
Sequence data described in this study are available in the National Center for Biotechnology Information's Sequence Read Archive under BioProject PRJNA628680 as BioSamples SAMN14749672 to SAMN14749719.
ACKNOWLEDGMENTS
This work was supported by U.S. Department of Agriculture Hatch Project IND011670 to S.R.L.
We declare no conflict of interest.
REFERENCES
- 1.Burton RA, Gidley MJ, Fincher GB. 2010. Heterogeneity in the chemistry, structure and function of plant cell walls. Nat Chem Biol 6:724–732. doi: 10.1038/nchembio.439. [DOI] [PubMed] [Google Scholar]
- 2.Hamaker BR, Tuncil YE. 2014. A perspective on the complexity of dietary fiber structures and their potential effect on the gut microbiota. J Mol Biol 426:3838–3850. doi: 10.1016/j.jmb.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 3.Laine RA. 1994. A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 1012 structures for a reducing hexasaccharide: the Isomer Barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology 4:759–767. doi: 10.1093/glycob/4.6.759. [DOI] [PubMed] [Google Scholar]
- 4.Martens EC, Kelly AG, Tauzin AS, Brumer H. 2014. The devil lies in the details: how variations in polysaccharide fine-structure impact the physiology and evolution of gut microbes. J Mol Biol 426:3851–3865. doi: 10.1016/j.jmb.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter NT, Martens EC. 2017. The critical roles of polysaccharides in gut microbial ecology and physiology. Annu Rev Microbiol 71:349–369. doi: 10.1146/annurev-micro-102215-095316. [DOI] [PubMed] [Google Scholar]
- 6.Abbott DW, van Bueren AL. 2014. Using structure to inform carbohydrate binding module function. Curr Opin Struct Biol 28:32–40. doi: 10.1016/j.sbi.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Koropatkin NM, Cameron EA, Martens EC. 2012. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deehan EC, Chen Y, Perez-Muñoz EP, Nguyen NK, Cheng CC, Triador L, Zhang Z, Bakal JA, Walter J. 2020. Precision microbiome modulation with discrete dietary fiber structures directs short-chain fatty acid production. Cell Host Microbe 27:389–316. doi: 10.1016/j.chom.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhou S, Liu X, Guo Y, Wang Q, Peng D, Cao L. 2010. Comparison of the immunological activities of arabinoxylans from wheat bran with alkali and xylanase-aided extraction. Carbohydr Polym 81:784–789. doi: 10.1016/j.carbpol.2010.03.040. [DOI] [Google Scholar]
- 10.Chassard C, Goumy V, Leclerc M, Del'homme C, Bernalier-Donadille A. 2007. Characterization of the xylan-degrading microbial community from human faeces. FEMS Microbiol Ecol 61:121–131. doi: 10.1111/j.1574-6941.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- 11.Dodd D, Moon YH, Swaminathan K, Mackie RI, Cann I. 2010. Transcriptomic analyses of xylan degradation by Prevotella bryantii and insights into energy acquisition by xylanolytic Bacteroidetes. J Biol Chem 285:30261–30273. doi: 10.1074/jbc.M110.141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, Larondelle Y, Delzenne NM. 2011. Prebiotic effects of wheat arabinoxylan related to the increase in Bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PLoS One 6:e20944. doi: 10.1371/journal.pone.0020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Despres J, Forano E, Lepercq P, Comtet-Marre S, Jubelin G, Chambon C, Yeoman CJ, Berg Miller ME, Fields CJ, Martens E, Terrapon N, Henrissat B, White BA, Mosoni P. 2016. Xylan degradation by the human gut Bacteroides xylanisolvens XB1A(T) involves two distinct gene clusters that are linked at the transcriptional level. BMC Genomics 17:326. doi: 10.1186/s12864-016-2680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen T, Long W, Zhang C, Liu S, Zhao L, Hamaker BR. 2017. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci Rep 7:2594. doi: 10.1038/s41598-017-02995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantu-Jungles TM, Hamaker BR. 2020. New view on dietary fiber selection for predictable shifts in gut microbiota. mBio 11:e02179-19. doi: 10.1128/mBio.02179-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Materials and methods. Download Text S1, DOCX file, 0.04 MB (36.3KB, docx) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Starch contents, molecular sizes, and dispersity values of the AXs used. Statistical analyses were done using two-tailed Student’s t test. Error bars represent the standard errors of three separate replicates. ns, not significant. Download FIG S1, TIF file, 0.6 MB (564.8KB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Linkage compositions of AXs (percent, mole basis). Download Table S1, DOCX file, 0.08 MB (83.3KB, docx) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Bray-Curtis dissimilarity of fecal microbial communities based on the relative abundances of OTUs at 97% similarity after in vitro fermentation for 12 and 24 h. Download FIG S2, TIF file, 0.5 MB (534.8KB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Changes in α-diversity of the fecal microbiota communities after in vitro fermentation for 12, 24, and 48 h, as measured by number of species observed and inverse Simpson, Simpson, Chao, and Shannon’s index calculators. Values with different letters in the same raw are statistically different (P < 0.05, Tukey’s test). Fructooligosaccharide (FOS) was used as a fast-fermenting, butyrate-producing positive control. The blank did not contain any substrate. Abbreviations: HRS, arabinoxylan extracted from hard red spring wheat; HRW, arabinoxylan extracted from hard red winter wheat; SRW, arabinoxylan extracted from soft red winter wheat. Download FIG S3, TIF file, 1.8 MB (1.8MB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative abundances (percentage of sequences) based on the top 50 OTUs in each sample after in vitro fermentation for 12 and 24 h. The top 50 OTUs accounted for more than 90% of the total sequences of all AX treatment groups at all time points. FOS was used as a fast-fermenting, butyrate-producing positive control. The blank did not contain any substrate. Download FIG S4, TIF file, 2.1 MB (2.1MB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative abundances (percentage of sequences) based on the top 50 OTUs in each sample after in vitro fermentation for 48 h. The top 50 OTUs accounted for more than 90% of the total sequences of all AX treatment groups at all time points. FOS was used as a fast-fermenting, butyrate-producing positive control. The blank did not contain any substrate. Download FIG S5, TIF file, 2.2 MB (2.2MB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Relative abundances (percentage of reads) of OTU20 (Roseburia) in each sample based on the top 50 OTUs after in vitro fermentation for 48 h. The top 50 OTUs accounted for more than 90% of the total sequences of all AX treatment groups at all time points. Error bars represent the standard errors of three separate replicates. SCFA, short-chain fatty acid. Download FIG S6, TIF file, 0.4 MB (431.1KB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
(a) Absolute amounts (mole basis) of total SCFAs, acetate, propionate, and butyrate produced after in vitro fermentation for 12, 24, 36, and 48 h. Total SCFA was calculated by summing up the amounts of acetate, propionate, and butyrate. (b) Proportions of acetate, propionate, and butyrate (relative to total SCFA; mole basis) produced after in vitro fermentation for 12, 24, 36, and 48 h. FOS was used as a fast-fermenting, butyrate-producing positive control. The blank did not contain any substrate. Error bars represent the standard errors of three separate replicates. Abbreviations: AXHRS, arabinoxylan extracted from hard red spring wheat; AXHRW, arabinoxylan extracted from hard red winter wheat; AXSRW, arabinoxylan extracted from soft red winter wheat. Download FIG S7, TIF file, 2.1 MB (2.1MB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Changes in pH of the treatments after in vitro fermentation for 0, 6, 12, 24, 36, and 48 h. Download FIG S8, TIF file, 0.5 MB (527.5KB, tif) .
Copyright © 2020 Tuncil et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
Sequence data described in this study are available in the National Center for Biotechnology Information's Sequence Read Archive under BioProject PRJNA628680 as BioSamples SAMN14749672 to SAMN14749719.