Abstract
Background
Residual kidney function (RKF) is thought to exert beneficial effects through clearance of uremic toxins. However, the level of native kidney function where clearance becomes negligible is not known.
Methods
We aimed to assess whether levels of nonurea solutes differed among patients with ‘clinically negligible’ RKF compared with those with no RKF. The hemodialysis study excluded patients with urinary urea clearance >1.5 mL/min, below which RKF was considered to be ‘clinically negligible’. We measured eight nonurea solutes from 1280 patients participating in this study and calculated the relative difference in solute levels among patients with and without RKF based on measured urinary urea clearance.
Results
The mean age of the participants was 57 years and 57% were female. At baseline, 34% of the included participants had clinically negligible RKF (mean 0.7 ± 0.4 mL/min) and 66% had no RKF. Seven of the eight nonurea solute levels measured were significantly lower in patients with RKF than in those without RKF, ranging from −24% [95% confidence interval (CI) −31 to −16] for hippurate, −7% (−14 to −1) for trimethylamine-N-oxide and −4% (−6 to −1) for asymmetric dimethylarginine. The effect of RKF on plasma levels was comparable or more pronounced than that achieved with a 31% higher dialysis dose (spKt/Vurea 1.7 versus 1.3). Preserved RKF at 1-year follow-up was associated with a lower risk of cardiac death and first cardiovascular event.
Conclusions
Even at very low levels, RKF is not ‘negligible’, as it continues to provide nonurea solute clearance. Management of patients with RKF should consider these differences.
Keywords: clearance, dialysis dose, ESRD, hemodialysis, uremic toxins
BACKGROUND
The association between residual kidney function (RKF) and improved survival in the dialysis population has been well established for more than two decades [1–4]. RKF, however small, contributes to ongoing electrolyte regulation, volume control, erythropoietin production and solute clearance [3, 5–8]. Solute clearance is of particular importance because native kidneys remain far superior to hemodialysis (HD) at removing various ‘nonurea’ solutes, particularly those that are large in molecular size, secreted by the native kidney or sequestered in the intracellular space [7, 9–11].
HD adequacy is routinely assessed using Kt/Vurea, the fractional clearance of urea from the body during dialysis [12, 13]. Despite its imperfections, urea kinetic modeling remains the principal way to measure HD adequacy, largely because a superior measure of adequacy does not exist yet and because it is a relatively simple calculation that relies on urea, an easy and inexpensive solute to assay throughout the world [12, 14–16]. As a result, dialyzers have evolved to the extent that they can efficiently remove urea without requiring a contribution from RKF. The disadvantage of this is 2-fold. First, dialyzers are less effective at clearing small, nonurea, organic solutes, many of which have pathologic implications in HD patients. Second, they have generated a reimbursement model in the USA incentivized on achieving a goal Kt/Vurea, which means US clinicians do not habitually measure RKF and adjust HD dosing accordingly, despite this being a recommendation in the Kidney Disease Outcomes Quality Initiative (KDOQI) HD clinical practice guidelines [12, 13]. Because the native kidneys remain uniquely superior to HD in removing various nonurea solutes from the body, we hypothesized that even a ‘clinically negligible’ degree of RKF would impact certain small, nonurea, organic solute levels.
The goal of our study was to determine the effect of RKF on eight nonurea solutes in patients with RKF considered to be clinically negligible. We addressed this question using data from the Hemodialysis (HEMO) study, a large, national, multicenter, randomized controlled trial of dialysis dose and membrane flux [17]. The HEMO study carefully measured RKF at baseline and excluded participants with a residual urea clearance >1.5 mL/min/35 L of urea, a threshold below which RKF was considered to be clinically negligible. This allowed us to compare solute levels in patients with RKF deemed clinically negligible with those without RKF.
MATERIALS AND METHODS
Study design
The HEMO study was a multicenter, randomized controlled trial that randomized 1846 prevalent HD patients in a 2 × 2 factorial design to either low- or high-flux membranes and to a dialysis prescription targeted to a standard- or high-dose single-pool Kt/Vurea (spKt/Vurea). The standard-dose goal was an spKt/Vurea of 1.25, whereas the high-dose goal was an spKt/Vurea of 1.65. In our analysis, we term this dialysis the ‘dose intervention group’ and refer to the group that a participant was randomized to in the parent study, that is, standard-dose goal versus high-dose goal. In our analysis, participants randomized to the standard-dose group achieved a mean spKt/Vurea of 1.32 ± 0.01 and those randomized to the high-dose goal achieved a mean spKt/Vurea of 1.72 ± 0.01. We term this dialysis ‘dose achieved’, which refers to the mean spKt/Vurea actually achieved by participants in each intervention arm. Patients 18–80 years of age who were undergoing in-center HD thrice weekly at 1 of 15 clinical centers associated with 72 participating dialysis units were enrolled between March 1995 and October 2000 and followed for prespecified outcomes until death, kidney transplantation or censoring of the study in December 2001. Major exclusion criteria included interdialytic urine collection with a residual urea clearance >1.5 mL/min/35 L urea volume of distribution, New York Heart Association Class IV congestive heart failure and severe malnutrition as measured by serum albumin <2.6 g/dL [17–19]. The participating institutions’ review boards reviewed and approved the study. Johns Hopkins Medicine (IRB00032559) and the University of California, San Francisco (10-00758) institutional review boards approved this study.
Data collection
In our study, we included 1280 participants with predialysis plasma samples available. Samples were collected 3–8 months after randomization in the HEMO study, a time point that allowed adequate separation between the study groups [20].
Urine collection measurements
Timed urine collections were performed once at baseline over 24–46 h in patients producing >50 mL of urine per day. This measurement was repeated annually at the discretion of the treating physician [19]. Residual kidney urea clearance was calculated from timed urine collections for each individual. Routine kinetic modeling took place at baseline and monthly thereafter and included predialysis and postdialysis urea concentration measurements. Extensive kinetic modeling that accounted for urea rebound occurred at follow-up visits at Months 4 and 36 and consisted of eight blood samples collected at designated times during and after dialysis, including a 30-min postdialysis sample [19].
We used urine collections at 1 year of follow-up for sensitivity analyses. These urine collections were collected at the discretion of the treating physician and were available for 173 of the 433 patients with RKF at baseline (40%). We excluded the remaining participants (n = 260) from our sensitivity analyses because we could not differentiate those that had truly lost RKF from those that retained RKF but did not have urine collected.
Solute measurements
Concentrations of p-cresol sulfate (PCS), indoxyl sulfate (IS), hippurate (HIPP) and phenylacetylglutamine (PAG) were assayed by stable isotope dilution liquid chromatography/tandem mass spectrometry (LC-MS/MS) using a modification of a previously described method [21]. Total plasma concentrations were measured after deproteination with methanol; further assay measurements and coefficients of variation for quality control have been described in detail previously [20]. Concentrations of trimethylamine-N-oxide (TMAO), asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and methylguanidine (MG) were also determined by LC-MS/MS. Recoveries and coefficients of variation for quality control have been previously described and published [20].
Other covariates
Demographics and clinical information for all participants were obtained at baseline at the time of enrollment. The index of coexisting disease (ICED), a composite index of 19 comorbid conditions and 9 physical impairments, was used to quantify the level of comorbidity at baseline. Scores are tabulated based on the severity or impairment of each comorbidity, with higher scores indicating a greater overall degree of comorbidity. Anthropometry was assessed once at baseline and included measurements such as height and weight. Normalized protein catabolic rate, a measure considered to reflect protein intake in a metabolically stable patient, was calculated using urea kinetic modeling from the predialysis and postdialysis urea measurements.
Statistical analysis
We examined baseline characteristics of the participants overall and by the presence or absence of RKF. Differences were compared using a chi-squared test for categorical variables and an unpaired Student’s t-test for continuous variables.
The primary outcome was a relative difference calculation of mean solute level in those with RKF (defined as residual urea clearance) compared with those without RKF {[(Mean solute level in those with RKF/mean solute level in those without RKF) − 1] × 100}. A negative value implied a lower solute level in the group with RKF. Confidence intervals (CIs) for this difference were calculated by bootstrapping with replacement and 2000 repetitions. Solutes were regressed on RKF using linear regression and then adjusted for age, sex, race and dialysis dose intervention group. To determine if the association between RKF and solutes was modified by spKt/Vurea, an interaction term between the dialysis dose randomization group and RKF (present or absent) was added to the model. In order to allow for direct comparison of the effect of residual urea clearance on the eight nonurea solutes, we performed linear regression of the natural log transformed and standardized solutes on residual urea clearance. To compare the effect of RKF on solute levels and the effect of dialysis dose intervention on solute levels, we calculated the relative difference of the mean solute level in those randomized to a high-dose HD versus a standard-dose HD {[(high Kt/Vurea group/standard Kt/Vurea group) – 1] × 100}, as previously described [20]. A negative value implied a lower solute level in the high-Kt/Vurea group. As in the primary analysis, 95% CIs were calculated using bootstrapping with 2000 replicates.
We used Cox proportional hazards models to analyze the association of RKF (defined as urine volume ≥250 mL/day) [3] with any-cause death, cardiac death and first cardiovascular event, adjusting for age, sex, race, comorbidities (as per the ICED score), cause of end-stage renal disease (ESRD), body mass index, diabetes and duration of prior dialysis. Outcomes analysis was performed using baseline RKF and preserved RKF, that is, RKF present at baseline and the 1-year follow-up. Stata/IC 14.1 (StataCorp, College Station, TX, USA) was used for all statistical analyses.
RESULTS
Participants and their characteristics
Our study sample was comprised of 1280 participants from the HEMO study, 433 (34%) of whom had RKF and 847 (66%) who had no RKF. Table 1 presents the baseline characteristics of the 1280 participants overall and by the presence or absence of RKF. Participants with RKF were more likely to be older (59 versus 57 years, P = 0.007), less likely to be black (57% versus 66%, P = 0.002) and had been on dialysis for a shorter period of time (1.8 versus 4.3 years, P < 0.001) compared with patients without RKF. Compared with excluded participants, those included in our analysis were less likely to have cardiac disease but otherwise differed only slightly (Supplementary data, Table S1).
Table 1.
Baseline characteristics of 1280 HD patients, overall and by RKF
| Characteristic | All | RKF | No RKF | P-value |
|---|---|---|---|---|
| Number | 1280 | 433 | 847 | |
| Demographics | ||||
| Age (years) | 57.5 ± 14.0 | 59.0 ± 13.6 | 56.7 ± 14.2 | 0.007 |
| Female, n (%) | 727 (56.8) | 229 (52.9) | 498 (58.8) | 0.049 |
| Black, n (%) | 804 (62.8) | 246 (56.8) | 558 (65.9) | 0.002 |
| Clinical characteristics | ||||
| ICED score | 1.95 ± 0.8 | 1.89 ± 0.9 | 1.98 ± 0.8 | 0.05 |
| Diabetes, n (%) | 578 (45.2) | 200 (46.2) | 378 (44.6) | 0.63 |
| Cardiac disease, n (%) | 1007 (78.7) | 334 (77.1) | 673 (79.5) | 0.35 |
| Gastrointestinal disease, n (%) | 480 (37.5) | 153 (35.3) | 327 (38.6) | 0.27 |
| Residual kidney urea clearance (mL/min/35 L TBW) | 0.24 ±0.4 | 0.72 ± 0.4 | 0 ± 0 | <0.001 |
| Urinary standard Kt/Vurea | 0.06 ± 0.1 | 0.19 ± 0.1 | 0 ± 0 | <0.001 |
| Urine volume (mL/24 h) | 78.0 ± 157.2 | 258.1 ± 187.8 | 0 ± 0 | <0.001 |
| BMI (kg/m2) | 25.6 ± 5.3 | 26.0 ± 5.1 | 25.4 ± 5.4 | 0.04 |
| Body surface area (m2) | 1.8 ± 0.2 | 1.8 ± 0.2 | 1.7 ± 0.2 | <0.001 |
| Dialysis characteristics | ||||
| Duration of prior dialysis (years) | 3.4 ± 4.1 | 1.8 ± 1.8 | 4.3 ± 4.7 | <0.001 |
| Predialysis SBP (mmHg) | 152.6 ± 21.8 | 154.0 ± 20.0 | 151.9 ± 22.7 | 0.1 |
| Predialysis weight (kg) | 72.5 ± 15.4 | 74.3 ± 15.2 | 71.6 ± 15.4 | 0.002 |
| Postdialysis weight (kg) | 69.6 ± 15.0 | 71.5 ± 14.8 | 68.7 ± 15.0 | 0.001 |
| Relative volume removeda (%) | 4.0 ± 1.5 | 3.8 ± 1.4 | 4.1 ± 1.5 | <0.001 |
| Absolute volume removed (L) | 2.9 ± 1.1 | 2.9 ± 1.1 | 2.8 ± 1.1 | 0.048 |
| Standardized volume removedb (L) | 3.0 ± 1.1 | 2.8 ± 1.1 | 3.0 ± 1.1 | <0.001 |
| High-dose HD intervention, n (%) | 638 (49.8) | 218 (50.3) | 420 (49.6) | 0.81 |
| High-flux intervention, n (%) | 638 (49.8) | 226 (52.2) | 412 (48.6) | 0.24 |
| Mean delivered dialysis (spKt/Vurea) | 1.5 ± 0.4 | 1.5 ± 0.5 | 1.5 ± 0.4 | 0.32 |
| Session length (min) | 206.8 ± 28.2 | 208.7 ± 28.7 | 205.8 ± 28.0 | 0.08 |
| Blood flow rate (mL/min) | 343.1 ± 60.7 | 345.7 ± 57.5 | 341.8 ± 62.3 | 0.29 |
| Dialysate flow rate (mL/min) | 673.2 ± 129.7 | 674.6 ± 133.5 | 672.6 ± 127.8 | 0.79 |
| Predialysis laboratory tests | ||||
| Serum urea nitrogen (mg/dL) | 56.7 ± 14.7 | 56.7 ± 14.0 | 56.7 ± 15.1 | 0.99 |
| Serum albumin (g/dL) | 3.6 ± 0.3 | 3.6 ± 0.3 | 3.6 ± 0.3 | 0.29 |
| Serum β2-microglobulin (mg/L) | 35.7 ± 13.7 | 30.7 ± 11.2 | 38.3 ± 14.1 | <0.001 |
| Equilibrated nPCR (g/kg/day) | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.2 | 0.003 |
Values are presented as mean ± SD unless stated otherwise. All values are prerandomization baseline data.
Relative volume removed is calculated as [(predialysis weight − postdialysis weight)/predialysis weight] × 100.
Mean volume removed per HD session as standardized to a 70-kg patient is calculated as [(predialysis weight − postdialysis weight)/postdialysis weight] × 70.
BMI, body mass index; nPCR, normalized protein catabolic rate; SBP, systolic blood pressure.
Among those with RKF, average residual kidney urea clearance was 0.7 ± 0.4 mL/min/35 L total body water (TBW) with an average urine volume of 1.8 L/week. Those with RKF had lower ultrafiltration requirements (relative volume removed 3.8% versus 4.1%, P < 0.001). When compared with those without RKF, this equated to 377 cc/week less ultrafiltration in absolute terms (P = 0.048) or 762 cc/week less ultrafiltration in a participant standardized to 70 kg (P < 0.001). Serum β2-microglobulin levels were also significantly lower in those with RKF compared with those without RKF, a finding that persisted when we controlled for dialysis dose and flux intervention group in the parent study (P < 0.001; Supplementary data, Table S2).
Association between RKF and solute concentrations
Table 2 describes the concentrations of urea and eight nonurea solutes overall by RKF. Seven of the eight nonurea solutes were lower in patients with RKF compared with those without. The difference ranged from 24% lower (95% CI −30.8 to −16.5) for HIPP to 3.7% lower (95% CI −6.4 to −0.9) for ADMA. Further adjustments for age, sex, race and dialysis dose intervention group in the parent study did not change these findings (Table 2).
Table 2.
Association of solute concentrations with RKF
| Solute | Overall (N = 1280) | RKF (n = 433) | No RKF (n = 847) | Mean difference | Relative differencea | Adjusted P-valueb | P-value for interactionc |
|---|---|---|---|---|---|---|---|
| Urea (mg/dL) | 59.7 ± 18.7 | 59.8 ± 18.5 | 59.6 ± 18.8 | 0.2 (−1.9–2.3) | 0.3 (−3.2–3.9) | 0.09 | 0.55 |
| PCS (mg/dL) | 3.3 ± 1.7 | 3.5 ± 1.7 | 3.3 ± 1.7 | 0.3 (0.1–0.5) | 8.2 (2.1–14.2) | 0.007 | 0.75 |
| IS (mg/dL) | 2.5 ± 1.2 | 2.3 ± 1.1 | 2.6 ± 1.2 | −0.3 (−0.4 to −0.2) | −11.1 (−16.0 to −6.2) | <0.001 | 0.57 |
| HIPP (mg/dL) | 5.4 ± 4.3 | 4.5 ± 3.7 | 5.9 ± 4.4 | −1.4 (−1.9 to −0.9) | −23.6 (−30.8 to −16.5) | <0.001 | 0.23 |
| PAG (mg/dL) | 4.5 ± 2.8 | 4.0 ± 2.6 | 4.7 ± 2.9 | −0.7 (−1.0 to −0.4) | −14.5 (−20.7 to −8.2) | <0.001 | 0.10 |
| TMAO (μM) | 102.2 ± 63.8 | 97.1 ± 61.4 | 104.8 ± 64.9 | −7.8 (−15.0 to −0.6) | −7.4 (−14.1 to −0.7) | 0.04 | 0.51 |
| MG (μM) | 7.7 ± 4.4 | 7.0 ± 3.8 | 8.1 ± 4.7 | −1.2 (−1.6 to −0.7) | −14.1 (−19.6 to −8.6) | <0.001 | 0.31 |
| ADMA (μM) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ±0.2 | −0.03 (−0.06 to −0.01) | −3.7 (−6.4 to −0.9) | 0.01 | 0.95 |
| SDMA (μM) | 4.3 ± 1.4 | 4.1 ± 1.3 | 4.4 ± 1.4 | −0.3 (−0.5 to −0.2) | −7.0 (−10.5 to −3.5) | 0.001 | 0.16 |
Relative difference was calculated as {[(Mean solute level in those with RKF/mean solute level in those without RKF) − 1] × 100}; 95% CIs were calculated using bootstrapping with 2000 replicates. Negative values imply a lower concentration in the group with RKF compared with the group without RKF.
P-values are from a linear regression model of solute on RKF adjusting for age, sex, race and dialysis dose intervention group.
P-value of the interaction term between dialysis dose intervention group and RKF (present or absent), adjusted for age, sex and race. The presence of statistically significant interaction would suggest that the association between RKF and the individual solute levels was modified by dialysis dose.
Among those with RKF, lower RKF was associated with higher concentrations of seven of eight uremic solutes (Table 3). Standardizing a natural log transformation of each of the solutes allowed for head-to-head comparison of solute level differences per 0.5 mL/min/35 L TBW decrease in residual urea clearance (Table 3). HIPP and MG were lower at 0.32 mg/dL and 0.34 μM, respectively, whereas TMAO was lower by 0.09 μM per each 0.5 mL/min/35 L TBW decrease in urea clearance. This association was similar when evaluated by baseline urine volume (Supplementary data, Table S3).
Table 3.
Association of solute concentration with measured urinary urea clearance in 433 HEMO study participants with RKF
| Solute | Absolute change in solute levela | P-valueb | Standardized, natural log change in solute levelc | P-valueb |
|---|---|---|---|---|
| PCS (mg/dL) | −0.08 ± 0.1 | 0.36 | −0.01 ± 0.05 | 0.8 |
| IS (mg/dL) | 0.24 ± 0.1 | <0.001 | 0.22 ± 0.05 | <0.001 |
| HIPP (mg/dL) | 1.10 ± 0.2 | <0.001 | 0.34 ± 0.05 | <0.001 |
| PAG (mg/dL) | 0.67 ± 0.1 | <0.001 | 0.25 ± 0.05 | <0.001 |
| TMAO (μM) | 6.07 ± 3.3 | 0.07 | 0.08 ± 0.05 | 0.1 |
| MG (μM) | 1.40 ± 0.2 | <0.001 | 0.33 ± 0.05 | <0.001 |
| ADMA (μM) | 0.03 ± 0.0 | 0.01 | 0.13 ± 0.05 | 0.011 |
| SDMA (μM) | 0.18 ± 0.1 | 0.01 | 0.12 ± 0.05 | 0.018 |
Per 0.5 mL/min/35 L TBW decrease in urea clearance.
Adjusted for age, sex race.
The natural log of each solute level was standardized among those patients with RKF and subsequently regressed on urea clearance allowing for a head-to-head comparison of solutes.
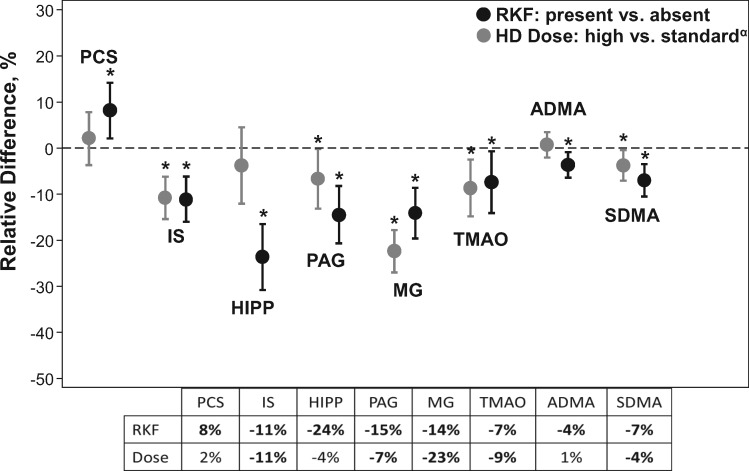
The relative differences in solute concentrations by RKF and dialysis dose randomization group are shown in Figure 1. PAG, TMAO, ADMA and SDMA levels were lower in those with RKF versus those without and the magnitude was greater when compared with the levels in those randomized to high-dose versus standard-dose HD. IS levels were equivalent, both lower by a relative difference of 11%. Among our study sample, the mean spKt/Vurea was 31% higher in those randomized to a high-dose dialysis group versus a standard-dose dialysis group (1.7 versus 1.3).
FIGURE 1.
Impact of RKF and HD dose on solute levels. The relative differences of the eight measured solute levels in those with RKF versus without RKF are juxtaposed with the relative differences in solute levels in those randomized to high-dose HD (mean spKt/Vurea = 1.7) versus standard-dose HD (mean spKt/Vurea = 1.3). Formulas used to calculate relative differences were [(mean solute level in those with RKF/mean solute level in those without RKF) – 1] × 100 and [(high Kt/Vurea group/standard Kt/Vurea group) – 1] ×100, respectively. The y-axis represents the relative difference in predialysis solute levels. A negative value implies a lower solute level in the group with RKF or high-dose HD in relation to those without RKF or standard-dose HD, respectively. The table in the figure shows the mean relative difference of each solute level rounded to the nearest whole number, with bolded values indicating statistical significance (P < 0.05). P-values and CIs were calculated by bootstrapping with replacement and 2000 repetitions. *Denotes P < 0.05 (RKF versus non-RKF and high versus low Kt/Vurea). αAs published by Meyer et al. [20].
Sensitivity analyses
Urine collection was performed in 40% of the 433 participants who had RKF at baseline and served as a sensitivity analysis cohort (Supplementary data, Table S4). Similar to our primary analysis, solute levels were lower in six of eight solutes in the group that maintained RKF over the 1 year of follow-up compared with the group without RKF. These results remained significant after adjustment for age, sex, race and dialysis dose intervention group (Supplementary data, Table S5).
Association between RKF and outcomes
The incidence of any-cause death, cardiac death or first cardiovascular event (composite of first cardiovascular hospitalization or death from any cause) per 1000 person-years was 152.4, 59.3 and 265.4, respectively (Table 4). RKF at baseline was associated with a trend toward 14% lower risk of cardiovascular events. Preserved RKF (presence of RKF at baseline and Year 1) was associated with a 19% lower risk of death, 25% lower risk of cardiac death and 16% lower risk of first cardiovascular event.
Table 4.
Association of outcomes and RKF at baseline and 1-year follow-up
| Residual kidney function at baseline | Any-cause death |
Cardiac death |
First cardiovascular event or any-cause death |
|||
|---|---|---|---|---|---|---|
| n = 1280 | n = 1280 | n = 1280 | ||||
| Events = 568 | Events = 221 | Events = 737 | ||||
| IR/1000 PY = 152.4 |
IR/1000 PY = 59.3 |
IR/1000 PY = 265.4 |
||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Unadjusted | 0.83 (0.63–1.10) | 0.19 | 0.84 (0.62–1.13) | 0.25 | 0.82 (0.67–1.00) | 0.05 |
| Age + sex + race | 0.81 (0.65–1.01) | 0.06 | 0.81 (0.61–1.06) | 0.12 | 0.83 (0.70–0.98) | 0.03 |
| Model 2 + ICED + cause + BMI + DM | 0.84 (0.66–1.07) | 0.16 | 0.78 (0.57–1.05) | 0.1 | 0.86 (0.73–1.01) | 0.07 |
| Residual kidney function at baseline and year 1 | Any-cause death |
Cardiac death |
First cardiovascular event or any-cause death |
|||
|---|---|---|---|---|---|---|
| n = 1212 | n = 1214 | n = 1214 | ||||
| Events = 548 | Events = 214 | Events = 702 | ||||
| IR/1000 PY = 155.2 |
IR/1000 PY = 60.6 |
IR/1000 PY = 267.7 |
||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Unadjusted | 0.81 (0.61–1.08) | 0.15 | 0.81 (0.60–1.09) | 0.16 | 0.81 (0.66–0.99) | 0.04 |
| Age + sex + race | 0.77 (0.62–0.97) | 0.02 | 0.76 (0.59–0.98) | 0.04 | 0.80 (0.68–0.95) | 0.01 |
| Model 2 + ICED + cause + BMI + DM | 0.81 (0.64–1.03) | 0.09 | 0.75 (0.56–0.99) | 0.04 | 0.84 (0.71–1.00) | 0.046 |
HR represents risk in patients with RKF compared with those without RKF. RKF was defined as urine volume ≥250 mL/24 h.
IR, incidence rate; PY, person-years; HR, hazard ratio; Cause, cause of ESRD; BMI, body mass index; DM, diabetes.
DISCUSSION
In this study from a large, national, multicenter study of prevalent HD patients, we demonstrate that seven of eight nonurea solute concentrations are lower in those with RKF compared with those without RKF, despite a mean urea clearance of only 0.7 mL/min/35 L TBW in those with RKF. The concentrations ranged from 24% lower for HIPP to 3.7% lower for ADMA. These findings are particularly significant since the HEMO study included only patients with a ‘clinically negligible’ degree of RKF as demonstrated by a mean residual urea clearance of 0.7 mL/min among those with RKF. The effect of RKF was confirmed in sensitivity analyses.
It is well established that mortality risk is lower among HD patients with RKF. Results from the Netherlands Cooperative Study on the Adequacy of Dialysis 2, the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) study and a recent study using data from a dialysis organization in the USA support the idea that all other factors being equal, patients that are best able to preserve their RKF after initiating HD are likely to live longer than those that are anuric near the start of dialysis or lose RKF at a rapid rate [2–4]. However, negligible RKF remains arbitrarily defined across study populations and dialysis adequacy guidelines. At times it has been defined by urine volume, whereas at other times it is defined by a minimal glomerular filtration rate or urea clearance [2, 3, 12, 17, 22].
When we compared the relative difference of uremic solute levels among those with RKF and those without RKF with patients randomized to high-dose HD (Kt/Vurea 1.7) versus standard-dose HD (Kt/Vurea 1.3), we found that uremic solute concentrations were significantly lower in those with RKF compared with those receiving standard-dose HD. We also found that preserved RKF at 1 year was associated with lower risk of death, cardiac death and first cardiovascular event. These findings are consistent with prior studies of RKF in the CHOICE study and analyses from a large dialysis organization [3, 4] and suggest that a lower burden of uremic toxins may be responsible for this observed association.
Previous studies have shown that secretory clearance may be preserved in the residual kidneys of HD patients [11, 23]. Our work supports the idea that RKF provides an added level of continuous clearance of solutes, either by filtration or tubular secretion, which is not provided by HD. This was demonstrated by higher levels of solutes that are normally secreted by the kidneys, including HIPP, PAG, IS, TMAO and ADMA, in anuric patients (Table 3) [24–26]. This was also true for IS, a solute whose excretion correlates highly with the degree of filtration and whose protein-bound state makes it less available for clearance by HD [23]. Additionally, both ADMA and TMAO levels were lower in those with RKF. Higher levels of ADMA and TMAO levels were associated with adverse outcomes in previous HEMO analyses; although no effect modification was seen by RKF, this could have been due to the sample size. The inability of conventional HD to effectively control the levels of these solutes may contribute to the increased risk of cardiovascular disease and all-cause mortality seen in the HD patient population and may provide an explanation for why even small amounts of RKF in prior studies were associated with better outcomes [2, 3]. In effect, native kidneys provide a level of clearance that is not provided by HD. This may partially explain the survival benefit seen in HD patients with RKF and should be studied in further research.
One solute in particular stands out from the rest, PCS. Notably, it was the only solute with a higher serum level in subjects with RKF compared with those without RKF. Our results are consistent with prior studies demonstrating that plasma PCS levels are largely independent of the rate of dialytic PCS removal in patients with ESRD [20, 27, 28]. By and large, increasing HD intensity to decrease plasma levels of uremic toxins has been largely unsuccessful and was recently reviewed in detail [29]. While the exact biological reason for this observation remains unexplained, it is hypothesized that the gut microbiome is altered in those with chronic kidney disease and ESRD, resulting in elevated PCS, IS, PAG and TMAO levels by increased generation or retention in the gut [25, 27, 28, 30–38]. As the relationship between the gut microbiome and kidney disease is further elucidated, it is becoming increasingly evident that the use of prebiotics and probiotics may play an important role in reducing systemic levels of uremic toxins [38–40]. Additionally, further attention is warranted to adsorbents such as AST-120 (Kremezin), an oral adsorbent of PCS and IS precursors [41], and to dialyzers incorporating a hollow fiber mixed matrix membrane capable of removing certain uremic toxins [42]. However, further studies are needed before these approaches can be widely accepted and implemented.
A few limitations of our study deserve mention. RKF was assessed once at baseline, whereas solutes were measured at a single time point 3–8 months later. Although a certain degree of differential misclassification is likely to have occurred as a result of loss of RKF during this time period, our methodology ensured the most conservative assessment of solute level differences in the patient population and suggests that the true effect size of RKF may actually be stronger. To address this limitation, we analyzed our data using baseline urine volume (≥250 mL/day) and residual urea clearance at 1-year follow-up and found similar effects on solute levels.
Although we assessed eight well-described uremic solutes based on prior known associations with increased cardiovascular disease or mortality in HD populations, other uremic solutes likely exist and may behave differently [20, 43–46]. Additionally, despite various adjustments, it remains possible that people with RKF are less sick in unmeasured ways and hence the possibility of residual confounding remains. These limitations are balanced by the major strengths of our study, including its large sample size and careful collection of samples [20, 43–46].
The clinical implications of our findings are multifold and highlight the idea that patients with RKF are unique and should be managed differently from those with no RKF. In current practice, dialyzers have become supremely efficient at urea clearance and allow a goal Kt/Vurea to be achieved without the need to account for RKF. Although the KDOQI HD guidelines support routine measurement of RKF, there is no reimbursement model to support the implementation of this practice. Given what is known about the survival benefit associated with RKF in HD patients, it may be worthwhile to incorporate RKF measurement as a quality and standard-of-care metric in order to ensure that RKF is measured (and possibly preserved) in this unique patient population. Additionally, implementing an individualized approach to HD that accounts for RKF in incident HD patients, such as incremental HD, should be more frequently employed [47].
In conclusion, a degree of RKF that was previously considered ‘clinically negligible’ provides a degree of uremic solute clearance that is not provided by HD. Clinical trials dedicated to investigating methods that prolong RKF in incident HD patients are warranted. In the meantime, clinicians caring for these patients should consider an individualized approach to HD prescription that takes into account routine RKF measurement.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the HEMO study participants, investigators and staff for their contributions to the HEMO study.
FUNDING
S.M.T-M. is supported by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) T32 DK 007732. T.L.S. is supported by a Veterans Affairs Career Development Award (CX001036-01A1). T.W.M. is supported by NIH R01 DK101674-01. T.S. is supported by RO3-DK-104012, R01-HL-132372, U01-DK-061022 and UH2-AG-056933. N.R.P. is supported by NIH NIDDK U01 DK106965. T.H.H., S.H., N.S.P., X.H. and J.C. do not report any funding sources.
CONFLICT OF INTEREST STATEMENT
T.W.M. received personal fees from Mitsubishi Tanabe Pharma. T.H. received personal fees from Tricida. The remaining authors declared no conflicts of interest.
(See related article by Koppe and Soulage. Preservation of residual kidney function to reduce non-urea solutes toxicity in haemodialysis. Nephrol Dial Transplant 2020; 35: 733--736)
AUTHORS’ CONTRIBUTIONS
All authors contributed to the conception or design, analysis, interpretation of data or a combination of the above. S.M.T-M. and T.S. drafted the article and all other authors contributed toward its revision. All authors provided intellectual content of critical importance to this work and gave final approval to the version submitted for publication. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol 1996; 7: 198–207 [DOI] [PubMed] [Google Scholar]
- 2. Termorshuizen F, Dekker FW, van Manen JG. et al. Relative contribution of residual renal function and different measures of adequacy to survival in hemodialysis patients: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD)-2. J Am Soc Nephrol 2004; 15: 1061–1070 [DOI] [PubMed] [Google Scholar]
- 3. Shafi T, Jaar BG, Plantinga LC. et al. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for End-Stage Renal Disease (CHOICE) study. Am J Kidney Dis 2010; 56: 348–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Obi Y, Rhee CM, Mathew AT. et al. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol 2016; 27: 3758–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vilar E, Farrington K.. Emerging importance of residual renal function in end-stage renal failure. Semin Dial 2011; 24: 487–494 [DOI] [PubMed] [Google Scholar]
- 6. Vilar E, Wellsted D, Chandna SM. et al. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant 2009; 24: 2502–2510 [DOI] [PubMed] [Google Scholar]
- 7. López-Menchero R, Miguel A, García-Ramón R. et al. Importance of residual renal function in continuous ambulatory peritoneal dialysis: its influence on different parameters of renal replacement treatment. Nephron 1999; 83: 219–225 [DOI] [PubMed] [Google Scholar]
- 8. Wang AY, Woo J, Wang M. et al. Important differentiation of factors that predict outcome in peritoneal dialysis patients with different degrees of residual renal function. Nephrol Dial Transplant 2005; 20: 396–403 [DOI] [PubMed] [Google Scholar]
- 9. Fry AC, Singh DK, Chandna SM. et al. Relative importance of residual renal function and convection in determining beta-2-microglobulin levels in high-flux haemodialysis and on-line haemodiafiltration. Blood Purif 2007; 25: 295–302 [DOI] [PubMed] [Google Scholar]
- 10. Bammens B, Evenepoel P, Verbeke K. et al. Removal of middle molecules and protein-bound solutes by peritoneal dialysis and relation with uremic symptoms. Kidney Int 2003; 64: 2238–2243 [DOI] [PubMed] [Google Scholar]
- 11. Marquez IO, Tambra S, Luo FY. et al. Contribution of residual function to removal of protein-bound solutes in hemodialysis. Clin J Am Soc Nephrol 2011; 6: 290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis 2015; 66: 884–930 [DOI] [PubMed] [Google Scholar]
- 13. Kt/V Dialysis Adequacy Measure Topic: Hemodialysis https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/ESRDQIP/Downloads/ESRDQIPPY2018finaltechnicalmeasurespecifications-.pdf (15 January 2018, date last accessed)
- 14. Eloot S, Schneditz D, Vanholder R.. What can the dialysis physician learn from kinetic modelling beyond Kt/Vurea? Nephrol Dial Transplant 2012; 27: 4021–4029 [DOI] [PubMed] [Google Scholar]
- 15. Vanholder R, Glorieux G, Eloot S.. Once upon a time in dialysis: the last days of Kt/V? Kidney Int 2015; 88: 460–465 [DOI] [PubMed] [Google Scholar]
- 16. Daugirdas JT. Kt/V (and especially its modifications) remains a useful measure of hemodialysis dose. Kidney Int 2015; 88: 466–473 [DOI] [PubMed] [Google Scholar]
- 17. Eknoyan G, Beck GJ, Cheung AK. et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 2002; 347: 2010–2019 [DOI] [PubMed] [Google Scholar]
- 18. Shafi T, Powe NR, Meyer TW. et al. Trimethylamine N-oxide and cardiovascular events in hemodialysis patients. J Am Soc Nephrol 2017; 28: 321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greene T, Beck GJ, Gassman JJ. et al. Design and statistical issues of the Hemodialysis (HEMO) study. Control Clin Trials 2000; 21: 502–525 [DOI] [PubMed] [Google Scholar]
- 20. Meyer TW, Sirich TL, Fong KD. et al. Kt/Vurea and nonurea small solute levels in the hemodialysis study. J Am Soc Nephrol 2016; 27: 3469–3478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sirich TL, Aronov PA, Plummer NS. et al. Numerous protein-bound solutes are cleared by the kidney with high efficiency. Kidney Int 2013; 84: 585–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clinical practice guidelines for peritoneal dialysis adequacy. Am J Kidney Dis 2006; 48(Suppl 1): S98–S129 [DOI] [PubMed] [Google Scholar]
- 23. Lowenstein J, Grantham JJ.. Residual renal function: a paradigm shift. Kidney Int 2017; 91: 561–565 [DOI] [PubMed] [Google Scholar]
- 24. Brooks DP, Rhodes GR, Woodward P. et al. Production of methylguanidine in dogs with acute and chronic renal failure. Clin Sci 1989; 77: 637–641 [DOI] [PubMed] [Google Scholar]
- 25. Tomlinson JAP, Wheeler DC.. The role of trimethylamine N-oxide as a mediator of cardiovascular complications in chronic kidney disease. Kidney Int 2017; 92: 809–815 [DOI] [PubMed] [Google Scholar]
- 26. Suchy-Dicey AM, Laha T, Hoofnagle A. et al. Tubular secretion in CKD. J Am Soc Nephrol 2016; 27: 2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Camacho O, Rosales MC, Shafi T. et al. Effect of a sustained difference in hemodialytic clearance on the plasma levels of p-cresol sulfate and indoxyl sulfate. Nephrol Dial Transplant 2016; 31: 1335–1341 [DOI] [PubMed] [Google Scholar]
- 28. Sirich TL, Fong K, Larive B. et al. Limited reduction in uremic solute concentrations with increased dialysis frequency and time in the Frequent Hemodialysis Network Daily Trial. Kidney Int 2017; 91: 1186–1192 [DOI] [PubMed] [Google Scholar]
- 29. Mair RD, Sirich TL, Meyer TW.. Uremic toxin clearance and cardiovascular toxicities. Toxins 2018; 10: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al Khodor S, Shatat IF.. Gut microbiome and kidney disease: a bidirectional relationship. Pediatr Nephrol 2017; 32: 921–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ramezani A, Raj DS.. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 2014; 25: 657–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rossi M, Johnson DW, Morrison M. et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol 2016; 11: 223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hida M, Aiba Y, Sawamura S. et al. Inhibition of the accumulation of uremic toxins in the blood and their precursors in the feces after oral administration of Lebenin, a lactic acid bacteria preparation, to uremic patients undergoing hemodialysis. Nephron 1996; 74: 349–355 [DOI] [PubMed] [Google Scholar]
- 34. Mishima E, Fukuda S, Shima H. et al. Alteration of the intestinal environment by lubiprostone is associated with amelioration of adenine-induced CKD. J Am Soc Nephrol 2015; 26: 1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakabayashi I, Nakamura M, Kawakami K. et al. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: a preliminary study. Nephrol Dial Transplant 2011; 26: 1094–1098 [DOI] [PubMed] [Google Scholar]
- 36. Mihai S, Codrici E, Popescu ID. et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res 2018; 2018: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xie A, Sheng J, Zheng F.. Intestinal microbiota and kidney diseases. Chin J Integr Med 2018; 24: 406–408 [DOI] [PubMed] [Google Scholar]
- 38. Cavalcanti Neto MP, Aquino JS, Romão da Silva LF. et al. Gut microbiota and probiotics intervention: a potential therapeutic target for management of cardiometabolic disorders and chronic kidney disease? Pharmacol Res 2018; 130: 152–163 [DOI] [PubMed] [Google Scholar]
- 39. Lopes R, Balbino KP, Jorge MP. et al. Modulation of intestinal microbiota, control of nitrogen products and inflammation by pre/probiotics in chronic kidney disease: a systematic review. Nutr Hosp 2018; 35: 722–730 [DOI] [PubMed] [Google Scholar]
- 40. Yang CY, Tarng DC.. Diet, gut microbiome and indoxyl sulphate in chronic kidney disease patients. Nephrology 2018; 23(Suppl 4): 16–20 [DOI] [PubMed] [Google Scholar]
- 41. Liu WC, Tomino Y, Lu KC.. Impacts of indoxyl sulfate and p-cresol sulfate on chronic kidney disease and mitigating effects of AST-120. Toxins 2018; 10: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tijink MS, Wester M, Glorieux G. et al. Mixed matrix hollow fiber membranes for removal of protein-bound toxins from human plasma. Biomaterials 2013; 34: 7819–7828 [DOI] [PubMed] [Google Scholar]
- 43. Sirich TL, Funk BA, Plummer NS. et al. Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J Am Soc Nephrol 2014; 25: 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schepers E, Speer T, Bode-Böger SM. et al. Dimethylarginines ADMA and SDMA: the real water-soluble small toxins? Semin Nephrol 2014; 34: 97–105 [DOI] [PubMed] [Google Scholar]
- 45. Vanholder R, Schepers E, Pletinck A. et al. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol 2014; 25: 1897–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tang WH, Wang Z, Kennedy DJ. et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res 2015; 116: 448–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Toth-Manikowski SM, Mullangi S, Hwang S. et al. Incremental short daily home hemodialysis: a case series. BMC Nephrol 2017; 18: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.