Abstract
Background
Phosphate (Pi) toxicity is a strong determinant of vascular calcification development in chronic kidney disease (CKD). Magnesium (Mg2+) may improve cardiovascular risk via vascular calcification. The mechanism by which Mg2+ counteracts vascular calcification remains incompletely described. Here we investigated the effects of Mg2+ on Pi and secondary crystalline calciprotein particles (CPP2)-induced calcification and crystal maturation.
Methods
Vascular smooth muscle cells (VSMCs) were treated with high Pi or CPP2 and supplemented with Mg2+ to study cellular calcification. The effect of Mg2+ on CPP maturation, morphology and composition was studied by medium absorbance, electron microscopy and energy dispersive spectroscopy. To translate our findings to CKD patients, the effects of Mg2+ on calcification propensity (T50) were measured in sera from CKD patients and healthy controls.
Results
Mg2+ supplementation prevented Pi-induced calcification in VSMCs. Mg2+ dose-dependently delayed the maturation of primary CPP1 to CPP2 in vitro. Mg2+ did not prevent calcification and associated gene and protein expression when added to already formed CPP2. Confirmatory experiments in human serum demonstrated that the addition of 0.2 mmol/L Mg2+ increased T50 from healthy controls by 51 ± 15 min (P < 0.05) and CKD patients by 44 ± 13 min (P < 0.05). Each further 0.2 mmol/L addition of Mg2+ led to further increases in both groups.
Conclusions
Our results demonstrate that crystalline CPP2 mediates Pi-induced calcification in VSMCs. In vitro, Mg2+ delays crystalline CPP2 formation and thereby prevents Pi-induced calcification.
Keywords: calciprotein particle, calcification propensity, chronic kidney disease, magnesium, vascular calcification
INTRODUCTION
Vascular calcification contributes to cardiovascular morbidity and mortality in chronic kidney disease (CKD) [1]. Currently treatment options are limited and persisting vascular calcification remains a clinical problem. In past years, several epidemiological studies have shown that a lower serum magnesium (Mg2+) status is independently associated with an increased risk of vascular calcification and cardiovascular mortality in CKD patients [2–4]. Therefore it is hypothesized that Mg2+ could be an effective tool to limit vascular calcification [5]. However, the mechanisms underlying the potent anti-calcification properties of Mg2+ are incompletely understood.
Hydroxyapatite- and protein-containing calciprotein particles (CPPs) are major drivers of calcification [6, 7]. The transition from calcium (Ca2+)- and phosphate (Pi)-containing amorphous or primary CPP1 towards crystalline or secondary CPP2 is key in the development of vascular smooth muscle cell (VSMC) calcification [6, 8]. Due to disturbances in the bone–mineral axis, resulting from in elevated Pi and decreased calcification inhibitors such as matrix gla protein (MGP) and fetuin-A, high Pi levels crystallize into CPP2 [7, 9]. These events result in active reprogramming of VSMCs, which in turn enhances the calcification process by up-regulating osteogenic genes, producing excess extracellular matrix, undergoing apoptosis and releasing pro-calcific exosomes [10–12].
Since VSMCs may activate osteogenic pathways that contribute to calcification, the capacity of Mg2+ to inhibit vascular calcification may rely on direct modulation of these cellular processes [13]. Indeed, multiple studies demonstrate that Mg2+ did not inhibit calcification upon transient receptor potential melastatin 7 blocking, an abundant Mg2+ channel in VSMCs, implying that intracellular Mg2+ prevents calcification [14, 15]. In addition, Mg2+ supplementation in vitro correlates with reduced expression of pro-calcification genes and with increased expression of calcification protectors [16–18]. On the contrary, in the extracellular compartment, Mg2+ has potent anti-crystallization properties, which have been shown to be of importance in its capacity to prevent VSMC calcification in vitro [19–22].
The importance of CPP2 in the development of vascular calcification has been exploited in a novel diagnostic tool where the intrinsic capacity of patient serum to prevent the transition from CPP1 to CPP2, or calcification propensity of the serum, can be measured using the T50 test [7, 23, 24]. The identification of factors affecting T50 is of interest in the context of clinical management of vascular calcification, as these factors may influence the development and progression of vascular calcification in renal disease patients [23, 25–27]. T50 is correlated with cardiovascular mortality and is affected by Pi [23]. Therefore, whether Pi toxicity resulting in increased risk for vascular calcification is determined by the presence of soluble Pi or that Pi toxicity is mediated by crystallization in CPP2 is important to consider.
In this study we aimed to delineate the mechanisms that explain the effects of Mg2+ on VSMC calcification. In our study we induced VSMC calcification using both Pi and CPP2, which allows comparison of the direct and indirect effects of Mg2+ supplementation on VSMC calcification. Using scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDX), we studied CPP transition, morphology and composition in the presence and absence of Mg2+in vitro. Furthermore, we studied the influence of Mg2+ on calcification propensity in serum from CKD patients and healthy controls ex vivo.
MATERIALS AND METHODS
Cell culture
Human aortic VSMCs (hVSMCs) were purchased from the American Type Culture Collection (Manassas, VA, USA, lot no. PCS-100-012) and cultured in Dulbecco’s Modified Eagle Medium (DMEM; Lonza, Basel, Switzerland) consisting of 20% (v/v) foetal bovine serum (FBS; BioWest, GE Healthcare, Little Chalfont, UK), 2 mmol/L L-glutamine and 10 μg/mL ciprofloxacin at 37°C in a humidified incubator containing 5% carbon dioxide (CO2; v/v). Cells were used until the 10th passage. For calcification experiments, cells were cultured in 12-well plates and switched to calcification medium at subconfluence. Calcification medium consisted of 5% FBS (v/v) and 3 mmol/L Pi (NaH2PO4), and 2 mmol/L Mg2+ when indicated. Cells were cultured for up to 8 days. The medium was changed every 2–3 days.
CPP synthesis, isolation and induction of calcification
Phenol red-free DMEM (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) was supplemented with 3.5 mmol/L NaH2PO4, 1 mmol/L calcium chloride, 10% FBS (v/v), 2 mmol/L l-glutamine and 10 μg/mL ciprofloxacin and stored in a humidified incubator at 37°C containing 5% CO2 (v/v) for 14 days. For isolation of CPP2, CPP-containing media were centrifuged at 16 000 g for 30 min at room temperature. The pellet was resuspended in DMEM containing 10 μg/mL ciprofloxacin and stored at 4°C. The Ca2+ content of the CPP2 suspension was measured using the o-cresolphthalein complexone method as described elsewhere [19]. Cells were treated with CPP2 at a final concentration ranging from 5 to 100 μg/mL Ca2+ (5–100 CPP). Cells were cultured in their designated media for up to 4 days without a change of media.
Analysis of VSMC calcification
Quantification of total cellular Ca2+ deposition using the o-cresolphthalein complexone method and alizarin red staining were performed as previously described [19].
Gene expression analysis
Total RNA was isolated from hVSMCs using TRIzol (Invitrogen, Thermo Fisher Scientific) according to the manufacturer’s instructions. Genomic DNA was removed by DNAse treatment prior to omplementary DNA synthesis from 1 μg total RNA (Promega, Madison, WI, USA). The primers used for polymerase chain reaction (PCR) amplification were equally efficient (Supplementary data, Table S1). Reverse transcription quantitative PCR was executed in duplicate using IQ SYBRGreen Mix (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s protocol in a Bio-Rad thermocycler. The expression levels of genes of interest were normalized to GAPDH expression levels.
Protein expression analysis
To prepare total lysate of hVSMCs, the hVSMC monolayer was scraped in a 1% (v/v) Triton-X100 lysis buffer containing protease inhibitors. The protein concentration was determined using the Pierce BCA Kit according to the manufacturer’s instructions (Thermo Fisher Scientific Waltham). Subsequently samples consisting of equal amounts of protein were denatured in Laemmli buffer containing 10 mmol/L dithiothreitol and applied to sodium dodecyl sulphate–polyacrylamide gel electrophoresis. Blots were incubated at 4°C overnight with primary antibodies against osteopontin (OPN; 1:1000; R&D Systems, Minneapolis, MN, USA; #MAB14331-100), transgelin (SM22α; 1:5000; Abcam, Cambridge, UK; #ab14106) and MGP (1:500; Proteintech, Rosemont, IL; #10734-1-AP). Blots were subsequently incubated with horseradish peroxidase conjugated secondary antibodies for 1 h at room temperature. Band intensity was measured using ImageJ software (National Institutes of Health, Bethesda, MD, USA) and expression was corrected for β-actin and expression levels per group were normalized to the control.
Electron microscopy
CPPs were transferred onto copper tape, coated with carbon and used for SEM analysis (GeminiSEM, Zeiss, Oberkochen, Germany) and EDX for elemental analysis (QUANTAX 200, Bruker, Billerica, MA, USA). Images were obtained using an Everhart–Thornley secondary electron detector (Bruker) at 5 kV for morphological observations and 20 kV for microelemental analyses. For transmission EM (TEM), the CPP solution was transferred onto a Formvar-coated copper grid and air dried. TEM was performed on a JEOL JEM 1400 microscope (JEOL USA, Peabody, MA, USA) with an accelerating voltage of 60 kV. Images were acquired at 15 000-fold magnification (Gatan, Pleasanton, CA, USA).
CPP maturation assay
CPP2 were generated in phenol red-free DMEM (Gibco) containing 10% FBS and MgCl2 was added to reach 1.0, 1.2, 1.4, 1.6, 1.8 and 2.0 mmol/L. As a reference, medium containing 10% FBS (v/v) was included. CPP2 maturation was monitored by daily measurement of the absorption at 570 nm for 14 days using a Benchmark Plus Microplate Spectrophotometer System (Bio-Rad) [6]. A separate group where MgCl2 was added only after absorption exceeded 0.15 (after 5 days) was included to study the effects of Mg2+ on already formed CPP2 and monitored until complete ripening after 14 days. To study the effects of high Pi on the inhibition of CPP2 by 2.0 mmol/L Mg2+, CPP2 maturation was monitored in the presence of 4 and 5 mmol/L Pi (final concentration) in addition to the standard CPP mixture in a separate experiment. To test a potential role for Mg2+ in altering the pH of the CPP mixtures, pH was measured for the CPP mixtures without Mg2+ and with 2.0 mmol/L Mg2+ at 0, 7 and 14 days of incubation.
Calcification propensity (T50 test) in serum samples supplemented with Mg2+
Serum samples collected prior to transplantation from renal transplant recipients (CKD patients) and donors (healthy controls) of the TransplantLines cohort study (METc UMCG No. 2014/077, NTC03272841) were used in this study [28]. Blood from 10 subjects per group was collected in 4 mL serum collection tubes and centrifuged at 1300 g for 10 min at room temperature. Serum was stored at −80°C until analysis. The T50 test (calcification propensity) has been described previously [24]. Serum was subjected to the T50 test without any additions (baseline) and after addition of solutions with MgCl2 resulting in increased concentrations of 0.2, 0.4, 0.6, 0.8 and 1.0 mmol/L. After 570 min, the test was terminated. Other laboratory measurements were performed during routine clinical visits prior to serum collection for this study (Supplementary data, Table S2).
Statistical analysis
Parametric data were analysed by one-way analysis of variance (ANOVA) with Tukey’s post hoc test to correct for multiple comparisons using PRISM software (GraphPad Software, San Diego, CA, USA). Non-parametric data as identified by the Shapiro–Wilk test for normality were analysed using Kruskal–Wallis analysis with Dunn’s correction for multiple comparisons. For grouped analysis, data were analysed using a two-way ANOVA. All data are shown as mean ± standard deviation (SD). P-values <0.05 were considered statistically significant.
RESULTS
Mg2+ prevents high Pi–induced VSMC calcification
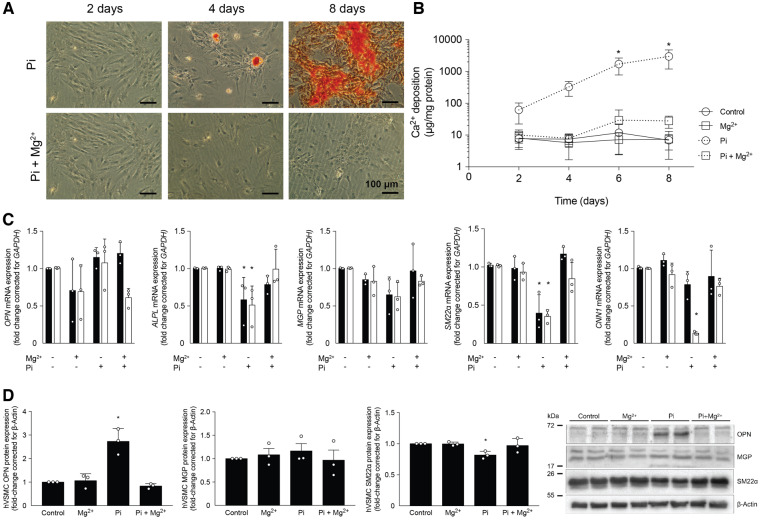
To study the preventive effect of Mg2+ on in vitro calcification, VSMCs were cultured in Pi medium and supplemented with MgCl2 (Figure 1). VSMCs cultured in high Pi showed calcification, as visualized by the alizarin red staining (Figure 1A) and as quantified by the o-cresolphthalein complexone method for Ca2+ (Figure 1B). VSMCs cultured in high Pi supplemented with Mg2+ stained negative for alizarin red and Ca2+ deposition was reduced by >100-fold versus high Pi–treated VSMCs after 8 days (28 ± 12 versus 2990 ± 1800 μg Ca2+/mg protein; P < 0.05). The preventive effect of Mg2+ on hVSMC calcification was preserved at 4 and 5 mmol/L Pi (Supplementary data, Figure S1). To evaluate the effect of Mg2+ on genetic changes induced by calcification in VSMCs, the gene expression of calcification inducers alkaline phosphatase (ALP) and OPN and calcification inhibitor MGP in addition to contractile markers calponin (CNN1) and SM22α were studied (Figure 1C). Compared with control VSMCs, VSMCs cultured in 3 mmol/L Pi had a 40–60% reduction in ALP and SM22α gene expression after both 6 and 8 days of incubation (P < 0.05). MGP mRNA expression remained stable. After high Pi treatment for 8 days, VSMCs expressed only ∼20% of CNN1 gene expression compared with control VSMCs (P < 0.05). Pi-treated VSMCs did not change OPN gene expression. Mg2+ supplementation in addition to high Pi preserved the levels of MGP, ALP, SM22α and CNN1 gene expression versus control VSMCs. VSMCs treated with 2 mmol/L Mg2+ alone and in combination with 3 mmol/L Pi showed a non-significant downregulation of OPN after 8 days. On a protein level, OPN was increased 2-fold by Pi treatment (P < 0.05). Furthermore, SM22α protein expression was decreased after 8 days of Pi treatment. In high Pi–treated hVSMCs supplemented with Mg2+, both the increased OPN and decreased SM22α protein expression were normalized. MGP protein expression remained stable in all treatment groups (Figure 1D).
FIGURE 1.
Mg2+ prevents Pi-induced calcification in hVSMCs. (A) Alizarin red staining was negative in Mg2+-supplemented Pi cultures, indicating the absence of calcification after 2, 4 and 8 days. (B) Ca2+ deposition on VSMCs was increased in Pi-treated cells compared with controls, but was absent after 2.0 mmol/L Mg2+ supplementation. (C) Pi-induced calcification decreased ALP and non-significantly decreased calcification inhibitor MGP and VSMC contractile genes SM22α and CNN1 after 6 days (black bars) and 8 days (white bars) of incubation, which was prevented by Mg2+. (D) Protein expression of OPN, SM22α and MGP was measured and quantified. Data are shown as fold changes compared with the day-matched controls and presented as the mean of three separate experiments, consisting of three replicate cultures ± SD. *P < 0.05 versus day-matched control.
CPPs induce VSMC calcification independent of Mg2+
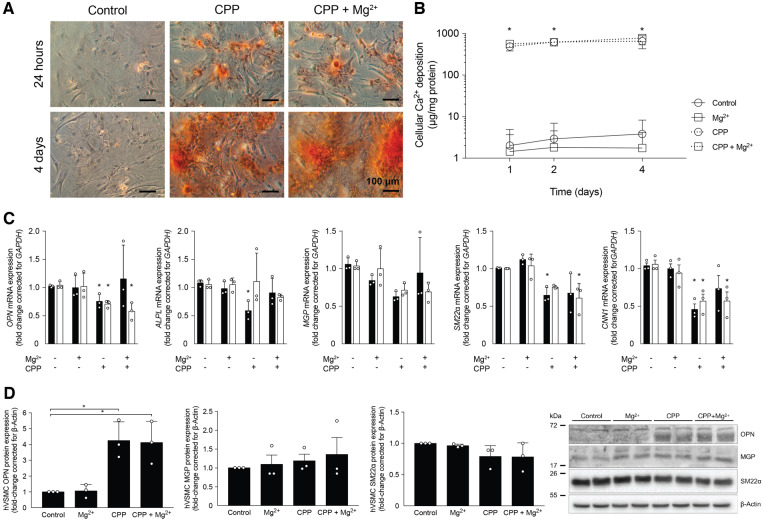
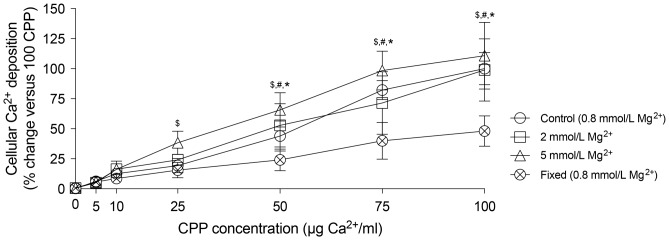
To be able to distinguish between intracellular and extracellular actions of Mg2+, the effects of Mg2+ on CPP2-induced VSMC calcification were assessed (Figure 2). Alizarin red staining (Figure 2A) and Ca2+ deposition measurements (Figure 2B) showed increased calcification after 24 h in VSMCs treated with 50 CPP2 (488 ± 62 versus 2 ± 2 μg Ca2+/mg protein; P < 0.05). Mg2+ supplementation to CPP2-treated VSMCs did not reduce Ca2+ deposition compared with CPP2-only treated VSMCs after 24 h (488 ± 62 versus 565 ± 62 μg Ca2+/mg protein) and 4 days (657 ± 132 versus 783 ± 142 μg Ca2+/mg protein, respectively). CPP2 treatment decreased CNN1 gene expression by almost 50% after both 2 days and 4 days (Figure 2C; P < 0.05). CPP2 treatment resulted in downregulation of OPN and ALP after 2 days, which seemed to be prevented by Mg2+. SM22α mRNA expression was decreased after 2 days of CPP treatment and after 4 days of Mg2+-supplemented CPP2 treatment. Protein concentrations of OPN were increased ∼4-fold in both CPP2-treated hVSMCs with and without 2 mmol/L Mg2+ (Figure 2D). SM22α protein expression showed a non-significant trend towards a reduction in CPP2-treated cells independent of Mg2+. MGP protein expression was stable in all treatment groups. To test whether Mg2+ might prevent CPP-induced calcification at lower CPP2 concentrations, Mg2+ was supplemented to VSMCs treated with a broad range of CPP2 dosages (Figure 3). Both 2 and 5 mmol/L Mg2+ did not reduce VSMC Ca2+ deposition at any of the CPP2 dosages, ranging from 5 to 100 CPP. Compared with CPP2 treatment containing no additional Mg2+, supplementation of 5 mmol/L Mg2+ resulted in increased Ca2+ deposition at 25, 50 and 75 CPP2 [82 ± 5% versus 99 ± 16% at 75 CPP (P < 0.05), respectively, where 100 CPP2 with no additional Mg2+ is set to 100% calcification].
FIGURE 2.
Mg2+ does not prevent CPP2-induced calcification in hVSMCs. (A) Alizarin red staining and (B) Ca2+ deposition quantification show that CPP2-induced calcification is not prevented by 2.0 mmol/L Mg2+ after 24 h and 4 days. (C) Gene expression related to calcification after CPP2 treatment was measured after 2 days (black bars) and 4 days (white bars) and remained unchanged by Mg2+. (D) Protein expression of OPN, SM22α and MGP was measured and quantified. Data are shown as fold changes compared with the day-matched controls and presented as the mean of three separate experiments, consisting of three replicate cultures ± SD. *P < 0.05.
FIGURE 3.
High Mg2+ concentration stimulates CPP2-induced calcification. Ca2+ deposition was measured after incubation of VSMCs for 24 h with varying CPP2 concentrations in the presence of 0.8 (control), 2 and 5 mmol/L Mg2+. Mg2+ did not prevent CPP2-induced calcification at any CPP concentration. At 5 mmol/L, Mg2+ increased cellular Ca2+ deposition at CPP2 concentrations of 25, 50 and 75 μg/mL Ca2+ versus CPP2 treatment without additional Mg2+. Data are shown as fold changes compared with 100 CPP2 (set at 100%). Presented data are the mean of three separate experiments consisting of three replicate cultures ± SD. P < 0.05 for CPP2 incubated with *0.8, #2 and $5 mmol/L Mg2+ versus CPP2 concentration-matched formalin-fixed cultures.
Mg2+ dose-dependently prevents secondary CPP maturation
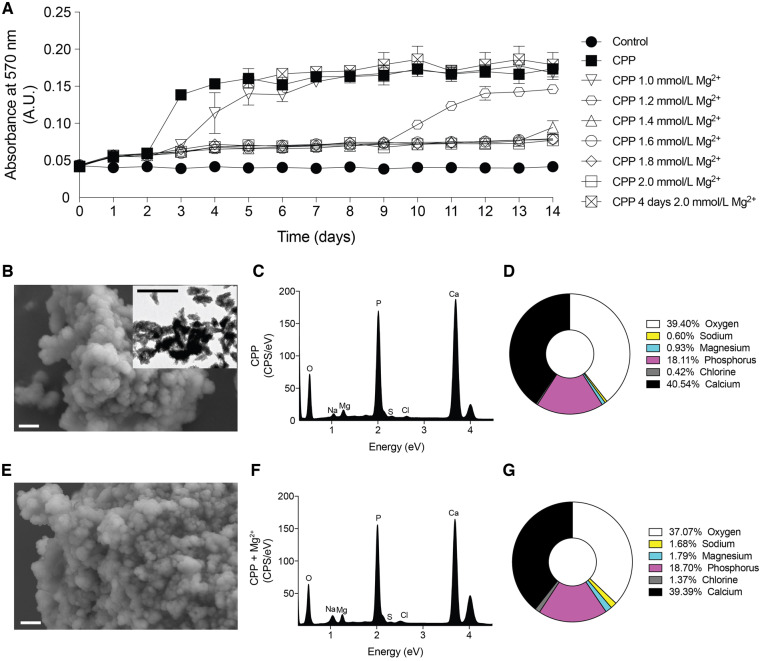
To study the action of Mg2+ on CPP transition in vitro, CPP medium was incubated to form CPP1 and CPP2 in the presence of a range of MgCl2 concentrations. CPP medium showed an initial increase in absorbance reflecting CPP1 formation after 1 day (absorbance change from 0.042 to 0.055) and the second increase in absorbance reflecting CPP2 formation after 3 days (absorbance change from 0.055 to 0.138; Figure 4A). Mg2+ supplementation resulted in a similar increase in absorbance after 1 day of culture (absorbance change of supernatant from 0.042 to 0.057 for 2.0 mmol/L Mg2+). Mg2+ final concentrations of 1.6, 1.8 and 2.0 mmol/L preserved the CPP1 state for 14 days (final absorbance 0.077). A final concentration of 1.0, 1.2 and 1.4 mmol/L delayed the second increase in absorbance by 1, 7 and 11 days, respectively. In the presence of 4 and 5 mmol/L Pi, 2 mmol/L Mg2+ inhibited CPP1 to CPP2 transition (Supplementary data, Figure S1). Increasing Mg2+ to a concentration of 2.0 mmol/L to CPP medium after CPP2 had formed (on Day 5) did not result in changes in the absorption of the CPP medium (final absorbance 0.170 versus 0.174). After 14 days, CPP2 and CPP2 supplemented with Mg2+ were isolated and analysed by EM and EDX to study CPP characteristics (Figure 4B–G). Mg2+ supplementation did not affect the pH of the CPP mixture (Supplementary data, Figure S2). SEM revealed similar morphology of the CPP2 independent of Mg2+ treatment (Figure 4B and E). EDX peaks were present at energy levels corresponding to enriched Pi and Ca2+ (Figure 4C and F). Quantification of the peaks showed that Mg2+-supplemented CPP2 contains 37.1% oxygen, 18.7% Pi and 39.4% Ca2+, which did not significantly differ from CPP2 without Mg2+ supplementation (Figure 4D and G). In addition, there was a slight trend towards increased Na+, Cl− and Mg2+ content in Mg2+-supplemented CPP2, but this was not statistically significant.
FIGURE 4.
Mg2+ dose-dependently inhibits the transition from CPP1 to CPP2 and does not affect CPP2 morphology after nucleation. (A) Absorbance of the CPP mixture containing different Mg2+ concentrations was measured at 570 nm as a readout for CPP1 (∼0.05 AU) to CPP2 (∼0.15 AU) transition. This graph is representative of five independently executed experiments each consisting of three replicates. Data are expressed as mean ± SD. A final concentration of 2.0 mmol/L Mg2+ after CPP2 (E–G) formation did not alter morphology (SEM) or composition (EDX) compared with control CPP2 (B–D). A TEM picture was inserted in B to confirm CPP2. Scale bars correspond to 300 nm (white) and 500 nm (black).
Mg2+ improves calcification propensity in serum from CKD patients and healthy controls
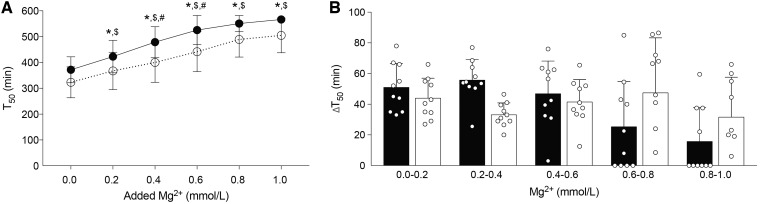
To examine the effects of Mg2+ on serum calcification propensity in CKD patients, the serum of CKD patients and healthy controls was supplemented with increasing concentrations of Mg2+ex vivo and subsequently T50 was measured. Serum Mg2+ concentration was not different between the groups (0.8 ± 0.2 versus 0.9 ± 0.5 mmol/L). There was a non-significant trend towards a lower baseline T50 in CKD patients compared with controls (372 ± 50 versus 323 ± 57 min). The addition of 0.2 mmol/L Mg2+ significantly improved T50 in donor patients by 51 ± 16 min and CKD patients by 44 ± 13 min (Figure 5A; P < 0.05). Each increment of 0.2 mmol/L addition of Mg2+ led to a similar increase of ∼40 min in CKD patients and 50 min in healthy controls (Figure 5B).
FIGURE 5.
Mg2+ dose-dependently increases T50 in CKD patient serum. Serum T50 is increased upon Mg2+ supplementation of serum from both healthy controls (solid line) and CKD patients (dotted line, A). (B) Change in T50 similar in CKD patients after each addition of Mg2+ compared with healthy controls. Black and white bars represent healthy controls and CKD patients, respectively. Data are shown as the mean of 10 patients in each group and are expressed as mean ± SD. P < 0.05 *for healthy controls versus baseline; $for CKD patients versus baseline and #for CKD patients versus healthy controls at specific Mg2+ concentration.
DISCUSSION
In this study we demonstrate that Mg2+ prevents high Pi–induced VSMC calcification. The preventive effect of Mg2+ is mediated via delayed CPP2 formation, because Mg2+ is unable to prevent calcification once CPP2 has formed. Specifically, we show that Mg2+ does not prevent CPP1 formation. Rather, Mg2+ prevents the transition from CPP1 to CPP2. In the sera of CKD patients and healthy controls, a small increase in Mg2+ of 0.2 mmol/L improved T50, reflecting a decreased calcification propensity. In addition, our results indicate that crystalline maturation of Pi towards CPP2 is an essential step in VSMC calcification.
The mechanism by which Mg2+ prevents high Pi–induced calcification in CKD models is incompletely understood. Using different in vitro models, we demonstrate that Mg2+ only prevents Pi-induced VSMC calcification prior to formation of CPP2. After CPP2 formation, Mg2+ supplementation did not alter crystal composition and morphology and did not prevent calcification of VSMCs. In addition, Mg2+ supplementation did not modify CPP2-induced expression profiles of osteogenic or VSMC contractility genes and proteins. In contrast, Mg2+ potently prevents Pi-induced calcification through indirect pathways, as an increased concentration of Mg2+ was insufficient to prevent VSMC calcification induced by CPP2. We have shown previously in a bovine model of VSMC calcification that Mg2+ blocks Ca2+-Pi crystal formation preceding Pi-induced calcification and independent of transcriptional changes in bovine VSMCs [19]. Other studies have identified the potent anti-crystallization properties of Mg2+ [20, 22, 29]. In aqueous solutions, Mg2+ is known to stabilize amorphous Ca2+-Pi particles and delay crystal nucleation [30]. Contrarily, another study suggested that the preventive action of Mg2+ on VSMC calcification did not depend on the inhibition of CPP2 maturation [31]. For instance, in the study by Louvet et al. [31], hydroxyapatite crystals were generated in the presence of hVSMC with 1% FBS only, whereas we used 5% FBS [31]. In low serum conditions, the availability of calcification inhibitors such as fetuin-A is lower, resulting in a milieu that is more prone to calcification. Thus the inhibitory effects of Mg2+ on CPP2 formation may potentially be overruled as Pi can precipitate more readily [24].
In our study, Mg2+ blocked calcification induced by high Pi, which is consistent with previous studies [15, 16, 18, 32]. When comparing our results, it is important to note that the reported experimental setups have used high Pi to induce VSMC calcification and have supplemented Mg2+ immediately in order to prevent calcification [15, 16, 18, 32]. Under these circumstances, it is possible that Mg2+ blocked crystal or CPP2 maturation that would otherwise have occurred naturally. Therefore inhibition of Pi-induced calcification may have been secondary to the prevention of CPP2 formation. With high Pi, medium crystals form spontaneously over time. The cellular effects of CPP2-induced calcification are already present 24 h after induction, while those of high Pi–induced calcification only occur after ∼1 week. Accordingly, it was shown that crystalline Pi–containing particles induce calcification and VSMC transdifferentiation rather than soluble Pi or amorphous Ca2+-Pi particles (or CPP1) [6, 8, 11].
Calcification propensity (T50) is a risk factor and indicator for cardiovascular morbidity and mortality in CKD patients [23, 33, 34]. To translate the effects of Mg2+ on CPP2 maturation to a more clinical setting, we investigated the effects of stepwise Mg2+ additions to serum from renal disease patients and healthy controls on T50. Our results demonstrate that Mg2+ increased T50 by ∼50 min per 0.2 mmol/L Mg2+ increase in serum from CKD patients and healthy controls. Interestingly, the dose-dependent effects of Mg2+ on calcification propensity are similar in both groups. These ex vivo results are consistent with a previous study in patients on dialysis [35, 36]. It was demonstrated that Mg2+ effectively and promptly increased T50 by 72 min once dialysate Mg2+ was adjusted from 0.5 to 1.0 mmol/L, which resulted in a serum increase of 0.36 mmol/L [36]. Dialysate Mg2+ supplementation increased T50 without modifying intracellular Mg2+ concentrations, consistent with the notion that serum Mg2+ is key to modify calcification propensity [36]. The ability of Mg2+ to potently modify T50 is of great clinical interest. Given the sensitivity of T50 for Mg2+, this relationship should be clinically exploited. Further clinical studies should investigate whether ex vivo determinations of calcification propensity could be used to define optimal dialysate or oral Mg2+ concentration for CKD patients.
Our results indicate that cellular Pi toxicity leading to calcification is determined by the presence of crystalline Pi in the form of CPP2. Efforts to prevent vascular calcification are currently aimed at lowering serum Pi concentrations in CKD patients. Importantly, management of serum Pi by Pi binders is insufficient to decrease the risk for cardiovascular disease and vascular calcification in CKD [37]. Instead, determinants affecting the transition from Pi towards crystalline Pi (CPP2) potentially prove to be more clinically relevant. Mg2+ prevented VSMC calcification in vitro despite high Pi concentrations and improved calcification propensity in humans without normalizing serum Pi concentrations [19, 36]. In addition, increased serum Mg2+ (>1.27 mmol/L) neutralizes the association between serum Pi concentration and the risk for cardiovascular mortality [38]. Therefore Mg2+ potentially disarms Pi toxicity in CKD without changing the soluble Pi concentration itself. As such, Pi toxicity, and therefore calcification risk, should be clinically determined based on Pi crystallinity.
This is the first study that uses CPP2-induced VSMC calcification to distinguish intracellular effects from extracellular effects of Mg2+. In addition, our approach includes in vitro models and an ex vivo study coupling the effects of Mg2+ to calcification propensity. Although we demonstrate that Mg2+ limits VSMC calcification primarily through the inhibition of CPP2 maturation already at small incremental concentrations, we cannot exclude potential contributing effects of intracellular pathways related to calcification. While the results presented here need to be verified in vivo, our conclusions may have important implications for clinical interpretation of the effects of Mg2+ on calcification. Recently a longer-term randomized clinical study using oral magnesium oxide supplements showed halted progression of coronary artery calcification in pre-dialysis CKD patients [39]. Another study is currently being initiated (ClinicalTrials.gov identifier NCT02542319) [40]. These studies are a major step towards improving cardiovascular outcomes in CKD patients using Mg2+ supplements.
In conclusion, our study demonstrates that Mg2+ prevents hVSMC calcification by inhibition of CPP2 maturation in vitro. In serum from CKD patients, Mg2+ increased T50 in a dose-dependent manner. Increasing serum Mg2+ may be a promising treatment to target pathological CPP2 maturation in CKD-induced vascular calcification.
SUPPLEMENTARY DATA
Supplementary data are available at ndt online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Huib Croes and Caro Bos (both from the Radboud University Medical Center, Nijmegen, The Netherlands) and Dr Swapna Karthik (Calciscon, Bern, Switzerland) for their wonderful technical assistance. The authors also thank Dr Jan-Luuk Hillebrands (University Medical Center Groningen, Groningen, The Netherlands) for sharing his expertise related to calciprotein particle synthesis.
FUNDING
This research was funded by grants from the Netherlands Organization for Scientific Research (NWO Veni 016.186.012 and VICI 016.130.668) and the Dutch Kidney Foundation (Kolff 14OKG17 and 15OP02). This work was furthermore supported by the NIGRAM2+ consortium, funded by Health Holland (LSHM17034) and the Dutch Kidney Foundation (16TKI02).
AUTHORS’ CONTRIBUTIONS
A.D.t.B., J.H.F.d.B., C.E., J.G.J.H. and M.H.d.B. were involved in the conception and design of the experiments. A.D.t.B., C.E., A.P. and L.W.Z. performed experiments and analysed data. S.J.L.B. recruited patients and founded the TransplantLines cohort study. A.D.t.B. drafted the manuscript. A.D.t.B., J.H.F.d.B., C.E., J.G.J.H., A.P. and M.H.d.B. critically revised the manuscript. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
M.H.d.B. has served as a consultant or received honoraria (to employer) from Amgen, Bayer, Kyowa Kirin Pharma, Sanofi Genzyme and Vifor Fresenius Medical Care Renal Pharma. A.P. is the inventor of the T50 test and co-founder, shareholder and employee of Calciscon.
REFERENCES
- 1. Sigrist MK, Taal MW, Bungay P. et al. Progressive vascular calcification over 2 years is associated with arterial stiffening and increased mortality in patients with stages 4 and 5 chronic kidney disease. Clin J Am Soc Nephrol 2007; 2: 1241–1248 [DOI] [PubMed] [Google Scholar]
- 2. Ishimura E, Okuno S, Yamakawa T. et al. Serum magnesium concentration is a significant predictor of mortality in maintenance hemodialysis patients. Magnes Res 2007; 20: 237–244 [PubMed] [Google Scholar]
- 3. Sakaguchi Y, Fujii N, Shoji T. et al. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int 2014; 85: 174–181 [DOI] [PubMed] [Google Scholar]
- 4. Salem S, Bruck H, Bahlmann FH.. Relationship between magnesium and clinical biomarkers on inhibition of vascular calcification. Am J Nephrol 2012; 35: 31–39 [DOI] [PubMed] [Google Scholar]
- 5. Floege J. Magnesium concentration in dialysate: is higher better? Clin J Am Soc Nephrol 2018; 13: 1309–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aghagolzadeh P, Bachtler M, Bijarnia R. et al. Calcification of vascular smooth muscle cells is induced by secondary calciprotein particles and enhanced by tumor necrosis factor-α. Atherosclerosis 2016; 251: 404–411 [DOI] [PubMed] [Google Scholar]
- 7. Viegas CSB, Santos L, Macedo AL. et al. Chronic kidney disease circulating calciprotein particles and extracellular vesicles promote vascular calcification. Arterioscler Thromb Vasc Biol 2018; 38:575–587 [DOI] [PubMed] [Google Scholar]
- 8. Sage AP, Lu J, Tintut Y. et al. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney Int 2011; 79: 414–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moe SM, Chen NX.. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol 2008; 19: 213–216 [DOI] [PubMed] [Google Scholar]
- 10. Kapustin AN, Chatrou MLL, Drozdov I. et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res 2015; 116: 1312–1323 [DOI] [PubMed] [Google Scholar]
- 11. Proudfoot D, Shanahan CM.. Nanocrystals seed calcification in more ways than one. Kidney Int 2011; 79: 379–382 [DOI] [PubMed] [Google Scholar]
- 12. Shanahan CM, Crouthamel MH, Kapustin A. et al. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res 2011; 109: 697–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Massy ZA, Drüeke TB.. Magnesium and cardiovascular complications of chronic kidney disease. Nat Rev Nephrol 2015; 11: 432–411 [DOI] [PubMed] [Google Scholar]
- 14. Herencia C, Rodriguez-Ortiz ME, Munoz-Castaneda JR. et al. Angiotensin II prevents calcification in vascular smooth muscle cells by enhancing magnesium influx. Eur J Clin Invest 2015; 45: 1129–1144 [DOI] [PubMed] [Google Scholar]
- 15. Montezano AC, Zimmerman D, Yusuf H. et al. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 2010; 56: 453–462 [DOI] [PubMed] [Google Scholar]
- 16. Kircelli F, Peter ME, Sevinc Ok E. et al. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol Dial Transplant 2012; 27: 514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Louvet L, Metzinger L, Büchel J. et al. Magnesium attenuates phosphate-induced deregulation of a microRNA signature and prevents modulation of Smad1 and Osterix during the course of vascular calcification. Biomed Res Int 2016; 2016: 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Oca AM, Guerrero F, Martinez-Moreno JM. et al. Magnesium inhibits wnt/b-catenin activity and reverses the osteogenic transformation of vascular smooth muscle cells. PLoS One 2014; 9: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ter Braake AD, Tinnemans PT, Shanahan CM. et al. Magnesium prevents vascular calcification in vitro by inhibition of hydroxyapatite crystal formation. Sci Rep 2018; 8: 2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Blumenthal NC, Betts F, Posner AS.. Stabilization of amorphous calcium phosphate by Mg and ATP. Calcif Tissue Res 1977; 23: 245–250 [DOI] [PubMed] [Google Scholar]
- 21. Boistelle R, Lopez-Valero I, Abbona F.. Cristallisation des phosphates de calcium en présence de magnésium. Nephrologie 1993; 14: 265–269 [PubMed] [Google Scholar]
- 22. Boskey AL, Posner AS.. Magnesium stabilization of amorphous calcium phosphate: a kinetic study. Mater Res Bull 1974; 9: 907–916 [Google Scholar]
- 23. Keyzer CA, de Borst MH, van den Berg E.. Calcification propensity and survival among renal transplant recipients. J Am Soc Nephrol 2015; 27: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pasch A, Farese S, Graber S. et al. Nanoparticle-based test measures overall propensity for calcification in serum. J Am Soc Nephrol 2012; 23: 1744–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith ER, Cai MM, McMahon LP. et al. Serum fetuin-A concentration and fetuin-A-containing calciprotein particles in patients with chronic inflammatory disease and renal failure. Nephrology (Carlton) 2013; 18: 215–221 [DOI] [PubMed] [Google Scholar]
- 26. Smith ER, Ford ML, Tomlinson LA. et al. Phosphorylated fetuin-A-containing calciprotein particles are associated with aortic stiffness and a procalcific milieu in patients with pre-dialysis CKD. Nephrol Dial Transplant 2012; 27: 1957–1966 [DOI] [PubMed] [Google Scholar]
- 27. Smith ER, Hewitson TD, Hanssen E. et al. Biochemical transformation of calciprotein particles in uraemia. Bone 2018; 110: 355–367 [DOI] [PubMed] [Google Scholar]
- 28. Eisenga MF, Gomes-Neto AW, Van Londen M. et al. Rationale and design of TransplantLines: a prospective cohort study and biobank of solid organ transplant recipients. BMJ Open 2018; 8: e024502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Apfelbaum F, Mayer I, Rey C. et al. Magnesium in maturing synthetic apatite: a Fourier transform infrared analysis. J Cryst Growth 1994; 144: 304–310 [Google Scholar]
- 30. Abbona F, Baronnet A.. A XRD and TEM study on the transformation of amorphous calcium phosphate in the presence of magnesium. J Cryst Growth 1996; 165: 98–105 [Google Scholar]
- 31. Louvet L, Bazin D, Büchel J. et al. Characterisation of calcium phosphate crystals on calcified human aortic vascular smooth muscle cells and potential role of magnesium. PLoS One 2015; 10: e0115342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Louvet L, Büchel J, Steppan S. et al. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant 2013; 28: 869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smith ER, Ford ML, Tomlinson LA. et al. Serum calcification propensity predicts all-cause mortality in predialysis CKD. J Am Soc Nephrol 2014; 25: 339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pasch A, Block GA, Bachtler M. et al. Blood calcification propensity, cardiovascular events, and survival in patients receiving hemodialysis in the EVOLVE Trial. Clin J Am Soc Nephrol 2017; 12: 315–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bressendorff I, Hansen D, Schou M. et al. Oral magnesium supplementation in chronic kidney disease stages 3 and 4: efficacy, safety, and effect on serum calcification propensity—a prospective randomized double-blinded placebo-controlled clinical trial. Kidney Int Rep 2017; 2: 380–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bressendorff I, Hansen D, Schou M. et al. The effect of increasing dialysate magnesium on serum calcification propensity in subjects with end stage kidney disease. Clin J Am Soc Nephrol 2018; 13: 1373–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ruospo M, Palmer SC, Natale P. et al. Phosphate binders for preventing and treating chronic kidney disease-mineral and bone disorder (CKD-MBD). Cochrane Database Syst Rev 2018; 8: CD006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sakaguchi Y, Fujii N, Shoji T. et al. Magnesium modifies the cardiovascular mortality risk associated with hyperphosphatemia in patients undergoing hemodialysis: a cohort study. PLoS One 2014; 9: e116273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sakaguchi Y, Hamano T, Obi Y. et al. A randomized trial of magnesium oxide and oral carbon adsorbent for coronary artery calcification in predialysis CKD. J Am Soc Nephrol 2019; 30:1073–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bressendorff I, Hansen D, Schou M. et al. The effect of magnesium supplementation on vascular calcification in chronic kidney disease-a randomised clinical trial (MAGiCAL-CKD): essential study design and rationale. BMJ Open 2017; 7: e016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.