Abstract
Acute respiratory distress syndrome (ARDS) remains a serious clinical problem with the main treatment being supportive in the form of mechanical ventilation. However, mechanical ventilation can be a double-edged sword: if set improperly, it can exacerbate the tissue damage caused by ARDS; this is known as ventilator-induced lung injury (VILI). To minimize VILI, we must understand the pathophysiologic mechanisms of tissue damage at the alveolar level. In this Physiology in Medicine paper, the dynamic physiology of alveolar inflation and deflation during mechanical ventilation will be reviewed. In addition, the pathophysiologic mechanisms of VILI will be reviewed, and this knowledge will be used to suggest an optimal mechanical breath profile (MBP: all airway pressures, volumes, flows, rates, and the duration that they are applied at both inspiration and expiration) necessary to minimize VILI. Our review suggests that the current protective ventilation strategy, known as the “open lung strategy,” would be the optimal lung-protective approach. However, the viscoelastic behavior of dynamic alveolar inflation and deflation has not yet been incorporated into protective mechanical ventilation strategies. Using our knowledge of dynamic alveolar mechanics (i.e., the dynamic change in alveolar and alveolar duct size and shape during tidal ventilation) to modify the MBP so as to minimize VILI will reduce the morbidity and mortality associated with ARDS.
Keywords: ventilator-induced lung injury, acute respiratory distress syndrome, acute lung injury, positive end-expiratory pressure, mechanical ventilation
pulmonary physiology and pathophysiology in positive pressure mechanical ventilation have been studied for decades (47). Preemptive application of adequate positive end-expiratory pressure (PEEP) can reduce pulmonary edema in animal models of high vascular pressure- (14), high alveolar surface tension- (39), and high permeability-induced (53) acute respiratory distress syndrome (ARDS; 47). Knowing that adjustments in the mechanical ventilator settings (i.e., PEEP) can protect the acutely injured lung is key to managing the critically ill patient, since the mainstay of ARDS treatment is still supportive in the form of mechanical ventilation. Indeed, animal studies suggested that protective ventilation can reduce pathology in the acutely injured lung (44), which led to a clinical trial demonstrating that protective mechanical ventilation can significantly reduce mortality (13). Paradoxically, the mechanical ventilator as a support strategy can have unintended effects that may accentuate lung pathology, depending on ventilator settings. The repetitive delivery of arbitrary settings will, in some patients, exacerbate the primary acute lung injury and cause a secondary ventilator-induced lung injury (VILI). However, even though application of PEEP has been shown to prevent or reduce edema in animal models of ARDS (14, 39, 53), PEEP has not been shown to improve outcome in patients with ARDS (12), which may be explained by the variable amount of recruitable lung with increased airway pressure in ARDS patients (23).
Historically, there have been three main mechanical breath parameters: tidal volume (Vt), plateau pressure (Pplat), and PEEP have been used for several decades in multiple combinations in an attempt to reduce VILI. More recently, the role of respiratory strain rate has also been shown to be an important contributor to VILI (48). Although mortality has been significantly reduced since ARDS was identified in 1967 (9), it remains at ~40% (64). Since the mortality rate in ARDS patients remains unacceptably high (63), further analysis of the entire mechanical breath profile (MBP; i.e., airway pressures, volumes, flows, rates, and the duration at which they are applied with each breath) and how each parameter impacts at the alveolar and alveolar duct is necessary.
In the current standard of care, ventilator adjustments (Vt, PEEP, and Pplat) are made on the basis of oxygenation and airway pressure, not in response to lung mechanics (13), and VILI may or may not be averted (5, 7). Although protective ventilation resulted in an initial drop in mortality, it has been disappointing that the current standard-of-care protective ventilation strategy has not further reduced mortality (47), and Villar et al. (63) underscore the critical need to better understand the impact of the mechanical breath on alveolar and alveolar duct inflation and deflation, and the mechanisms of ARDS and VILI at the alveolar level, to develop protective ventilator strategies that work.
WHAT PHYSIOLOGIC PARAMETERS CAN DEFINE THE OPTIMALLY PROTECTIVE MECHANICAL BREATH?
The goal of the protective breath is not to optimize oxygenation but rather to minimize damage to pulmonary epithelial and vascular endothelial cells and their associated connective tissues. To design the optimally protective breath, we must 1) consider the complex microanatomy of interconnected alveoli with shared alveolar walls, 2) consider the physiologic mechanisms of VILI in the microenvironment of the terminal air space (the alveoli and alveolar ducts), 3) consider the dynamic alveolar physiology (i.e., the dynamic change in alveolar size and shape during tidal ventilation) and how changes in dynamic alveolar physiology cause tissue damage resulting in VILI, and 4) apply this information to postulate an optimal mechanical breath profile (MBP), necessary to a ventilation protocol capable of blocking VILI. A mechanical breath comprises airway pressures, volumes, flows, rates, and the duration over which they are applied throughout inspiration and expiration. Critical consideration of VILI mechanisms and dynamic alveolar physiology can unveil MBP parameter combinations that are lung protective, thus enabling the practitioner to adjust protocols and minimize or avert VILI. Our approach is to identify an optimal combination of these physical parameters over the course of the ventilator cycle that is adaptive in real time to each individual breath.
Key Points
Current protective mechanical ventilation strategies necessary to reduce ventilator-induced lung injury (VILI) in ARDS patients have focused on three components of the mechanical breath: tidal volume, plateau pressure, and PEEP.
Although ARDS mortality has been reduced with these strategies, it remains unacceptably high at ~40%.
To improve protective mechanical ventilation, the dynamic physiology of the alveolus in both normal and pathologic conditions must be known.
Once we identify the alveolar pathophysiology, we can modify the components of the mechanical breath necessary to normalize physiology and protect the lung.
PATHOPHYSIOLOGY OF VILI
Normal dynamic alveolar anatomy consists of interdependent, homogeneous inflated alveoli (42) with little movement in alveolar volume during tidal ventilation (Fig. 1 and Supplemental Material, Supplemental Animation S1; Supplemental Material for this article is available at the Journal of Applied Physiology Web site; 15, 46). An important feature of ARDS pathophysiology is loss of surfactant function (2, 21) causing alveoli to either remain collapsed throughout ventilation or sequentially collapse and reopen with each breath. This lung pathophysiology is believed to set the lung up for a secondary VILI. The mechanism of VILI is complex, with two primary mechanisms that can subsequently contribute to two secondary mechanisms, which can only occur in the presence of the primary mechanism.
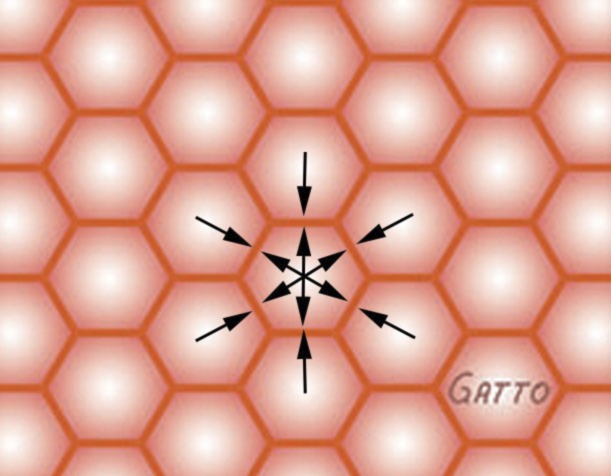
Fig. 1.
Interdependent alveoli with shared alveolar walls represented as hexagons. This homogeneous anatomical design assists in stabilizing alveoli preventing collapse during expiration and overdistension during inspiration, since there is no pressure (arrows) gradient between alveoli (opposing arrows of equal force). Only ~2% change in alveolar area is measured between inspiration (Supplemental Animation S1A) and expiration (Supplemental Animation S1B) using in vivo microscopy.
Primary VILI mechanisms are as follows: 1) collapsed or edema-filled alveoli that are surrounded by open alveoli causing stress risers (S-R; 18, 49; Fig. 2, A and B, and Supplemental Animation S2) and 2) alveoli that recruit and derecruit (R/D) with tidal ventilation resulting in an increase in dynamic strain (Fig. 3, A and B, and Supplemental Animation S3; 1, 17).
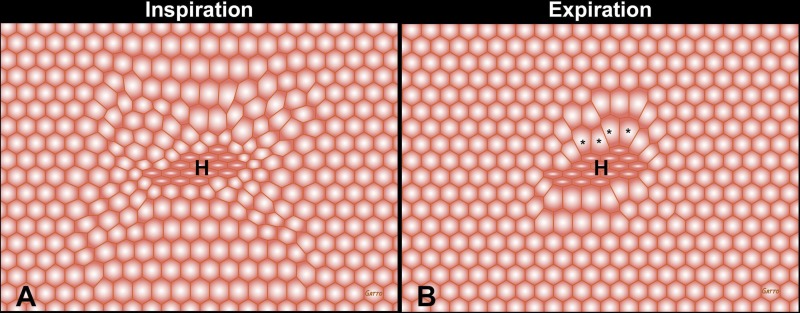
Fig. 2.
Heterogeneous alveolar injury depicted as a cluster of collapsed alveoli in the center of the field (H) surrounded by open interdependent alveoli (hexagons). Note the distortion and overdistension of the patent alveoli adjacent to the collapsed alveolar cluster (asterisks), with more overdistension alveoli at expiration (B) compared with inspiration (A). The larger the change in size of these alveoli with each breath, the larger the dynamic strain-induced tissue damage. These dynamic changes can be seen in Supplemental Animation S2.
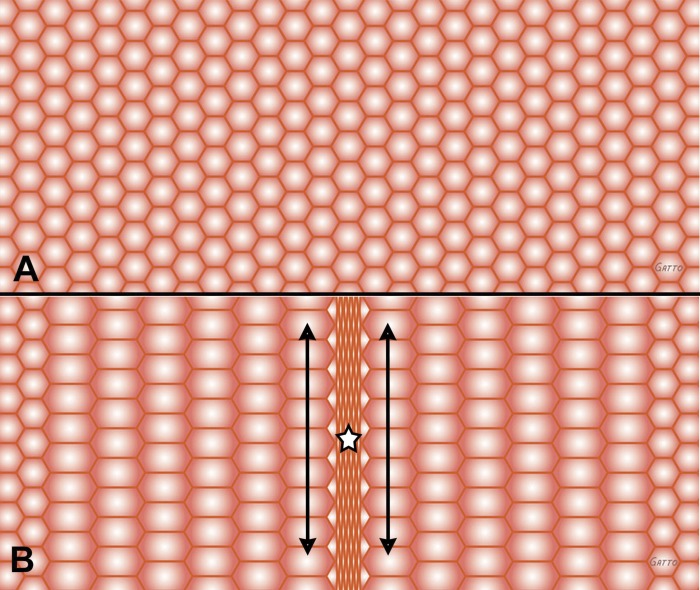
Fig. 3.
Alveoli depicted as hexagons are homogeneously inflated at inspiration (A). Acute lung injury can cause heterogeneous collapse of a group of alveoli during expiration (B, star), while the remaining alveoli remain open. Collapsed alveoli are depicted in the center of the field (B, star). Alveolar instability causes alveoli to open (A, all alveoli homogeneously recruited) and collapse (B, central alveoli collapse causing heterogeneous ventilation) with each breath. Alveolar instability results in excessive dynamic shear stress on alveolar walls of the unstable alveoli (B, star). In addition, alveoli adjacent to the collapsing area are overdistended during expiration with significant dynamic strain during each breath (arrows). These dynamic changes can be seen in Supplemental Animation S3.
Secondary VILI mechanisms are as follows: 1) ARDS causes a reduction in functional lung volume by a combination of partially and fully collapsed (i.e., atelectasis) alveoli. If lung volume loss is severe, even small tidal volumes can result in VILI caused by overdistension (OD; 24) of normal alveoli and alveolar ducts, in the remaining aerated lung regions (36). However, if the lung is fully recruited, even large tidal volumes set on the ventilator will not cause alveolar OD since this volume is shared by millions of alveoli, and thus alveolar Vt remains very small. 2) Stress/strain-induced injury secondary to the above mechanisms causes release of inflammatory mediators, which exacerbate the physical damage, termed “biotrauma” (54, 58).
Stress risers.
Although even the normal lung is not homogeneous throughout, because of variation in gas distribution, conducting airway asymmetry, and anisotropic alveolar expansion, stress transmission is still relatively well distributed in most lung tissue (Fig. 1 and Supplemental Animation S1). However, alveolar collapse and edema result in a heterogeneous inflation pattern such that the stress during cyclic volume change becomes concentrated onto the open lung tissue adjacent to collapsed tissue (18, 49; Fig. 2, A and B, and Supplemental Animation S2). It has been shown that the homogeneous lung is stable but local atelectasis, causing stress risers, can occur if alveolar surface tension is high and insensitive to alveolar surface area (57).
Recruitment/derecruitment.
Normal alveoli are stable and do not have marked changes in volume with each tidal breath (<4–6% change with each cycle; Fig. 1 and Supplemental Animation S1). Thus dynamic strain is minimal in normal alveoli (15, 16, 45). Loss of surfactant function with acute lung injury renders alveoli unstable, greatly reducing the alveolar collapse time constant, resulting in a rapid collapse of the alveolus with each expiration (58; Fig. 3, A and B, and Supplemental Animation S3). This leads to a large dynamic alveolar strain, which is known to be a primary VILI mechanism (1, 17). Pathologic changes in alveolar stability can be identified using a deep inflation (DI) and measuring the change in elastance (H) and hysteresivity (η). H and η not returning to normal following a DI correlates strongly with alveolar instability viewed directly using in vivo microscopy (4). Stabilizing alveoli and preventing derecruitment with adequate PEEP, measured directly by in vivo microscopy, significantly reduce acute lung injury (58).
Overdistension and biotrauma.
In a homogenous, stable lung, it is postulated that alveolar OD sufficient to cause VILI histopathology does not exist in the absence of S-R or R/D, unless extremely high airway pressures are delivered. We hypothesize that because of interdependence (Fig. 1), alveolar OD can only occur adjacent to collapsed or unstable alveoli (42, 55). Mead et al. demonstrated that the shared alveolar walls would be stretched in response to a single overdistended or collapsing alveolus (42). We have seen that loss of interdependence (i.e., collapse of 1 or more alveoli) can cause instability and OD of adjacent alveoli, using in vivo microscopy. Supplemental Animation S4 depicts how the collapse of a single “alveolus” results in instability and OD of adjacent alveoli, stressing the importance of maintaining a fully recruited lung. Biotrauma occurs secondary to alveolar S-R- and R/D-induced tissue trauma causing a release of inflammatory mediators. Thus VILI will be minimized if both S-R and R/D are blocked. OD of a fully inflated lung can cause airways to rupture (e.g., pneumothorax) if sufficiently elevated yet does not cause lung histopathology typical of VILI (56). Alveolar ducts, rather than alveolar sacs, may be the site of OD and the possible site of airway rupture in a heterogeneously ventilated lung (36). In summary, VILI is minimized in an open and stable lung, even with high lung volumes and pressures. In summary, VILI has most often been viewed as an alteration of the barrier between the alveolar space and capillary, which is closely associated with an increase in inflammation (biotrauma). However, physical failure of the load-bearing cells and tissues during ventilation is now understood to also play a major role in VILI (60).
Key Points
VILI can occur when the normal, homogenous lung becomes heterogeneousx.
The heterogeneous lung is a combination of normal alveoli, alveoli flooded with edema, and unstable alveoli that collapse and reopen during tidal ventilation.
The primary mechanism of VILI is an alteration of normal dynamic alveolar physiology resulting in excessive alveolar volume change (strain) with each breath.
The secondary mechanism of VILI is alveolar overdistension and generation of inflammatory mediators caused by excessive alveolar strain.
DYNAMIC CHANGES IN ALVEOLAR VOLUME
Although a great deal of literature has been devoted to understanding the physiology of normal and abnormal alveolar mechanics (i.e., the dynamic change in alveolar size and shape during tidal ventilation), there is still no consensus on alveolar size change in the normal lung with each breath (26). It is well accepted that alveolar microanatomy is complex, with alveoli sharing walls in a honeycomb fashion forming a structurally stable design (Fig. 1; 42). Because of this alveolar interdependence and the fact that alveolar walls are only one or two cells thick (a vascular endothelial cell and an alveolar epithelial cell), it is proposed that that alveolar volume change is not simply stretching and contracting, similar to a rubber balloon, although this has never been directly proven, with the more likely mechanism being folding and unfolding like a paper bag (62). The three most logical mechanisms by which alveoli and alveolar ducts can change size during ventilation are as follows: 1) an all-or-none alveolar opening and closing during each breath, such that there are more open alveoli at inspiration than at expiration; 2) fluctuations in alveolar size owing to crumpling and uncrumpling of alveolar walls, similar to how a paper bag would change volume; and 3) change in the size of the alveolar duct with minimal change in alveolar size (26, 50).
Alveolar volume change is often perceived to be elastic in nature such that for a given force causing stress [i.e., tidal volume (Vt)], alveoli would be expected to change size (i.e., strain) immediately on both inspiration and expiration. However, alveoli do not behave as an elastic structure, but rather as a viscoelastic system. A viscoelastic system would not exhibit an immediate change in alveolar size during tidal ventilation (10, 19, 31, 40, 59). The delay in strain following the applied force-induced stress is represented graphically as a hysteresis in the stress (σ)-strain (ε) curve. Direct visualization of the whole lung and subpleural alveoli during lung inflation has demonstrated this lag between the time that the Vt force delivers the stress until the time alveoli actually begin to expand or recruit (strain; Supplemental Movie S1; 3). Similar assessments of alveolar mechanics and stability have been previously published (61). Although in vivo microscopy is one of the few methods to measure the dynamic change in alveolar size during ventilation in real time, it must be remembered that subpleural alveoli are unique in that they are not totally surrounded by alveoli and thus they may inflate or deflate differently from the majority of alveoli that compose the lung. Also, measuring dynamic inflation and deflation of a single alveolus in an animal ARDS model demonstrated that there was no significant reduction in alveolar size until airway pressure fell from 13 to 6 mmHg (46). A second time lag would be generated by collapsed alveoli with different opening pressure/duration constants, such that for “stickier” alveoli, a higher pressure or longer duration at a pressure would be necessary for recruitment. This would generate a time lag from when the pressure was applied and when the alveolus was recruited. Thus time is a critical component in determining alveolar inflation and deflation during each mechanical breath.
ALVEOLAR VISCOELASTICITY: THE SPRING AND DASHPOT
The change in alveolar size with ventilation is complex and has been depicted as viscoelastic in nature. This viscoelasticity has been described on a molecular basis using a mathematical framework (20, 59). In this paper, we will use a very basic model to analyze viscoelastic behavior using a spring attached to a dash in a filled pot (Supplemental Animation S5). Using this model, the force, which in the case of the lung would be the pressure generated with the Vt, is applied to the tissue represented by the spring and dashpot. Supplemental Animation S5 shows that in response to this stress, in such a viscoelastic system the spring would move rapidly whereas there would be a significant delay and a much slower movement of the dashpot. Conversely, when the stress is released (expiratory phase of lung ventilation), the spring compresses rapidly followed by a slow reverse strain of the dashpot (Supplemental Animation S4). Using this spring-and-dashpot model to describe dynamic alveolar inflation, we can postulate that the rate of dashpot movement on both inspiration and expiration could be altered by lung pathology. For example, with loss of surfactant function during ARDS, collapsed alveoli would be very sticky (25), and thus it would take a great deal of stress over time to recruit them. This would be depicted by a very slowly moving dashpot. Once these surfactant-depleted alveoli open, they would be unstable and derecruit very rapidly during expiration (28), and this would be represented by a fast collapsing dashpot.
Viscoelastic physiology implies that the time at inspiration and expiration is a key MBP parameter that has the potential to “open the lung and keep it open” (37). Unfortunately, time has been overlooked in most protective ventilation strategies (13). An extended duration at inspiration would apply the pressure to the alveolus for a longer period of time, gradually “nudging” open alveoli with each breath (Supplemental Animation S5 and Supplemental Movie S1). Minimal duration at expiration would quickly release the pressure, allowing for ventilation and CO2 removal, but minimize alveolar collapse because the dashpot would not have time to move (Supplemental Animation S6). Using this physiologic knowledge, a mechanical breath that may better open and stabilize the lung can be constructed.
Considering viscoelasticity as a key component of dynamic alveolar mechanics is critical when undertaking the design of a mechanical breath that will minimize VILI. Since the two primary mechanisms of VILI are alveolar R/D and S-R, a protective breath must be designed to prevent or treat both of these pathologies. Considering that alveoli are viscoelastic, we postulate that a mechanical breath with an extended inspiratory and short expiratory duration would recruit alveoli with each breath (Supplemental Animation S5), as well as prevent alveolar collapse during expiration (Supplemental Animation S6). This would minimize VILI by reducing both S-R and alveolar R/D (Figs. 2 and 3 and Supplemental Animations S2 and S3). Thus our protective mechanical breath strategy uses the component of time, in addition to the airway pressures, volumes, and flows being delivered with each breath, to block VILI. If lung volume increase were slow, the corresponding pressure would be near the elastic limit of the tissue. Higher ventilation rates would generate higher stresses exacerbating tissue injury, and if this were true, it would offer a second benefit of the extended inspiratory duration.
Key Points
Alveoli do not inflate and deflate in an ideal elastic spring-like fashion (elastic), but rather are a viscoelastic system.
In a viscoelastic system there is a time lag between the time the force (Vt) is applied or removed and when the alveolus opens or collapses.
This knowledge of dynamic alveolar physiology suggests that an extended time at inspiration would recruit alveoli and a minimal time at expiration would prevent alveolar collapse.
Recruiting alveoli and preventing their collapse would convert the heterogeneous lung to homogeneous ventilation and reduce VILI.
TRANSLATING DYNAMIC ALVEOLAR PHYSIOLOGY TO THE BEDSIDE
The current standard-of-care protective mechanical ventilation in patients with established ARDS uses low Vt and a sliding scale of PEEP based on oxygenation (13). This strategy is based on the knowledge that a significant volume of lung is either collapsed or edema filled and thus if a normal Vt were forced into this lung, the remaining normal lung tissue would be injured by overdistension. We showed evidence earlier that OD does not occur in the homogeneously ventilated lung, and thus it seems that a better strategy would be initially opening the lung, which would eliminate heterogeneous ventilation and stress risers (33, 43). PEEP would then be applied to keep the newly recruited alveoli open, eliminating the final mechanical mechanism of VILI, that of alveolar R/D. Indeed, this strategy of initially recruiting the ARDS lung has been shown to improve oxygenation and driving pressure without detrimental effects on mortality, ventilator-free days, or mortality in a pilot study (32). In that study a decremental PEEP trial was conducted as the pressure in the recruited lung was decreased in 2-cmH2O increments with the optimal PEEP identified by the best dynamic lung compliance (32). A meta-analysis has recently shown that this open lung approach significantly reduces the mortality in ARDS patients (38).
The open lung approach makes sound physiologic sense as a method to minimize the mechanisms of VILI at the alveolar level. Indeed, a link has been shown between optimal compliance/elastance and alveolar overdistension and instability in an animal model (6) and the role of driving pressure, which is calculated using compliance, as an indicator of mortality in ARDS patients (5). However, using compliance/elastance does not take advantage of the viscoelastic component of alveolar inflation and deflation. In theory, an extended time at inflation would progressively recruit alveoli, and a short time at expiration would prevent alveolar collapse. There are three US Food and Drug Administration-approved mechanical breath strategies that incorporate an extended inspiratory and minimal expiratory duration: 1) inverse inspiratory-to-expiratory ratio (I:E; 11, 43), 2) high-frequency oscillatory ventilation (HFOV; 22, 66), and 3) airway pressure release ventilation (APRV; 27, 30). Relatively little study has been directed at inverse I:E in ARDS, and the results have been mixed (11, 43). HFOV has been extensively studied; however, recent work has shown that HFOV may increase mortality in ARDS patients (22, 65), although the reasons for this failure may not be due to the impact of HFOV on alveolar mechanics (41). The failure of HFOV to reduce mortality may be due to a misdistribution of ventilation in the heterogeneously injured lung (29). A very specific strategy of personalized APRV (30) has been shown to be effective at stabilizing alveoli (35, 36) and protecting from the development of ARDS in a clinically applicable porcine model (34, 51, 52). In a meta-analysis this personalized APRV strategy significantly reduced ARDS incidence and mortality in a surgical intensive care unit (8), but it has not been tested in a prospective clinical trial. It is also possible that a novel method of multifrequency oscillatory ventilation that would better personalize the HFOV breath to the patient’s lung pathology would improve outcome (33).
Key Point
Ventilation strategies that 1) greatly extend the inspiratory time, continually recruiting the alveoli with slow opening time constants; and 2) dramatically reduce expiratory duration to a time less than the alveolar collapse time constant would be lung protective on the basis of our knowledge that dynamic alveolar physiology is viscoelastic in nature.
CONCLUSIONS
Although controversy still exists, there is strong evidence suggesting that the two primary mechanisms of VILI at the alveolar level are stress risers and repetitive alveolar opening and collapsing. If these primary injury mechanisms are left untreated, they will result in a secondary mechanical mechanism of injury, alveolar overdistension. All three mechanical injuries cause inflammation resulting in biotrauma, which is the fourth component of the VILI tetrad. Logic dictates that if a mechanical breath can be applied that eliminates both of these primary VILI mechanisms, lung tissue damage would be significantly reduced. Thus the goal for protective mechanical ventilation is to open the lung and keep it open (37) or, if applied early, “never let the lung collapse.”
The normal lung on mechanical ventilation inflates via increased pressure delivered by the ventilator and deflates passively because of elastic lung recoil. Alveolar inflation and deflation are viscoelastic, and thus there is a short but distinct time lag between the time that the force of airway pressure is delivered and the time that alveolar inflation occurs. Conversely, there is a distinct time lag between the point that stress is removed during lung deflation and when alveoli begin to collapse. In the acutely injured lung with loss of surfactant function, a longer time is required at inspiration to open alveoli and a much shorter time at expiration to prevent alveolar collapse.
Understanding that alveoli are viscoelastic structures suggests that a greatly extended inspiratory duration would open alveoli and a very short duration at expiration would keep alveoli open. By modifying the time of the applied breath during both inspiration and expiration in an attempt to maintain a homogeneously ventilated lung, the incidence of VILI may be reduced, which may significantly lower the morbidity and mortality associated with ARDS.
DISCLOSURES
P. Andrews, G. F. Nieman, and N. M. Habashi have presented and received honoraria and/or travel reimbursement at event(s) sponsored by Dräger Medical Systems, Inc., outside of the published work. P. Andrews, G. F. Nieman, N. M. Habashi, and L. A. Gatto have lectured for Intensive Care Online Network, Inc. (ICON). N. M. Habashi is the founder of ICON, of which P. Andrews is an employee. N. M. Habashi holds patents on a method of initiating, managing, and/or weaning airway pressure release ventilation, as well as controlling a ventilator in accordance with the same, but these patents are not commercialized, licensed, or royalty producing. The authors maintain that industry had no role in the design and conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
AUTHOR CONTRIBUTIONS
G.F.N. analyzed data; J.S. and L.A.G. prepared figures; G.F.N. and L.A.G. drafted manuscript; G.F.N., J.S., M.K.-S., P.A., H.A., N.M.H., and L.A.G. edited and revised manuscript; G.F.N., J.S., M.K.-S., P.A., H.A., N.M.H., and L.A.G. approved final version of manuscript.
REFERENCES
- 1.Albaiceta GM, Blanch L. Beyond volutrauma in ARDS: the critical role of lung tissue deformation. Crit Care : 304, 2011. doi: 10.1186/cc10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert RK. The role of ventilation-induced surfactant dysfunction and atelectasis in causing acute respiratory distress syndrome. Am J Respir Crit Care Med : 702–708, 2012. doi: 10.1164/rccm.201109-1667PP. [DOI] [PubMed] [Google Scholar]
- 3.Albert SP, DiRocco J, Allen GB, Bates JH, Lafollette R, Kubiak BD, Fischer J, Maroney S, Nieman GF. The role of time and pressure on alveolar recruitment. J Appl Physiol (1985) : 757–765, 2009. doi: 10.1152/japplphysiol.90735.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen GB, Pavone LA, DiRocco JD, Bates JH, Nieman GF. Pulmonary impedance and alveolar instability during injurious ventilation in rats. J Appl Physiol (1985) : 723–730, 2005. doi: 10.1152/japplphysiol.01339.2004. [DOI] [PubMed] [Google Scholar]
- 5.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med : 747–755, 2015. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 6.Amini R, Herrmann J, Kaczka DW. Intratidal overdistention and derecruitment in the injured lung: a simulation study. IEEE Trans Biomed Eng : 681–689, 2017. doi: 10.1109/TBME.2016.2572678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrews P, Sadowitz B, Kollisch-Singule M, Satalin J, Roy S, Snyder K, Gatto L, Nieman G, Habashi N. Alveolar instability (atelectrauma) is not identified by arterial oxygenation predisposing the development of an occult ventilator-induced lung injury. Intensive Care Med Exp : 54, 2015. doi: 10.1186/s40635-015-0054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews PL, Shiber JR, Jaruga-Killeen E, Roy S, Sadowitz B, O’Toole RV, Gatto LA, Nieman GF, Scalea T, Habashi NM. Early application of airway pressure release ventilation may reduce mortality in high-risk trauma patients: a systematic review of observational trauma ARDS literature. J Trauma Acute Care Surg : 635–641, 2013. doi: 10.1097/TA.0b013e31829d3504. [DOI] [PubMed] [Google Scholar]
- 9.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet : 319–323, 1967. doi: 10.1016/S0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 10.Bates JH. Lung Mechanics. An Inverse Modeling Approach. Cambridge, UK: Cambridge University Press, 2009. doi: 10.1017/CBO9780511627156 [DOI] [Google Scholar]
- 11.Boehme S, Bentley AH, Hartmann EK, Chang S, Erdoes G, Prinzing A, Hagmann M, Baumgardner JE, Ullrich R, Markstaller K, David M. Influence of inspiration to expiration ratio on cyclic recruitment and derecruitment of atelectasis in a saline lavage model of acute respiratory distress syndrome. Crit Care Med : e65–e74, 2015. doi: 10.1097/CCM.0000000000000788. [DOI] [PubMed] [Google Scholar]
- 12.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT; National Heart, Lung, and Blood Institute ARDS Clinical Trials Network . Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med : 327–336, 2004. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 13.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A; Acute Respiratory Distress Syndrome Network . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med : 1301–1308, 2000. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 14.Bshouty Z, Ali J, Younes M. Effect of tidal volume and PEEP on rate of edema formation in in situ perfused canine lobes. J Appl Physiol (1985) : 1900–1907, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Carney DE, Bredenberg CE, Schiller HJ, Picone AL, McCann UG II, Gatto LA, Bailey G, Fillinger M, Nieman GF. The mechanism of lung volume change during mechanical ventilation. Am J Respir Crit Care Med : 1697–1702, 1999. doi: 10.1164/ajrccm.160.5.9812031. [DOI] [PubMed] [Google Scholar]
- 16.Cereda M, Xin Y, Kadlecek S, Hamedani H, Rajaei J, Clapp J, Rizi RR. Hyperpolarized gas diffusion MRI for the study of atelectasis and acute respiratory distress syndrome. NMR Biomed : 1468–1478, 2014. doi: 10.1002/nbm.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen ZL, Song YL, Hu ZY, Zhang S, Chen YZ. An estimation of mechanical stress on alveolar walls during repetitive alveolar reopening and closure. J Appl Physiol (1985) : 190–201, 2015. doi: 10.1152/japplphysiol.00112.2015. [DOI] [PubMed] [Google Scholar]
- 18.Cressoni M, Cadringher P, Chiurazzi C, Amini M, Gallazzi E, Marino A, Brioni M, Carlesso E, Chiumello D, Quintel M, Bugedo G, Gattinoni L. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med : 149–158, 2014. doi: 10.1164/rccm.201308-1567OC. [DOI] [PubMed] [Google Scholar]
- 19.D’Angelo E, Calderini E, Torri G, Robatto FM, Bono D, Milic-Emili J. Respiratory mechanics in anesthetized paralyzed humans: effects of flow, volume, and time. J Appl Physiol (1985) : 2556–2564, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Faffe DS, Zin WA. Lung parenchymal mechanics in health and disease. Physiol Rev : 759–775, 2009. doi: 10.1152/physrev.00019.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fanelli V, Mascia L, Puntorieri V, Assenzio B, Elia V, Fornaro G, Martin EL, Bosco M, Delsedime L, Fiore T, Grasso S, Ranieri VM. Pulmonary atelectasis during low stretch ventilation: “open lung” versus “lung rest” strategy. Crit Care Med : 1046–1053, 2009. doi: 10.1097/CCM.0b013e3181968e7e. [DOI] [PubMed] [Google Scholar]
- 22.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, Zhou Q, Matte A, Walter SD, Lamontagne F, Granton JT, Arabi YM, Arroliga AC, Stewart TE, Slutsky AS, Meade MO; OSCILLATE Trial Investigators; Canadian Critical Care Trials Group . High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med : 795–805, 2013. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 23.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med : 1775–1786, 2006. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 24.Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med : 776–784, 2005. doi: 10.1007/s00134-005-2627-z. [DOI] [PubMed] [Google Scholar]
- 25.Gattinoni L, Vagginelli F, Carlesso E, Taccone P, Conte V, Chiumello D, Valenza F, Caironi P, Pesenti A; Prone-Supine Study Group . Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med : 2727–2733, 2003. doi: 10.1097/01.CCM.0000098032.34052.F9. [DOI] [PubMed] [Google Scholar]
- 26.Gatto LA, Fluck RR Jr. Alveolar mechanics in the acutely injured lung: role of alveolar instability in the pathogenesis of ventilator-induced lung injury. Respir Care : 1045–1055, 2004. [PubMed] [Google Scholar]
- 27.Habashi NM. Other approaches to open-lung ventilation: airway pressure release ventilation. Crit Care Med , Suppl: S228–S240, 2005. doi: 10.1097/01.CCM.0000155920.11893.37. [DOI] [PubMed] [Google Scholar]
- 28.Halter JM, Steinberg JM, Schiller HJ, DaSilva M, Gatto LA, Landas S, Nieman GF. Positive end-expiratory pressure after a recruitment maneuver prevents both alveolar collapse and recruitment/derecruitment. Am J Respir Crit Care Med : 1620–1626, 2003. doi: 10.1164/rccm.200205-435OC. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann J, Tawhai MH, Kaczka DW. Regional gas transport in the heterogeneous lung during oscillatory ventilation. J Appl Physiol (1985) : 1306–1318, 2016. doi: 10.1152/japplphysiol.00097.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain SV, Kollisch-Singule M, Sadowitz B, Dombert L, Satalin J, Andrews P, Gatto LA, Nieman GF, Habashi NM. The 30-year evolution of airway pressure release ventilation (APRV). Intensive Care Med Exp : 11, 2016. doi: 10.1186/s40635-016-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson BD, Sieck GC. Differential susceptibility of diaphragm muscle fibers to neuromuscular transmission failure. J Appl Physiol (1985) : 341–348, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Kacmarek RM, Villar J, Sulemanji D, Montiel R, Ferrando C, Blanco J, Koh Y, Soler JA, Martínez D, Hernández M, Tucci M, Borges JB, Lubillo S, Santos A, Araujo JB, Amato MB, Suárez-Sipmann F; Open Lung Approach Network . Open lung approach for the acute respiratory distress syndrome: a pilot, randomized controlled trial. Crit Care Med : 32–42, 2016. doi: 10.1097/CCM.0000000000001383. [DOI] [PubMed] [Google Scholar]
- 33.Kaczka DW, Herrmann J, Zonneveld CE, Tingay DG, Lavizzari A, Noble PB, Pillow JJ. Multifrequency oscillatory ventilation in the premature lung: effects on gas exchange, mechanics, and ventilation distribution. Anesthesiology : 1394–1403, 2015. doi: 10.1097/ALN.0000000000000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kollisch-Singule M, Emr B, Jain SV, Andrews P, Satalin J, Liu J, Porcellio E, Kenyon V, Wang G, Marx W, Gatto LA, Nieman GF, Habashi NM. The effects of airway pressure release ventilation on respiratory mechanics in extrapulmonary lung injury. Intensive Care Med Exp : 35, 2015. doi: 10.1186/s40635-015-0071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kollisch-Singule M, Emr B, Smith B, Roy S, Jain S, Satalin J, Snyder K, Andrews P, Habashi N, Bates J, Marx W, Nieman G, Gatto LA. Mechanical breath profile of airway pressure release ventilation: the effect on alveolar recruitment and microstrain in acute lung injury. JAMA Surg : 1138–1145, 2014. doi: 10.1001/jamasurg.2014.1829. [DOI] [PubMed] [Google Scholar]
- 36.Kollisch-Singule M, Emr B, Smith B, Ruiz C, Roy S, Meng Q, Jain S, Satalin J, Snyder K, Ghosh A, Marx WH, Andrews P, Habashi N, Nieman GF, Gatto LA. Airway pressure release ventilation reduces conducting airway micro-strain in lung injury. J Am Coll Surg : 968–976, 2014. doi: 10.1016/j.jamcollsurg.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lachmann B. Open up the lung and keep the lung open. Intensive Care Med : 319–321, 1992. doi: 10.1007/BF01694358. [DOI] [PubMed] [Google Scholar]
- 38.Lu J, Wang X, Chen M, Cheng L, Chen Q, Jiang H, Sun Z. An open lung strategy in the management of acute respiratory distress syndrome: a systematic review and meta-analysis. Shock (January 25, 2017). doi: 10.1097/SHK.0000000000000822, in press. [DOI] [PubMed] [Google Scholar]
- 39.Luecke T, Roth H, Herrmann P, Joachim A, Weisser G, Pelosi P, Quintel M. PEEP decreases atelectasis and extravascular lung water but not lung tissue volume in surfactant-washout lung injury. Intensive Care Med : 2026–2033, 2003. doi: 10.1007/s00134-003-1906-9. [DOI] [PubMed] [Google Scholar]
- 40.Lumb AB. Nunn’s Applied Respiratory Physiology. New York: Elsevier, 2017, p. xii. [Google Scholar]
- 41.Malhotra A, Drazen JM. High-frequency oscillatory ventilation on shaky ground. N Engl J Med : 863–865, 2013. doi: 10.1056/NEJMe1300103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol : 596–608, 1970. [DOI] [PubMed] [Google Scholar]
- 43.Mercat A, Titiriga M, Anguel N, Richard C, Teboul JL. Inverse ratio ventilation (I/E = 2/1) in acute respiratory distress syndrome: a six-hour controlled study. Am J Respir Crit Care Med : 1637–1642, 1997. doi: 10.1164/ajrccm.155.5.9154869. [DOI] [PubMed] [Google Scholar]
- 44.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med : 1327–1334, 1994. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 45.Namati E, Thiesse J, de Ryk J, McLennan G. Alveolar dynamics during respiration: are the pores of Kohn a pathway to recruitment? Am J Respir Cell Mol Biol : 572–578, 2008. doi: 10.1165/rcmb.2007-0120OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nieman GF, Bredenberg CE, Clark WR, West NR. Alveolar function following surfactant deactivation. J Appl Physiol Respir Environ Exerc Physiol : 895–904, 1981. [DOI] [PubMed] [Google Scholar]
- 47.Nieman GF, Gatto LA, Habashi NM. Impact of mechanical ventilation on the pathophysiology of progressive acute lung injury. J Appl Physiol (1985) : 1245–1261, 2015. doi: 10.1152/japplphysiol.00659.2015. [DOI] [PubMed] [Google Scholar]
- 48.Protti A, Maraffi T, Milesi M, Votta E, Santini A, Pugni P, Andreis DT, Nicosia F, Zannin E, Gatti S, Vaira V, Ferrero S, Gattinoni L. Role of strain rate in the pathogenesis of ventilator-induced lung edema. Crit Care Med : e838–e845, 2016. doi: 10.1097/CCM.0000000000001718. [DOI] [PubMed] [Google Scholar]
- 49.Retamal J, Bergamini BC, Carvalho AR, Bozza FA, Borzone G, Borges JB, Larsson A, Hedenstierna G, Bugedo G, Bruhn A. Non-lobar atelectasis generates inflammation and structural alveolar injury in the surrounding healthy tissue during mechanical ventilation. Crit Care : 505, 2014. doi: 10.1186/s13054-014-0505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roan E, Waters CM. What do we know about mechanical strain in lung alveoli? Am J Physiol Lung Cell Mol Physiol : L625–L635, 2011. doi: 10.1152/ajplung.00105.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy S, Habashi N, Sadowitz B, Andrews P, Ge L, Wang G, Roy P, Ghosh A, Kuhn M, Satalin J, Gatto LA, Lin X, Dean DA, Vodovotz Y, Nieman G. Early airway pressure release ventilation prevents ARDS: a novel preventive approach to lung injury. Shock : 28–38, 2013. doi: 10.1097/SHK.0b013e31827b47bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roy S, Sadowitz B, Andrews P, Gatto LA, Marx W, Ge L, Wang G, Lin X, Dean DA, Kuhn M, Ghosh A, Satalin J, Snyder K, Vodovotz Y, Nieman G, Habashi N. Early stabilizing alveolar ventilation prevents acute respiratory distress syndrome: a novel timing-based ventilatory intervention to avert lung injury. J Trauma Acute Care Surg : 391–400, 2012. doi: 10.1097/TA.0b013e31825c7a82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russell JA, Hoeffel J, Murray JF. Effect of different levels of positive end-expiratory pressure on lung water content. J Appl Physiol Respir Environ Exerc Physiol : 9–15, 1982. [DOI] [PubMed] [Google Scholar]
- 54.Santos CC, Zhang H, Liu M, Slutsky AS. Bench-to-bedside review: biotrauma and modulation of the innate immune response. Crit Care : 280–286, 2005. doi: 10.1186/cc3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schiller HJ, McCann UG II, Carney DE, Gatto LA, Steinberg JM, Nieman GF. Altered alveolar mechanics in the acutely injured lung. Crit Care Med : 1049–1055, 2001. doi: 10.1097/00003246-200105000-00036. [DOI] [PubMed] [Google Scholar]
- 56.Seah AS, Grant KA, Aliyeva M, Allen GB, Bates JHT. Quantifying the roles of tidal volume and PEEP in the pathogenesis of ventilator-induced lung injury. Ann Biomed Eng : 1505–1516, 2011. doi: 10.1007/s10439-010-0237-6. [DOI] [PubMed] [Google Scholar]
- 57.Stamenović D, Wilson TA. Parenchymal stability. J Appl Physiol (1985) : 596–602, 1992. [DOI] [PubMed] [Google Scholar]
- 58.Steinberg JM, Schiller HJ, Halter JM, Gatto LA, Lee HM, Pavone LA, Nieman GF. Alveolar instability causes early ventilator-induced lung injury independent of neutrophils. Am J Respir Crit Care Med : 57–63, 2004. doi: 10.1164/rccm.200304-544OC. [DOI] [PubMed] [Google Scholar]
- 59.Suki B, Barabási AL, Lutchen KR. Lung tissue viscoelasticity: a mathematical framework and its molecular basis. J Appl Physiol (1985) : 2749–2759, 1994. [DOI] [PubMed] [Google Scholar]
- 60.Suki B, Hubmayr R. Epithelial and endothelial damage induced by mechanical ventilation modes. Curr Opin Crit Care : 17–24, 2014. doi: 10.1097/MCC.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 61.Suki B, Stamenović D, Hubmayr R. Lung parenchymal mechanics. Compr Physiol : 1317–1351, 2011. doi: 10.1002/cphy.c100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol (1985) : 2026–2033, 1999. [DOI] [PubMed] [Google Scholar]
- 63.Villar J, Blanco J, Kacmarek RM. Current incidence and outcome of the acute respiratory distress syndrome. Curr Opin Crit Care : 1–6, 2016. doi: 10.1097/MCC.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 64.Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr Opin Crit Care : 3–9, 2014. doi: 10.1097/MCC.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 65.Young D. High-frequency oscillation for ARDS. N Engl J Med : 2234, 2013. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 66.Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, Rowan K, Cuthbertson BH; OSCAR Study Group . High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med : 806–813, 2013. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]