Abstract
The mechanical properties of lung tissue are important determinants of lung physiological functions. The connective tissue is composed mainly of cells and extracellular matrix, where collagen and elastic fibers are the main determinants of lung tissue mechanical properties. These fibers have essentially different elastic properties, form a continuous network along the lungs, and are responsible for passive expiration. In the last decade, many studies analyzed the relationship between tissue composition, microstructure, and macrophysiology, showing that the lung physiological behavior reflects both the mechanical properties of tissue individual components and its complex structural organization. Different lung pathologies such as acute respiratory distress syndrome, fibrosis, inflammation, and emphysema can affect the extracellular matrix. This review focuses on the mechanical properties of lung tissue and how the stress-bearing elements of lung parenchyma can influence its behavior.
I. INTRODUCTION
The mechanical properties of lung tissue are important determinants of the overall mechanical behavior of the respiratory system, contributing significantly to both elastic and dissipative (resistive) properties of the lung. In fact, the dissipative properties of lung tissue are the major determinants of lung resistance within the breathing frequency range (11, 14, 143). The biomechanical properties of connective tissues play fundamental roles in the functioning of almost every organ and are critical determinants of how mechanical forces acting on the body/organ produce physical changes at the cellular level (144). It is recognized that mechanical interactions between cells and the extracellular matrix (ECM) have regulatory effects on cellular physiology, leading to reorganization and remodeling of the ECM, which in turn influences lung function (19, 144).
The act of breathing entails the cyclic application of physical stresses at the pleural surface as well as the transmission of those stresses throughout the lung tissue and its adherent cells (40). The applied stresses result in length changes of parenchymal structures (strain), yielding volume variation. To support extreme strain variations, while maintaining the minimal tissue diffusional barrier necessary for adequate gas exchange, lung parenchyma presents important stress-bearing systems, including the gas-liquid interface, the connective tissue matrix, and the contractile apparatus (38). These stress-bearing elements can be significantly changed during pathological states, thus affecting lung mechanics. Excellent reviews can be found on macroscale (90) and microscale (138, 144) lung mechanics, as well as on transmission of mechanical stresses from the pulmonary macroscale to the cell microenvironment (40). This review focuses on the mechanical properties of lung tissue and how the stress-bearing elements of lung parenchyma can influence its behavior in normal and pathological conditions.
II. TISSUE MECHANICS: ENERGY DISSIPATION AND STORAGE DURING VENTILATION
A. Elastance and Resistance
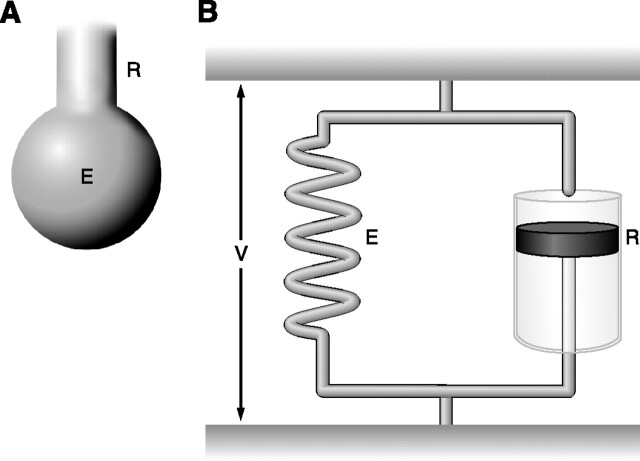
During quiet breathing, energy is used to overcome elastic and resistive forces. The classic equation of motion describes the relationship among pressure, volume, and flow as P(t) = EV(t) + RV′(t) + P0, where at any instant t, P is applied pressure, E is elastance, V is volume, R is resistance, V′ is flow, and P0 is pressure corresponding to transpulmonary pressure at end expiration. Thus resistive pressure (Pres) refers to pressure dissipated to overcome resistance (R); whenever flow is absent, Pres equals zero (112). The representative model for this equation is the resistive-elastic model, characterized by a single resistive compartment connected to a single elastic compartment, and represented by a dashpot in parallel with a spring. To increase volume in this resistive-elastic model, part of the applied energy would be dissipated by the dashpot (resistance), whereas another fraction of the total energy would be stored in the spring (elastance), providing the driving pressure for expiration (Fig. 1). The resistive-elastic model, however, does not explain the pressure-time profile observed after interruption of a constant flow inflation, where a slow pressure decay can be found even after flow has ceased (stress relaxation).
FIG. 1.
Linear unicompartmental model (resistive-elastic model). A: anatomical representation: tube with resistance (R) and ballon with elastance (E). B: mechanical representation characterized by a dashpot with Newtonian resistance (R) in parallel with a spring with elastance (E) submitted to the same deformation volume (V).
It has been consistently observed that recoil pressures at the same lung volumes are always less during deflation than inflation (hysteresis), meaning that the mechanical energy (work) expended during inflation exceeds that recovered during deflation. With repeated cycling, loops enclose an area representing the energy lost per cycle (59). During quiet breathing, the area enclosed by the pressure-volume or stress-strain curves is nearly independent of frequency. Thus, under constant amplitude cycling, energy dissipation is nearly independent of frequency (1). However, the dissipation is proportional to the product of resistance and frequency. Taken together, these findings imply that resistance must be inversely proportional to frequency (6, 43). Tissue resistance sharply contrasts with airway resistance, which exhibits much less frequency dependence (59).
B. Viscoelasticity
The relationship between stress and strain, e.g., the constitutive equation, contains information on the underlying mechanisms and structure contributing to such a behavior (43). Closely related to the hysteresis seen during volume cycling of the lung are the phenomena of stress adaptation and creep. In this line, soft biological tissues are known to be highly viscoelastic in nature. In contrast to perfect elastic materials, viscoelastic substances do not maintain a constant stress under constant deformation; the stress, instead, slowly relaxes (stress relaxation). On the other hand, under constant stress, the material undergoes a continuous deformation in time, i.e., creep (15, 23, 97, 142) (Fig. 2). Indeed, viscoelasticity represents an important component of respiratory mechanics, being responsible, in some cases, for most of the pressure dissipated during breathing (4, 88, 129). The viscoelastic model adds a third component to the resistive-elastic one, the Maxwell body, in which a dashpot (viscous element) lies in series with a spring (elastic element) (55, 56) (Fig. 3). In this model, the spring can transfer energy to the dashpot that burns it as heat; hence, the energy of the system may diminish even when it is not moving. The slow decay in pressure observed after end-inflation occlusion can be perfectly superimposed to alveolar pressure measured by alveolar capsules, thus representing energy dissipation at the lung parenchyma level (119). The linear viscoelastic model predicts dynamic elastance and its frequency dependence quite well (26, 142), but it cannot account for about one-third of the energy loss (hysteresis), suggesting that additional nonviscous plastoelastic elements are required in the model. They do not impart time and frequency dependence (stress relaxation and dynamic elastance) but do consume energy (59).
FIG. 2.
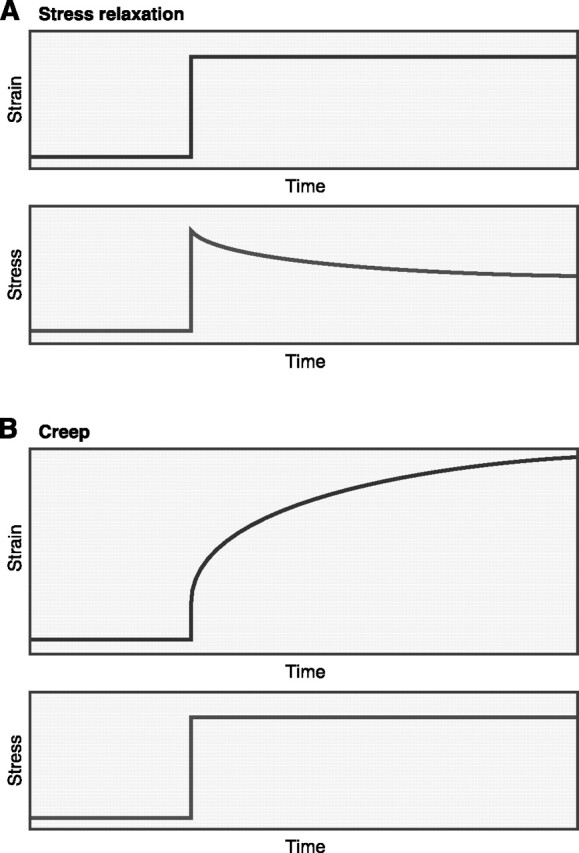
Viscoelastic materials, such as soft biological tissues, when held at a constant deformation (strain) show a progressive decrease in stress, called stress relaxation (A), and when held at a constant stress they show a progressive increase in deformation (strain), i.e., creep (B).
FIG. 3.
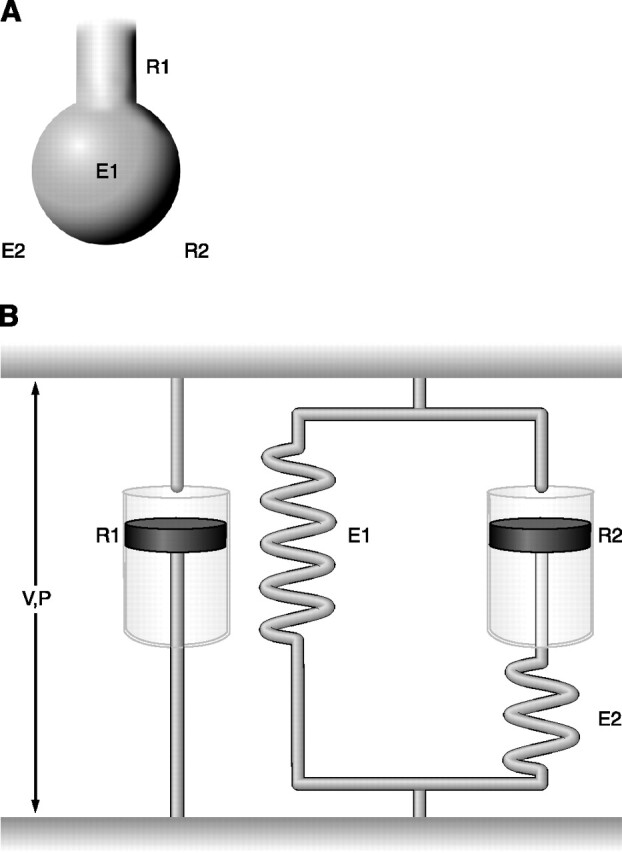
Viscoelastic model. A: anatomical representation with two resistive components (R1 and R2) and two elastic components (E1 and E2). B: mechanical representation characterized by a resistive component (R1) in parallel with a Kelvin body, which consists of an elastic component (E1), representing the static elastance, in parallel with a Maxwell body, a dashpot assembled in series with a spring, representing the viscoelastic behavior. The distance between the two horizontal bars is analog to lung volume (V), and the tension between them represents the airway opening pressure (P).
Lung parenchyma displays prominent viscoelastic behavior. However, resistive pressures across tissue, in contrast to airways, are non-Newtonian (55, 56). It is recognized that the major part of energy dissipation associated with cyclic lung expansion depends on the amount of expansion but not the rate of expansion, demonstrating that lung tissue shows a stress incompatible with the notion of a viscous stress (41). During cycling, the stress that develops in the viscoelastic body displays a component in phase with strain, which is the elastic stress contributing to the storage modulus (or dynamic elastance in the lung), and a component out of phase with strain, corresponding to the viscous dissipation and contributing to the loss modulus (or lung tissue resistance, or damping) (6, 55). Thus the relative contribution of tissue resistance to overall lung resistance can be magnified by large volume changes and by low flow rates, the opposite effect being observed in the airway component (83).
The viscoelastic properties of lung tissue cause the effective tissue resistance (Rti) to be high at very low frequencies but then decrease quasi-hyperbolically to zero at higher frequencies (6, 12, 41, 51, 146). As a consequence, in many species, Rti of the healthy lung represents the main contributor to total lung resistance during breathing (85). In this line, study using alveolar capsules disclosed that from 0.5 to 2.0 Hz and in the face of physiological tidal volumes, lung tissue resistance represents a major component of total pulmonary resistance (83). At breathing frequencies in the range of 0.2–0.4 Hz, tissue resistance can account for ∼40% of lung resistance (6).
During a physiological tidal excursion, there is little or no influence of airway inhomogeneities on measurements of lung mechanics in the healthy lung. On the other hand, true static lung elastance (EL) is approached at very low frequencies, and, as frequency increases, EL augments owing to either tissue viscoelasticity (i.e., a real tissue phenomenon) or airway inhomogeneities (i.e., an airway phenomenon) (85). In general, although Rti varies widely with tidal volume, lung volume, and lung size, tissue hysteresivity (η) remains remarkably constrained to a narrow range. Indeed, η exhibits small variability, frequency invariance, and numerical consistency across species (41).
Numerous studies have used models mainly in the context of describing oscillatory behavior of lung tissue where the hysteresis area of the P-V curve is nearly independent of frequency and the stress relaxation function follows a power law dependence on time (103, 142, 146). The constant-phase model by Hantos et al. (52), describing the viscoelastic properties of lung tissues, has been considered superior to the classic spring and dashpot representation. Although the electrical analog of viscoelastic processes as well as phenomenological and mechanical approaches yield good quantitative correspondence with data, they lack anatomic and mechanistic specificities (52).
Later models tried to deal with dynamic tissue behavior on a mechanistic basis (96). Some mechanisms have been proposed as contributors to the constant-phase tissue viscoelasticity, such as the structural disposition of fibers and their instantaneous configuration during motion (the interaction between fibers in close proximity), since elastic fibers dissipate energy as they slip with respect to each other (96). Additionally, lung tissue might exhibit molecular mechanisms similar to those proposed for polymer rheology (142). Maksym and Bates (86) suggested a role for the relative stress-bearing contributions of collagen and elastin fibers based on the differential elastic properties of these two types of fibers, in which collagen fibers were progressively recruited with strain. Bates (11) also proposed that the nonlinear elastic properties and linear elastic behavior of lung tissues arise from different physical processes, whereas elastic recoil is linked to geometry as fibers rearrange themselves; stress adaptation would reflect a process of diffusion due to the thermal motion of the fibers with respect to each other and to the ground substance (11).
Micromechanical studies have tried to determine tissue microrheological behavior, that is, the measurement of its complex elastic modulus G. G represents the complex ratio between stress and strain in the frequency domain and can be split into an elastic component G′ and a viscous or frictional component G′′. Fredberg and Stamenovic (41) showed that dissipative and elastic processes are coupled in lung tissue, and hysteresivity (η) denotes the empirical variable that quantifies the dependence of dissipative processes on elastic processes, a tax that must be spent any time elastic energy is stored. These authors also postulated that dissipative and elastic processes are coupled at the level of the stress-bearing element, which represents a structure composed of anatomically distinct materials. Thus a modification in η reflects either a change in hysteretic behavior within one of those materials or an alteration in the mechanical interaction among them (41). However, the structural damping law is only descriptive; it does not specify the mechanisms through which the coupling of dissipative and elastic processes might come about, and thus the molecular basis of η remains obscure (62). Nevertheless, four processes are implicated in this phenomenon: the kinetics of cross-bridge attachment-detachment within the contractile element, the kinetics of the surface-active molecule adsorption-desorption to the surface film, the kinetics of fiber-fiber networking within the connective tissue matrix, and the kinetics of recruitment-derecruitment (41).
C. Stress-Strain Relationship and Lung Tissue Components
The elastic recoil of the lung at normal breathing frequencies is dominated by the nonlinear stress-strain characteristics of the lung tissue (139), whereas the main stress-bearing constituents are collagen and elastin fibers (43). In the lung parenchyma elastin provides elasticity, especially at lower stress levels, while the collagen fibers are loosely arranged and wavy, not becoming tight until the parenchyma is distended (43). In other words, elastin responds for load bearing at low strains; as strain increases, the collagen fibers progressively take up more load, thereby stiffening the tissue (86). These fibers significantly differ in their mechanical properties: elastin is highly stretchable before rupturing, while collagen is much stiffer and significantly less stretchable than elastin. Indeed, collagen represents the basic structural element for soft and hard tissues that provides mechanical integrity and strength to a variety of tissues and organs. On the other hand, elastin is the most “linear” elastic biosolid material. Both fiber types show a complex organization and are integrated with cells and intercellular substance in the tissues. This intercellular substance, called ground substance, contains proteoglycans, glycosaminoglycans, and tissue fluid. Depending on how the fibers, cells, and ground substance are organized into a structure, the mechanical properties of the tissue vary (43). The importance of fiber organization is especially evident for collagen that is organized into many different kinds of structures according to the organ/tissue function, varying from the simplest parallel fibered structure found in tendon and ligaments to the most complex structures of blood vessels (43).
Loading and unloading of animal tissues result in two different stress-strain curves (hysteresis loop), deviating from the linear Hooke's law and disclosing the existence of an energy dissipation mechanism (43). Furthermore, when held at constant strain, these tissues show stress relaxation and creep. Beyond physiological ranges, soft tissues usually have a large reserve of strength before they rupture and fail (43). These features of hysteresis, relaxation, and creep at lower stress ranges (“physiological”) indicate that biological tissues are inelastic, with their stress-strain relationship nonlinear. Soft tissues, however, can be treated as one elastic material in loading, and another elastic material in unloading (43).
In the physiological state, lung tissues are not unstressed. In fact, the lung does not have a defined stress-free state; unlike most other organs, it is a pressure-supported structure (77). The chest wall and associated respiratory muscles provide the external distending stress to maintain the lung state of inflation, whereas the transpulmonary pressure may vary during breathing but always remains positive. Thus the mechanical behavior of the lung depends on the level of the prestress transpulmonary pressure, as well as on the mechanical behavior of its microstructural elements (138). The parenchymal microstructure functions as a tension-supported lattice (78, 91), and the transpulmonary pressure (distending stress) is transmitted throughout the parenchymal tissues, thus distending all intrapulmonary structures (91).
In the lung, airways and blood vessels are embedded in and attached to a surrounding tissue that consists of an interconnected network of thin walls (168). Intraparenchymal vessels and airways exhibit mechanical properties different from those of the parenchymal tissues in which they are embedded; therefore, parenchymal distortions arise during the act of breathing (40, 91). Transpulmonary pressure acts directly on the elastic surface of the lung, and this stress is transmitted to internal points of the organ by the elastic elements. Although the lung is exposed to transpulmonary pressure, its air spaces are distended solely by the force applied on them by surrounding tissues, as a result of mechanical interdependence. In a homogeneous lung, all distensible regions are exposed to transpulmonary pressure, while in nonuniformly expanded lungs, the effective distending pressure differs from transpulmonary pressure (91, 168) (Fig. 4).
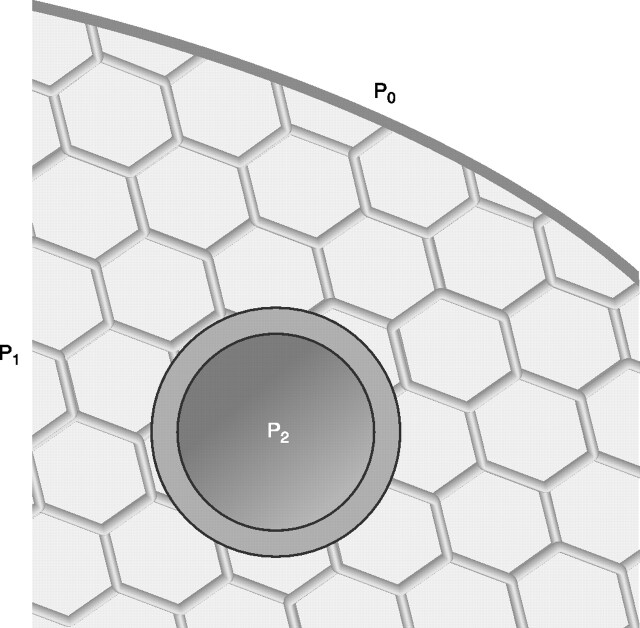
FIG. 4.
Schematic representation of the shear stress mechanism. A thin-walled elastic tube (airway or blood vessel) is embedded in spring-network material, whereas pressures P0, P1, and P2 act on the walls of the tube. P1 is uniform throughout the spring network. In the initial pretensed state, P1 and P2 are assumed to be equal and greater than P0, thus rendering uniform the initial state of the material. If the pressures in the three regions change slightly, a nonuniform deformation can occur, and the mechanics of the thin-walled tube are affected by the surrounding material. [Adapted from Wilson (168).]
Many efforts have been focused on establishing a mathematical relation, called the constitutive equation, which describes the mechanical behavior of the lung, i.e., its stress-strain relationship (138, 144). The simplest equation is the linear relationship (Hooke's law), which contains only two independent coefficients: the bulk modulus, indicating the ability to resist a small uniform expansion, and the shear modulus, indicating the ability to resist a small isovolume shape distortion (138).
In the continuum elasticity model suggested for lung parenchyma (168), the shear modulus increases with augmenting distending stress. The effects of a local disturbance are spread out by the surrounding material if the shear modulus is low, whereas the effects of the surrounding material on the mechanics of a local element are greater if the shear modulus is high.
The microscopic properties of the parenchymal network influence both elastic and resistive properties of the lung. Hysteresis, which can be measured as the area enclosed by the pressure-volume loop of the lung, closely relates to tissue resistance (20, 41). Indeed, the potential sources of hysteresis in the normal lung include the air-liquid interface (92, 102, 169), parenchymal contractile cells (38), and the behavior of the collagen-elastin network (20, 96, 175), that is, the main stress-bearing systems (38).
D. Stress-Bearing Elements: Mechanical Contributions of Elastin, Collagen, Fiber Network, and Interstitial Cells
The principal stress-bearing elements of septal tissues have been commonly assumed to be the connective tissue fibers. Collagen, elastin, proteoglycans, and glycosaminoglycans constitute the major components of the connective tissue (93, 166). Although the precise contribution of each of these elements remains unknown, the mechanical behavior of the collagen-elastin-proteoglycan matrix likely accounts for the main proportion of energy dissipation during breathing (3, 41, 95). It should be kept in mind that when fibers are arranged in a network, the latter can display hysteretic and viscoelastic properties (21, 22, 123). Furthermore, the elastic properties of collagen and elastic fibers differ to a great extent. Collagen, the most abundant component, admits only minimal stretching, showing great tensile strength but low compliance. Conversely, elastic fibers may be stretched by a factor of 2–2.3 in relation to their unloaded lengths, presenting lower tensile strength than collagen but higher compliance (59, 96, 166).
Different theories try to explain tissue viscoelastic behavior in terms of the movement of fibers within the connective matrix. The fiber-fiber interaction theory proposes that energy dissipation occurs not at the molecular level within collagen and elastin, but, instead, at the level of fiber-fiber contact and by the shearing of glycosaminoglycans that provide the lubricating film between adjacent fibers (96). A second theory suggests that the mechanical properties of the tissue matrix can be understood as a polymer, whereas viscoelastic behavior occurs because of conformational changes in the molecules, thus being secondary to the structural disposition of fibers and their instantaneous configuration during motion (142). This theory proposes that external stresses cause polymer reptation, a sort of snakelike unfolding of fibers that alters the configuration of molecules and creates distortions that dissipate energy (142).
Evidences of distinct tissue mechanical properties between mouse strains suggest a key role for specific extracellular fiber composition and the amount of contractile structures in the lung parenchyma. The total amount of collagen fibers in lung parenchyma strips has been positively correlated with tissue resistance and elastance, while elastic fiber content has been positively associated with tissue elastance (34). In addition, specific components of the elastic system may also play a role in the observed differences in tissue mechanics among species (35, 44, 94). Altogether these evidences suggest that collagen and elastic fibers directly contribute to lung tissue stiffness and viscosity. It is noteworthy, however, that not only the absolute amount of fibers constitutes an important factor affecting mechanical behavior, but rather the organization and/or interaction among these fibers (96, 174).
The better understanding of tissue resistance behavior requires the unfolding of the complexity of interactions between the different structures comprising this material. The anatomic elements responsible for tissue hysteresivity, resistance, and elastance are thus far unknown (82). As underlined before, the potential factors contributing to tissue hysteresis include contractile elements in the lung parenchyma (73, 166), the collagen elastin matrix (96), the air-liquid interface or surfactant (92), and recruitment-derecruitment of atelectatic alveolar spaces (135). Schurch et al. (126) showed that after few consecutive cycles, the hysteresis of the surface film during small-amplitude cycling becomes negligible, suggesting that during tidal breathing the contributions of the surface film to lung hysteresis are small. In addition, dynamic properties of parenchymal tissue strips do not change after loss of cell viability (174). Taken together, these studies suggest that lung parenchyma mechanics are most likely dominated by the extracellular matrix (175). Furthermore, elastance of lung tissue strips is far less than that of collagen or elastic fibers alone, which represents a network effect where the total stiffness of a large network of interconnected springs can be smaller than the spring constant of individual springs (175). When matrix strains under an applied load, relative movement occurs among neighboring fibers, implying that strains at the microstructural level may not follow strains at the macroscopic level (96). Yuan et al. (175) showed that digesting both elastin and collagen resulted in a large decrease in lung tissue elastance and indicated that the actual topology of the network must play an important role in its macroscopic behavior. Tissue mechanics at the macroscopic level are dominated by the connective fiber network, whereas interstitial cells play a less significant role (174). Various network models have been proposed in the literature, including fiber recruitment type models (86, 87, 113), the line element model of the alveolar duct (170), or other geometric models (30).
Suki et al. (142) proposed a mechanistic basis for the constant-phase-type tissue viscoelastic behavior, i.e., the so-called “reptation” motion of the collagen-elastin fibers. The distribution of fiber width and length has been identified as a candidate mechanism at the level of the fiber network responsible for the macroscopic viscoelasticity (174). On the basis of the architecture of the microstructure of the lung, slow reptation of fibers could contribute to the viscoelastic properties of lung tissue (142).
Mijailovich et al. (95) also identified the connective tissue network as the primary source of lung macroscopic behavior, whereas a stick-and-slip motion transfers the load between fibers in close contact. On the other hand, distinct mechanical states can be observed owing to contractile cells in lung parenchyma (38). Experimental evidence supports that hysteresivity is directly associated with the cross-bridge cycling rate and the metabolic state of the cells (39). However, Yuan et al. (174) found nearly identical mechanical parameters in viable and nonviable tissue samples, implying that the extracellular matrix may also play an important role in the mechanical properties of intact lung tissues. Nevertheless, the authors concluded that this finding does not imply that the cellular components do not influence parenchymal mechanics, since tissue stiffness changes in response to different contractile agents owing to cell contraction (174).
How specific remodeling features affect lung tissue mechanical properties can be partially unshadowed by lung maturation studies. The maturing lung represents a naturally occurring model of altered extracellular matrix that changes rapidly during the postnatal stage. The alveolarization process, characterized by an increase in the number and size of alveolar walls and a decrease in alveolar thickness, is followed by a marked increase in the amount of collagen and elastic fibers during the first several weeks of life (101). Proteoglycans and glycoproteins also change, including a decrease in hyaluronic acid and chondroitin sulfate proteoglycan concentrations (148, 149).
Nardell and Brody (101) correlated morphological changes and extracellular appearance of collagen and elastin with lung mechanical properties in a rat model of lung growth. The potential influences of alveolar or airway surface forces and of chest wall were eliminated by performing saline volume-pressure curves on excised rat lungs from the perinatal period (day 4) to early adulthood (day 40). Postnatal lung growth in the rat can be divided in four stages: lung expansion (between birth and 4 days), alveolar proliferation (between 4 and 12 days), elastin accumulation (between 12 and 20 days), and equilibrated growth (between 20 and 40 days). The authors showed that changes in lung elastin concentration are associated with an increase in lung recoil, while lung collagen is more closely associated with the structural integrity of the lung as measured by resistance to lung rupture. Furthermore, lung volume tends to follow the course of lung elastin concentration rather than collagen's (101).
Another study, examining the viscoelastic behavior of isolated parenchymal strips from rat lungs at different ages, showed that tissue resistance and elastance decreases with maturation, while hysteresivity (η) increases (149). Since the amount of collagen and elastin rises with maturation (101), it may be concluded that fiber structure, orientation, and/or alveolar geometry, rather than the absolute amount of collagen and elastin per se may be fundamental in determining tissue viscoelastic behavior (149). Indeed, maturational changes in tissue viscoelastic behavior in rats are accompanied by changes in the composition and configuration of the alveolar wall, with increasing collagen and elastic fiber contents and diminishing hyaluronic acid and α-actin smooth muscle-positive cells (148). These findings support the concept that the total amount of extracellular matrix fibers does not respond entirely for the mechanical behavior of the elastic elements. Indeed, changes in fiber subtype, topology, or cross-linking with maturation could contribute to it (148). In addition, proteoglycans and glycosaminoglycans may add to viscoelastic behavior because they coat the stress-bearing fibers and can affect tissue turgor. These molecules may act as “lubricant,” modulating the energy dissipation between fibers and thereby altering tissue mechanics (148). Indeed, the rise in η with maturation could be possibly explained either by the increase in the absolute number of fibers interacting with each other or, alternatively, by changes in the ground substance (148, 149).
E. Mechanical Interactions Between Collagen and Proteoglycans
Proteoglycans consist of a protein core with large polysaccharide side chains, the glycosaminoglycans. Glycosaminoglycans interact with collagen, elastin, and cells probably modulating the formation and/or remodeling of the connective tissue, but their mechanical roles remain largely unknown (61). Decorin, a small leucine-rich proteoglycan present in tissues such as the lung with fibrillar collagen, plays a key role in regulating collagen fibril formation and the spatial arrangement of collagen fibers in the matrix (67). It may also take part in the remodeling associated with respiratory diseases such as pulmonary fibrosis and asthma, by means of decorin-regulated collagen fibrillogenesis (172). Decorin binds transforming growth factor-β, a cytokine implicated in remodeling (158). Additionally, decorin-deficient mice show altered lung tissue mechanics with increased compliance at higher pressures and stresses, and smaller airway resistance (45). The alterations in tissue properties observed in decorin-deficient mice are probably secondary to abnormal collagen fibril formation (28, 48).
Proteoglycans can also potentially alter tissue turgor and viscoelasticity (124). Since it has been proposed that tissue viscoelasticity reflects the mechanical friction generated as adjacent fibers slide across each another (96), the nature of this mechanical interaction could be modified by the lubricating film between them. Proteoglycans coat individual collagen and elastic fibers; hence, alterations in these molecules could possibly affect energy dissipation at the fiber interface (3). The hydrophilic nature of proteoglycans and glycosaminoglycans attracts ions and fluid into the matrix (124). Indeed, the swelling of charged proteoglycans influences the macroscopic mechanical properties of lung tissue strips (3, 25). Although it seems that the network organization of collagen and elastic fibers constitutes the dominant factor in the elastic behavior of lung tissue, interaction of the fibers with proteoglycans can affect fiber network stability, thus contributing to the tissue mechanical profile (3, 25, 34).
F. Contractile Elements and Tissue Mechanics During Induced Constriction
Subpleural lung parenchymal strips are commonly used as a model for the study of mechanical and pharmacological properties of the lung periphery (34, 39, 115, 121, 173), being considered a good proxy of the peripheral lung tissue (38, 120).
The gas-free isolated parenchymal strip preparation abolishes the gas-liquid interface, thus eliminating an important stress-bearing system, as well as the effects of recruitment-derecruitment of atelectatic alveolar spaces. This preparation brings the experimenter a step closer to the contractile system and to the extracellular matrix behavior (38). The attachment-detachment of cross bridges during volume cycling (41) or the fiber network of the connective tissue (22, 123) could account for the hysteresis observed in the length-tension curve (41, 96).
The relevance of the parenchymal strip as a proxy for small airways has been questioned because the strip presents a complex morphology and contains a diversity of contractile cell types (33, 48). However, it can be considered as a proxy for the integrated contractile response of the parenchyma itself (38). Indeed, many authors have used the parenchymal strip as a means of investigating dynamic mechanical responses to constrictor challenge at the parenchymal level. Lung periphery constitutes a complex system comprising alveolar walls, bronchioles, and small vessels. The determination of the precise elements that respond to contractile stimulus is rather difficult, and it has been shown that the volume proportions of different anatomic structures, such as amounts of airway, vasculature, and alveolar wall, do not correlate with oscillatory mechanics in subpleural parenchymal lung strips under baseline conditions (82).
It is also noteworthy that in parenchymal strip preparations lung tissue undergoes uniaxial loading, and hence, microstructural elements are presumed to deform only unidimensionally, in contrast to the three-dimensional deformation present in the normally breathing lung. In the whole lung, macroscale hysteresivity is proportional but smaller than microscale hysteresivity, and microstructural elements respond both by reorienting and lengthening. On the other hand, during uniaxial deformation of lung strips, macroscale and microscale hysteresivity are similar, and microstructural components can respond only by lengthening; thus macroscale hysteresitivy (η) is a direct reflection of hysteresivity of the microstructural element (38).
The pressure-volume behavior of the lungs can be changed by several contractile interventions (29, 66, 81). Many lung responses that have initially been ascribed to changes in airway caliber have lately been shown to reflect ongoing events at the lung tissue level (79, 83, 117, 159). Lung parenchyma can express diverse mechanical responses according to the specific agonist present in the cellular microenvironment, which reveal themselves mainly in the hysteretic nature of the tissue (38, 84). Indeed, it has been demonstrated that the hysteretic behavior of pulmonary tissues increases with activation of the contractile machinery (38). Three types of contractile elements can participate: smooth muscle of small airways, smooth muscle in alveolar ducts, and interstitial contractile cells (59, 73).
It is currently considered that connective matrix is passively driven by contractile cells along its functional kernel characteristics to a new operating level without intrinsic structural changes, thus without significant changes in the transfer of tissue mechanical properties (115). On the other hand, contractile cell systems show prominent hysteretic properties, presenting structural changes during constriction because of bridge dynamics (40) and plastic remodeling of the cytoskeleton (49). Authors agree that the connective tissue network dominates parenchymal mechanics, which can be influenced by the tone or contraction of interstitial cells (38, 82, 115). Recent findings suggest that mechanical properties of lung tissue in baseline conditions are actually dominated by the connective fiber network, whereas interstitial cells play a less significant role (174). Interestingly, constriction modifies intrinsic mechanical properties of connective matrix by mechanisms diverse from passive stretching (114, 115), suggesting that constriction of some element at the tissue level occurs (98, 99). The mechanical response to passive length changes obeys the constant-phase behavior in the frequency domain (44, 115), while the Newtonian component of resistance increases only during constrictor challenge, but not during passive stretch (115). The mechanical coupling of contractile cells and fibers depends on the amount of constriction or characteristics of the machinery involved. Contractile cells modulate the mechanical properties of the connective matrix (115). Thus energy dissipation at tissue level derives from a complex combination of changes in small airways, modifications in alveolar ducts, and distortion of parenchymal tissues secondary to activation of contractile elements (80, 98).
It has also been suggested that tissue response to bronchoconstrictor agents can be secondary to increased inhomogeneities in peripheral airway resistance, which produce an artifactual increase in tissue resistance, suggesting the failure of the model to describe the lungs under highly heterogeneous constriction (13, 85). Although there is little or no influence of airway inhomogeneities on measurements of lung mechanics in healthy lungs during a physiological tidal excursion, airway inhomogeneities significantly increase tissue resistance during agonist-induced constriction (85). Lutchen et al. (85) also demonstrated that the frequency-dependent behavior of lung elastance (EL) is markedly influenced by inhomogeneities, rather than a strict consequence of altered tissue viscoelasticity. Indeed, methacholine challenge increases lung resistance at all frequencies and also the positive frequency dependence of EL with minute alterations in its low-frequency values, thus suggesting little changes in the true tissue elastance (85). These findings, however, can be model dependent, since they were observed in a single-compartment homogeneous model used to characterize lung constriction.
III. TISSUE MECHANICS IN LUNG DISEASES
A. Lung Parenchyma Remodeling: Micromechanics of Injured Lungs
During disease states, small-scale heterogeneity in mechanical properties (such as local shear moduli) increases considerably, which contributes importantly to local stress distributions (64). The determinants of the lung parenchymal stress and strain distributions in the intact thorax depend critically on the lung resistance to a shape change. Thus the effects of injury on lung mechanical properties become of paramount importance. Several mechanisms have been suggested to explain the larger shear modulus of injured lungs, such as interfacial tensions associated with alveolar exudates, increased surface tension, and the consequently increased prestress of the axial elastin and collagen network, interstitial edema and matrix remodeling, and scar formation and fibrosis (64).
Damaged lungs present two attributes that account for their increased risk of deformation injury. First, the number of airspaces capable of expanding during inspiration diminishes (“baby lung”), increasing the risk of injury from regional overexpansion, since units that do not expand during breathing undergo a stronger deforming stress (47, 64). Second, local impedance to lung expansion is heterogeneous because of uneven distribution of liquid and surface tension in distal airspaces, which generates shear stress between neighboring, interdependent units that operate at different volumes (64, 91). Subpleural alveoli of edematous isolated rat lungs present wavy, flooded walls and contain air pockets of different sizes and shapes (63). The presence of differently sized air pockets with diverse radii of curvature implies a nonuniform alveolar gas pressure and/or uneven surface tensions (64). Bachofen and co-workers (8, 9) suggested that regional differences in the physicochemical properties of the surfactant could be the source of the nonuniform surface tension. A maintained nonuniform alveolar gas pressure may suggest that the air pockets are trapped by liquid and foam in conducting airways (64).
One should not forget the important phenomenon of interdependence between elements of the lung parenchyma network, which promotes uniform expansion of individual units. Stress increases whenever the sum of forces of the surrounding tissue acts over a smaller surface area. Consequently, when an obstructed unit (e.g., an alveolus, a lobule, or a lung segment) resists expansion, the neighboring units exert a large inflationary stress on it. Moreover, the tension and strain of individual connective tissue elements onto the collapsed segment increase out of proportion in relation to those of more remote network structures (64).
Analysis by histochemistry demonstrated that lung collagen content augments in both acute and chronic interstitial diseases, suggesting that significant remodeling of alveolar tissue occurs even in acute situations (118). The extracellular matrix remodeling process occurs as a response to lung injury involving all of its components in a kind of chain reaction. Indeed, the elastic system, a major component of the extracellular matrix, that plays an important role in maintaining the patency of the airways and lung elastic recoil is also altered, with elastic fiber deposition, both in animal models of pulmonary fibrosis (42, 106, 111) and human lung fibrosis (104). On the other hand, increased elastin destruction occurs in certain pathological conditions owing to the release of powerful elastolytic proteases by inflammatory cells (130). Reactivation of elastin synthesis occurs in response to increased destruction, but in a disordered manner. Thus elastosis could also partially respond for the loss of the normal architecture of the alveolar walls, contributing to the alveolar mechanical dysfunction and remodeling present in acute and chronic interstitial lung diseases (104).
The small leucine-rich proteoglycan decorin regulates collagen fibril formation and spatial arrangement in the matrix (67). Thus decorin may also play a role in the remodeling associated with respiratory diseases such as pulmonary fibrosis and asthma (16). In fact, decorin-deficient mice present decreased airway resistance and increased respiratory compliance in vivo, while in vitro length-stress curves similarly show increased compliance of lung strips. The alterations in tissue properties are likely the result of abnormal collagen fibril formation (45).
Mechanical forces applied to normal lung parenchyma, such as during mechanical ventilation, can also induce structural and functional changes (19, 165). Lung cells submitted to different types of force produce proinflammatory mediators (107, 162). In addition, mechanical forces induce expression of extracellular matrix proteins by lung tissue (19, 147). Evidences gathered from lung parenchymal strip oscillation disclosed that tissue elastance and resistance augmented with increasing operating force and decreased with increasing amplitude. Additionally, a rise in force induced early tissue remodeling (46). In this model, force and amplitude variations simulate in vivo pressure and volume changes, respectively (65). Moreover, pathophysiological conditions of the lung may shift the balance of forces so as to chronically alter the amount of strain imposed on the airways (100, 164). Tepper et al. (150) demonstrated, in isolated rabbit intrapulmonary bronchial segments and lung parenchymal tissue slices, that the imposition of physiological levels of chronic mechanical strain resulted in significant changes in the passive and active physiological responses of these tissues. The absence of chronic strain on intraparenchymal airways resulted in smaller, stiffer airways that generated greater pressures and narrowed to a greater extent in response to contractile stimulation than airways that had been subjected to chronic strain. In addition, the effects of strain were most prominent in the smaller, more compliant airways (150).
B. Lung Fibrosis
Pulmonary fibrosis is characterized by abnormal lung physiology and by the excessive production of extracellular matrix molecules such as collagen, elastin, and proteoglycans (136, 140). Since Rti accounts for a major proportion of energy dissipation during breathing, defining dynamic tissue behavior in fibrotic diseases represents an important goal (32). Since 1967 it is recognized that Rti increases in patients with lung fibrosis (7). More recently, a bleomycin-induced rat model of pulmonary fibrosis disclosed that Rti rises both in intact animals and in isolated lung parenchyma strips oscillated in an organ bath, with similar magnitude changes in vitro and in vivo (31). Besides the documented increases in collagen and elastin (136, 140), investigators have focused on changes in proteoglycans, the macromolecules that comprise the ground substance of the pulmonary matrix (32). Three major subclasses of proteoglycans have been described: hyalectins, which are large molecules such as versican that form aggregates with hyaluronic acid; basement membrane proteoglycans such as perlecan; and the small leucine-rich ones, which include lumican, biglycan, fibromodulin, and decorin (68). The proteoglycans present many different biological functions: they affect mechanical behavior, influence collagen fiber formation and assembly, modulate cell migration, and interact with various cytokines and growth factors (68). Indeed, a prominent deposition of these molecules is observed in both granulomatous and nongranulomatous forms of fibrotic lung disease (16, 17). Evidences suggest that the large proteoglycan versican (16, 17) or the small fibromodulin decorin and biglycan are altered in the early stages of fibrosis (167).
Links between changes in lung function and structure have been described in fibrotic disease. A modest correlation was observed between the degree of fibrosis in idiopathic pulmonary fibrosis and the shape of the lung pressure-volume curve (122). Moreover, positive correlations between volume proportion of collagen and parenchymal strip resistance and elastance were demonstrated in bleomycin-induced fibrosis in rats, with no correlation between collagen or elastin and hysteresivity (31). Ebihara et al. (32) described, in bleomycin-instilled rats, changes in oscillatory and quasi-static mechanics prior to measurable changes in volume fraction of either collagen or elastic fibers. Actually, those authors reported important positive associations between changes in structure and function, namely, biglycan and the three oscillatory parameters (resistance, elastance, and hysteresivity), suggesting that biglycan acts on lung mechanics by means of its influence on the matrix assembly (32, 125).
Oscillatory mechanics of lung strips in a murine model of silicosis showed time-dependent functional changes, with increased tissue resistance and elastance, followed by a later rise in hysteresivity. Tissue mechanical behavior was correlated with lung parenchyma inflammation and remodeling, characterized by a process of fibroelastosis, thus supporting a key role for the elastic system and its specific components on tissue mechanical properties (36).
C. Lung Inflammation: Asthma
Various lung diseases are characterized by underlying acute and/or chronic inflammation. Moreover, lung inflammation has been associated with structural changes collectively referred to as remodeling. This complex and variable mechanism occurs in conditions such as acute lung injury and fibrotic disorders, as described above, as well as in chronic obstructive pulmonary diseases.
Asthma is characterized by reversible airway obstruction, airway hyperresponsiveness, and airway inflammation. As in many chronic inflammatory disorders, asthmatic airway inflammation is also believed to cause tissue injury and subsequent structural changes (58, 133). These changes have attracted a great deal of interest because they may account for pathophysiological aspects in asthma that are poorly addressed with current anti-inflammatory strategies (58).
There are evidences that asthmatic inflammation extends to distal airways and lung parenchyma (76, 155, 156, 171). Prolonged inflammation may lead to thickening of the airway wall, which can be found throughout the bronchial tree including airways <2 mm in diameter (116). In nonfatal asthma, where airways are only slightly thickened, changes are most pronounced in the small airways (24, 57). Another characteristic structural change, the reticular basement membrane thickening with collagen deposition in the subepithelial space, constitutes an almost pathogenomic feature of asthma, although its clinical significance has not yet been established (133). Moreover, distal airway and lung parenchyma remodeling features have been described in a murine model of chronic allergic sensitization displaying increased tissue elastance and resistance (127, 128, 171).
Other elements of the extracellular matrix, such as proteoglycans and glycosaminoglycans, are altered by remodeling and have been related to changes in tissue mechanics, cellular activity, cytokine function, and fluid balance. Deposition of the proteoglycans versican, biglycan, and lumican, as well as of the glycosaminoglycan hyaluronan has been described in asthma (62, 110, 132, 161). The finding of increased amounts of proteoglycans in the airways of asthmatics and ovalbumin-sensitized/challenged mice is of particular interest. Proteoglycans bind with fibrillar collagen and influence the interaction of collagen fibrils and their assembly (53). They also bind to other components of the extracellular matrix and growth factors, affecting cell adhesion and extracellular matrix deposition (53, 108). The importance of increased airway extracellular matrix deposition in the pathogenesis of asthma remains uncertain. However, asthma severity and decline in FEV1 have been correlated with subepithelial fibrosis (60). Furthermore, a significant association was observed between enhanced proteoglycan deposition and increased airway responsiveness (61).
Abnormal elastic fiber network also occurs in the asthmatic airway, perhaps caused by changes in the elastolytic process (57, 160). Fragmentation of central airway fibers and marked elastolysis are described in fatal asthma (57). Furthermore, increased levels of elastase were found in sputum of asthmatic patients (160). Elastic fiber fragmentation has also been observed in an animal model of chronic allergic inflammation (171). Airway geometry, function, and dynamics are subject to these remodeling processes (133).
Data exist suggesting that epithelial damage participates in the proinflammatory process by releasing cytokines, growth factors, and mediators (57, 133). Epithelial injury can result in a self-sustaining phenotype of wound repair modulation, by activation/reactivation of the epithelial-mesenchymal trophic unit (18, 70, 151). Collagen, reticular and elastic fibers, proteoglycans and glycoproteins, all of which contribute to lung remodeling, can be produced by mesenchymal cells influenced by epithelial cells. Thus epithelial-mesenchymal interactions play an important role in extracellular matrix production and deposition (18, 57, 133). The presence of remodeling features in bronchial biopsy specimens of young children, in the absence of eosinofilic infiltrates, suggests an early role for epithelial-mesenchymal trophic interactions, secondary to epithelial injury and stress (37, 105).
Tomioka et al. (152) studied airway and tissue mechanics at baseline and after methacholine challenge in an allergic murine model that simulates some features of asthma. They observed that allergic inflammation significantly increased the responsiveness of the lung periphery. In their model, the rheological properties of lung parenchyma were not altered to any significant extent by inflammation. In contrast, the inflammation caused a sizeable increase in the responsiveness of tissue elastance and resistance to methacholine measured by the alveolar capsule, as well as of tissue dissipative (Gti) and conservative (Hti) parameters determined by forced oscillations (152). The effects of allergen itself on lung parenchyma have also been investigated (50). Indeed, the notion that mechanical abnormalities of the lung parenchyma are also important in asthma has progressively attracted more and more attention (127, 128), as suggested by the finding that parenchymal lung resistance may be significantly elevated even in patients with spirometric data within normal limits (71, 72). Lung biopsies also provide evidence that asthmatic inflammatory processes involve the lung parenchyma (24, 75).
Finally, it is noteworthy that the mechanical stress developed during asthmatic bronchoconstriction may also induce remodeling. Epithelial cells and fibroblasts respond to mechanical stress by increasing collagen synthesis, as well as the ratio between matrix metalloproteinases and tissue inhibitors of metalloproteinases (147).
D. Emphysema
Pulmonary emphysema has been defined by pathological criteria as an irreversible destruction of lung parenchyma distal to the terminal bronchioles with airway wall destruction and airspace enlargement, not associated with significant fibrosis (137). The most common cause of emphysema is cigarette smoking, and the central elements in its definition are the concepts of destruction and irreversibility of the pathological process (173). Although environmental toxins affect both proximal airways and alveolar lung tissue, the two anatomic compartments respond differently: airways present cell proliferation, while alveolate lung tissue disappears (173). The major mechanisms thought to be responsible for the development and progression of emphysema include the protease-antiprotease hypothesis, inflammation, oxidative stress, alveolar cell apoptosis, and matrix remodeling (10, 145, 173). Probably, these are not distinct processes and each can contribute to disease complexity (145).
The most widely accepted hypothesis for tissue destruction in emphysema rests on the imbalance of protease and antiprotease activities, leading to enzymatic degradation of elastin (10). The concept that tissue destruction results from protease burden, mainly neutrophil elastase, has relied on the findings in patients deficient in α1-antitrypsin, the major inhibitor of neutrophil elastase (141, 173), in concert with the use of intrapulmonary instillation of elastolitic enzymes to develop animal models of emphysema (131). However, experimental findings suggest that neutrophil elastase may not be the principal protease regulating tissue destruction in emphysema (145). Macrophage elastase was demonstrated to be sufficient to cause emphysema after chronic inhalation of cigarette smoke (54), while experimental emphysema can also be induced by overexpression of collagenase (27) or impaired synthesis of specific proteoglycans (157).
Inflammatory cells, including macrophages, T lymphocytes, B lymphocytes, and neutrophils represent the best-known source of proteases in the lungs. In addition, lung tissue destruction can be further enhanced by inflammatory cells recruited by elastin fragments liberated during elastic fibers degradation (131). Cigarette smoke inhalation induces neutrophils, lymphocytes, and macrophages infiltration in the lung, but the specific pathological role of each inflammatory cell in emphysema remains to be determined. Lung infiltration of inflammatory cells in emphysema has been attributed to either cigarette smoke/pollutants or oxidative stress as the result of exposure to environmental hazards (173). Recent findings suggest, however, that inflammation could be the result of an autoimmune attack to lung tissue (2).
Neutrophil elastase and matrix metalloproteinases (MMPs) are the most studied candidates for protease/antiprotease imbalance in emphysema. Evidences support a significant role for the macrophage metalloelastase (MMP-12) and the collagenase MMP-1 in emphysema, while a role for an imbalance between MMP and tissue inhibitor of metalloproteinases (TIMP) has also been suggested (173). The targets of proteolitic enzymes and free radicals liberated by polymorphonuclear cells and monocytes are collagen, elastin, proteoglycans, and α1-antitrypsin (163). In addition to proteases, other factors have been implicated in tissue destruction leading to emphysema, such as oxidative stress and apoptosis of lung structural cells (131). However, one interesting question emerges: do the inflammatory elements target directly components of cigarette smoke or is inflammation, rather, a secondary response to cellular and molecular alterations induced by cigarette smoke? Each puff of cigarette smoke contains numerous toxic compounds, free radicals, and potent oxidants such as hydrogen peroxide, hydroxyl anion, and organic radicals (154). Tuder et al. (154) underlined the important similarities between the pathophysiological responses to aging and emphysema owing to chronic cigarette smoking, both presenting oxidative stress and apoptosis as common molecular mechanisms. The authors suggested that emphysema may recapitulate events underlying accelerated lung aging processes resulting from failure of lung maintenance and repair (154). In this context, inflammation might represent the response to unrepaired organ injury, rather than the first line of response to environmental stresses (153, 154).
The finding that decreased vascular endothelial growth factor (VEGF) or VEGF signaling, an obligatory lung maintenance factor throughout lung development, can induce emphysema suggests that alveolar maintenance is required for the structural preservation of the lung (74, 154, 173). Thus alveolar cell destruction in emphysematous lungs might be caused by apoptosis, possibly unrelated to preceding matrix protease degradation. Indeed, apoptotic cells retrieved by bronchoalveolar lavage after the induction of acute apoptosis and alveolar enlargement were able to degrade elastin (5, 173). On the other hand, oxidative stress decreases the expression of VEGF coreceptor and an accessory molecule that potentiates VEGF binding (89). In emphysema, free radical species can be generated by exogenous sources, such as cigarette smoke, and by endogenous origins, particularly pulmonary inflammatory cells. In addition, data from chronic obstructive pulmonary disease (COPD) patients support the occurrence of an overwhelming oxidative burden from reactive oxygen species in moderate or severe disease, while healthy smokers and stable patients show adaptive responses against oxidative stress, probably mediated by glutamate cystenyl ligase, the main regulator of the most important antioxidant glutathione (173). In fact, the interactions among apoptosis, matrix proteolysis, and oxidative stress have been proposed as the final conduit of alveolar destruction in emphysema (153).
Although most studies suggest that elastin constitutes the major target of destruction in emphysema, pathophysiological changes can also result from abnormalities in the collagen matrix (69). Indeed, collagen presents higher stiffness and failure threshold than elastin, protecting the lung from rupture at high volumes. Thus alveolar wall failure suggests that the collagen network in the emphysematous lung cannot be normal. Several MMPs, including MMP-1, -8, and -13, are capable of degrading collagen (145). In addition, cigarette smoke can undermine the repair function of fibroblasts, epithelial cells, and mesenchymal cells, yielding persistent abnormalities in tissue structure (109). Elastin and collagen seem to be the major constituents of the extracellular matrix, accounting for lung tissue viscoelastic mechanical properties, and, interestingly, elastic fibers behave more linearly than collagen fibers (69). In a mouse model of elastase-induced emphysema, Ito et al. (69) observed increased total collagen content and collagen-related dynamic nonlinearity, while failure stress of isolated parenchymal tissues decreased. These findings suggest that, despite the increased collagen content, the alveolar walls and the collagen fibers were likely to be weaker in the emphysematous lung as a consequence of the process of degrading and remodeling. In this model, emphysematous mice also showed increased hysteresivity and lower elastance. Although in normal lung tissue a positive association between hysteresivity and elastin-to-collagen ratio can be found, emphysematous mice show increased hysteresivity, whereas collagen content also increased. Based on these results, the authors suggested that remodeling in emphysema produces weak and viscous alveolar walls that also fail at lower stress. Thus abnormal collagen remodeling may play a significant role in lung functional changes in emphysema (69). Indeed, according to Brewer et al. (20), normal lungs show a fall in hysteresivity with increasing transpulmonary pressure (PL), while emphysematous rat lungs display constant hysteresivity at all PL levels. Lung elastance also showed a significantly reduced dependence on PL in the treated animals. In normal lungs, the volume-dependent nature of hysteresivity can be attributed to the progressive contribution of collagen at higher lung volumes (134), since the hysteresivity of collagen is less than that of elastin (95, 175). Thus, taken together, the results suggest that the PL dependence of elastance and hysteresivity can signal the early remodeling of elastin and collagen fibers in the alveolar walls during the development of emphysema (20).
IV. CONCLUSION
In this review we summarized the basic concepts involved in energy dissipation during breathing, focusing on those pertaining to lung tissue behavior. The potential participating mechanisms, the different models available for the determination of tissue properties, as well as the participation of the stress-bearing elements were reviewed. The mechanical properties of lung tissue are important determinants of lung physiological functions, being affected in diverse diseases. More recently, models have tried to deal with dynamic tissue behavior on a mechanistic basis, and evidences suggest that events at the microstructural level may not follow those at the macroscopic level. However, the precise molecular basis for an integrative biological phenomenon remains unknown.
Acknowledgments
Address for reprint requests and other correspondence: W. A. Zin, Laboratório de Fisiologia da Respiração, Instituto de Biofísica Carlos Chagas Filho-C.C.S., Universidade Federal do Rio de Janeiro, Ilha do Fundão, 21941-902 Rio de Janeiro, Brazil (e-mail: wazin@biof.ufrj.br).
REFERENCES
- 1.Agostoni E, Hyatt RE. Static behavior of the respiratory system. In: Handbook of Physiology. The Respiratory System. Mechanics of Breathing. Bethesda, MD: Am. Physiol. Soc. 1986, sect. 3, vol. III, pt. 1, chapt. 9, p. 113–130.
- 2.Agusti A, MacNee W, Donaldson K, Cosio M. Hypothesis: does COPD have an autoimmune component? Thorax : 832–834, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Jamal R, Roughley PJ, Ludwig MS. Effect of glycosaminoglycan degradation on lung tissue viscoelasticity. Am J Physiol Lung Cell Mol Physiol : L306–L315, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Antonaglia V, Peratoner A, De Simoni L, Gullo A, Milic-Emili J, Zin WA. Bedside assessment of respiratory viscoelastic properties in ventilated patients. Eur Respir J : 302–308, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Aoshiba K, Yokohori N, Nagai A. Alveolar wall apoptosis causes lung destruction and emphysematous changes. Am J Respir Cell Mol Biol : 555–562, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Bachofen H. Lung tissue resistance and pulmonary hysteresis. J Appl Physiol : 296–301, 1968. [DOI] [PubMed] [Google Scholar]
- 7.Bachofen H, Scherrer M. Lung tissue resistance in diffuse insterstitial pulmonary fibrosis. J Clin Invest : 133–140, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachofen H, Schurch S, Michel RP, Weibel ER. Experimental hydrostatic pulmonary edema in rabbit lungs. Am Rev Respir Dis : 989–996, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Bachofen H, Schurch S, Weibel ER. Experimental hydrostatic pulmonary edema in rabbit lungs: barrier lessons. Am Rev Respir Dis : 997–1004, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med : 269–280, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Bates JHT. A micromechanical model of lung tissue rheology. Ann Biomed Eng : 679–687, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Bates JHT, Decramer M, Zin WA, Harf A, Milic-Emili J, Chang HK. Respiratory resistance with histamine challenge by single breath and forced oscillation methods. J Appl Physiol : 873–880, 1986. [DOI] [PubMed] [Google Scholar]
- 13.Bates JHT, Lauzon AM, Dechman GS, Maksym GN, Schuessler TF. Temporal dynamics of pulmonary response to intravenous histamine in dogs: effects of dose and lung volume. J Appl Physiol : 616–626, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Bates JHT, Maksym GN, Navajas D, Suki B. Lung tissue rheology and 1/f noise. Ann Biomed Eng : 674–681, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Bayliss LE, Robertson GW. The visco-elastic properties of the lungs. Q J Exp Physiol Cogn Med Sci : 27–47, 1939. [Google Scholar]
- 16.Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med : 1819–1828, 1996. [DOI] [PubMed] [Google Scholar]
- 17.Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycans in granulomatous lung diseases. Eur Respir J : 2731–2737, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Boxall C, Holgate ST, Davies DE. The contribution of transforming growth factor-beta and epidermal growth factor signalling to airway remodelling in chronic asthma. Eur Respir J : 208–229, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Breen EC. Mechanical strain increases type I collagen expression in pulmonary fibroblasts in vitro. J Appl Physiol : 203–209, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Brewer KK, Sakai H, Alencar AM, Majumdar A, Arold SP, Lutchen KR, Ingenito EP, Suki B. Lung and alveolar wall elastic and hysteretic behavior in rats: effects of in vivo elastase treatment. J Appl Physiol : 1926–1936, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Brown RE, Butler JP, Rogers RA, Leith DE. Mechanical connections between elastin and collagen. Connect Tissue Res : 295–308, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Bull HB. Protein structure and elasticity. In: Tissue Elasticity. Washington, DC: Am Physiol Soc, 1957, p.33–42.
- 23.Butler J. The adaptation of the relaxed lungs and chest wall to changes in volume. Clin Sci : 421–433, 1957. [PubMed] [Google Scholar]
- 24.Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis : 405–410, 1993. [DOI] [PubMed] [Google Scholar]
- 25.Cavalcante FS, Ito S, Brewer K, Sakai H, Alencar AM, Almeida MP, Andrade JS Jr, Majumdar A, Ingenito EP, Suki B. Mechanical interactions between collagen and proteoglycans: implications for the stability of lung tissue. J Appl Physiol : 672–679, 2005. [DOI] [PubMed] [Google Scholar]
- 26.D'Angelo E, Calderini E, Torri G, Robatto FM, Bono D, Milic-Emili J. Respiratory mechanics in anesthetized paralyzed humans: effects of flow, volume, and time. J Appl Physiol : 2556–2564, 1989. [DOI] [PubMed] [Google Scholar]
- 27.D'Armiento J, Dalal SS, Okada Y, Berg RA, Chada K. Collagenase expression in the lungs of transgenic mice causes pulmonary emphysema. Cell : 955–961, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol : 729–743, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Troyer A, Yernault JC, Rodenstein D. Influence of beta-2 agonist aerosol on pressure-volume characteristics of the lungs. Am Rev Respir Dis : 987–995, 1978. [DOI] [PubMed] [Google Scholar]
- 30.Denny E, Schroter RC. Relationships between alveolar size and fibre distribution in a mammalian lung alveolar duct model. J Biomech Eng : 289–297, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Dolhnikoff M, Mauad T, Ludwig MS. Extracellular matrix and oscillatory mechanics of rat lung parenchyma in bleomycin-induced fibrosis. Am J Respir Crit Care Med : 1750–1757, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Ebihara T, Venkatesan N, Tananka R, Ludwig MS. Changes in extracellular matrix and tissue viscoelasticity in bleomycin-induced lung fibrosis. Am J Respir Crit Care Med : 1569–1576, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Evans JN, Adler KB. The lung strip: evaluation of a method to study contractility of pulmonary parenchyma. Exp Lung Res : 187–195, 1981. [DOI] [PubMed] [Google Scholar]
- 34.Faffe DS, D'Alessandro ES, Xisto DG, Antunes MA, Romero PV, Negri EM, Rodrigues NRD, Capelozzi VL, Zin WA, Rocco PRM. Mouse strain dependence of lung tissue mechanics: role of specific extracellular matrix composition. Respir Physiol Neurobiol : 186–196, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Faffe DS, Rocco PR, Negri EM, Zin WA. Comparison of rat and mouse pulmonary tissue mechanical properties and histology. J Appl Physiol : 230–234, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Faffe DS, Silva GH, Kurtz PMP, Negri EM, Capelozzi VL, Rocco PRM, Zin WA. Lung tissue mechanics and extracellular matrix composition in a murine model of silicosis. J Appl Physiol : 1400–1406, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Fedorov IA, Wilson SJ, Davies DE, Holgate ST. Epithelial stress and structural remodelling in childhood asthma. Thorax : 389–394, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fredberg JJ, Bunk D, Ingenito E, Shore SA. Tissue resistance and the contractile state of lung parenchyma. J Appl Physiol : 1387–1397, 1993. [DOI] [PubMed] [Google Scholar]
- 39.Fredberg JJ, Jones KA, Nathan M, Raboudi S, Prakash YS, Shore SA, Butler JP, Sieck GC. Friction in airway smooth muscle mechanism: mechanism, latch and implications in asthma. J Appl Physiol : 2703–2712, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Fredberg JJ, Kamm RD. Stress transmission in the lung: pathways from organ to molecule. Annu Rev Physiol : 505–541, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Fredberg JJ, Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol : 2408–2419, 1989. [DOI] [PubMed] [Google Scholar]
- 42.Fukuda Y, Ferrans VJ, Schoenberger CI, Rennard SI, Crystal RG. Patterns of pulmonary structural remodeling after experimental paraquat toxicity. The morphogenesis of intraalveolar fibrosis. Am J Pathol : 452–475, 1985. [PMC free article] [PubMed] [Google Scholar]
- 43.Fung YC. Biomechanics. New York: Springer-Verlag, 1993.
- 44.Fust A, Bates JHT, Ludwig MS. Mechanical properties of mouse distal lung: in vivo versus in vitro comparison. Respir Physiol Neurobiol : 77–86, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Fust A, LeBellego F, Iozzo RV, Roughley PJ, Ludwig MS. Alterations in lung mechanics in decorin-deficient mice. Am J Physiol Lung Cell Mol Physiol : L159–L166, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Garcia CSNB, Rocco PRM, Facchinetti LD, Lassance RM, Caruso P, Deheinzelin D, Morales MM, Romero PV, Faffe DS, Zin WA. What increases type III procollagen mRNA levels in lung tissue: stress induced by changes in force or amplitude? Respir Physiol Neurobiol : 59–70, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M. Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis : 730–736, 1987. [DOI] [PubMed] [Google Scholar]
- 48.Goldie RG, Bertram JF, Papadimitriou Paterson JW. The lung parenchyma strip. Trends Pharmacol Sci : 7–9, 1984. [Google Scholar]
- 49.Gunst SJ, Meiss RA, Wu MF, Rowe M. Mechanisms for the mechanical plasticity of tracheal smooth muscle. Am J Physiol Cell Physiol : C1267–C1276, 1995. [DOI] [PubMed] [Google Scholar]
- 50.Hall GL, Petak F, McMenamin C, Sly PD. The route of antigen delivery determines the airway and lung tissue mechanical responses in allergic rats. Clin Exp Allergy : 562–568, 1999. [DOI] [PubMed] [Google Scholar]
- 51.Hantos Z, Daróczy B, Suki B, Galgoczy G, Csendes T. Forced oscillatory impedance of the respiratory system at low frequencies. J Appl Physiol : 123–132, 1986. [DOI] [PubMed] [Google Scholar]
- 52.Hantos Z, Daróczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol : 168–178, 1992. [DOI] [PubMed] [Google Scholar]
- 53.Hardingham TE, Fosang AJ. Proteoglycans: many forms and many functions. FASEB J : 861–870, 1992. [PubMed] [Google Scholar]
- 54.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science : 2002–2004, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Hildebrandt J. Dynamic properties of air-filled excised cat lung determined by liquid plethysmograph. J Appl Physiol : 246–250, 1969. [DOI] [PubMed] [Google Scholar]
- 56.Hildebrandt J. Pressure-volume data of cat lung interpreted by a plastoelastic, linear viscoelastic model. J Appl Physiol : 246–250, 1970. [DOI] [PubMed] [Google Scholar]
- 57.Homer RJ, Elias JA. Consequences of long-term inflammation: airway remodeling. Clin Chest Med : 331–343, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Homer RJ, Elias JA. Airway remodeling in asthma: therapeutic implications of mechanisms. Physiology : 28–35, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Hoppin FG Jr, Stothert JC Jr, Greaves IA, Lai YL, Hildebrandt J. Lung recoil: elastic and rheological properties. In: Handbook of Physiology. The Respiratory System. Mechanics of Breathing. Bethesda, MD: Am. Physiol. Soc. 1986, sect. 3, vol. III, pt. 1, chapt. 13, p. 195–215.
- 60.Hoshino M, Nakamura Y, Sim J, Shimojo J, Isogai S. Bronchial subepithelial fibrosis and expression of matrix metalloproteinase-9 in asthmatic airway inflammation. J Allergy Clin Immunol : 783–788, 1998. [DOI] [PubMed] [Google Scholar]
- 61.Huang J, Olivenstein R, Taha R, Hamid Q, Ludwig M. Enhanced proteoglycan deposition in the airway wall of atopic asthmatics. Am J Respir Crit Care Med : 725–729, 1999. [DOI] [PubMed] [Google Scholar]
- 62.Hubmayr RD. Biology lessons from oscillatory cell mechanics. J Appl Physiol : 1617–1618, 2000. [DOI] [PubMed] [Google Scholar]
- 63.Hubmayr RD. Perspective on lung injury and recruitment: a skeptical look at the opening and collapse story. Am J Respir Crit Care Med : 1647–1653, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Hubmayr RD. Pulmonary micromechanics of injured lungs. In: Lung Biology in Health and Disease. Ventilator-Induced Lung Injury, edited by C. Lenfant. Bethesda, MD: Am. Physiol. Soc., 2006, vol. 215, chapt. 2, p. 21–44.
- 65.Ingenito EP, Mark L, Davison B. Effects of acute lung injury on dynamic tissue properties. J Appl Physiol : 2689–2697, 1994. [DOI] [PubMed] [Google Scholar]
- 66.Ingram RH Jr, McFadden ER Jr. Localization and mechanisms of airway responses. N Engl J Med : 596–600, 1977. [DOI] [PubMed] [Google Scholar]
- 67.Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol : 141–174, 1997. [DOI] [PubMed] [Google Scholar]
- 68.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem : 609–652, 1998. [DOI] [PubMed] [Google Scholar]
- 69.Ito S, Ingenito EP, Brewer KK, Black LD, Parameswaran H, Lutchen KR, Suki B. Mechanics, nonlinearity, and failure strength of lung tissue in a mouse model of emphysema: possible role of collagen remodeling. J Appl Physiol : 503–511, 2005. [DOI] [PubMed] [Google Scholar]
- 70.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest : 1776–1784, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaminsky DA, Bates JHT, Irvin CG. Effects of cool, dry air stimulation on peripheral lung mechanics in asthma. Am J Respir Crit Care Med : 179–186, 2000. [DOI] [PubMed] [Google Scholar]
- 72.Kaminsky DA, Wenzel SE, Corcano C, Gurka DA, Feldsien DC, Irvin CG. Hyperpnea induced changes in parenchymal lung mechanics in normal subjects and in asthmatics. Am J Respir Crit Care Med : 1260–1266, 1997. [DOI] [PubMed] [Google Scholar]
- 73.Kapanci Y, Assimacopoulos A, Irle C, Zwahlen A, Gabbiani G. “Contractile interstitial cells” in pulmonary alveolar septa: a possible regulator of ventilation/perfusion ratio? Ultrastructural immunofluorescence and in vitro studies. J Cell Biol : 375–392, 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voekel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest : 1311–1319, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kraft M, Djukanovic R, Wilson S, Holgate ST, Martin RJ. Alveolar tissue inflammation in asthma. Am J Respir Crit Care Med : 1505–1510, 1996. [DOI] [PubMed] [Google Scholar]
- 76.Kuwano K, Bosken CH, Pare PD, Bai TR, Wiggs BR, Hogg JC. Small airways dimensions in asthma and in chronic obstructive pulmonary disease. Am Rev Respir Dis : 1220–1225, 1993. [DOI] [PubMed] [Google Scholar]
- 77.Lai-Fook SJ. Lung parenchyma described as a prestressed compressible material. J Biomechanics : 357–365, 1977. [DOI] [PubMed] [Google Scholar]
- 78.Lai-Fook SJ, Wilson TA, Hyatt RE, Rodarte JR. Elastic constants of inflated lobes of dog lungs. J Appl Physiol : 508–513, 1976. [DOI] [PubMed] [Google Scholar]
- 79.Lim TK, Pride NB, Ingram RH Jr. Effects of volume history during spontaneous and acutely induced air-flow obstruction in asthma. Am Rev Respir Dis : 591–596, 1987. [DOI] [PubMed] [Google Scholar]
- 80.López-Aguilar J, Romero PV. Effect of elastase pretreatment on rat lung strip induced constriction. Respir Physiol : 239–246, 1998. [DOI] [PubMed] [Google Scholar]
- 81.Loring SH, Ingram RH Jr, Drazen JM. Effects of lung inflation on airway and tissue responses to aerosol histamine. J Appl Physiol : 806–811, 1981. [DOI] [PubMed] [Google Scholar]
- 82.Ludwig MS, Dallaire MJ. Structural composition of lung parenchymal strip and mechanical behavior during sinusoidal oscillation. J Appl Physiol : 2029–2035, 1994. [DOI] [PubMed] [Google Scholar]
- 83.Ludwig MS, Dreshaj I, Solway J, Munoz A, Ingram RH Jr. Partitioning of pulmonary resistance during constriction in the dog: effects of volume history. J Appl Physiol : 807–815, 1987. [DOI] [PubMed] [Google Scholar]
- 84.Ludwig MS, Robatto FM, Simard S, Stamenovic D, Fredberg JJ. Lung tissue resistance during contractile stimulation: structural damping decomposition. J Appl Physiol : 1332–1337, 1992. [DOI] [PubMed] [Google Scholar]
- 85.Lutchen KR, Hantos Z, Peták F, Adamicza A, Suki B. Airway inhomogeneities contribute to apparent lung tissue mechanics during constriction. J Appl Physiol : 1841–1849, 1996. [DOI] [PubMed] [Google Scholar]
- 86.Maksym GN, Bates JHT. A distributed nonlinear model of lung tissue elasticity. J Appl Physiol : 32–41, 1997. [DOI] [PubMed] [Google Scholar]
- 87.Maksym GN, Fredberg JJ, Bates JHT. Force heterogeneity in a two-dimensional network model of lung tissue elasticity. J Appl Physiol : 1223–1229, 1998. [DOI] [PubMed] [Google Scholar]
- 88.Marshall R, Widdicombe JG. Stress relaxation of the human lung. Clin Sci : 19–31, 1960. [PubMed] [Google Scholar]
- 89.Marwick JA, Stevenson CS, Giddings J, MacNee W, Butler K, Rahman I, Kirkham PA. Cigarette smoke disrupts VEGF(165)-VEGFR-2 receptor signaling complex in rat lungs and patients with COPD: morphological impact of VEGFR-2 inhibition. Am J Physiol Lung Cell Mol Physiol : L897–L908, 2006. [DOI] [PubMed] [Google Scholar]
- 90.Mead J. Respiration: pulmonary mechanics. Annu Rev Physiol : 169–192, 1973. [DOI] [PubMed] [Google Scholar]
- 91.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol : 596–608, 1970. [DOI] [PubMed] [Google Scholar]
- 92.Mead J, Whittenberger L, Radford EP Jr. Surface tension as a factor in pulmonary volume-pressure hysteresis. J Appl Physiol : 191–196, 1957. [DOI] [PubMed] [Google Scholar]
- 93.Mercer RR, Crapo JD. Spatial distribution of collagen and elastin fibers in the lungs. J Appl Physiol : 756–765, 1990. [DOI] [PubMed] [Google Scholar]
- 94.Mercer RR, Russell ML, Crapo JD. Alveolar septal structure in different species. J Appl Physiol : 1060–1066, 1994. [DOI] [PubMed] [Google Scholar]
- 95.Mijailovich SM, Stamenovic D, Brown R, Leith DE, Fredberg JJ. Dynamic moduli of rabbit lung tissue and pigeon ligamentum propatagiale undergoing uniaxial cyclic loading. J Appl Physiol : 773–782, 1994. [DOI] [PubMed] [Google Scholar]
- 96.Mijailovich SM, Stamenovic D, Fredberg JJ. Toward a kinetic theory of connective tissue micromechanics. J Appl Physiol : 665–681, 1993. [DOI] [PubMed] [Google Scholar]
- 97.Mount LE. The ventilation flow-resistance and compliance of rat lungs. J Physiol : 157–167, 1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nagase T, Lei M, Robatto FM, Eidelman DH, Ludwig MS. Tissue viscance during induced constriction in rabbit lungs: morphological-physiological correlations. J Appl Physiol : 1900–1907, 1992. [DOI] [PubMed] [Google Scholar]
- 99.Nagase T, Matsui H, Aoki T, Ouchi Y, Fukuchi Y. Lung tissue behavior in the mouse during constriction induced by methacholine and endothelin-1. J Appl Physiol : 2373–2378, 1996. [DOI] [PubMed] [Google Scholar]
- 100.Naghshin J, Wang L, Pare PD, Seow CY. Adaptation to chronic length change in explanted airway smooth muscle. J Appl Physiol : 448–453, 2003. [DOI] [PubMed] [Google Scholar]
- 101.Nardell EA, Brody JS. Determinants of mechanical properties of rat lung during postnatal development. J Appl Physiol : 140–148, 1982. [DOI] [PubMed] [Google Scholar]
- 102.Navajas D, Maksym GN, Bates JHT. Dynamic viscoelastic nonlinearity of lung parenchymal tissue. J Appl Physiol : 348–356, 1995. [DOI] [PubMed] [Google Scholar]
- 103.Navajas D, Mijailovich S, Glass GM, Stamenovic D, Fredberg JJ. Dynamic response of the isolated passive rat diaphragm strip. J Appl Physiol : 2681–2692, 1992. [DOI] [PubMed] [Google Scholar]
- 104.Negri EM, Montes GS, Saldiva PHN, Capelozzi VL. Architectural remodelling in acute and chronic interstitial lung disease: fibrosis or fibroelastosis? Histopathology : 393–401, 2000. [DOI] [PubMed] [Google Scholar]
- 105.Payne DN, Rogers AV, Adelroth E, Bandi V, Guntupalli KK, Bush A, Jeffery PK. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med : 78–82, 2003. [DOI] [PubMed] [Google Scholar]
- 106.Pierce RA, Albertine KH, Starcher BC, Bohnsack JF, Carlton DP, Bland RD. Chronic lung injury in preterm lambs: disordered pulmonary elastin deposition. Am J Physiol Lung Cell Mol Physiol : L452–L460, 1997. [DOI] [PubMed] [Google Scholar]
- 107.Pugin J, Dunn I, Jolliet P, Tassaux D, Magnenat JL, Nicod LP, Chevrolet JC. Activation of human macrophages by mechanical ventilation in vitro. Am J Physiol Lung Cell Mol Physiol : L1040–L1050, 1998. [DOI] [PubMed] [Google Scholar]
- 108.Reinhardt AK, Bottoms SE, Laurent GJ, McAnulty RJ. Quantification of collagen and proteoglycan deposition in a murine model of airway remodelling. Respir Res : 30, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rennard SI, Togo S, Holz O. Cigarette smoke inhibits alveolar repair. Proc Am Thorac Soc : 703–708, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roberts C, Burke A. Remodeling of the extracellular matrix in asthma: proteoglycan synthesis and degradation. Can Respir J : 48–50, 1998. [PubMed] [Google Scholar]
- 111.Rocco PRM, Negri EM, Kurtz PM, Vasconcellos FP, Silva GH, Capelozzi VL, Romero PV, Zin WA. Lung tissue mechanics and extracellular matrix remodeling in acute lung injury. Am J Respir Crit Care Med : 1067–1071, 2001. [DOI] [PubMed] [Google Scholar]
- 112.Rodarte JR, Rehder K. Dynamics of respiration. In: Handbook of Physiology. The Respiratory System. Mechanics of Breathing. Bethesda, MD: Am. Physiol. Soc. 1986, sect. 3, vol. III, pt. 1, chapt. 10, p. 131–144.
- 113.Romero FJ, Pastor A, Lopez J, Romero PV. A recruitment-based rheological model for mechanical behavior of soft tissues. Biorheology : 17–35, 1998. [DOI] [PubMed] [Google Scholar]
- 114.Romero PV, Robatto FM, Simard S, Ludwig MS. Lung tissue behavior during methacholine challenge in rabbits in vivo. J Appl Physiol : 207–212, 1992. [DOI] [PubMed] [Google Scholar]
- 115.Romero PV, Zin WA, Lopez-Aguilar J. Frequency characteristics of lung tissue during passive stretch and induced pneumoconstriction. J Appl Physiol : 882–890, 2001. [DOI] [PubMed] [Google Scholar]
- 116.Saetta M, Di Stefano A, Rosina C, Thiene G, Fabbri LM. Quantitative structural analysis of peripheral airways and arteries in sudden fatal asthma. Am Rev Respir Dis : 138–143, 1991. [DOI] [PubMed] [Google Scholar]
- 117.Sakae RS, Leme AS, Dolhnikoff M, Pereira PM, Warth MPTN, Zin WA, Saldiva PHN, Martins MA. Neonatal capsaicin treatment decreases airway and pulmonary tissue responsiveness to methacholine. Am J Physiol Lung Cell Mol Physiol : L23–L29, 1994. [DOI] [PubMed] [Google Scholar]
- 118.Saldiva PHN, Delmonte VC, Carvalho CRR, Kairalla RA, Auler JOC Jr. Histochemical evaluation of lung collagen content in acute and chronic interstitial diseases. Chest : 953–957, 1989. [DOI] [PubMed] [Google Scholar]
- 119.Saldiva PHN, Zin WA, Santos RL, Eidelman DH, Milic-Emili J. Alveolar pressure measurement in open-chest rats. J Appl Physiol : 302–306, 1992. [DOI] [PubMed] [Google Scholar]
- 120.Salerno FG, Dallaire M, Ludwig MS. Does the anatomic makeup of parenchymal lung strips affect oscillatory mechanics during induced constriction? J Appl Physiol : 66–72, 1995. [DOI] [PubMed] [Google Scholar]
- 121.Salerno FG, Kurosawa H, Eidelman DH, Ludwig MS. Characterization of the anatomical structures involved in the contractile response of the rat lung periphery. Br J Pharmacol : 734–740, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sansores RH, Ramirez-Venegas A, Perez-Padilla R, Montano M, Ramos C, Becerril C, Gaxiola M, Pare P, Selman M. Correlation between pulmonary fibrosis and the lung pressure-volume curve. Lung : 315–323, 1996. [DOI] [PubMed] [Google Scholar]
- 123.Sasaki N, Nakayama Y, Yoshikawa M, Enyo A. Stress relaxation function of bone and bone collagen. J Biomech : 1369–1376, 1993. [DOI] [PubMed] [Google Scholar]
- 124.Schmidt MB, Mow VC, Chun LE, Eyer DR. Effects of proteoglycan extraction on the tensile behavior of articular cartilage. J Orthop Res : 353–363, 1990. [DOI] [PubMed] [Google Scholar]
- 125.Schonherr E, Witsch-Prehm P, Harrach B, Robenek H, Rauterberg J, Kresse H. Interaction of biglycan with type I collagen. J Biol Chem : 2776–2783, 1995. [DOI] [PubMed] [Google Scholar]
- 126.Schurch S, Bachofen H, Goerke J, Green F. Surface properties of rat pulmonary surfactant studied with the captive bubble method: adsorption, hysteresis, stability. Biochim Biophys Acta : 127–136, 1992. [DOI] [PubMed] [Google Scholar]
- 127.Schutz N, Petak F, Barazzone-Argiroffo C, Fontao F, Habre W. Effects of volatile anaesthetic agents on enhanced airway tone in sensitized guinea pigs. Br J Anaesth : 254–260, 2004. [DOI] [PubMed] [Google Scholar]
- 128.Schutz N, Petak F, Barazzone-Argiroffo C, Sly PD, Habre W. Prevention of bronchoconstriction in sensitized guinea pigs: efficacy of common prophylactic drugs. Respir Physiol Neurobiol : 167–178, 2004. [DOI] [PubMed] [Google Scholar]
- 129.Sharp JT, Johnson FN, Goldberg NB, Van Lith P. Hysteresis and stress adaptation in the human respiratory system. J Appl Physiol : 296–307, 1967. [DOI] [PubMed] [Google Scholar]
- 130.Shepherd KE, Lynch KE, Wain JC, Brown EN, Wilson RS. Elastin fibres and the diagnosis of bacterial pneumonia in ARDS. Crit Care Med : 1829–1834, 1995. [DOI] [PubMed] [Google Scholar]
- 131.Shifren A, Mecham RP. The stumbling block in lung repair of emphysema: elastic fiber assembly. Proc Am Thorac Soc : 428–433, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shute J, Parmar J, Holgate ST, Howarth PH. Urine glycosaminoglycan levels are increased in acute severe asthma: a role for eosinophil gelatinase B? Int Arch Allergy Immunol : 366–367, 1997. [DOI] [PubMed] [Google Scholar]
- 133.Slade DJ, Kraft M. Airway remodeling from bench to bedside: current perspectives. Clin Chest Med : 71–86, 2006. [DOI] [PubMed] [Google Scholar]
- 134.Sly PD, Collins RA, Thamrin C, Turner DJ, Hantos Z. Volume dependence of airway and tissue impedances in mice. J Appl Physiol : 1460–1466, 2003. [DOI] [PubMed] [Google Scholar]
- 135.Smaldone GC, Mitzner W, Itoh H. Role of alveolar recruitment in lung inflantion: influence on pressure-volume hysteresis. J Appl Physiol : 1321–1332, 1983. [DOI] [PubMed] [Google Scholar]
- 136.Snider GL, Celli BR, Goldstein RH, O'Brien JJ, Lucey EC. Chronic interstitial pulmonary fibrosis produced in hamsters by endotracheal bleomycin. Am Rev Respir Dis : 289–297, 1978. [DOI] [PubMed] [Google Scholar]
- 137.Snider GL, Kleinerman JL, Thurlbeck WM, Bengali ZH. The definition of emphysema: report of a National-Heart-Lung-and-Blood-Institute. Division of Lung-Diseases Workshop. Am Rev Respir Dis : 182–185, 1985. [DOI] [PubMed] [Google Scholar]
- 138.Stamenovic D. Micromechanical foundations of pulmonary elasticity. Physiol Rev : 1117–1128, 1990. [DOI] [PubMed] [Google Scholar]
- 139.Stamenovic D, Yager D. Elastic properties of air- and liquid-filid lung parenchyma. J Appl Physiol : 2565–2570, 1988. [DOI] [PubMed] [Google Scholar]
- 140.Starcher BC, Kuhn C, Overton JE. Increased elastin and collagen content in the lungs of hamsters receiving an intratracheal injection of bleomycin. Am Rev Respir Dis : 299–305, 1978. [DOI] [PubMed] [Google Scholar]
- 141.Stoller JK, Aboussouan LS. Alpha 1-antitrypsin deficiency. Lancet : 2225–2236, 2005. [DOI] [PubMed] [Google Scholar]
- 142.Suki B, Barabasi AL, Lutchen KR. Lung tissue viscoelasticity: a mathematical framework and its molecular basis. J Appl Physiol : 2749–2759, 1994. [DOI] [PubMed] [Google Scholar]
- 143.Suki B, Bates JHT. A nonlinear viscoelastic model of lung tissue mechanics. J Appl Physiol : 826–833, 1991. [DOI] [PubMed] [Google Scholar]
- 144.Suki B, Ito S, Stamenovic D, Lutchen KR, Ingenito EP. Biomechanics of the lung parenchyma: critical roles of collagen and mechanical forces. J Appl Physiol : 1892–1899, 2005. [DOI] [PubMed] [Google Scholar]
- 145.Suki B, Lutchen KR, Ingenito EP. On the progressive nature of emphysema. Roles of proteases, inflammation, and mechanical forces. Am J Respir Crit Care Med : 516–521, 2003. [DOI] [PubMed] [Google Scholar]
- 146.Suki B, Peslin R, Duvivier C, Farré R. Lung impedance in healthy humans measured by forced oscillations from 0.01 to 01 Hz. J Appl Physiol : 1623–1629, 1989. [DOI] [PubMed] [Google Scholar]
- 147.Swartz MA, Tschumperlin DJ, Kamm RD, Drazen JM. Mechanical stress is communicated between different cell types to elicit matrix remodelling. Proc Natl Acad Sci USA : 6180–6185, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tanaka R, Al-Jamal R, Ludwig MS. Maturational changes in extracellular matrix and lung tissue mechanics. J Appl Physiol : 2314–2321, 2001. [DOI] [PubMed] [Google Scholar]
- 149.Tanaka R, Ludwig MS. Changes in viscoelastic properties of rat lung parenchymal strips with maturation. J Appl Physiol : 2081–2089, 1999. [DOI] [PubMed] [Google Scholar]
- 150.Tepper RS, Ramchandani R, Argay E, Zhang L, Xue Z, Liu Y, Gunst SJ. Chronic strain alters the passive and contractile properties of rabbit airways. J Appl Physiol : 1949–1954, 2005. [DOI] [PubMed] [Google Scholar]
- 151.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol : 131–142, 2006. [DOI] [PubMed] [Google Scholar]
- 152.Tomioka S, Bates JHT, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs. forced oscillations. J Appl Physiol : 263–270, 2002. [DOI] [PubMed] [Google Scholar]
- 153.Tuder RM, Petrache I, Elias JA, Voekel NF, Henson PM. Apoptosis and emphysema: the missign link. Am J Respir Cell Mol Biol : 551–554, 2003. [DOI] [PubMed] [Google Scholar]
- 154.Tuder RM, Yoshida T, Arap W, Pasqualini R, Petrache I. Cellular and molecular mechanisms of alveolar destruction in emphysema. An evolutionary perspective. Proc Am Thorac Soc : 503–511, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tulic MK, Christodoulopoulos P, Hamid Q. Small airway inflammation in asthma. Respir Res : 333–339, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tulic MK, Hamid Q. Contribution of the distal lung to the pathologic and physiologic changes in asthma: potential therapeutic target Roger S. Mitchell lecture. Chest : 348S–355S, 2003. [DOI] [PubMed] [Google Scholar]
- 157.Van Kuppevelt TH, van de Lest CH, Versteeg EM, Dekhuijzen PN, Veerkamp JH. Induction of emphysematous lesions in rat lung by beta-d-xyloside, an inhibitor of proteoglycan synthesis. Am J Respir Cell Mol Biol : 75–84, 1997. [DOI] [PubMed] [Google Scholar]
- 158.Venkatesan N, Roughley PJ, Ludwig MS. Proteoglycan expression in bleomycin lung fibroblasts: role of transforming growth factor-β1 and interferon-γ. Am J Physiol Lung Cell Mol Physiol : L806–L814, 2002. [DOI] [PubMed] [Google Scholar]
- 159.Vettermann J, Warner DO, Brichant JF, Rehder K. Halothane decrease both tissue and airway resistances in excised canine lungs. J Appl Physiol : 2698–2703, 1989. [DOI] [PubMed] [Google Scholar]
- 160.Vignola AM, Bonanno A, Mirabella A, Riccobono L, Mirabella F, Profite M, Bellia V, Bousquet J, Bonsignore G. Increase levels of elastase and alpha-1 antitrypsin in sputum of asthmatic patients. Am J Respir Crit Care Med : 505–511, 1998. [DOI] [PubMed] [Google Scholar]
- 161.Vignola AM, Chanez P, Campbell AM, Souques F, Lebel B, Enander I, Bousquet J. Airway inflammation in mild intermittent and in persistent asthma. Am J Respir Crit Care Med : 403–409, 1998. [DOI] [PubMed] [Google Scholar]
- 162.Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol : L167–L173, 1999. [DOI] [PubMed] [Google Scholar]
- 163.Vlahovic G, Russel ML, Mercer RR, Crapo JD. Cellular and connective tissue changes in alveolar septal wall in emphysema. Am J Respir Crit Care Med : 2086–2092, 1999. [DOI] [PubMed] [Google Scholar]
- 164.Wang L, Pare PD, Seow CY. Effect of chronic passive length change on airway smooth muscle length-tension relationship. J Appl Physiol : 734–740, 2001. [DOI] [PubMed] [Google Scholar]
- 165.Webb HH, Tierney DE. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis : 556–565, 1974. [DOI] [PubMed] [Google Scholar]
- 166.Weibel ER. Functional morphology of lung parenchyma. In: Handbook of Physiology. The Respiratory System. Mechanics of Breathing. Bethesda, MD: Am. Physiol. Soc., 1986, sect. 3, vol. III, chapt. 2, p. 47–91.
- 167.Westergren-Thorsson G, Hernnas J, Sarnstrand B. Altered expression of small proteoglycans, collagen, and transforming growth factor-β1 in developing bleomycin-induced pulmonary fibrosis in rats. J Clin Invest : 632–637, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wilson TA. A continuum analysis of a two-dimensional mechanical model of the lung parenchyma. J Appl Physiol : 472–478, 1972. [DOI] [PubMed] [Google Scholar]
- 169.Wilson TA. Relations among recoil pressure, surface area, and surface tension in the lung. J Appl Physiol : 921–926, 1981. [DOI] [PubMed] [Google Scholar]
- 170.Wilson TA, Bachofen H. A model for mechanical structure of the alveolar duct. J Appl Physiol : 1064–1070, 1982. [DOI] [PubMed] [Google Scholar]
- 171.Xisto DG, Farias LL, Ferreira HC, Picanço MR, Amitrano D, Lapa e Silva JR, Negri EM, Mauad T, Carnielli D, Silva LF, Capelozzi VL, Faffe DS, Zin WA, Rocco PR. Lung parenchyma remodeling in a murine model of chronic allergic inflammation. Am J Respi Crit Care Med : 829–837, 2005. [DOI] [PubMed] [Google Scholar]
- 172.Yamaguchi Y, Mann DM, Rouslahti E. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature : 281–284, 1990. [DOI] [PubMed] [Google Scholar]
- 173.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev : 1047–1062, 2007. [DOI] [PubMed] [Google Scholar]
- 174.Yuan H, Ingenito EP, Suki B. Dynamic properties of lung parenchyma: mechanical contributions of fiber network and interstitial cells. J Appl Physiol : 1420–1431, 1997. [DOI] [PubMed] [Google Scholar]
- 175.Yuan H, Kononov S, Cavalcante FSA, Lutchen KR, Ingenito EP, Suki B. Effects of collagenase and elastase on the mechanical properties of lung tissue strips. J Appl Physiol : 3–14, 2000. [DOI] [PubMed] [Google Scholar]