Abstract
Background
Tinnitus due to hyperactivity across neuronal ensembles along the auditory pathway is reported. We hypothesized that trigeminal neuralgia patients may subsequently suffer from tinnitus. Using nationwide, population-based data and a retrospective cohort study design, we investigated the risk of tinnitus within 1 year following trigeminal neuralgia.
Methods
We used the Taiwan National Health Insurance Research Dataset, a claims database, to identify all patients diagnosed with trigeminal neuralgia from January 2001 to December 2014, 12,587 patients. From the remaining patients, we identified 12,587 comparison patients without trigeminal neuralgia by propensity score matching, using sex, age, monthly income, geographic region, residential urbanization level, and tinnitus-relevant comorbidities (hyperlipidemia, diabetes, coronary heart disease, hypertension, cervical spondylosis, temporomandibular joint disorders and injury to head and neck and index year). All study patients (n = 25,174) were tracked for a one-year period to identify those with a subsequent diagnosis of tinnitus over 1-year follow-up.
Results
Among total 25,174 sample patients, the incidence of tinnitus was 18.21 per 100 person-years (95% CI = 17.66 ~ 18.77), the rate being 23.57 (95% CI = 22.68 ~ 24.49) among patients with trigeminal neuralgia and 13.17 (95% CI = 12.53 ~ 13.84) among comparison patients. Furthermore, the adjusted Cox proportional hazard ratio for tinnitus in the trigeminal neuralgia group was 1.68 (95% CI = 1.58 ~ 1.80) relative to the comparison cohort.
Conclusions
We found a significantly increased risk of tinnitus within 1 year of trigeminal neuralgia diagnosis compared to those without the diagnosis. Further studies in other countries and ethnicities are needed to explore the relationship between trigeminal neuralgia and subsequent tinnitus.
Keywords: Tinnitus, Trigeminal neuralgia, Trigeminal nerve
Introduction
Tinnitus is the auditory phantom sensation of sound in the absence of external stimuli. About 10% of the population suffers from tinnitus, making it one of the most common health conditions in the world [1, 2]. The most common cause of tinnitus is tinnitus associated with hearing loss caused by noise overexposure and aging. However, tinnitus can result from non-otologic causes, such as head and neck trauma [3], temporomandibular disorders [4–7], and cervical spine disorders [8, 9]. A certain percentage of patients find their tinnitus provoked by movement of or applying pressure to the head and neck region [10, 11]. Research has shown that the somatic origins of tinnitus may be due to interactions between somatic and auditory neuronal pathways in the central nervous system, indicating the role of somatosensory components in some cases of tinnitus [10, 12–16].
Trigeminal neuralgia is a common cause of chronic orofacial pain due to inflammation or other pathology of the trigeminal nerve. The trigeminal nerve, which divides into three branches, the ophthalmic (V1), maxillary (V2), and mandibular (V3) branches, is responsible for the sensory supply of the orofacial region. Trigeminal neuralgia is characterized by extremely disturbing, sporadic, and recurrent episodes of burning facial pain. While it is typically characterized by paroxysmal attacks of facial pain, atypical trigeminal neuralgia may manifest as a less intense condition associated with a constant background pain without intervals of relief.
Trigeminal neuralgia, like other pain disorders of the head and neck region such as temporomandibular disorders (TMD) and cervical spine disorders (CSD), is thought to be associated with transmission of nociceptive inputs through the trigeminal nerve which converge with other somatosensory pathways in the brainstem [17–19]. There is increasing evidence of tinnitus originating from hyperactivity across neuronal ensembles along the auditory pathway [20–22], and from functional and anatomical interchanges between the somatosensory and auditory pathways in the brainstem [14, 23–25]. Previous animal studies have demonstrated that the trigeminal nerve input interacts with the neural activity of the central auditory pathways related to sound perception at the level of dorsal cochlear nucleus [13, 26]. This may explain the mechanism of tinnitus experienced by some patients with trigeminal nerve pathology. Pathophysiologic involvement of the trigeminal system in tinnitus is also supported by clinical observations that tinnitus sensation can be elicited or is reported to be triggered by certain face and jaw movements in some patients [10, 11, 27].
Therefore, it is reasonable to hypothesize a prospective or comorbid association between trigeminal neuralgia and tinnitus. To the best of our knowledge, there is no documented study that explored tinnitus risk following a diagnosis of trigeminal neuralgia. This nationwide, population-based, retrospective cohort study was carried out to investigate the risk of tinnitus within 1 year following a diagnosis of trigeminal neuralgia.
Methods
Database
Data for this study was accessed from the Taiwan National Health Insurance Research Dataset (NHIRD). The NHIRD is a claims database extracted from Taiwan’s National Health Insurance (NHI) system claims data which has all medical claims of about 23.95 million NHI enrollees in 2018 (99% of the Taiwanese population). The NHIRD allows researchers in Taiwan access to de-identified longitudinal claims data on all medical care utilized by 1 million randomly selected enrollees, all care utilization since the initiation of NHI in 1995. The data present an opportunity to explore the relationship between trigeminal neuralgia and subsequent occurrence of tinnitus. The study was approved by the Institutional Review Board of Taipei Medical University (TMU-JIRB N201712044).
Study sample
The study was designed as a retrospective cohort study. To select study patients, we first identified 132,803 patients who received their first diagnosis of trigeminal neuralgia (ICD-9-CM code 350.1) at outpatient facilities (doctor’s offices or outpatient departments of hospitals) between 1 January 2001 and 31 December 2014. We assigned the date of their first-time diagnosis of trigeminal neuralgia as their index date. We excluded 1859 patients less than 18 years of age, and 118,357 patients who had received a diagnosis of tinnitus (ICD-9-CM code 388.3) within the year prior to the index date. Ultimately, the selected trigeminal neuralgia (study) cohort included 12,587 patients.
We identified comparison patients from the Registry of beneficiaries included in the NHIRD. After excluding patients with trigeminal neuralgia and those aged less than 18 years of age, we used propensity-score matching to select 12,587 comparison patients who matched the study patients on sex, age group, monthly income, geographic region, urbanization level of the patient’s residence (5 levels, 1 = most urbanized, 5 = least urbanized), vertigo-relevant co-morbidities (hyperlipidemia, diabetes, coronary heart disease, hypertension, cervical spondylosis, temporomandibular joint disorders and injury to head and neck), and index year. The index year for patients of the study cohort was the year when they first received the diagnosis of trigeminal neuralgia, and the date was their index date. For comparison cohort patients, their index year was the year of their matched trigeminal neuralgia case after ensuring they had at least one episode of medical care utilization in the index year. We assigned their index date as the date of first utilization of medical care in the index year. We further screened the comparison patients to exclude those who had ever received a diagnosis of tinnitus in the year prior to their index date.
Outcome variable
The outcome variable of interest was tinnitus occurring during one-year follow-up from the index date. Patient (n = 25,174) were tracked for a one-year period following their index date to identify any claim with a diagnosis of tinnitus.
Statistical analysis
We used the SAS statistical package (SAS System for Windows, Version 8.2, Cary NC, USA) for statistical analyses. We used the Kaplan-Meier curves with log-rank test to examine the differences in one-year, tinnitus-free survival between the study cohort and comparison cohort. Cox proportional hazards regression analysis was used to estimate the one-year adjusted risk of tinnitus following trigeminal neuralgia. We verified that the proportional hazards assumption was satisfied based on survival curves for both strata (study and comparison cohorts) showing hazard functions that were proportional over time. Adjusted hazard ratio (HR) along with 95% confidence intervals (CI) was used to estimate the risk of tinnitus, with a two-sided p value < 0.05 considered as statistically significant.
Results
Table 1 presents the sample distribution, categorized by trigeminal neuralgia status on sociodemographic characteristics and co-morbidities. Of the total sampled patients, the mean age was 54.6 years old (standard deviation = 14.7 years old); 54.5 and 54.7 for study patients and comparison patients, respectively (p = 0.237). After propensity-score matching, we found statistically significant differences in sex (p = 0.016), monthly income (p = 0.011) hypertension (p = 0.008) and hyperlipidemia (p < 0.001), cervical spondylosis (p < 0.001), temporomandibular joint disorders (p < 0.001) and injury to head and neck (p < 0.001) between study and comparison patients. The two groups were similar on age, geographic region, diabetes and CHD.
Table 1.
Demographic characteristics of patients with trigeminal neuralgia and comparison patients in Taiwan, 2001–2014 (total patients = 25,174)
| Variable | Patients with trigeminal neuralgia (n = 12,587) |
Comparison patients (n = 12,587) | P value | ||
|---|---|---|---|---|---|
| Total No. | % | Total No. | % | ||
| Males | 4495 | 35.7 | 4680 | 37.2 | 0.016 |
| Age, Mean (SD) | 54.5 (14.8) | 54.7 (14.7) | 0.237 | ||
| Hypertension | 4910 | 39.0 | 4703 | 37.4 | 0.008 |
| Coronary heart disease | 2261 | 18.0 | 2199 | 17.5 | 0.314 |
| Hyperlipidemia | 3405 | 27.1 | 3655 | 29.0 | < 0.001 |
| Diabetes | 2160 | 17.2 | 2153 | 17.1 | 0.920 |
| Cervical spondylosis | 1611 | 12.8 | 712 | 5.7 | < 0.001 |
| Injury to head and neck | 118 | 0.9 | 59 | 0.5 | < 0.001 |
| Temporomandibular joint disorders | 1126 | 8.9 | 161 | 1.3 | < 0.001 |
D standard deviation
Table 2 shows the overall incidence of tinnitus during one-year follow-up, and incidence among study patients and comparison patients. Among the total 25,174 sample patients, incidence of tinnitus was 18.21 per 100 person-years (95% CI = 17.66 ~ 18.77), 23.57 (95% CI = 22.68 ~ 24.49) among the trigeminal neuralgia group, and 13.17 (95% CI = 12.53 ~ 13.84) among the comparison group. The Kaplan-Meier log-rank test suggested that patients with trigeminal neuralgia have a significantly lower, 1-year tinnitus-free survival compared to the comparison group (p < 0.001). Figure 1 presents the Kaplan-Meier survival curves showing tinnitus-free survival among the two groups.
Table 2.
Incidence rate, crude and adjusted hazard ratios for tinnitus among the sampled patients
| Tinnitus occurrence over 1-year follow-up | Total (n = 25,174) | Patients with trigeminal neuralgia (n = 12,587) | Comparison groups (n = 12,587) | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Yes | 4168 | 16.6 | 2612 | 20.8 | 1556 | 12.4 |
| Incidence per 100 person-years (95%CI) | 18.2 (17.7 ~ 18.8) | 23.6 (22.7 ~ 24.5) | 13.2 (12.5 ~ 13.8) | |||
| Crude HR (95% CI) | – | 1.78 (1.67, 1.90)*** | 1.00 | |||
| Adjusted a HR (95% CI) | – | 1.68 (1.58, 1.80)*** | 1.00 | |||
Notes: *** indicates p < 0.001. HZ = hazard ratio
aAdjustment for patient’s sex, age, urbanization level, monthly income and geographic region, and hypertension, hyperlipidemia, coronary heart disease, diabetes, cervical spondylosis, temporomandibular joint disorders and injury to head and neck
Fig. 1.
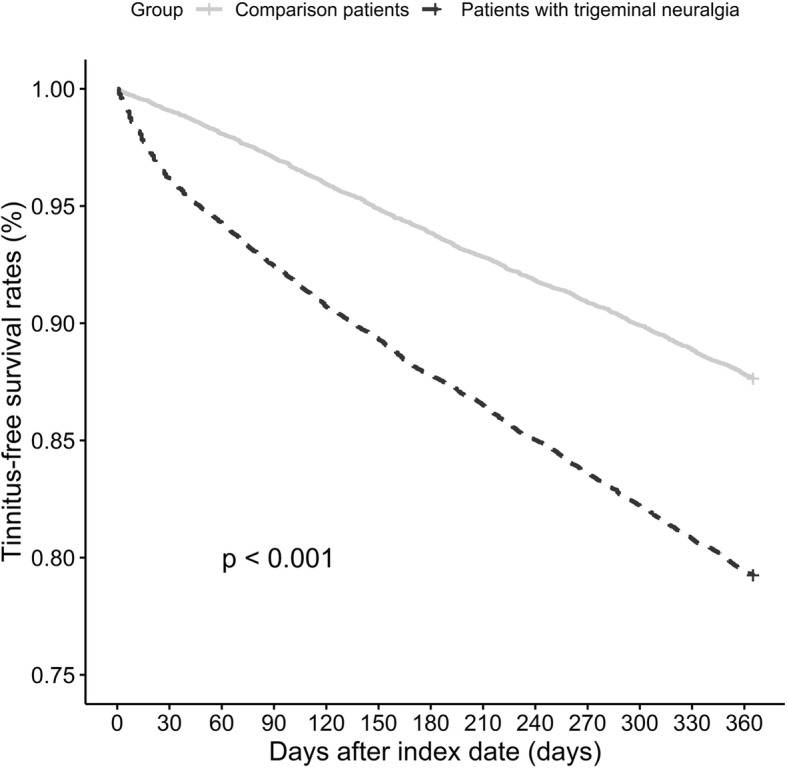
Tinnitus-free survival rates for patients with trigeminal neuralgia and comparison patients (x axis = days after index date, y axis = tinnitus-free survival rate)
Table 2 also shows that the unadjusted HR for tinnitus of the trigeminal neuralgia group relative to the comparison group was 1.78 (95% CI = 1.67 ~ 1.90) during 1-year follow-up. The adjusted HR was 1.68 (95% CI = 1.58 ~ 1.80) after adjusting for age, sex, monthly income, geographic region, hyperlipidemia, diabetes, coronary heart disease, hypertension, cervical spondylosis, temporomandibular joint disorders and injury to head and neck.
Table 3 shows the relationship between trigeminal neuralgia and tinnitus stratified by surgical intervention. We found that the unadjusted HR for tinnitus for patients with trigeminal neuralgia who underwent surgical interventions relative to those without intervention was 2.91 (95% CI = 1.31 ~ 6.49). However, statistical significance of the association was not sustained after adjusting for age, sex, monthly income, geographic region, hyperlipidemia, diabetes, coronary heart disease, hypertension, cervical spondylosis, temporomandibular joint disorders and injury to head and neck (adjusted HR = 2.20, 95% CI = 0.98 ~ 4.91). It is possible however, that loss of statistical significance with adjustment for covariates may be due to the inadequate statistical power.
Table 3.
Incidence rate, crude and adjusted hazard ratios for tinnitus among the sampled patients stratified by surgical intervention
| Tinnitus occurrence over 1-year follow-up | Patients with trigeminal neuralgia who underwent surgical interventions (n = 19) | Patients with trigeminal neuralgia who did not undergo surgical interventions (n = 12,568) | Comparison groups (n = 12,587) | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Yes | 6 | 31.6 | 2606 | 20.7 | 1556 | 12.4 |
| Incidence per 100 person-years (95%CI) | 38.5 (15.6 ~ 80.1) | 23.6 (22.7 ~ 24.5) | 13.2 (12.5 ~ 13.8) | |||
| Crude HR (95% CI) | 2.91 (1.31, 6.49)** | 1.78 (1.67, 1.9)*** | 1.00 | |||
| Adjusted a HR (95% CI) | 2.20 (0.98, 4.91) | 1.68 (1.58, 1.8)*** | 1.00 | |||
Notes: ** indicates p < 0.01; *** indicates p < 0.001; HZ hazard ratio
aAdjustment for patient’s sex, age, urbanization level, monthly income and geographic region, and hypertension, hyperlipidemia, coronary heart disease, diabetes, cervical spondylosis, temporomandibular joint disorders and injury to head and neck
Table 4 presents the incidence and HR of tinnitus between study and comparison patients by age group. It indicates that there was a consistent significant relationship between trigeminal neuralgia and tinnitus across all age groups. We found that the relationship to be the most pronounced among those < 45 years, with an adjusted HR of 1.91 (95% CI: 1.65 ~ 2.22) for patients with trigeminal neuralgia relative to the comparison group.
Table 4.
Incidence rate, crude and adjusted hazard ratios for tinnitus among the sampled patients according to age group
| Tinnitus occurrence over 1-year follow-up | < 45 | 45 ~ 64 | > 64 | |||
|---|---|---|---|---|---|---|
| Patients with trigeminal neuralgia (n = 2933) | Comparison groups (n = 2862) | Patients with trigeminal neuralgia (n = 6195) | Comparison groups (n = 6196) | Patients with trigeminal neuralgia (n = 3459) | Comparison groups (n = 3529) | |
| n, % | n, % | n, % | ||||
| Yes | 544 (18.5) | 270 (9.4) | 1261 (20.4) | 711 (11.5) | 807 (23.3) | 575 (16.3) |
| Incidence per 100 person-years (95%CI) | 20.9 (19.2 ~ 22.7) | 9.9 (8.8 ~ 11.1) | 23.1 (21.8 ~ 24.3) | 12.2 (11.3 ~ 13.1) | 26.9 (25.1 ~ 28.8) | 17.8 (16.3 ~ 19.3) |
| Crude HR (95% CI) | 2.09 (1.81, 2.42)*** | 1.00 | 1.89 (1.72, 2.07)*** | 1.00 | 1.51 (1.36, 1.68)*** | 1.00 |
| Adjusted a HR (95% CI) | 1.91 (1.65, 2.22)*** | 1.00 | 1.78 (1.62, 1.95)*** | 1.00 | 1.45 (1.30, 1.62)*** | 1.00 |
Notes: *** indicates p < 0.001. HZ hazard ratio
aAdjustment for patient’s sex, age, urbanization level, monthly income and geographic region, and hypertension, hyperlipidemia, coronary heart disease, diabetes, cervical spondylosis, temporomandibular joint disorders and injury to head and neck
Table 4 presents the incidence and HR of tinnitus between the study and comparison patients classified by age group. The results show a consistent and significant relationship between trigeminal neuralgia and tinnitus within all age groups. The association showed highest effect size among the age group of < 45 years, with an adjusted HR of 1.91 (95% CI: 1.65 ~ 2.22) compared to a HR of 1.78 for the age groups of 45 ~ 64 and 1.45 for the > 65 age group.
Discussion
To our knowledge, this is the first population-based study to investigate the relationship between trigeminal neuralgia and subsequent appearance of tinnitus. Based on longitudinal data available in the National Health Insurance Research Database (NHIRD) of Taiwan, we find that trigeminal neuralgia is significantly associated with tinnitus in the year following the diagnosis.
While tinnitus has been traditionally associated with otologic conditions such as noise-induced hearing loss, a growing body of evidence has shown that convergence of auditory and somatosensory pathways in the brain stem also plays an important role in the pathogenesis of tinnitus [26–29]. The auditory system processes sound signals arising in the ear and transmits them to the cortex. The cochlear nucleus is the central site for multisensory integration of inputs originating in sources other than the auditory nerve, namely, somatosensory inputs from the trigeminal ganglia, cervical dorsal root ganglia, spiral trigeminal nucleus, and dorsal column nuclei [15, 23–25, 30]. Therefore, pathological changes in the peripheral somatosensory structures in the head and neck region may influence the auditory processing of inputs at the level of the cochlear nucleus. Trigeminal neuralgia and other tinnitus-causing somatosensory disorders such as TMD or CSD share some neurophysiological characteristics in transmitting the nociceptive inputs in the central nervous system. Therefore, disruptions in trigeminal inputs that are relayed in the cochlear nucleus may significantly influence the higher-order neuronal output, causing changes to the output transmitted on to the higher auditory pathways, causing phantom sound that is experienced as tinnitus.
Microvascular constriction within the inner ear has been proposed as a possible mechanism for tinnitus caused by disruption of sensorineural input from the auditory pathway [31–37]. Microvascular constriction affecting the inner ear may also affect the trigeminal nerve in its anatomical course through the inner ear, causing both trigeminal neuralgia and tinnitus as part of the same pathophysiology. Potentially this could underlie the observed comorbid occurrence of tinnitus and trigeminal neuralgia in some patients [31–37]. Disruptions of the trigeminal nerve caused by neuralgia may also induce or contribute to tinnitus by affecting the vasculature of the inner ear. The trigeminal nerve is the source of innervation for blood vessels around the spiral modiolus and the stria vascularis of inner ear [31, 38]. The major function of the stria vascularis is to regulate the ionic composition of the endolymph, to maintain high potassium concentrations to sustain the endolymphatic potential necessary for sound transduction. Disruption of the stria vascularis causes reduced perception of sounds and altered auditory processing, potentially leading to tinnitus [32].
A strength of this study is that more than 99% of Taiwan’s population is enrolled in the NHI, and the health system, both provider availability and health insurance features are designed to be maximally affordable and accessible to all citizens regardless of socio-economic status. Therefore, apart from being representative of the entire Taiwanese population, the study has minimal potential for bias due to differential healthcare utilization rates among the study and control groups. The advantage of a population-based study is the ability to achieve a large sample size with minimal selection bias and participation bias.
Despite its strengths, a cautionary note due to some limitations is warranted. First, epidemiological association does not prove biological causation. Biomedical studies are needed to explain the causal mechanisms underlying the observed association. Second, under-ascertainment of tinnitus is a potential limitation because only subjects who sought medical care can be identified from a claims database. Such bias would lead to underestimation of the association between trigeminal neuralgia and tinnitus. Finally, the dataset used in this study does not provide information on types of TN, clinical response to the typical drugs used for TN, treatment strategy, cranial autonomic features and neurovascular compression which could provide some insight into the pathophysiological context and etiology of the observed association. Additionally, the absence of data on other potential risk factors and comorbidities, such as lifestyle, tobacco or other psychoactive substance use, anxiety, depression, attention disorders and sleep disorders also limits the strength of study findings.
Conclusion
Our findings suggest that trigeminal neuralgia may be associated with subsequent development of tinnitus in the following year. Further studies in other regions and ethnicities are needed to confirm the observed association. Biomedical studies are needed to investigate the causal pathways involved in the development of tinnitus among trigeminal neuralgia patients.
Acknowledgements
None.
Authors’ contributions
Y.F., H.C. and S participated in the design of the study and helped to draft the manuscript. Y.W. and B.C. performed the statistical analysis and helped to draft the manuscript. T.H. and C.S. conceived of the study, participated in its design and coordination and helped to draft the manuscript. All authors reviewed the manuscript. The author(s) read and approved the final manuscript.
Funding
None.
Availability of data and materials
The National Health Insurance Research Database, which has been transferred to the Health and Welfare Data Science Center (HWDC). Interested researchers can obtain the data through formal application to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yen-Fu Cheng, Email: entist@gmail.com.
Sudha Xirasagar, Email: sxirasagar@sc.edu.
Tzong-Han Yang, Email: tzonghannyang@gmail.com.
Chuan-Song Wu, Email: seancswu@seed.net.tw.
Yi-Wei Kao, Email: kyw498762030@gmail.com.
Ben-Chang Shia, Email: stat1001@tmu.edu.tw.
Herng-Ching Lin, Email: henry11111@tmu.edu.tw.
References
- 1.Bhatt JM, Lin HW, Bhattacharyya N. Prevalence, severity, exposures, and treatment patterns of tinnitus in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(10):959–965. doi: 10.1001/jamaoto.2016.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jastreboff PJ, Hazell JW. A neurophysiological approach to tinnitus: clinical implications. Br J Audiol. 1993;27(1):7–17. doi: 10.3109/03005369309077884. [DOI] [PubMed] [Google Scholar]
- 3.Folmer RL, Griest SE. Chronic tinnitus resulting from head or neck injuries. Laryngoscope. 2003;113(5):821–827. doi: 10.1097/00005537-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Bousema EJ, Koops EA, van Dijk P, Dijkstra PU. Association between subjective tinnitus and cervical spine or Temporomandibular disorders: a systematic review. Trends Hear. 2018;22:2331216518800640. doi: 10.1177/2331216518800640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.HILGENBERG PB, SALDANHA ADD, CUNHA CO, RUBO JH, CONTI PCR. Temporomandibular disorders, otologic symptoms and depression levels in tinnitus patients. J Oral Rehabil. 2012;39(4):239–244. doi: 10.1111/j.1365-2842.2011.02266.x. [DOI] [PubMed] [Google Scholar]
- 6.Buergers R, Kleinjung T, Behr M, Vielsmeier V. Is there a link between tinnitus and temporomandibular disorders? J Prosthet Dent. 2014;111(3):222–227. doi: 10.1016/j.prosdent.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Lee CF, Lin MC, Lin HT, Lin CL, Wang TC, Kao CH. Increased risk of tinnitus in patients with temporomandibular disorder: a retrospective population-based cohort study. Eur Arch Otorhinolaryngol. 2016;273(1):203–208. doi: 10.1007/s00405-015-3491-2. [DOI] [PubMed] [Google Scholar]
- 8.Kuttila S, Kuttila M, Le Bell Y, Alanen P, Suonpää J. Recurrent tinnitus and associated ear symptoms in adults. Int J Audiol. 2005;44(3):164–170. doi: 10.1080/14992020500057608. [DOI] [PubMed] [Google Scholar]
- 9.Pezzoli M, Ugolini A, Rota E, Ferrero L, Milani C, Pezzoli L, et al. Tinnitus and its relationship with muscle tenderness in patients with headache and facial pain. J Laryngol Otol. 2015;129(7):638–643. doi: 10.1017/S0022215115001425. [DOI] [PubMed] [Google Scholar]
- 10.Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol. 1999;20(6):351–362. doi: 10.1016/S0196-0709(99)90074-1. [DOI] [PubMed] [Google Scholar]
- 11.Levine RA, Nam EC, Oron Y, Melcher JR. Evidence for a tinnitus subgroup responsive to somatosensory based treatment modalities. Elsevier. 2007;166:195–207. doi: 10.1016/S0079-6123(07)66017-8. [DOI] [PubMed] [Google Scholar]
- 12.Heeringa AN, Wu C, Shore SE. Multisensory integration enhances temporal coding in ventral Cochlear nucleus bushy cells. J Neurosci. 2018;38(11):2832–2843. doi: 10.1523/JNEUROSCI.2244-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koehler SD, Shore SE. Stimulus timing-dependent plasticity in dorsal cochlear nucleus is altered in tinnitus. J Neurosci. 2013;33(50):19647–19656. doi: 10.1523/JNEUROSCI.2788-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks KL, Martel DT, Wu C, Basura GJ, Roberts LE, Schvartz-Leyzac KC, et al. Auditory-somatosensory bimodal stimulation desynchronizes brain circuitry to reduce tinnitus in guinea pigs and humans. Sci Transl Med. 2018;10(422):eaal3175. doi: 10.1126/scitranslmed.aal3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shore SE. Multisensory integration in the dorsal cochlear nucleus: unit responses to acoustic and trigeminal ganglion stimulation. Eur J Neurosci. 2005;21(12):3334–3348. doi: 10.1111/j.1460-9568.2005.04142.x. [DOI] [PubMed] [Google Scholar]
- 16.Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci. 2008;27(1):155–168. doi: 10.1111/j.1460-9568.2007.05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henssen DJHA, Kurt E, Kozicz T, van Dongen R, Bartels RHMA, van Cappellen van Walsum A-M. New insights in trigeminal anatomy: a double Orofacial tract for nociceptive input. Front Neuroanat. 2016;10:53. doi: 10.3389/fnana.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montano N, Conforti G, Di Bonaventura R, Meglio M, Fernandez E, Papacci F. Advances in diagnosis and treatment of trigeminal neuralgia. Ther Clin Risk Manag. 2015;11:289–299. doi: 10.2147/TCRM.S37592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu XM, Mense S. Somatotopical arrangement of rat spinal dorsal horn cells processing input from deep tissues. Neurosci Lett. 1990;108(1–2):43–47. doi: 10.1016/0304-3940(90)90703-c. [DOI] [PubMed] [Google Scholar]
- 20.Parra LC, Pearlmutter BA. Illusory percepts from auditory adaptation. J Acoust Soc Am. 2007;121(3):1632–1641. doi: 10.1121/1.2431346. [DOI] [PubMed] [Google Scholar]
- 21.Wei L, Ding D, Sun W, Xu-Friedman MA, Salvi R. Effects of sodium salicylate on spontaneous and evoked spike rate in the dorsal cochlear nucleus. Hear Res. 2010;267(1–2):54–60. doi: 10.1016/j.heares.2010.03.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res. 2005;206(1–2):200–226. doi: 10.1016/j.heares.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Mizuno N. Single neurons in the spinal trigeminal and dorsal column nuclei project to both the cochlear nucleus and the inferior colliculus by way of axon collaterals: a fluorescent retrograde double-labeling study in the rat. Neurosci Res. 1997;29(2):135–142. doi: 10.1016/S0168-0102(97)00082-5. [DOI] [PubMed] [Google Scholar]
- 24.Haenggeli CA, Pongstaporn T, Doucet JR, Ryugo DK. Projections from the spinal trigeminal nucleus to the cochlear nucleus in the rat. J Comp Neurol. 2005;484(2):191–205. doi: 10.1002/cne.20466. [DOI] [PubMed] [Google Scholar]
- 25.Schofield BR, Coomes DL. Auditory cortical projections to the cochlear nucleus in Guinea pigs. Hear Res. 2005;199(1–2):89–102. doi: 10.1016/j.heares.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Shore S, Zhou J, Koehler S. Neural mechanisms underlying somatic tinnitus. Prog Brain Res. 2007;166:107–123. doi: 10.1016/S0079-6123(07)66010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simmons R, Dambra C, in ELS . Head, neck, and eye movements that modulate tinnitus. thieme-connectcom. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryugo DK, Haenggeli CA, Doucet JR. Multimodal inputs to the granule cell domain of the cochlear nucleus. Exp Brain Res. 2003;153(4):477–485. doi: 10.1007/s00221-003-1605-3. [DOI] [PubMed] [Google Scholar]
- 29.Dehmel S, Cui YL, Shore SE. Cross-modal interactions of auditory and somatic inputs in the brainstem and midbrain and their imbalance in tinnitus and deafness. Am J Audiol. 2008;17(2):S193–S209. doi: 10.1044/1059-0889(2008/07-0045). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright DD, Ryugo DK. Mossy fiber projections from the cuneate nucleus to the cochlear nucleus in the rat. J Comp Neurol. 1996;365(1):159–172. doi: 10.1002/(SICI)1096-9861(19960129)365:1<159::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 31.Calbucci F, Tognetti F, Bollini C, Cuscini A, Michelucci R, Tassinari CA. Intracranial microvascular decompression for “cryptogenic” hemifacial spasm, trigeminal and glossopharyngeal neuralgia, paroxysmal vertigo and tinnitus: I. surgical technique and results. Ital J Neurol Sci. 1986;7(3):359–366. doi: 10.1007/BF02340876. [DOI] [PubMed] [Google Scholar]
- 32.De Ridder D, Vanneste S, Adriaenssens I, Lee APK, Plazier M, Menovsky T, et al. Microvascular decompression for tinnitus: significant improvement for tinnitus intensity without improvement for distress. A 4-year limit. Neurosurgery. 2010;66(4):656–660. doi: 10.1227/01.NEU.0000366110.87836.53. [DOI] [PubMed] [Google Scholar]
- 33.Michelucci R, Tassinari CA, Samoggia G, Tognetti F, Calbucci F. Intracranial microvascular decompression for “cryptogenic” hemifacial spasm, trigeminal and glossopharyngeal neuralgia, paroxysmal vertigo and tinnitus: II. Clinical study and long-term follow up. Ital J Neurol Sci. 1986;7(3):367–374. doi: 10.1007/BF02340877. [DOI] [PubMed] [Google Scholar]
- 34.Okamura T, Kurokawa Y, Ikeda N, Abiko S, Ideguchi M, Watanabe K, et al. Microvascular decompression for cochlear symptoms. J Neurosurg. 2000;93(3):421–426. doi: 10.3171/jns.2000.93.3.0421. [DOI] [PubMed] [Google Scholar]
- 35.Didier A, Miller JM, Nuttall AL. The vascular component of sodium salicylate ototoxicity in the Guinea pig. Hear Res. 1993;69(1–2):199–206. doi: 10.1016/0378-5955(93)90108-D. [DOI] [PubMed] [Google Scholar]
- 36.Jannetta PJ. Observations on the etiology of trigeminal neuralgia, hemifacial spasm, acoustic nerve dysfunction and glossopharyngeal neuralgia. Definitive microsurgical treatment and results in 117 patients. Neurochirurgia. 1977;20(5):145–154. doi: 10.1055/s-0028-1090369. [DOI] [PubMed] [Google Scholar]
- 37.Ryu H, Yamamoto S, Sugiyama K, Uemura K, Nozue M. Neurovascular decompression of the eighth cranial nerve in patients with hemifacial spasm and incidental tinnitus: an alternative way to study tinnitus. J Neurosurg. 1998;88(2):232–236. doi: 10.3171/jns.1998.88.2.0232. [DOI] [PubMed] [Google Scholar]
- 38.Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the Guinea pig. J Comp Neurol. 2006;495(1):100–112. doi: 10.1002/cne.20863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The National Health Insurance Research Database, which has been transferred to the Health and Welfare Data Science Center (HWDC). Interested researchers can obtain the data through formal application to the HWDC, Department of Statistics, Ministry of Health and Welfare, Taiwan (http://dep.mohw.gov.tw/DOS/np-2497-113.html).


