Abstract
Background:
The ankle-brachial index (ABI) may underestimate the severity of peripheral arterial disease (PAD) in patients with noncompressible vessels. This study analyzed limitations of the ABI and toe-brachial index (TBI), if done alone, in patients with symptomatic PAD, diagnosed by duplex ultrasound (DUS) examination, particularly in patients with diabetes and chronic kidney disease (CKD).
Methods:
This is a retrospective review of prospectively collected data. All patients underwent resting ABIs, TBI, and/or DUS. An ABIs of 0.90 or less in either leg was considered abnormal, and the term inconclusive ABIs (noncompressibility) was used if the ABI was 1.3 or greater. The sensitivity, specificity, positive predictive value, negative predictive value, and overall accuracy (OA) of ABIs in detecting 50% or greater stenosis of any arterial segment based on DUS were determined. A TBI of less than 0.7 was considered abnormal.
Results:
We included 2226 ABIs and 1383 DUS examinations: 46% of patients had diabetes, 16% had CKD, and 39% had coronary artery disease. Fifty-three percent of the ABIs were normal, 34% were abnormal, and 13% were inconclusive. For patients with limb-threatening ischemia, 40% had normal ABIs, 40% abnormal ABIs, and 20% were inconclusive. The sensitivity and OA for ABIs in detecting 50% or greater stenosis in the whole series were 57% (95% confidence interval [CI], 53.7–61.2) and 74% (95% CI, 71.9–76.6); for diabetics 51% (95% CI, 46.1–56.3) and 66% (95% CI, 62.3–69.8); nondiabetics 66% (95% CI, 59.9–70.9) and 81% (95% CI, 78.2–83.9). For patients with CKD, the sensitivity and OA for ABIs in detecting 50% or greater stenosis was 43% (95% CI, 34.3–52.7) and 67% (95% CI, 60.2–73.0) versus patients with no CKD 60% (95% CI, 56.3–64.6) and 76% (95% CI, 73.1–78.1). If patients with inconclusive ABIs were excluded, these values were 69% (95% CI, 65.2–72.9) and 80% (95% CI, 77.2–81.9) in the whole series; 67% (95% CI, 61.6–72.7) and 75% (95% CI, 70.5–78.4) for diabetics; and 63% (95% CI, 51.3–73.0) and 78% (95% CI, 70.6–83.9) for patients with CKD. Thirty-three percent of TBIs were normal and 67% were abnormal. The sensitivity and OA for abnormal TBI in detecting 50% or greater stenosis were 85% (95% CI, 78.9–90.0) and 75% (95% CI, 70.1–80.2) in the whole series; 84% (95% CI, 76.0–90.3) and 74% (95% CI, 67.1–80.2) for diabetics; and 77% (95% CI, 61.4–88.2) and 72% (95% CI, 59.9–82.3) for patients with CKD. For those with inconclusive ABIs, these values for TBI were 75% and 69%.
Conclusions:
Of symptomatic patients with PAD with 50% or greater stenosis on DUS examination, 43% had normal/inconclusive resting ABIs (49% in diabetics and 57% in CKD). TBI may help in patients with inconclusive ABIs. These patients should undergo further imaging to determine proper treatment.
Keywords: Ankle-brachial index, Peripheral arterial disease, Toe-brachial index, Diabetes mellitus, Chronic kidney disease
The ankle-brachial index (ABI) is commonly used as a noninvasive screening tool to detect peripheral artery disease (PAD) of the lower extremity. Because lower limbs normally have ankle pressures equal to or greater than ipsilateral arm pressures, a ratio of 1.0 or greater is considered normal. PAD is usually considered to be present when the resting ABI is 0.90 or less with a normal range of 1.0 to 1.3, where values of 0.91 to 0.99 are considered borderline low or when the resting ABIs is normal, but the postexercise ABI is 0.9 or less or there is a 20% or greater decrease in the postexercise ABI.1 Resting ABIs are often used as the sole screening modality to determine the need for intervention in patients with suspected symptomatic PAD.
There are significant data suggesting the diagnostic shortcomings of resting ABIs in patients with confounding factors, such as diabetes or chronic kidney disease (CKD). The ABIs may underestimate the severity of PAD in patients with noncompressible vessels secondary to arterial calcification,2 which is commonly seen in diabetics, CKD, and advanced age.3,4 Despite of this limitation, a significant number of patients with symptomatic PAD are seen by other nonvascular colleagues, specifically, cardiologists, radiologists, and even family practitioners who obtain resting ABIs and, if normal, they are content and presume that no significant disease exists.
Duplex ultrasound (DUS) examination is a noninvasive imaging modality often used to further delineate the extent of PAD. Published data has shown a sensitivity of 85% to 90% and specificity of greater than 95% for DUS in detection 50% or greater stenosis.5 Unlike ABIs, DUS examination is not subject to the same limitations of vessel noncompressibility frequently encountered in diabetics and patients with CKD.
Therefore, we present one of the largest studies to analyze the limitations of resting ABIs with or without toe-brachial index (TBI), if done alone, in patients with symptomatic PAD, as diagnosed by DUS, particularly in diabetics and patients with CKD.
METHODS
This is a retrospective review of prospectively collected data of patients tested between October 1, 2016, and March 1, 2017, for PAD of the lower extremity in our Intersocietal Accreditation Commission–accredited vascular laboratory. This study was only confined to patients who were referred to our vascular center with symptomatic PAD, that is, vascular claudication, limb-threatening ischemia (LTI; rest pain and trophic changes). No patients with asymptomatic PAD were included in this study. The study was approved by the Institutional Review Board of Charleston Area Medical Center/West Virginia University and informed consent was not required.
Each patient had resting ABIs, with or without TBI, alone and/or DUS, according to physician preference. Demographic/clinical characteristics, and indications for testing, including claudication or LTI were recorded. Patients were screened for diabetes, CKD, and coronary artery disease. Informed consent was not necessary because all data were anonymous and no personal patient information was identified.
ABIs measurement.
After resting supine for 5 minutes, ABIs were measured using a Doppler probe to obtain systolic pressures at the following locations: right and left brachial, and posterior tibial and dorsalis pedis arteries, in that order.6 The higher of the posterior tibial or dorsalis pedis artery systolic pressure values were taken as the numerator for each leg and the higher of the right and left brachial systolic pressure readings as the denominator.7 An ABI of 0.90 or less in either leg was considered abnormal. The term arterial noncompressibility was used when Doppler systolic signal was not obliterated at ankle cuff inflation pressures of 250 mm Hg or greater. The term inconclusive ABI was used if it was 1.3 or greater.
TBI measurement.
This was done by applying a cuff to the base of the toe(s) that was at least 1.2 times wider than the toe; usually a 2.5- to 3.0 cm cuff was used for the great toe. The digital pulse was examined using Doppler probe, and a similar technique was applied to measure the Doppler toe pressure.
Normal toe pressures vary from 60% to 80% of the ankle pressures. Toe pressures that are significantly lower than this signify digital arterial occlusive disease. The exception to this criteria is when ankle pressure is artificially high (arterial calcinosis), in which case the toe pressure may be much lower than 80% of the ankle pressure in the absence of digital artery disease. A TBI of greater than 0.7 is generally considered normal.
Arterial DUS examination.
This was done according to our previously published protocol.8 In brief, bilateral duplex waveforms (monophasic, biphasic, or triphasic) or occlusion of the following arteries were recorded: common and external iliacs, common femoral, superficial and deep femoral, popliteal, anterior and posterior tibial, and peroneal. The peak systolic and end-diastolic velocities of these arteries were also obtained. The percentage of stenosis or occlusion was noted. Patients were considered to have PAD if DUS showed 50% or greater stenosis in the major arterial tree, for example, the aortoiliac, common femoral artery, femoral popliteal arterial disease or at least two vessel runoff with greater than 50% stenosis (anterior tibial, posterior tibial, or cranial). We also analyzed data for detecting total arterial occlusion in any of these segments. Greater than 50% stenosis on DUS examination was defined as PSV of 300 c/s or greater and/or a velocity ratio of less than 2.8
Data analysis.
Data analysis was performed using SAS 9.3 (SAS, Inc, Cary, NC). Basic descriptive statistics, such as means and standard deviations for continuous variables and proportions and frequencies for categorical variables was used. Comparisons of categorical variables were performed using contingency table analysis with a χ2 test to determine statistically significant differences. Area under the receiver operating characteristic curve was used to determine the diagnostic accuracy of resting ABIs in our entire population as well as in diabetics and patients with CKD. Only patients who had ABI and/or TBI who also had DUS were analyzed. The 50% or greater stenosis or occlusion were determined based on DUS. An alpha level of 0.05 or less was used to determine statistical significance.
RESULTS
This study of 1162 patients included 2226 ABIs and 1383 concomitant DUS examinations, with a mean age of 65.4 years (range, 18–98 years). Five hundred patients (43%) had LTI and 662 (57%) had claudication. There were 537 (46%) with diabetes, 183 (16%) with, CKD and 450 (39%) with coronary artery disease. There were 1178 limbs with normal ABIs (53%), 754 abnormal ABIs (34%), and 294 inconclusive (13%). Ninety-eight limbs (6.9%) did not have an ABI completed, secondary to various reasons, including patient refusal, limb amputation, and/or machine failure.
Fig shows the association between resting ABIs values in relation to indications and comorbidities. It is interesting to see that, for patients with LTI, 40% had normal ABIs and 20% had inconclusive ABIs. For diabetics, 41% had normal ABIs and 23.7% had inconclusive ABIs.
Fig.
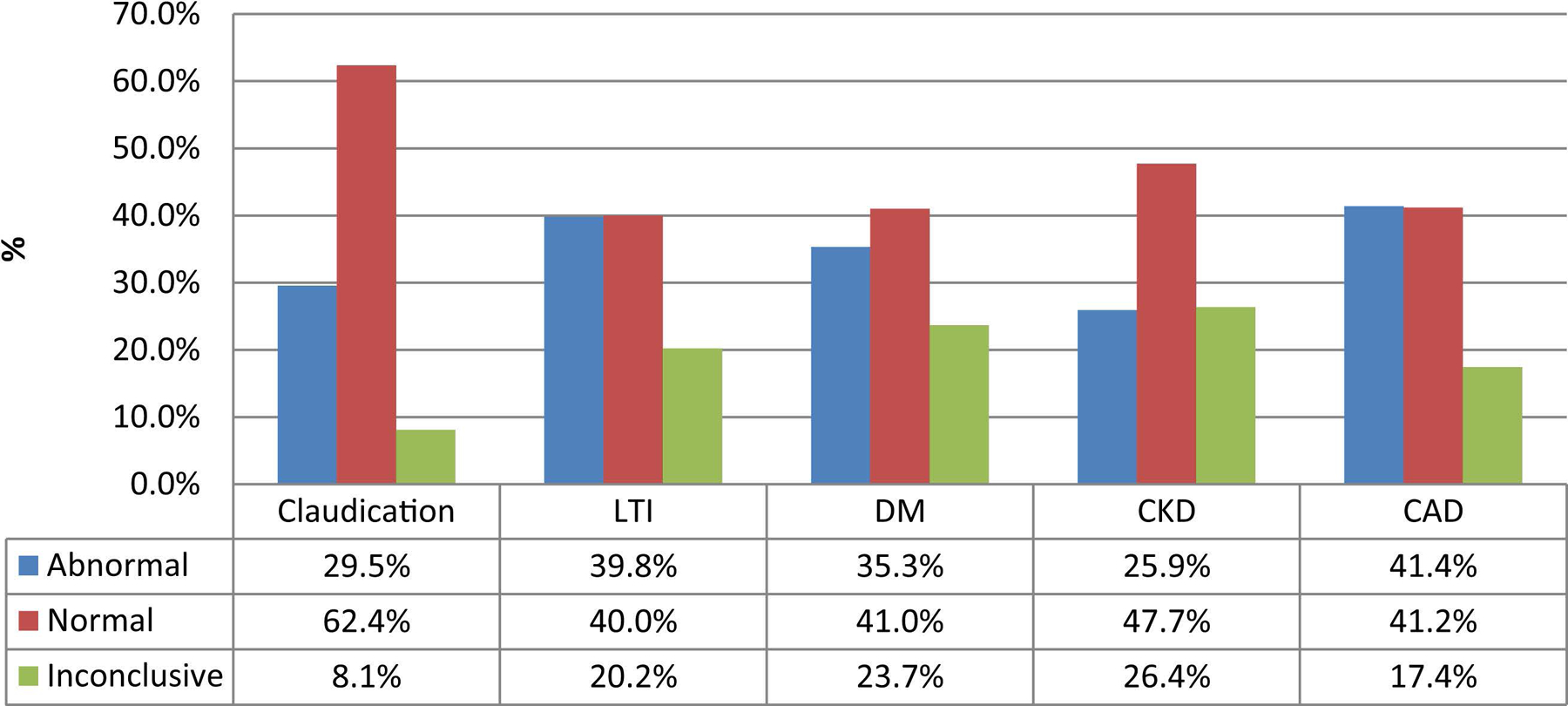
Resting ankle-brachial index (ABI) values in relation to indication and comorbidities. CAD, coronary artery disease; CKD, chronic kidney disease; DM, diabetes mellitus; LTI, limb-threatening ischemia.
Accuracy of resting ABIs in detecting 50% of greater stenosis according to DUS.
Table I summarizes the overall accuracy (OA) of resting ABIs in detecting 50% or greater stenosis of any segment of the major arterial tree (aortoiliac, common femoral, superficial femoral, popliteal, anterior tibial, posterior tibial, or peroneal arteries). As noted in this table, the sensitivity was 57% (95% CI, 53.7–61.2) with an OA of 74% (95% CI, 71.9–76.6) in the whole series. However, for diabetic patients the sensitivity was 51% (95% CI, 46.1–56.3) with OA of 66% (95% CI, 62.3–69.8) in contrast with nondiabetics, where the sensitivity was 66% (95% CI, 59.9–70.9) with an OA of 81% (95% CI, 78.2–83.9; P < .0001).
Table I.
Accuracy of the resting ankle-brachial index (ABI) in detecting 50% or greater stenosis according to duplex ultrasound (DUS) imaging (whole series)
| 50% Stenosis or greater | Sensitivity | Specificity | PPV | NPV | Accuracya | P value for accuracyb |
|---|---|---|---|---|---|---|
| All patients | 57 (53.7–61.2) | 91 (88.2–92.6) | 86 (82.4–88.3) | 69 (66.7–70.6) | 74 (71.9–76.6) | |
| Diabetics | ||||||
| Diabetics | 51 (46.1–56.3) | 89 (84.3–92.5) | 88 (83.0–91.0) | 54 (51.6–57.1) | 66 (62.3–69.8) | <.0001 |
| Nondiabetics | 66 (59.9–70.9) | 92 (88.6–94.0) | 84 (79.0–87.6) | 80 (77.3–82.4) | 81 (78.2–83.9) | |
| CKD | ||||||
| CKD | 43 (34.3–52.7) | 95 (88.7–98.4) | 91 (81.2–96.2) | 58 (54.3–62.2) | 67 (60.2–73.0) | .0059 |
| Non-CKD | 60 (56.3–64.6) | 90 (87.2–92.2) | 85 (81.3–87.7) | 71 (68.6–73.0) | 76 (73.1–78.1) | |
| Diabetics and CKD | ||||||
| Diabetics and CKD | 36 (26.1–46.5) | 95 (85.9–98.9) | 92 (77.9–97.2) | 49 (44.6–52.8) | 59 (50.7–66.9) | <.0001 |
| Nondiabetics and CKD | 61 (56.8–64.8) | 90 (87.6–92.4) | 85 (81.7–87.9) | 71 (69.3–73.6) | 76 (73.7–78.5) | |
| <80 Years of age | 60 (55.6–63.8) | 91 (88.2–92.9) | 85 (81.7–88.1) | 72 (69.5–73.8) | 76 (73.6–78.6) | <.0001 |
| ≥80 Years of age | 47 (38.1–56.4) | 89 (79.5–95.2) | 88 (78.6–93.5) | 50 (45.4–54.6) | 63 (55.6–69.5) |
CKD, Chronic kidney disease; NPV, negative predictive value; PPV, positive predictive value.
Values are presented as percentages (95% confidence interval).
Accuracy = (True positive + True negative)/(True positive + True negative + False positive + False negative).
χ2 test.
The sensitivity for patients with CKD was 43% (95% CI, 34.3–52.7) with an OA of 67% (95% CI, 60.2–73.0) in contrast with 60% (95% CI, 56.3–64.6) for patients with no CKD and OA of 76% (95% CI, 73.1–78.1; P = .0059). When patients who have both diabetes and CKD were combined, the sensitivity was only 36% (95% CI, 26.1–46.5) with an OA of 59% (95% CI, 50.7–66.9; P < .0001). Similarly, patients who were less than 80 years of age had a sensitivity of 60% (95% CI, 55.6–63.8) with an OA of 76% (95% CI, 73.6–78.6) versus 47% (95% CI, 38.1–56.4) and 63% (95% CI, 55.6–69.5; P < .0001) for patients 80 years of age or older, respectively.
When patients with inconclusive ABI were excluded, because these patients might undergo further imaging, regardless, the sensitivity was 69% (95% CI, 65.2–72.9) with an OA of 80% (95% CI, 77.2–81.9). For diabetics, it was 67% (95% CI, 61.6–72.7) with an OA of 75% (95% CI, 70.5–78.4) in contrast with nondiabetic patients, where the sensitivity was 71% (95% CI, 65.3–76.3) with an OA of 83% (95% CI, 80.1–85.8; P = .0003) and for patients with CKD the sensitivity was 63% (95% CI, 51.3–73.0) with an OA of 78% (95% CI, 70.6–83.9) in contrast with patients with no CKD the sensitivity was 70% (95% CI, 66.0–74.3) with OA of 80% (95% CI, 77.3–82.3; P = .5291). Patients less than 80 years of age had a sensitivity of 69% (95% CI, 64.3–72.7) with an OA of 80% (95% CI, 77.4–82.3), whereas patients 80 years of age or older had a sensitivity of 73% (95% CI, 61.4–81.9) with an OA of 77% (95% CI, 69.4–84.2; P = .5067; Table II).
Table II.
Accuracy of resting ankle-brachial index (ABI) in detecting 50% or greater stenosis according to duplex ultrasound (DUS) imaging (excluding inconclusive ABI)
| ≥50 Stenosis | Sensitivity | Specificity | PPV | NPV | Accuracya | P value for accuracyb |
|---|---|---|---|---|---|---|
| All patients | 69 (65.2–72.9) | 89 (86.6–91.6) | 86 (82.5–88.3) | 76 (73.5–78.1) | 80 (77.2–81.9) | |
| Diabetics | ||||||
| Diabetics | 67 (61.6–72.7) | 85 (79.7–90.1) | 88 (83.1–90.9) | 63 (59.3–67.4) | 75 (70.5–78.4) | .0003 |
| Nondiabetics | 71 (65.3–76.3) | 91 (87.9–93.6) | 84 (79.1–87.6) | 83 (80.0–85.3) | 83 (80.1–85.8) | |
| CKD | ||||||
| CKD | 63 (51.3–73.0) | 94 (85.8–97.9) | 91 (81.4–96.1) | 70 (64.2–76.0) | 78 (70.6–83.9) | .5291 |
| Non-CKD | 70 (66.0–74.3) | 89 (85.7–91.2) | 85 (81.4–87.7) | 77 (74.2–79.2) | 80 (77.3–82.3) | |
| Diabetics and CKD | ||||||
| Diabetics and CKD | 57 (43.2–69.8) | 93 (80.1–98.5) | 92 (78.3–97.1) | 60 (52.8–67.4) | 72 (61.8–80.3) | .0412 |
| Nondiabetics and CKD | 71 (66.4–74.5) | 89 (86.2–91.5) | 85 (81.8–87.9) | 77 (74.8–79.7) | 80 (77.9–82.7) | |
| <80 Years of age | 69 (64.3–72.7) | 90 (86.9–92.1) | 85 (81.8–88.1) | 77 (74.3–79.1) | 80 (77.4–82.3) | .5067 |
| ≥80 Years of age | 73 (61.4–81.9) | 85 (72.4–93.3) | 88 (79.1–93.3) | 67 (58.5–74.8) | 77 (69.4–84.2) |
CKD, Chronic kidney disease; NPV, negative predictive value; PPV, positive predictive value.
Values are presented as percentages (95% confidence interval).
Accuracy = (True positive + True negative)/(True positive + True negative + False positive + False negative).
χ2 test.
Value of resting ABIs in detecting total arterial occlusion.
Overall, 358 arterial occlusions (aorta, iliacs, common femoral, superficial femoral, popliteal, tibial, and/or peroneal arteries) were noted on DUS, 239 (67%) of which only had an abnormal ABI; meanwhile, 119 (33%) had a normal and/or inconclusive ABI.
Table III summarizes the results in patients with total arterial occlusion. As noted, the sensitivity for the whole series was 67% (95% CI, 61.6–71.6) with an OA of 76% (95% CI, 73.2–77.8). For diabetics, the sensitivity was 58% (95% CI, 51.3–65.3) with an OA of 70% (95% CI, 66.3–73.6) versus nondiabetics 78% (95% CI, 70.2–83.9) and 80% (95% CI, 77.2–83.0; P < .001) and for patients with CKD the sensitivity was 53% (95% CI, 39.1–65.7) with an OA of 75% (95% CI, 69.2–81.0) versus 70% (95% CI, 64.0–74.7) and 76% (95% CI, 73.0–78.0; P = .9682) for patients who had no CKD.
Table III.
Accuracy of resting ankle-brachial index (ABI) in detecting any segment with total occlusion according to duplex ultrasound (DUS) imaging (whole series)
| Any segment with 100% occlusion | Sensitivity | Specificity | PPV | NPV | Accuracya | P value for accuracyb |
|---|---|---|---|---|---|---|
| All patients | 67 (61.6–71.6) | 79 (76.0–81.1) | 52 (48.7–55.6) | 87 (85.4–88.7) | 76 (73.2–77.8) | |
| Diabetics | ||||||
| Diabetics only | 58 (51.3–65.3) | 75 (71.1–79.5) | 53 (47.6–57.7) | 80 (76.6–82.2) | 70 (66.3–73.6) | <.0001 |
| Nondiabetics only | 78 (70.2–83.9) | 81 (77.6–84.0) | 52 (47.1–56.3) | 93 (91.1–94.9) | 80 (77.2–83.0) | |
| CKD | ||||||
| CKD only | 53 (39.1–65.7) | 84 (77.2–89.2) | 54 (43.7–64.6) | 83 (78.5–86.4) | 75 (69.2–81.0) | .9682 |
| Non-CKD only | 70 (64.0–74.7) | 78 (74.7–80.4) | 52 (48.2–55.5) | 88 (86.1–89.8) | 76 (73.0–78.0) |
CKD, Chronic kidney disease; NPV, negative predictive value; PPV, positive predictive value.
Values are presented as percentages (95% confidence interval).
Accuracy = (True positive + True negative)/(True positive + True negative + False positive + False negative).
χ2 test.
When patients with inconclusive ABIs were excluded, the sensitivity for the whole series was 82% (95% CI, 77.5–86.6) with an OA of 77% (95% CI, 74.7–79.5). For diabetics, the sensitivity was 80% (95% CI, 72.3–85.9) with an OA of 72% (95% CI, 67.7–75.9) versus nondiabetics 85% (95% CI, 78.3–90.6) and 81% (95% CI, 77.7–83.7; P = .0003). For patients with CKD, the sensitivity was 70% (95% CI, 54.8–83.2) with an OA of 76% (95% CI, 68.6–82.3) versus 85% (95% CI, 79.4–88.8) and 77% (95% CI, 74.7–79.9) for patients who had no CKD (P = .6831; Table IV).
Table IV.
Accuracy of resting ankle-brachial index (ABI) in detecting any segment with total occlusion according to duplex ultrasound (DUS) imaging (excluding inconclusive ABI)
| Any segment with 100% occlusion | Sensitivity | Specificity | PPV | NPV | Accuracya | P value for accuracyb |
|---|---|---|---|---|---|---|
| All patients | 82 (77.5–86.6) | 75 (72.5–78.3) | 52 (49.0–55.3) | 93 (91.1–94.5) | 77 (74.7–79.5) | |
| Diabetics | ||||||
| Diabetics only | 80 (72.3–85.9) | 68 (63.2–73.4) | 53 (48.3–57.1) | 88 (84.7–91.4) | 72 (67.7–75.9) | .0003 |
| Nondiabetics only | 85 (78.3–90.6) | 80 (76.1–83.0) | 52 (47.3–56.1) | 95 (93.4–96.9) | 81 (77.7–83.7) | |
| CKD | ||||||
| CKD only | 70 (54.8–83.2) | 78 (69.4–85.1) | 54 (44.7–63.8) | 88 (81.6–91.9) | 76 (68.6–82.3) | .6831 |
| Non-CKD only | 85 (79.4–88.8) | 75 (71.9–78.1) | 52 (48.5–55.2) | 94 (91.9–95.4) | 77 (74.7–79.9) |
CKD, Chronic kidney disease; NPV, negative predictive value; PPV, positive predictive value.
Values are presented as percentages (95% confidence interval).
Accuracy = (True positive + True negative)/(True positive + True negative + False positive + False negative).
χ2 test.
Value of adding the TBI to the ABI.
Five hundred ninety-one limbs had TBI; 33% were normal and 67% were abnormal (<0.7). Overall, 189 patients had an abnormal ABI and almost all had a TBI of less than 0.7 (n = 188). Meanwhile, 244 had a normal ABIs where 133 were associated with a normal TBI (55%) and 111 (45%) had an abnormal TBI. One hundred fifty-three patients had an inconclusive ABI, 58 of which (38%) had a normal TBI and 95 (62%) had an abnormal TBI.
Two hundred ninety-seven limbs had both TBI and DUS. Among the patients who had a TBI, there was a significantly greater number of patients with diabetes mellitus, CKD, and threatening-limb ischemia. Table V shows the accuracy of resting TBI in detecting 50% or greater stenosis according to DUS. As noted, the sensitivity for the whole series was 85% (95% CI, 78.9–90.0) with an OA of 75% (95% CI, 70.1–80.2). For diabetics, the sensitivity was 84% (95% CI, 76.0–90.3) with an OA of 74% (95% CI, 67.1–80.2) versus nondiabetics where it was 87% (95% CI, 75.8–94.2) and 78% (95% CI, 68.8–85.0; P= .482), respectively. The sensitivity for patients with CKD was 77% (95% CI, 61.4–88.2) with an OA of 72% (95% CI, 59.9–82.3) versus patients with no CKD where the sensitivity was 88% (95% CI, 80.9–92.9) with an OA of 76% (95% CI, 70.4–81.8; P = .4634).
Table V.
Accuracy of resting toe-brachial index (TBI) (<0.7 vs ≥0.7) in detecting 50% or greater stenosis according to duplex ultrasound (DUS) imaging
| ≥50 Stenosis | Sensitivity | Specificity | PPV | NPV | Accuracya | P value for accuracyb |
|---|---|---|---|---|---|---|
| All patients | 85 (78.9–90.0) | 62 (52.6–70.4) | 76 (71.4–79.9) | 75 (66.6–81.1) | 75 (70.1–80.2) | |
| Diabetics | ||||||
| Diabetics | 84 (76.0–90.3) | 58 (46.1–69.9) | 76 (70.4–80.8) | 70 (59.4–78.8) | 74 (67.1–80.2) | .482 |
| Nondiabetics | 87 (75.8–94.2) | 67 (52.1–79.2) | 76 (44.8–63.9) | 81 (68.4–89.3) | 78 (68.8–85.0) | |
| CKD | ||||||
| CKD | 77 (61.4–88.2) | 64 (42.5–82.0) | 79 (68.0–86.4) | 62 (46.3–74.8) | 72 (59.9–82.3) | .4634 |
| Non-CKD | 88 (80.9–92.9) | 61 (50.9–70.9) | 75 (70.1–79.6) | 79 (69.8–85.9) | 76 (70.4–81.8) |
CKD, Chronic kidney disease; NPV, negative predictive value; PPV, positive predictive value.
Values are presented as percentages (95% confidence interval).
Accuracy = (True positive + True negative)/(True positive + True negative + False positive + False negative).
χ2 test
Table VI summarizes the accuracy of resting ABI and TBI in detecting greater than 50% stenosis according to DUS in the whole series. As noted in this table, the sensitivity for the whole series was 68% (95% CI, 64.7–71.9) with an OA of 77% (95% CI, 74.6–79.1). Meanwhile, the sensitivity for diabetic patients was 64% (95% CI, 59.0–89.9) with an OA of 69% (95% CI, 65.7–73.0) versus nondiabetics where the sensitivity was 74% (95% CI, 68.6–78.8) with an OA of 83% (95% CI, 80.3–85.8; P < .0001). For patients with CKD, the sensitivity was 63% (95% CI, 53.2–71.2) with an OA 73% (95% CI, 66.8–78.9) versus 70% (95% CI, 65.6–73.4) and 78% (95% CI, 75.1–79.9; P = .1562) for patients with no CKD, respectively.
Table VI.
Accuracy of Resting ankle-brachial index (ABI) and toe-brachial index (TBI) in detecting 50% or greater stenosis according to duplex ultrasound (DUS) imaging (whole series)a
| ≥50 Stenosis | Sensitivity | Specificity | PPV | NPV | Accuracyb | P value for accuracyc |
|---|---|---|---|---|---|---|
| All patients | 68 (64.7–71.9) | 85 (82.3–87.7) | 82 (78.9–84.4) | 73 (71.1–75.6) | 77 (74.6–79.1) | |
| Diabetics | ||||||
| Diabetics | 64 (59.0–89.9) | 78 (72.0–82.7) | 81.5 (77.5–84.9) | 59 (54.9–62.1) | 69 (65.7–73.0) | <.0001 |
| Nondiabetics | 74 (68.6–78.8) | 89 (86.1–92.0) | 82 (77.8–85.9) | 84 (80.9–86.2) | 83 (80.3–85.8) | |
| CKD | ||||||
| CKD | 63 (53.2–71.2) | 86 (77.6–92.1) | 84 (76.4–89.9) | 66 (60.0–70.9) | 73 (66.8–78.9) | .1562 |
| Non-CKD | 70 (65.6–73.4) | 85 (81.9–87.8) | 81 (78.1–84.2) | 75 (72.4–77.3) | 78 (75.1–79.9) |
CKD, Chronic kidney disease; NPV, negative predictive value; PPV, positive predictive value.
Values are presented as percentages (95% confidence interval).
Abnormal is defined as follows. If the ABI is abnormal, it is abnormal. If the ABI is normal/inconclusive and the TBI is abnormal then it was coded as abnormal. All others (ABI would be normal/inconclusive) are coded normal/inconclusive.
Accuracy = (True positive + True negative)/(True positive + True negative + False positive + False negative).
χ2 test.
Value of the TBI in patients with inconclusive ABIs or normal or abnormal resting ABIs in detecting 50% or greater stenosis.
Ninety-five limbs had both TBIs and DUS, 38 had normal TBIs, and 57 had abnormal TBIs with a sensitivity of 75% and OA of 69% for patients with inconclusive ABIs only.
DISCUSSION
The ABI has been used as a screening tool in detecting PAD of lower extremity. It also has been used as a predictor for risk of foot ulcer healing, level of leg amputation, and cardiovascular disease-related events.9 Moyer10 in a recommendation statement to the US Preventive Services Task Force concluded that for asymptomatic patients who do not have a known diagnosis of PAD, severe CKD, diabetes, or CVD, the ABI was a reliable indicator of PAD of lower extremity.
The sensitivity and specificity of ABIs in detecting PAD have been reported to be 95% and 56%, respectively.11 However, among diabetics, the ABI has been shown to have limited sensitivity for detection of PAD. Potier and Halbron12 published data showing a 57% prevalence of PAD among high-risk diabetics with ABIs between 0.9 and 1.3. In the same sample, they also found a PAD prevalence of 58% among diabetics with an ABI of greater than 1.3. This false elevation of the ABI among diabetics has been attributed to subclinical medial calcification of the arteries, which causes noncompressibility, resulting in a falsely elevated Doppler systolic blood pressure.13
Stein et al4 showed that, among patients presenting with suspected PAD, 46% were found to have normal resting ABIs.4 They concluded that additional testing is needed to detect PAD in symptomatic patients presenting with normal resting ABIs. They did not, however, use DUS examination.
Patients with CKD are also at increased risk for PAD.14 These patients often have falsely elevated ABIs owing to microcalcification of arteries.15 Adragao et al16 found an association between elevation of ABIs above the normal range and increased cardiovascular mortality in patients with end-stage renal disease. Similarly, both low and high ABIs were shown to be associated with increased cardiovascular disease and all-cause mortality in a study by Criqui et al.17
Because of this artefactual elevation in resting ABIs, it is not uncommon to miss the diagnosis of PAD in patients with diabetes and CKD during ABI screening. As a result, patients with symptomatic PAD but an ABI within the normal range may be deprived of the benefits of intervention.
Recently, several authorities raised significant reservations in using ABIs only for the diagnosis of PAD. Hinchliffe et al,18 in a comprehensive study on diabetics with PAD, found low evidence to indicate that ABIs in the normal range excluded PAD. Similarly, Tehan et al19 concluded that ABIs have a poor relationship to PAD and felt it was a worthless indicator of PAD of lower extremities. Similar conclusions were made by Al-Qaisi et al20 and others,21–23 who also found that, in diabetics with calcified ankle arteries, ABIs can give artificially higher values (>1.3) and was considered to be nondiagnostic.
Similar findings were noted in a Cochran systematic review by Crawford et al11 and it was felt that the use of the TBI or some other imaging might be needed in diabetics.
Calcification in the ankle arteries may inflate lower values of ABIs and may produce an ABI that seems to be normal, when actually lower extremity circulation might be inadequate. Ix et al24 analyzed the relationship between medial arterial calcification and ABIs in 185 diabetics and concluded that although MAC is highly likely to present in people with ABIs of greater than 1.3, it is also present in patients with values of less than 1.3, which make ABIs somewhat inconclusive in these patients.24 Similar findings were noted by Brooks et al.25 These studies point to the fact that many ABI that falls within normal range may mask the presence of PAD. These misleading ABI data seem to be produced in diabetics and may also be present in nondiabetics. They felt that, because calcification is less likely to occur in digital arteries, the TBI may be capable of providing more accurate assessment.
Our present study, which is one of the largest to address these issues, analyzed 2226 ABIs in 1383 patients who had concomitant DUS. Almost one-half were diabetics and 16% had CKD, only amplifying these findings. As noted in our study, the sensitivity and OA for resting ABI in detective 50% or greater stenosis for the whole series were 57% (95% CI, 53.7–61.2) and 74% (95% CI, 71.9–76.6), respectively; for diabetics, 51% (95% CI, 46.1–56.3) and 66% (95% CI, 62.3–69.8); and for nondiabetics, 66% (95% CI, 59.9–70.9) and 81% (95% CI, 78.2–83.9) (statistically significant difference, P < .0001). These values for patients with CKD were 43% (95% CI, 34.3–52.7) and 67% (95% CI, 60.2–73.0), respectively, versus 60% (95% CI, 56.3–64.6) and 76% (95% CI, 73.1–78.1) for patients with no CKD (P = .0059).
When we excluded patients with inconclusive ABI, because some of these patients might undergo further imaging, the OA for ABIs for the whole series was 80% with a negative predictive value of 76%. However, the OA for diabetics was 75% with a negative predictive value of 63% and for CKD 78% and 70%, respectively.
Some studies suggested that postexercise ABIs may be used for detecting PAD in suspected claudicant with and without diabetes.26 These authorities found that the overall diagnostic accuracy of postexercise ABIs in detecting PAD of the lower extremities was not greatly improved compared with resting ABIs. They also felt that the overall diagnostic accuracy of ABIs in diabetics was low for both resting and postexercise ABIs and should be interpreted with caution. We did not perform postexercise ABIs in our patients because many of our patients had LTI and severe claudication and they could not exercise. Second, the protocol of performing exercise in our vascular laboratory requires the presence of a physician in the same room while the test is being done, which was not practical or available in most circumstances.
Value of the TBI.
Because some studies found that ABIs may often yield a false-negative outcome, they recommended the use of TBI as complementary or supplementing ABIs.18,19,22,23,27–29 Hoyer et al29 found that diabetes was an independent risk factor for high ABIs and that high ABIs may mask the frequency of PAD of lower extremities. It was also noted that elevated ABIs was noted in 8% of their patients; however, among those patients 62% had PAD of the lower extremities.
The American College of Cardiology/American Heart Association 2005 guidelines define a TBI of less than 0.7 as diagnostic for PAD of lower extremities. However, the overall sensitivity and specificity for this index may vary according to the reference or study group.29 It is generally recommended to use TBI in evaluating patients with falsely elevated ABIs, specifically in diabetics and patients with CKD because of underlying medial calcification. Spangeus et al30 reported on a retrospective cross-sectional study of 161 diabetics and 160 nondiabetics who had ABIs in the normal range, which was defined in their study as between 0.9 and 1.4, and found that the median ABI value was similar for both groups, except that the TBI value was slightly higher for diabetic patients. However, there was no significance in using TBI over ABI in these groups unless the ABI was elevated.
An abnormal TBI in our study had a sensitivity and OA in detecting 50% or greater stenosis of 85% (95% CI, 78.9–90.0) and 75% (95% CI, 70.1–80.2) in the whole series; however, these values were 84% (95% CI, 76.0–90.3) and 74% (95% CI, 67.1–80.2) for diabetics and 77% (95% CI, 61.4–88.2) and 72% (95% CI, 59.9–82.3) for patients with CKD; for patients with inconclusive ABIs, these values were 75% and 69%, respectively.
Our study has some limitations, including being a retrospective analysis; however, the data were collected prospectively. It also relied on the use of color DUS as the reference standard for the accuracy of ABI/TBI in detecting significant PAD and not conventional catheter directed arteriography. However, the accuracy of DUS in detecting significant PAD has been validated extensively in literature, including by our group.8,31 Also, this study relies on DUS as the gold standard. However, it is well-known that DUS might be somewhat limited in some patients with aortoiliac occlusive disease, particularly in obese patients or in the presence of excessive bowel gas. To be noted, our study excluded patients with these limitations.
Hur et al31 analyzed 324 diabetics who underwent ABI testing and DUS to determine the degree of stenosis in patients with PAD, and found that 25% of patients had an ABI of 0.91 to 1.40 were diagnosed with PAD by DUS. They also demonstrated that the prevalence rate of PAD varied according to the method used; it was present in 5.6% by ABI versus 29% by DUS. They concluded that DUS was a useful tool for detecting PAD in diabetics with an ABI of 0.91 to 1.40.
This study did not analyze the ankle waveform analysis. A significant number of our patients, particularly with LTI, had monophasic/biphasic signals because many of them had long-standing chronic ischemia with accompanied vasodilation.
CONCLUSIONS
The present study confirmed that a resting ABI can mask the presence of PAD of the lower extremities. of symptomatic patients with PAD with 50% or greater stenosis on DUS, 43% had a normal or inconclusive resting ABI, which was 49% in diabetics and 57% in patients with CKD. TBI may help in patients with an inconclusive ABI. This finding suggests that patients with symptomatic PAD should undergo further imaging to determine proper treatment.
Supplementary Material
ARTICLE HIGHLIGHTS.
Type of Research: Single-center retrospective analysis of prospectively collected data
Key Findings: The sensitivity and overall accuracy for resting ankle-brachial indices (ABIs) in detecting 50% or greater stenosis in patients with symptomatic peripheral artery disease were 57% (95% confidence interval [CI], 53.7–61.2) and 74% (95% CI, 71.9–76.6); for diabetics 51% (95% CI, 46.1–56.3) and 66% (95% CI, 62.3–69.8); for nondiabetics 66% (95% CI, 59.9–70.9) and 81% (95% CI, 78.2–83.9); for patients with CKD: 43% (95% CI, 34.3–52.7) and 67% (95% CI, 60.2–73.0) versus no CKD 60% (95% CI, 56.3–64.6) and 76% (95% CI, 73.1–78.1).
Take Home Message: Resting ABIs can mask PAD of the lower extremities in symptomatic patients. The toe-brachial index may help in patients with inconclusive ABIs and further imaging to determine treatment may be necessary.
Acknowledgments
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Footnotes
Author conflict of interest:
none.
Presented at the Fourty-seventh Annual Symposium of the Society for Clinical Vascular Surgery, Boca Raton, Fla, March 16-20, 2019.
REFERENCES
- 1.Kullo IH, Rooke TW. Peripheral arterial disease. N Engl J Med 2016;374:861–71. [DOI] [PubMed] [Google Scholar]
- 2.Quigley FG, Faris IB, Duncan HJ. A comparison of Doppler ankle pressures and skin perfusion pressure in subjects with and without diabetes. Clin Physiol 1991;11:21–5. [DOI] [PubMed] [Google Scholar]
- 3.Taguchi JT, Suwangool P. “Pipe-stem” brachial arteries: a cause of pseudohypertension. JAMA 1974;228:733. [PubMed] [Google Scholar]
- 4.Stein R, Hriljac I, Halperin JL, Gustavson SM, Teodorescu V, Olin JW. Limitation of the resting ankle-brachial index in symptomatic patients with peripheral arterial disease. Vasc Med 2006;11:29–33. [DOI] [PubMed] [Google Scholar]
- 5.Koelemay MJ, den Hartog D, Prins MH, Kromhout JG, Legemate DA, Jacobs MJ. Diagnosis of arterial disease of the lower extremities with duplex ultrasonography. Br J Surg 1996;83:404–9. [DOI] [PubMed] [Google Scholar]
- 6.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. ; American Heart Association Council on Peripheral Vascular Disease; Council on Epidemiology and Prevention; Council on Clinical Cardiology; Council on Cardiovascular Nursing; Council on Cardiovascular Radiology and Intervention; and Council on Cardiovascular Surgery and Anesthesia. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012;126:2890–909. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Peripheral Arterial Disease). J Am Coll Cardiol 2006;47:e1–192.16386656 [Google Scholar]
- 8.AbuRahma AF, Khan S, Robinson PA. Selective use of segmental Doppler pressures and color duplex imaging in the localization of arterial occlusive disease of the lower extremity. Surgery 1995;118:496–503. [DOI] [PubMed] [Google Scholar]
- 9.Trevethan R Subjecting the ankle-brachial index to timely scrutiny: is it time to say goodbye to the ABI? Scand J Clin Lab Invest 2018;78:94–101. [DOI] [PubMed] [Google Scholar]
- 10.Moyer VA. Screening for peripheral artery disease and cardiovascular disease risk assessment with the ankle-brachial index in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2013;159:342–8. [DOI] [PubMed] [Google Scholar]
- 11.Crawford F, Welch K, Andras A, Chappell FM. Ankle brachial index for the diagnosis of lower limb peripheral arterial disease. Cochrane Database Syst Rev 2016;9:CD010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Potier L, Halbron M. Ankle-to-brachial ratio index underestimates the prevalence of peripheral occlusive disease in diabetic patients at high risk for arterial disease. Diabetes Care 2009;32:e44. [DOI] [PubMed] [Google Scholar]
- 13.Emanuele MA, Buchanan BJ, Abraira C. Elevated leg systolic pressures and arterial calcification in diabetic occlusive vascular disease. Diabetes Care 1981;4:289–92. [DOI] [PubMed] [Google Scholar]
- 14.O’Hare AM, Glidden DV, Fox CS, Hsu CY. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999–2000. Circulation 2004;109:320–3. [DOI] [PubMed] [Google Scholar]
- 15.Ix JH, Katz R, De Boer IH, Kestenbaum BR, Allison MA, Siscovick DS, et al. Association of chronic kidney disease with the spectrum of ankle brachial index the CHS (Cardiovascular Health Study). J Am Coll Cardiol 2009;54:1176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adragao T, Pires A, Branco P, Castro R, Oliveira A, Nogueira C, et al. Ankle-brachial index, vascular calcifications and mortality in dialysis patients. Nephrol Dial Transplant 2012;27: 318–25.21551082 [Google Scholar]
- 17.Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, et al. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2010;56: 1506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinchliffe RJ, Brownrigg JRW, Apelqvist J, Boyko EJ, Ritridge R, Mills JL, et al. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab Res Rev 2016;32(Suppl 1):37–44. [DOI] [PubMed] [Google Scholar]
- 19.Tehan P, Bray A, Keech R, Rounsley R, Carruthers A, Chuter VH. Sensitivity and specificity of the toe-brachial index for detecting peripheral arterial disease. J Ultrasound Med 2015;34:1737–43. [DOI] [PubMed] [Google Scholar]
- 20.Al-Qaisi M, Nott DM, King DH, Kaddoura S. Ankle brachial pressure index (ABPI): an update for practitioners. Vasc Health Risk Manag 2009;5:833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, et al. Medial vascular calcification revisited: review and perspectives. Eur Heart J 2014;35:1515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suominen V, Rantanen T, Vanermo M, Saarinen J, Salenius J. Prevalence and risk factors of PAD among patients with elevated ABI. Eur J Vasc Endovasc Surg 2008;35:709–14. [DOI] [PubMed] [Google Scholar]
- 23.Bunte MC, Jacob J, Nudelman B, Shishehbor MH. Validation of the relationship between ankle-brachial and toe-brachial indices and infragenicular arterial patency in critical limb ischemia. Vasc Med 2015;20:23–9. [DOI] [PubMed] [Google Scholar]
- 24.Ix JH, Miller RG, Criqui MH, Orchard TJ. Test characteristics of the ankle-brachial index and ankle-brachial difference for medial arterial calcification on X-ray in type 1 diabetes. J Vasc Surg 2012;56:721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brooks B, Dean R, Patel S, Wu B, Molyneaus L, Yue DK. TBI or not TBI: that is the question. Is it better to measure toe pressure than ankle pressure in diabetic patients? Diabetes Med 2001;18:528–32. [DOI] [PubMed] [Google Scholar]
- 26.Tehan PE, Barwick AL, Sebastian M, Chuter VH. Diagnostic accuracy of the postexercise ankle-brachial index for detecting peripheral artery disease in suspected claudicants with and without disease. Vasc Med 2018;23:116–25. [DOI] [PubMed] [Google Scholar]
- 27.Xu D, Li J, Zou L, Xu Y, Hu D, Pagoto SL, et al. Sensitivity and specificity of the ankle-brachial index to diagnose peripheral artery disease: a structure review. Vasc Med 2010;15:361–9. [DOI] [PubMed] [Google Scholar]
- 28.Hyun S, Forbang NI, Allison MA, Denenberg JO, Criqui MH, Ix JH. Ankle-brachial index, toe-brachial index, and cardiovascular mortality in persons with and without diabetes mellitus. J Vasc Surg 2014;60:390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoyer C, Sandermann J, Petersen LJ. The toe-brachial index in the diagnosis of peripheral arterial disease. J Vasc Surg 2013;58:231–8. [DOI] [PubMed] [Google Scholar]
- 30.Spangeus A, Wijkman M, Lindstrom T, Engvall JE, Ostgren CJ, Nystrom FH, et al. Toe brachial index in middle aged patients with diabetes mellitus type 2: not just a peripheral issue. Diabetes Res Clin Pract 2013;100: 195–202. [DOI] [PubMed] [Google Scholar]
- 31.Hur KY, Jun JE, Choi YJ, Lee Y, Kim DJ, Park SW, et al. Color Doppler ultrasonography is a useful tool for diagnosis of peripheral artery disease in type 2 diabetes mellitus patients with ankle-brachial index 0.91 to 1.40. Diabetes Metab J 2018;42:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


