Abstract
Purpose
Clinical pharmacists in primary care clinics can potentially help manage chronic pain and opioid prescriptions by providing services similar to those provided within their scope of practice to patients with diabetes and hypertension. We evaluated the feasibility and acceptability of a pharmacist-physician collaborative care model for patients with chronic pain.
Methods
The program consisted of an in-person pharmacist consultation and optional follow-up visits over 4 months in 2 primary care practices. Eligible patients had chronic pain and a long-term prescription for opioids or buprenorphine or were referred by their primary care physician (PCP). Pharmacist recommendations were communicated to PCPs via the electronic medical record (EMR) and direct communication. Mixed-methods evaluation included baseline and follow-up surveys with patients, EMR review of opioid-related clinical encounters, and provider interviews.
Results
Between January and October 2018, 47 of the 182 eligible patients enrolled, with 46 completing all follow-up; 43 patients (91%) had received opioids over the past 6 months. The pharmacist recommended adding or switching to a nonopioid pain medication for 30 patients, switching to buprenorphine for pain and complex persistent opioid dependence for 20 patients, and tapering opioids for 3 patients. All physicians found the intervention acceptable but wanted more guidance on prescribing buprenorphine for pain. Most patients found the intervention helpful, but some reported a lack of physician follow-up on recommended changes.
Conclusion
The study demonstrated that comanagement of patients with chronic pain is feasible and acceptable. Policy changes to increase pharmacists’ authority to prescribe may increase physician willingness and confidence to carry out opioid tapers and prescribe buprenorphine for pain.
Keywords: chronic pain, collaborative care, opioid, pharmacist
KEY POINTS.
Clinical pharmacist comanagement of chronic conditions such as hypertension and diabetes has been shown to improve clinical outcomes and reduce costs.
In a pilot study, the comanagement model of care for treatment of chronic pain was applied in a fee-for-service setting through use of a pharmacist embedded in 2 primary care clinics.
The intervention was feasible and well accepted by both patients and physicians in a fee-for-service primary care setting.
Despite increasing awareness of the opioid epidemic, 68% of the approximately 70,000 reported drug overdose deaths in the United States in 2017 involved an opioid.1 Because many of these deaths involved prescription opioids, multiple health agencies have promoted policies to reduce opioid prescribing.2-4 These policies have contributed to a decline in overall opioid prescribing since 2012.5,6 However, the amount of opioids being prescribed today still remains greater than it was 20 years ago, and many of these prescriptions are by primary care physicians (PCPs) for patients with chronic pain.5 In addition, despite decreases in new opioid prescriptions, there remains a significant number of patients who receive long-term opioid therapy.7
As PCPs seek to restrict their opioid prescribing, they are encouraging patients to engage in opioid tapers and consider nonopioid treatment options to reduce their potential risk of harm.3,4 However, pain medication management is not always straightforward, and this issue represents an opportunity for enhanced outcomes through a more multidisciplinary approach to care for this population. Changing pharmacologic therapy can require patient buy-in, frequent appointments, close monitoring of withdrawal symptoms, coordination of behavioral health counseling, referral for treatment with other nonmedication modalities for pain, and regulatory checks for prescription misuse. To overcome these barriers, effective integrated care is necessary, and PCPs may benefit from greater involvement by pharmacists in these cases.
Pharmacist-based collaborative care models (PCCMs), wherein clinical pharmacists provide in-depth clinical support to physicians in primary care and mental health, have been shown to lead to a range of positive outcomes in other chronic conditions, such as atrial fibrillation, diabetes, hypertension, and depression.8-17 Positive outcomes include safer prescribing, reduced costs, and high patient and physician satisfaction.18-24 However, to date there are few high-quality models in fee-for-service settings outside the Veterans Health Administration (VHA) that provide multidisciplinary pharmacist support to PCPs to identify and treat patients with chronic pain who may be at high risk for comorbid opioid use disorder (OUD).25-30 Therefore, we aimed to develop and conduct a pilot trial to apply the proven model of physician-pharmacist coordinated care to a population of patients with chronic pain receiving opioid therapy.
We hypothesized that a physician-pharmacist model of care is feasible and acceptable in an ambulatory care setting and pilot tested this model in 2 primary care clinics serving diverse patient populations in an academic medical center. We measured overall feasibility and acceptability amongst primary care providers and their participating patients as well as recommendations given by the pharmacist for this population (eg, dose changes, medication switches, use of buprenorphine for pain and/or OUD).
Methods
During the period November 2017 through November 2018, we conducted a 4-month intervention at 2 participating sites. These included an internal medicine ambulatory clinic with 7 PCPs and a family practice facility with 17 PCPs. Both clinics are located in Ann Arbor, MI, and are affiliated with a large academic medical center. Physicians and staff were oriented to the study by the research team. A mixed-methods evaluation including survey data collection, chart review, and semistructured interviews was conducted to evaluate the feasibility and acceptability of the intervention. This study was reviewed and approved by the institutional review board at the University of Michigan.
Identification of participants
To identify potential participants, the study team used DataDirect (Progress Software Corporation, Bedford, MA), a self-serve tool that searches electronic medical records using the University of Michigan’s Electronic Medical Record Search Engine (EMERSE)31 to create a patient data set based on specific criteria. To be eligible for study participation, patients must have been receiving care at the intervention sites and must have had a documented chronic pain diagnosis code and at least 1 active opioid prescription. After receiving a list of patients meeting these criteria, all records were screened again by the research team to capture a cohort of patients on long-term opioid therapy (ie, patients with an opioid prescription for a more than 30-day supply, with a refill) and to remove patients receiving opioids for short durations. Prospective participants were also excluded if they received opioids related to cancer therapy or end-stage palliative care. Additionally, we accepted all patients with a direct referral from the site PCPs, irrespective of whether they were currently on opioid therapy. We allowed these direct referrals to increase provider buy-in and to also assess demand for pharmacist consultations for management of non–opioid-related therapy.
After identifying a list of eligible patients, participating PCPs were provided with a list of their eligible patients and were asked to comment on whether the patients would be suitable candidates. All candidate patients deemed appropriate by the PCP were mailed an introduction letter that included an outline of the study and the study team contact information. Patients could contact the study staff directly to schedule an appointment. However, most eligible participants were recruited in person by the research team. Research assistants identifed upcoming primary care visits and met with patients before or after their scheduled appointment to discuss the study and schedule an appointment with the pharmacist if the patient was interested. Patients who were referred by their PCPs were phoned to schedule an appointment with the pharmacist. When the participant presented for the initial pharmacist meeting, a research assistant described the study, requested written informed consent, and had the patient complete a baseline survey prior to meeting with the pharmacist. Participants were provided a $20 gift card at their initial visit and at the completion of their 4-month follow-up survey.
Pharmacist intervention
The study pharmacist was a clinical pharmacist with expertise in pain management who devoted 5 hours a week to the intervention. Patients met with the pharmacist in a private clinic office at one of the clinic sites for their initial 60-minute visit. The pharmacist used a template note to review pain history, medication history, response to prior medication trials, and risk factors for substance use disorders or other mental health conditions. The pharmacist was allowed flexibility to make recommendations to patients based upon evidence-based guidelines and not upon a specific protocol (eg, a collaborative care or stepped medication management protocol). If a medication change or referral was needed, the pharmacist discussed this recommendation with the patient prior to contacting the PCP. If follow-up was warranted or requested by the patient, the pharmacist conducted follow-up visits with the patient via phone.
The pharmacist contacted the patient’s PCP after each interaction if a recommendation was made and agreed to by the patient. In addition, the pharmacist visit was documented as an encounter in the electronic medical record. The pharmacist then offered to support the physician with patient follow-up, education, and dosing considerations and to be available based on the level of support the physician determined was needed. The pharmacist also interacted with the physicians via email, phone, and in clinic as needed.
Data collection
Data collection included a baseline and 4-month follow-up survey with all patients, review of electronic medical records, and interviews with providers.
Survey data
The patient surveys included questions from the self-reported pain scores using the PEG (Pain, Enjoyment, General Activity) Scale Assessing Pain Intensity and Interference32; the Tobacco, Alcohol, Prescription medications, and other Substance (TAPS) Tool33; the Short Form 12-Item Health Survey (SF-12)34; the Current Opioid Misuse Measure (COMM)35; and a validated instrument to measure satisfaction with their primary care providers, the Patient Satisfaction Questionnaire Short Form (PSQ-18).36 Participants were also asked about emergency room utilization and knowledge of buprenorphine and naloxone. These surveys were completed at baseline on paper or tablet computer and at 4-month follow-up by phone or an email survey link. The 4-month follow-up survey also included 2 open-ended questions requesting patients’ perspectives on how the intervention was helpful or not helpful and suggestions for improvement.
Electronic medical record data
All included patients’ medical records from the 4 months preceding the initial pharmacist contact through the 4 months following contact were reviewed. The review captured information including any changes made to prescribed pain medication regimens and/or daily oral morphine equivalents. In addition, we captured instances when a patient contacted the clinic to discuss pain and/or pain medications during this period, PCP acknowledgment of follow-up to address the pharmacist recommendations, whether urine toxicology screens were performed, and whether patients received prescriptions for naloxone. We did not capture whether prescription drug monitoring programs were checked prior to prescriptions, as that practice was mandated by the State of Michigan during the study period, forcing mandatory completion by all prescribers independent of the intervention. Data were entered into REDCap (Research Electronic Data Capture; Vanderbilt University, Nashville, TN), a HIPAA (Health Insurance Portability and Accountability Act)–compliant, Web-based data management system.
Qualitative data collection
To evaluate experiences with and perceptions of the pharmacist collaborative, we contacted all physicians and clinic staff involved with the intervention via email at both clinic sites. Eight providers responded and were interviewed (5 physicians, 2 medical assistants, and 1 pharmacist). Interviews were completed using a semistructured guide created upon constructs from the Consolidated Framework in Implementation Research (CFIR)37,38 and aimed to understand the advantages of the intervention, its sustainability, and overall acceptability. In-person interviews were conducted in a private conference room at each primary care clinic and were audio-recorded and transcribed for analysis.
Quantitative data analysis
Survey and electronic medical record data were reviewed using SAS 9.4 (SAS Institute, Cary, NC). All continuous and categorical measures were reviewed for missing data and other distributional characteristics. Four participants had 1 missing data point among the SF-12 questions; prior to scoring, each missing data point was replaced by the median value for the applicable SF-12 question in the study cohort. Scores for the SF-12 summary scales, the TAPS measure, and the PEG score were derived as specified in previous studies.38,39,31 Text responses (eg, pharmacist-to-PCP recommendations) were examined and categorized for analysis. Descriptive analysis was comprised of means with SD values or as frequencies with percentages, as appropriate. Paired t tests were used to compare within-person continuous measures at baseline and after follow-up. Categorical measures were evaluated via logistic regression. Within-person matched-pair dichotomous measures at baseline and follow-up were evaluated with McNemar’s test of agreement.
Qualitative data analysis
Qualitative data were analyzed using Dedoose (SocioCultural Research Consultants, LLC, Manhattan Beach, CA), a Web-based qualitative coding software. Our overall approach used what Miller and Crabtree referred to as the “editing analysis style,” which contains both deductive and inductive elements.39 Two research assistants independently read interview transcripts, organized responses into individual segments that express a single concept, and labeled these phrases with the appropriate codes. An iterative process was used to compare coding results until agreement on the criteria for inclusion was reached.40 The codebook included deductive codes identified prior to coding and based on the study’s goals (eg, relative advantage and disadvantage, sustainability, patient needs). Coders met regularly to discuss and resolve any discrepancies until agreement was reached. Data were then aggregated and organized by code and broader category. Through analysis of this aggregated data, a set of major emergent themes were identified.
Results
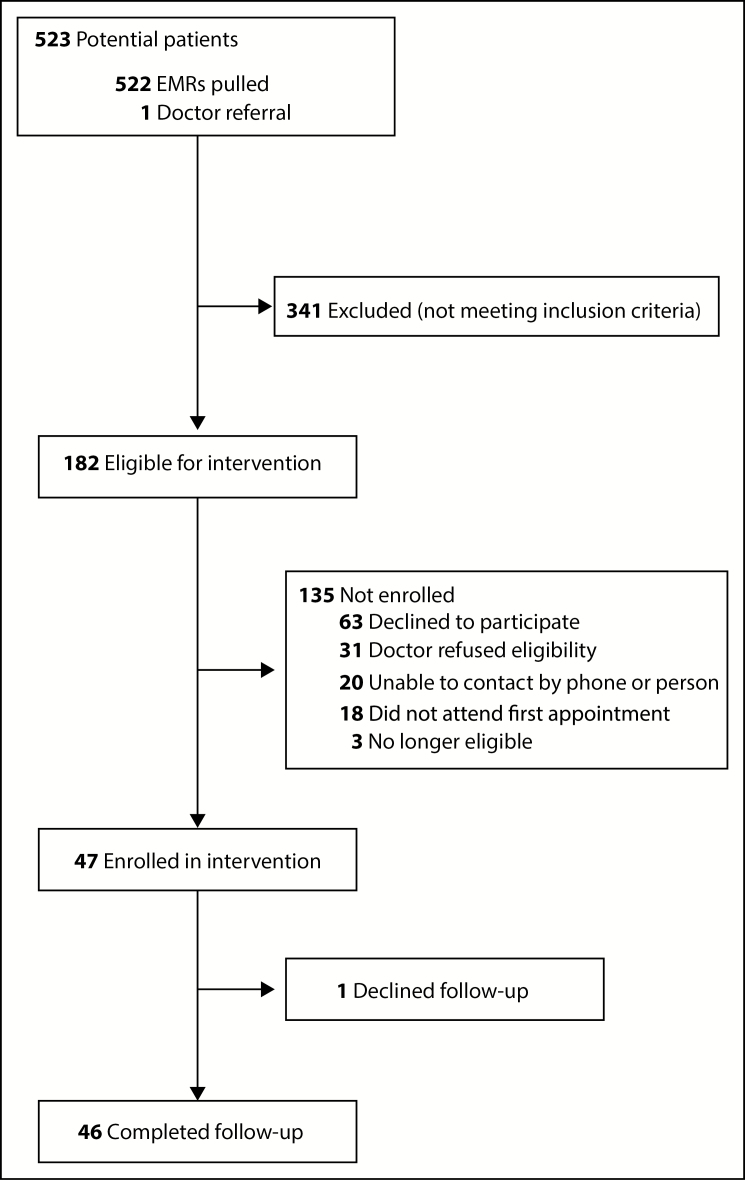
DataDirect identified 523 potential subjects, of whom 182 were eligible for the intervention. Of these eligible patients, 47 were enrolled (26% of eligible patients), with 46 patients completing all subsequent interventions over the 4-month study period (Figure 1). The remaining patients declined to participate (35%), could not be contacted by phone or in person (11%), did not attend the first appointment after initially agreeing (10%), or were no longer eligible by the time of the appointment (2%), or their PCP felt they would not be good candidates (17%). Reasons commonly cited by PCPs for feeling that a patient would not be a good candidate included situations where the physician felt that the existing physician-patient relationship was tenuous and that having another person discuss pain management could lead to patient dissatisfaction or distrust. In some cases, there was a worry that study participation would add an additional appointment burden for a patient who had difficulty attending primary care appointments or already had multiple specialists involved in his/her care. Common reasons for patients declining to participate included feeling the intervention was not needed and travel barriers to attending clinic appointments.
Figure 1.
Flowchart of patient eligibility screening and follow-up. EMR indicates electronic medical record.
Participants’ mean age was 55.8 years, and 55% were female. As defined by their PCS score, 90% of patients had moderate to severe disability. Of the cohort, 38% reported unemployment due to disability, 26% were retired, and 32% were employed at least part-time. Prior to the intervention, the mean number of oral morphine equivalents (OME) among study participants was 36, placing them at low-moderate risk of overdose (Table 1).
Table 1.
Baseline Characteristics of Study Cohort (n = 47)
| Characteristic | No. (%)a |
|---|---|
| Age, mean (SD), y | 55.8 (11.9) |
| Female | 26 (55) |
| Race | |
| Black or African American | 10 (21) |
| White | 33 (70) |
| All others | 4 (9) |
| Ethnicity | |
| Hispanic | 2 (4) |
| Non-Hispanic | 39 (83) |
| Other/unknown | 6 (13) |
| Employment status | |
| Full or part-time employment | 15 (32) |
| Unemployed and/or disabled | 20 (42) |
| Retired | 12 (26) |
| Health insurance type | |
| Medicare and/or Medicaid | 25 (53) |
| Private | 22 (47) |
| PCS category | |
| No to mild disability (40–50+) | 4 (8) |
| Moderate disability (30–39.99) | 14 (30) |
| Severe disability (<30) | 28 (60) |
| Unknown/missing data | 1 (2) |
| MCS category | |
| No to mild disability (40–50+) | 34 (72) |
| Moderate disability (30–39.99) | 9 (19) |
| Severe disability (<30) | 3 (6) |
| PEG score, mean (SD) | 6.5 (2.2) |
| Current or past use of pain therapiesb | |
| Nondrug pain therapies | 7 (15) |
| Opiates | 42 (89) |
| Gabapentinoids | 22 (47) |
| TCA and/or SNRI | 15 (32) |
| Adjuvant therapies | 27 (57) |
| Buprenorphine | 9 (19) |
| Other | 5 (11) |
| Naloxone prescribed or recommended | 7 (15) |
| Oral morphine equivalents, mean (SD) | 36.0 (6.8) |
Abbreviations: PCS, Physical Component Summary of Short Form 12-Item Health Survey (SF-12); MCS, Mental Component Summary of SF-12; PEG, Pain, Enjoyment, General Activity Scale Assessing Pain Intensity and Interference; TCA, tricyclic antidepressant; SNRI, serotonin- norepinephrine reuptake inhibitor.
aAll values are number (percentage) unless otherwise indicated.
bDuring 4 months leading up to initial pharmacist visit.
All participants had at least 1 in-person visit with the pharmacist, 8% had 1 or 2 follow-ups by phone, and another 8% had 3 to 7 follow-up phone visits. The pharmacist often made 1 or more recommendations for the patients. For 43% of patients, the pharmacist recommended switching to buprenorphine for pain; other recommendations included adding or switching to a nonopioid pain medication (64%) and adding nonpharmacological measures (17%) (Table 2). In 35 cases (74%), the PCP acknowledged the pharmacist recommendations, and in 34 of those cases, at least 1 recommendation was implemented. The recommendations most likely to be implemented were those advising the addition of nonopioid medications (45%). Of the 20 cases in which switching to buprenorphine was recommended, only 2 patients were actually transitioned during the 4 months after the intervention.
Table 2.
Pharmacists’ Patient Encounters, Recommendations, and Follow-up Actions
| No. (%) of Patients | |
|---|---|
| Patient encounters | |
| At least 1 in-person visit | 47 (100) |
| Follow-up by phone | 8 (16) |
| Recommendations to PCPs | |
| Add or change nonopioid pain medication | 30 (64) |
| Switch to buprenorphine | 20 (43) |
| Add nonpharmacological measures | 8 (17) |
| Switch to other opioid formulation | 6 (13) |
| Pharmacist agreement with or support for current plan | 4 (8) |
| Opioid taper | 3 (6) |
| Refer to specialist for pain, mental health disorders, or substance use disorder | 2 (4) |
| PCP notification and follow-up actions | |
| PCP notified of pharmacist recommendations | 46 (98) |
| PCP acknowledged recommendations | 35 (76 of 46) |
| PCP accepted/followed at least 1 part of recommendations | 34 (74 of 46) |
| PCP instituted switch to buprenorphine as recommended | 2 (10 of 20) |
Abbreviation: PCP, primary care physician.
Notably, in the 4 months after having the initial in-person visit with the pharmacist, participants initiated fewer calls and healthcare visits related to pain (Table 3). In the 4 months prior to the study, participants initiated a mean (SD) of 10.4 (8.5) encounters, which was reduced to 8.9 (7.0) encounters (P = 0.06) in the 4 months following the intervention. Participants also had a nonsignificant opioid dose reduction of 7 OME (19%), on average, without worsening pain (P = 0.23). There was also a notable change in patient-reported attitudes towards buprenorphine. Prior to the intervention, only 30% of participants believed buprenorphine was used to treat pain, and this number increased to 43% (P = 0.06) after the intervention.
Table 3.
Pre-Post Analysis of Impact of Intervention
| Variable | Baselinea | Postinterventionb | Paired t or S Statistic |
|---|---|---|---|
| Oral morphine equivalents, mean (SD) | 36.0 (6.8) | 29.1 (5.6) | t = 1.21 (P = 0.23) |
| Average PEG pain score in past week, mean (SD) | 6.2 (1.9) | 6.1 (2.1) | t = –0.21 (P = 0.84) |
| No. of patient-initiated healthcare encounters for pain, mean (SD) | 10.4 (8.5) | 8.9 (7.0) | t = 1.93 (P = 0.062) |
| Heard of buprenorphine, No. (%) | 22 (47) | 30 (65) | S = 5.33 (P = 0.021) |
| Believe buprenorphine is used for detox and/or OUD treatment, No. (%) | 17 (36) | 13 (28) | S = 6.23 (P = 0.013) |
| Believe buprenorphine is used to treat pain, No. (%) | 14 (30) | 20 (43) | S = 3.60 (P = 0.06) |
Abbreviations: OUP, opioid use disorder; PEG, Pain, Enjoyment, General Activity Scale Assessing Pain Intensity and Interference.
a4 months before initial pharmacist visit.
b4 months after initial pharmacist visit.
Four major themes emerged from the qualitative data: a present need for the intervention, that the intervention worked well for participants, suggested improvements to the process, and sustainability concerns. The intervention was well received by PCPs, who welcomed support in weaning patients with chronic pain off opioids and appreciated the focus on nonprocedural pain management offered by the pharmacist (Table 4). Because the collaborative model of care was familiar to physicians who had previously used it to help manage diabetes and hypertension, physicians thought that the pilot study’s intervention integrated well into the clinic’s existing workflow. One commented:
Table 4.
Feedback From Project Participants on Feasibility and Acceptability of Intervention
| Theme | Subtheme | Role | Selected Quote |
|---|---|---|---|
| Need for intervention present | Advantage over more procedurally based pain specialists | Physician | “I think his [the pharmacist’s] approach was . . . different than the ‘physician approach’ of a chronic pain clinic . . . they [pain clinic] are just looking for something to inject, which is a whole different approach than trying to talk to you about [options for] pain management . . . .” |
| Value of pharmacist involvement | Physician | “. . . the pharmacist is the expert in drugs and medication side effects . . . being able to explain the pain pattern and help patients respond to long-term narcotics . . . . I’m not sure that much education is given out at the other places.” | |
| Effectiveness in primary care | Physician | “I think getting out into primary care is definitely an advantage because that is where the majority of the chronic pain population on opioids resides and is prescribed.” | |
| Intervention worked well for participants | Easy to understand for physicians | Physician | “I think it worked well. I mean, I don’t think it particularly created any new work, and it is a model that we are already familiar with based on our participation with the diabetes and hypertension projects.” |
| Well perceived by patients | Patient | “I found that the pharmacists’ recommendations were the most helpful to me—informing me of medications that could be beneficial alternatives to my previous medications.” | |
| Suggested improvements to process | More communication with patients | Physician | “I think having closer follow-up . . . would have been helpful . . . it’s very common for me to tell a patient to do something and see them back in 3 months and absolutely nothing has changed . . . having someone who could do that instant follow . . . would help tremendously.” |
| Increased use of protocols, algorithms | Physician | “Can’t stress enough how we need a very simplistic, [perhaps] bulleted or numbered [set of] directions . . . it needs to be as simple as ‘[first] do this; after this much time, do this.’” | |
| Refine how patients are referred to reach difficult population | Pharmacist | “I think the next step would be either expanding it to other available clinics or building in a structure by which for certain patients . . . it would be required to see me before going to their [PCP] for a refill or for their next visit. I think that we have seen a good number of patients, but I think that there are many patients [who declined to participate in the study but] also need help—and it’s a tough population to get in.” | |
| Sustainability concerns | Access to qualified pharmacists | Physician | “. . . the pool of pharmacists out there who have much experience or expertise in chronic pain management, I suspect, is small.” |
Abbreviation: PCP, primary care physician.
“I think it worked well. I mean, I don’t think it particularly created any new work, and it is a model that we are already familiar with based on our participation with the diabetes and hypertension projects.”
Patients also reported positive feedback about the intervention in their open-ended survey responses. For example, one commented:
“I found that the pharmacists’ recommendations were the most helpful to me—informing me of medications that could be beneficial alternatives to my previous medications.”
When probed about ways to improve the intervention, physicians felt that they could have benefitted from more detailed guidance and/or protocols when managing medications that may be new and unfamiliar, such as buprenorphine. As one physician commented:
“Can’t stress enough how we need a very simplistic, [perhaps] bulleted or numbered [set of] directions . . . it needs to be as simple as ‘[First] do this; after this much time, do this.’”
The pharmacist also expressed that referrals should potentially be mandatory, as many patients who would have benefitted opted out of the intervention because participation was optional as part of the study:
“I think the next step would be either expanding it to other available clinics or building in a structure by which for certain patients . . . it would be required to see me before going to their [PCP] for a refill or for their next visit. I think that we have seen a good number of patients, but I think that there are many patients [who declined to participate in the study but] also need help—and it’s a tough population to get in.”
From a sustainability standpoint, there was concern about how we would train and/or find other clinical pharmacists with pain expertise if this intervention were to be scaled up. One physician said:
“. . . the pool of pharmacists out there who have much experience or expertise in chronic pain management, I suspect, is small.”
Discussion
The pilot study showed that collaborative care by physicians and pharmacists is a feasible and acceptable model for the management of chronic pain in an ambulatory care setting. Patients with chronic pain receiving opioid therapy can often be a difficult population to engage in an opioid taper, as there is often patient-level fear about mismanagement of pain or opioid dose reductions.41 However, within 8 months, we were able to enroll over 25% of eligible patients, showing buy-in from both patients and providers. Additionally, both groups felt satisfied with the intervention, and only 1 participant declined follow-up.
To date, there have been few studies exploring how clinical pharmacists can improve pain management within primary care settings.26,29,30 These studies took place in a VHA setting, focused on patients with chronic pain who had long-term opioid prescriptions, and reported positive outcomes for several metrics: decreased opioid prescribing without an increase in reported pain, increased urine drug toxicology testing, and high levels of satisfaction among participating PCPs. In addition, one study involved use of clinical pharmacists to help manage OUD.42 However, to our knowledge, the pilot project was the first to explore the use of pharmacists for that purpose in a fee-for-service model. While the study was grant funded, prior work by our team has identified ways to bill for pharmacist management of chronic diseases through private insurers, such as Blue Cross Blue Shield.43 Theoretically, if a pharmacist model were shown to be efficacious for chronic pain, a similar reimbursement pattern could be established for pain management.
During the study, patient attitudes towards buprenorphine changed markedly, with more patients reporting an understanding that buprenorphine could be used to treat pain; notably, pharmacist recommendations included switching to buprenorphine for pain management in 43% of cases. Multiple studies have shown that buprenorphine therapy may be an efficacious way to manage pain, especially for patients receiving high doses of opioids or with complex persistent dependence.44,45 Using buprenorphine for pain management also does not require that a physician undergo the 8-hour training needed to receive an “X-waiver,” or special license to prescribe the medication. The pharmacist in the study provided this knowledge and wrote out a clear plan for transition. However, physicians, particularly those without an X-waiver, indicated a desire for more specific instructions and increased guidance; this highlights the need for more detailed protocols in future programs seeking to change PCP buprenorphine prescribing behaviors. For other medications, such as warfarin and insulin, which also require close dose titration and monitoring, pharmacists can adjust medication dosages and prescriptions based upon collaborative care agreements.46,47 Thus far, collaborative care agreements have rarely included controlled substances, such as full opioid agonists or partial agonists like buprenorphine.42 Additionally, the State of Michigan does not allow physicians to delegate controlled substances prescribing authority to pharmacists as a part of these collaborative care agreements. These types of agreements could potentially allow for closer monitoring and medication adjustment by pharmacists that will alleviate physician burden when prescribing complex medications like buprenorphine, which requires frequent titration upon initiation.
Limitations of the pilot study included that it was designed only to assess feasibility and acceptability and, therefore, was not powered to assess efficacy in terms of outcomes such as decreased opioid prescribing. Second, our study did not include a control group, so it is difficult to ascertain to what degree the decrease in opioid prescribing was due to our intervention as opposed to ongoing opioid regulatory changes. However, we did include an electronic medical record review to make comparisons regarding healthcare utilization before and after the intervention, and there were no new regulatory changes over the period of our program. In addition, the intervention was carried out in 2 primary care clinics in a large academic medical center and may not be generalizable to other primary care settings. Lastly, the clinical pharmacist in the intervention specialized in pain medicine. He followed a structured note template to gather patient histories but was allowed flexibility in making recommendations based upon his expertise. Future efforts to implement such models will need to include ways to train general clinical pharmacists who may be less knowledgeable about medications used to treat pain and OUD.
Our pilot study was successful in implementing a model of collaborative care by pharmacists and physicians to help manage chronic pain. Larger studies will be needed to assess for efficacy, to determine if this model of care translates into fewer adverse events, and to assess financial impact. Barriers to larger-scale implementation of pharmacist-physician comanagement for the treatment of chronic pain include ensuring there are enough pharmacists with the appropriate expertise and securing funding for such programs. Future studies could focus on increasing the frequency of pharmacist follow-up and implementing more detailed protocols that could increase physician comfort with transitioning patients to buprenorphine and tapering opioids.
Conclusion
The study demonstrated that comanagement of patients with chronic pain is feasible and acceptable. Policy changes to increase pharmacists’ authority to prescribe may increase physician willingness and confidence to carry out opioid tapers and prescribe buprenorphine for pain.
Disclosures
The project was funded by an investigator-initiated research grant to Dr. Lagisetty from the Blue Cross Blue Shield of Michigan Foundation (grant number 2442.II) and by the National Institute of Diabetes and Digestive and Kidney Diseases (grant number P30DK092926); the funders played no role in the design, conduct, or analysis of the project or the decision to submit the manuscript of this article for publication. Dr Lagisetty also receives funding from the National Institute on Drug Abuse of the National Institutes of Health (under award number K23DA047475). The other authors have declared no potential conflicts of interest.

An audio interview that supplements the information in this article is available on AJHP’s website at www.ajhpvoices.org.
References
- 1. Centers for Disease Control and Prevention. Understanding the epidemic.https://www.cdc.gov/drugoverdose/epidemic/index.html. Accessed February 27, 2019.
- 2. McNairy SD, Keple R, Thuras T, Westermeyer J, et al. Benefits of combined buprenorphine medication management and support group. Am J Addict. 2011;20(4):392. [Google Scholar]
- 3. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65(1):1-49. [DOI] [PubMed] [Google Scholar]
- 4. US Department of Health and Human Services. Strategy to combat opioid abuse, misuse, and overdose.https://www.hhs.gov/opioids/sites/default/files/2018-09/opioid-fivepoint-strategy-20180917-508compliant.pdf. Accessed April 13, 2019.
- 5. Guy GP Jr, Zhang K, Schieber LZ, Young R, Dowell D. County-level opioid prescribing in the United States, 2015 and 2017. JAMA Intern Med. 2019;179(4):574-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guy GP Jr, Zhang K, Bohm MK, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schieber LZ, Guy GP Jr, Seth P, et al. Trends and Patterns of geographic variation in opioid prescribing practices by state, United States, 2006–2017. JAMA Netw Open. 2019;2(3):e190665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Al-Arifi MN, Al-Omar HA. Impact of a multidisciplinary intensive education program on type 2 diabetes mellitus patients’ glycemic control and cardiovascular risk factors. Saudi Med J. 2018;39(7):705-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38(3):309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benedict AW, Spence MM, Sie JL, et al. Evaluation of a pharmacist-managed diabetes program in a primary care setting within an integrated health care system. J Manag Care Spec Pharm. 2018;24(2):114-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brunisholz KD, Olson J, Anderson JW, et al. “ Pharming out” support: a promising approach to integrating clinical pharmacists into established primary care medical home practices. J Int Med Res. 2018;46(1):234-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gatwood JD, Chisholm-Burns M, Davis R, et al. Impact of pharmacy services on initial clinical outcomes and medication adherence among veterans with uncontrolled diabetes. BMC Health Serv Res. 2018;18(1):855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kharjul M, Braund R, Green J. The influence of pharmacist-led adherence support on glycaemic control in people with type 2 diabetes. Int J Clin Pharm. 2018;40(2):354-359. [DOI] [PubMed] [Google Scholar]
- 14. Oser CS, Fogle CC, Bennett JA. A project to promote adherence to blood pressure medication among people who use community pharmacies in rural Montana, 2014–2016. Prev Chronic Dis. 2017;14:E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Prudencio J, Cutler T, Roberts S, Marin S, Wilson M. The effect of clinical pharmacist-led comprehensive medication management on chronic disease state goal attainment in a patient-centered medical home. J Manag Care Spec Pharm. 2018;24(5):423-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sarayani A, Mashayekhi M, Nosrati M, et al. Efficacy of a telephone-based intervention among patients with type-2 diabetes; a randomized controlled trial in pharmacy practice. Int J Clin Pharm. 2018;40(2):345-353. [DOI] [PubMed] [Google Scholar]
- 17. Sitbon M, Corny J, Beaussier H, Bezie Y. The effect of partial patients’ adherence to antihypertensive drugs: scope for pharmacists’ role in hypertension care. Int J Clin Pharm. 2018;40(1):1-2. [DOI] [PubMed] [Google Scholar]
- 18. Asayut N, Sookaneknun P, Chaiyasong S, Saramunee K. Outcomes, costs and stakeholders’ perspectives associated with the incorporation of community pharmacy services into the National Health Insurance System in Thailand: a systematic review. Int J Pharm Pract. 2018;26(1):16-27. [DOI] [PubMed] [Google Scholar]
- 19. Kennelty KA, Polgreen LA, Carter BL. Team-based care with pharmacists to improve blood pressure: a review of recent literature. Curr Hypertens Rep. 2018;20(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hwang AY, Gums TH, Gums JG. The benefits of physician-pharmacist collaboration. J Fam Pract. 2017;66(12):E1-E8. [PubMed] [Google Scholar]
- 21. Nelissen HE, Cremers AL, Okwor TJ, et al. Pharmacy-based hypertension care employing mHealth in Lagos, Nigeria—a mixed methods feasibility study. BMC Health Serv Res. 2018;18(1):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ourth H, Nelson J, Spoutz P, Morreale AP. Development of a pharmacoeconomic model to demonstrate the effect of clinical pharmacist involvement in diabetes management. J Manag Care Spec Pharm. 2018;24(5):449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siaw MYL, Malone DC, Ko Y, Lee JY. Cost-effectiveness of multidisciplinary collaborative care versus usual care in the management of high-risk patients with diabetes in Singapore: short-term results from a randomized controlled trial. J Clin Pharm Ther. 2018;43(6):775-783. [DOI] [PubMed] [Google Scholar]
- 24. Siaw MYL, Toh JH, Lee JY. Patients’ perceptions of pharmacist-managed diabetes services in the ambulatory care and community settings within Singapore. Int J Clin Pharm. 2018;40(2):403-411. [DOI] [PubMed] [Google Scholar]
- 25. Krebs EE, Gravely A, Nugent S, et al. Effect of opioid vs nonopioid medications on pain-related function in patients with chronic back pain or hip or knee osteoarthritis pain: the SPACE randomized clinical trial. JAMA. 2018;319(9):872-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Westanmo A, Marshall P, Jones E, Burns K, Krebs EE. Opioid dose reduction in a VA health care system—implementation of a primary care population-level initiative. Pain Med (Malden, Mass.). 2015;16(5):1019-1026. [DOI] [PubMed] [Google Scholar]
- 27. Boscarino JA, Hoffman SN, Han JJ. Opioid-use disorder among patients on long-term opioid therapy: impact of final DSM-5 diagnostic criteria on prevalence and correlates. Subst Abuse Rehabil. 2015;6:83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Speed TJ, Parekh V, Coe W, Antoine D. Comorbid chronic pain and opioid use disorder: literature review and potential treatment innovations. Int Rev Psychiatry (Abingdon, England). 2018;30(5):136-146. [DOI] [PubMed] [Google Scholar]
- 29. Harden P, Ahmed S, Ang K, Wiedemer N. Clinical implications of tapering chronic opioids in a veteran population. Pain Med. 2015;16(10):1975-1981. [DOI] [PubMed] [Google Scholar]
- 30. Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8(7):573-584. [DOI] [PubMed] [Google Scholar]
- 31. Hanauer DA, Mei Q, Law J, Khanna R, Zheng K. Supporting information retrieval from electronic health records: a report of University of Michigan’s nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE). J Biomed Inform. 2015;55:290-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24(6):733-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gryczynski J, McNeely J, Wu LT, et al. Validation of the TAPS-1: a four-item screening tool to identify unhealthy substance use in primary Care. J Gen Intern Med. 2017;32(9):990-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Larson CO. Use of the SF-12 instrument for measuring the health of homeless persons. Health Serv Res. 2002;37(3):733-750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130(1-2):144-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marshall GN, Hays RD. The Patient Satisfaction Questionnaire Short-form (PSQ-18). Santa Monica, CA: RAND. [Google Scholar]
- 37. Keith RE, Crosson JC, O’Malley AS, Cromp D, Taylor EF. Using the Consolidated Framework for Implementation Research (CFIR) to produce actionable findings: a rapid-cycle evaluation approach to improving implementation. Implement Sci. 2017;12(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crabtree B, Miller W.. Doing Qualitative Research. Thousand Oaks, CA: Sage Publications; 1999. [Google Scholar]
- 40. Armstrong P, Cashman NR, Cook DJ, et al. A letter from CMAJ’s editorial board to the CMA. CMAJ. 2002;167(11):1230; author reply, 1230-1231. [PMC free article] [PubMed] [Google Scholar]
- 41. Penney LS, Ritenbaugh C, DeBar LL, Elder C, Deyo RA. Provider and patient perspectives on opioids and alternative treatments for managing chronic pain: a qualitative study. BMC Fam Pract. 2017;17(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. DiPaula BA, Menachery E.. Physician–pharmacist collaborative care model for buprenorphine-maintained opioid-dependent patients. J Am Pharm Assoc (2003). 2015;55(2):187-192. [DOI] [PubMed] [Google Scholar]
- 43. Choe HM, Lin AT, Kobernik K, et al. Michigan pharmacists transforming care and quality: developing a statewide collaborative of physician organizations and pharmacists to improve quality of care and reduce costs. J Manag Care Spec Pharm. 2018;24(4):373-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fishman MA, Kim PS. Buprenorphine for chronic pain: a systemic review. Curr Pain Headache Rep. 2018;22(12):83. [DOI] [PubMed] [Google Scholar]
- 45. Oldfield BJ, Edens EL, Agnoli A, et al. Multimodal treatment options, including rotating to buprenorphine, within a multidisciplinary pain clinic for patients on risky opioid regimens: a quality improvement study. Pain Med (Malden, Mass.). 2018;19(suppl 1):S38-S45. [DOI] [PubMed] [Google Scholar]
- 46. Centers for Disease Control and Prevention. Collaborative practice agreements and pharmacists’ patient care services: a resource for pharmacists.https://www.cdc.gov/dhdsp/pubs/docs/Translational_Tools_Pharmacists.pdf. Accessed May 4, 2019.
- 47. Centers for Disease Control and Prevention. Advancing team-based care through collaborative practice agreements: a resource and implementation guide for adding pharmacists to the care team.2017. https://www.cdc.gov/dhdsp/pubs/docs/CPA-Team-Based-Care.pdf. Accessed May 4, 2019.