Abstract
Aims
We assessed the performance of modelsf (risk scores) for predicting recurrence of atrial fibrillation (AF) in patients who have undergone catheter ablation.
Methods and results
Systematic searches of bibliographic databases were conducted (November 2018). Studies were eligible for inclusion if they reported the development, validation, or impact assessment of a model for predicting AF recurrence after ablation. Model performance (discrimination and calibration) measures were extracted. The Prediction Study Risk of Bias Assessment Tool (PROBAST) was used to assess risk of bias. Meta-analysis was not feasible due to clinical and methodological differences between studies, but c-statistics were presented in forest plots. Thirty-three studies developing or validating 13 models were included; eight studies compared two or more models. Common model variables were left atrial parameters, type of AF, and age. Model discriminatory ability was highly variable and no model had consistently poor or good performance. Most studies did not assess model calibration. The main risk of bias concern was the lack of internal validation which may have resulted in overly optimistic and/or biased model performance estimates. No model impact studies were identified.
Conclusion
Our systematic review suggests that clinical risk prediction of AF after ablation has potential, but there remains a need for robust evaluation of risk factors and development of risk scores.
Keywords: Atrial fibrillation, Catheter ablation, Recurrence, Prognostic model, Model performance, Systematic review
What’s new?
Several prognostic models have been developed to predict individual risk of recurrence of atrial fibrillation (AF) after catheter ablation. To the best of our knowledge this is the first comprehensive systematic review of such models to (i) include detailed risk of bias assessment of model development and validation studies and (ii) provide a descriptive summary of measures of model performance in forest plots.
Model discriminatory ability based on the c-statistic was highly variable; no model had consistently poor or good discriminatory ability. Model calibration (i.e. how well predicted risk agrees with observed risk) was rarely reported. Thus overall assessment of model performance remains incomplete.
Risks of bias were substantial and included a lack of internal validation in model development studies, flawed variable selection and weighting, low event rates and poor reporting of missing data.
Robust evaluation of risk factors and development of clinically useful risk scores is still needed.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia diagnosed in clinical practice, and worldwide incidence and prevalence is increasing.1 Atrial fibrillation is predicted to affect between 1.3 and 1.8 million patients in the UK and 18 million people in Europe by 2060.2,3 Drivers for this increase include an ageing population, better survival from conditions such as ischaemic heart disease and increasing multimorbidity.4,5 Atrial fibrillation is associated with increased morbidity and mortality, particularly cardiovascular related.4,5 Currently available treatments can reduce this, particularly via anticoagulation for stroke prevention,5 but many patients remain symptomatic even on optimal rate control therapy. Furthermore, these patients remain at high risk of cardiovascular complications, often manifesting as heart failure or sudden death.6,7 To mitigate this epidemic of AF-related disease, efforts are underway to improve primary and secondary prevention.8,9
Unfortunately, recurrent AF is common: approximately 70% of patients experience recurrence after a cardioversion.10,11 This proportion can be somewhat reduced with the use of antiarrhythmic drugs.10,11 Atrial fibrillation ablation, mainly via pulmonary vein isolation, is an effective and safe intervention to restore and maintain sinus rhythm.12,13 Recurrence of AF after catheter ablation is estimated to be between 20% and 45%.14,15 Catheter ablation seems to achieve a better quality of life than antiarrhythmic drug therapy.16,17 Furthermore, recent data suggest that AF ablation could have a positive effect on left ventricular function in patients with heart failure.18 These benefits are better sustained in patients who remain free of AF and need to be balanced against the discomfort and complication risk of AF ablation.5 Hence, there is a growing clinical need to identify patients at risk of developing recurrent AF after AF ablation.
Numerous risk factors are associated with the development of AF, including age, hypertension, diabetes mellitus, and heart failure.19,20 Less validated risk factors include subclinical hyperthyroidism, obesity, and sleep apnoea syndrome.19 Risk factors associated with recurrence are less well-established but likely include type of AF (chronic or paroxysmal) and echocardiographic parameters.21,22
Prognostic models, which combine several predictors to generate an individualized risk estimate have been developed for AF prediction in different populations. We identified two systematic reviews on prognostic models for predicting recurrent AF after ablation23,24; these reviews had limited search strategies and did not include formal risk of bias appraisal. We therefore performed a comprehensive systematic review on predicting recurrent AF in patients who underwent AF ablation.
Methods
The systematic review protocol was registered with PROSPERO (CRD42018111649). Full details of methods have been published.25
Study eligibility criteria
Study design
Published or unpublished studies reporting (i) prediction model development with internal validation, (ii) prediction model development with external validation, (iii) external model validation with or without model updating, or (iv) model impact assessment were eligible for inclusion. Studies that developed a new model with no subsequent validation were recorded but not assessed. A prognostic model was defined as a combination of two or more predictors within a statistical model used to predict an individual’s risk of the outcome.26 An impact study quantifies the impact of the model on clinical decision-making and patient outcome.
Population
Patients undergoing single or repeat ablation using any method were eligible for inclusion. There were no restrictions on previous treatments.
Outcomes
The clinical outcome of interest was recurrent AF at any time post-ablation. We excluded models that were developed for predicting a different outcome (e.g. the CHADS2 score for stroke prediction). Model performance measures of interest were calibration measures (e.g. calibration slope, calibration-in-the-large), which indicate how well the predicted risk compares to the observed risk, and discrimination measures (e.g. c-statistic), which indicate how well the model differentiates between those with and without the outcome.27 Measures that quantify the added discriminative value of one model over another, such as the net reclassification index (NRI) and/or integrated discrimination index (IDI), were also extracted.
Search strategy
Bibliographic databases (MEDLINE, MEDLINE In-Process, Embase, and Cochrane CENTRAL) were searched from inception to November 2018 using combinations of text and index terms relating to AF and models (Supplementary material online, File S1). The ‘model’ component of the search strategy was informed by a validated search filter.28 There were no date or language restrictions. Reference lists of relevant articles were checked and subject experts consulted. ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform were searched for ongoing studies and the Conference Proceedings Citation Index for conference abstracts.
Study selection
A sample of records was screened by two reviewers to pilot the screening criteria. In a change from the protocol, the remainder of the title and abstract screening was undertaken by one reviewer only (J.D., N.C., or C.H.) to process the large volume of records retrieved (n = 16 023). Records, where eligibility for inclusion was unclear, were discussed by a panel of reviewers (J.D., N.C., Y.T., and C.H.), and disagreements on study eligibility were resolved through discussion. Full texts (n = 150) were reviewed where a decision could not be made based on title and abstract.
Data extraction
Data extraction was undertaken by one reviewer (J..D.) using a pre-defined and piloted data extraction form (Excel 2016). Data items to extract were based on the CHARMS29 checklist, and included:
Participants (e.g. proportion with paroxysmal/persistent AF, ablation procedure).
Study design (e.g. prospective or retrospective cohort, sample size, length of follow-up).
Outcome measures (e.g. definition and frequency of outcome assessment).
Model development (e.g. method for selection of predictors, validation method).
Model performance (e.g. c-statistic, ratio of observed and expected events (E/O)).
Assessment of risk of bias
Risk of bias was assessed using the Prediction Study Risk Of Bias Assessment Tool (PROBAST).30 This assesses criteria within five domains: participant selection; predictors; outcomes; sample size; and patient flow and analysis (Supplementary material online, File S2). Risk of bias assessment was performed by one reviewer (J.D.) and checked by a further two (Y.T. and R.A.).
Synthesis
All studies were narratively described, with key findings tabulated and results presented with confidence intervals (CIs) when reported. Several studies reported the c-statistic. However, quantitative pooling was not possible due to differences in populations (e.g. different approaches to ablation, single vs. repeat ablation), variable electrocardiogram (ECG) monitoring intensity for recurrent AF,31 length of follow-up, possible overlap between patient cohorts, and a lack of uncertainty measures such as CIs. The c-statistics, grouped by type of model or by study, were instead presented in forest plots; this included subgroup analyses. A c-statistic of ≥0.7 was considered good and ≥0.8 very good discriminative ability; values <0.7 were considered weak, and <0.5 as very weak.32 These cut-offs are arbitrary and intended as a rough guide only. Lack of meta-analysis precluded formal exploration of publication bias using funnel plots.
The body of evidence identified was considered in the context of the Grading of Recommendations, Assessment, Development and Evaluations (GRADE)33 domains (risk of bias, imprecision, inconsistency, indirectness, and publication bias). As there is no specific guidance on how to apply GRADE to systematic reviews of prognostic models, we did not produce a GRADE summary of findings table or generate a quality score. PRISMA guidelines34 were followed for the reporting of the systematic review.
Results
Search results
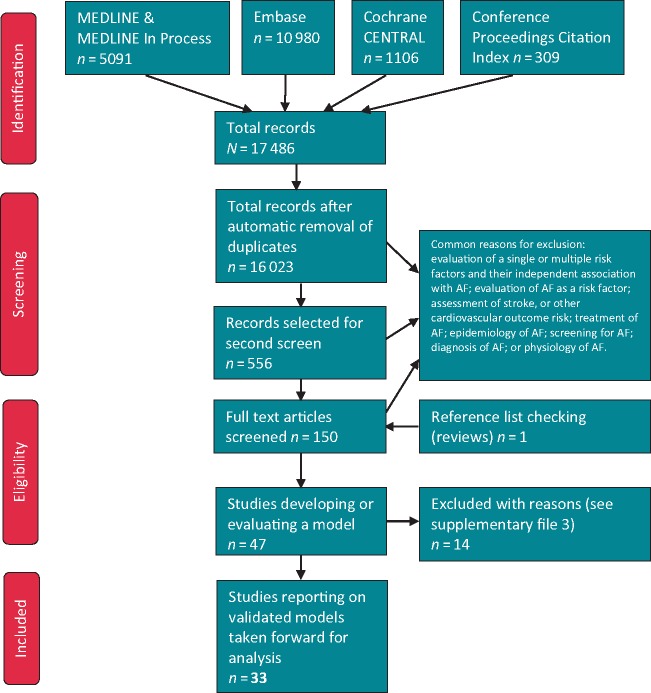
Thirty-three studies of 13 models were included (Figure 1). Six studies35–40 included two separate cohorts. Studies that developed a model, which was not validated (either in the same or another study) were documented but not analysed (Supplementary material online, File S3). One study (Kosiuk et al.41) developed and externally validated a score (DR-FLASH) primarily to predict low-voltage areas rather than AF; this study was not included but findings have been presented in Supplementary material online, File S4.
Figure 1.
PRISMA flow diagram. AF, atrial fibrillation.
Study characteristics
Twelve studies37–40,42–49 described the development (or modification) of a model, and 28 studies35–40,44,46,47,50–68 (including 31 patient cohorts) undertook external model validation. Seven37–40,44,46,47 of these studies undertook both model development and external validation. Twenty-five studies reported a single relevant model, and eight studies36,37,44,46,55,64,66,67 evaluated two or more. No model impact studies were identified. Most studies were retrospective analyses of consecutive patients; detailed study characteristics are provided in Supplementary material online, File S5.
Variables included in models
Twenty-five variables were included across 13 models (Table 1). Models included between three and six variables. The most common variables were left atrial parameters (nine models), type of AF (eight models), age (seven models), sex (four models), and estimated glomerular filtration rate (eGFR, four models).
Table 1.
Model variables
| Variables | Risk score Berkowitsch 2012 | ALARMEc | APPLE | SUCCESS | ATLAS | BASE-AF2 | CAAP-AF | HATCH | HATCH + OSA | B-HATCH | MB-LATER | FER2CI | Risk score Jarman 2012 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | √ | √ | √ | √ | √ | √ | √ | ||||||
| Sex | √ | √ | √ | √ | |||||||||
| Type of AF | √ | √ | √ | √ | √ | √ | √ | √ | |||||
| Duration of persistent AF | √ | ||||||||||||
| Previous ablations | √ | ||||||||||||
| MetS | √ | √ | |||||||||||
| eGFR | √ | √ | √ | √ | |||||||||
| Left atrial parameters | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||
| Min coupling interval of APC | √ | ||||||||||||
| Cardiomyopathy | √ | ||||||||||||
| Heart failure | √ | √ | √ | ||||||||||
| LVEF | √ | √ | |||||||||||
| BMI | √ | ||||||||||||
| Current smoking | √ | √ | |||||||||||
| AF history | √ | ||||||||||||
| Early recurrence | √ | √ | √ | ||||||||||
| CAD | √ | ||||||||||||
| Antiarrhythmics failed | √ | ||||||||||||
| Hypertension | √ | √ | √ | ||||||||||
| OSA | √ | ||||||||||||
| COPD | √ | √ | √ | ||||||||||
| Stroke or TIA | √ | √ | √ | ||||||||||
| BNP | √ | ||||||||||||
| Bundle branch block | √ | ||||||||||||
| Presence of severe comorbiditya | √ | ||||||||||||
| Point range and cut-offs;1 point for each variable unless otherwise stated | Point range: 0–4; √ non-PAF; √ ≤68 mL/min eGFR; √ NLA >11.5 | Point range: 0–5; √ non-PAF; √ ≤68 mL/min eGFR; √ NLA >11.5 or >10.25 depending on study | Point range: 0–5; √ >65 years; √ PAF; √ <60 mL/min eGFR; √ LAD ≥43 mm; √ <50% LVEF | Point range as APPLE score + additional point for each previous ablation | Possible points: 15+; 1 for age (>60); 4 for female gender; 7 for current smoker; 2 for non-PAF; 1 for each 10 mL/m2 LAV indexed for body surface area | Point range: 0–6; √ non-PAF; √ LAD >40 mm; √ >28 kg/m2 BMI; √ >6 years AF | Point range: 0–13; 1 for <50, 2 for 50 to <60, 3 for 60 to <70, and 4 for ≥70; √ female; 2 for persistent or long-standing AF; 0 for LAD <4; 1 for 4 to <4.5; 2 for 4.5 to <5; 3 for 5 to <5.5; and 4 for ≥5.5 cm; 0 for none, 1 for 1 or 2, 2 for >2 (AADs failed) | Point range: 0–7; √ >75 years; 2 for stroke and heart failure | No details on point range; √ >75 years; 2 for stroke and heart failure | Point range: 0–10; √ >75 years; 2 for stroke and heart failure; 3 points for BNP ≥100 pg/dL | Point range: 0–6; 1 for PAF and 2 for long-standing AF; √ male; √ LAD ≥47 mm | Point range: 0–4; 1 point for female; 2 for early recurrence of AF; 1 for coupling interval <49% | Point range: 0–7; √ duration of continuous AF >1 year (1 point); √ LAD 40–45 mm (1 point), 46–50 mm (2 points), >50 (3 points); √ any severe comorbiditya 3 points |
√, variable included in model; AADs, antiarrhythmic drugs; AF, atrial fibrillation; APC, atrial premature contraction; BMI, body mass index; BNP, brain natriuretic peptide; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; MetS, metabolic syndrome; NLA, normalized left atrial area; OSA, obstructive sleep apnoea; PAF, paroxysmal atrial fibrillation; TIA, transient ischaemic attack.
Severe comorbidity defined as severe mitral regurgitation, moderate mitral stenosis, mitral valvotomy, mitral valve replacement, hypertrophic cardiomyopathy, or structural congenital heart disease.
Risk of bias
Population, predictors, and outcomes
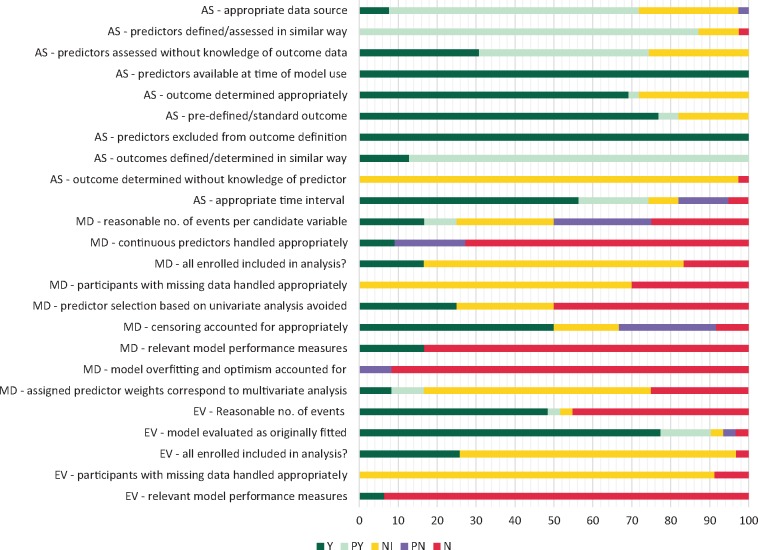
There was poor reporting of whether AF recurrence was determined without knowledge of predictor information (97% of study cohorts; Figure 2). Only one study46 specifically noted that treating physicians were not blinded to one of the variables [brain natriuretic peptide (BNP) status] which may have influenced frequency or intensity of screening. Studies did not always report how AF recurrence was assessed (28% of study cohorts), whether a standard outcome definition was used (18%) or whether predictors were assessed without knowledge of outcome information (26%). An assumption was made that single-centre studies would have a consistent approach to defining and assessing predictors, although this may not always be the case (e.g. for left atrial parameters). Studies used a combination of ECG and Holter monitoring for assessing recurrence, with around 60% of studies reporting that additional investigations were scheduled if patients reported symptoms. There was variation both within and between studies in intensity of monitoring which can influence outcome detection (e.g. monitoring between two and four times in the first year). Only one study44 reported the proportion of patients who received Holter monitoring. Three studies56,60,67 had a proportion of patients with implantable recorders and one40 a proportion of patients with pacemaker data. Follow-up time was variable (6 months to >5 years, Supplementary material online, File S5).
Figure 2.
Risk of bias summary. Chart shows percentage of study cohorts meeting/not meeting criteria: AS, all studies (39 study cohorts); EV, external validation studies (31 study cohorts); MD, model development studies (12 study cohorts); N, no; NI, no or insufficient information; PN, probably no; PY, probably yes; Y, yes. There are more evaluations than studies, as some studies included more than one cohort and/or analysis; the criterion ‘participants with missing data handled appropriately’ is only applicable where there was missing data.
Analysis—model development studies
Model development was subject to substantial risk of bias and/or poor reporting (Figure 2; Supplementary material online, File S2). Three studies (25%)40,42,47 had an adequate (>10) number of events per candidate variable, and three studies38,40,46 used appropriate methods for selecting predictors (i.e. based on multivariable modelling). One study46 stated that a variable cut-off was chosen on the basis of prior research; the remaining studies appeared to dichotomize at least one variable based on study data. Two studies47,49 (17%) appeared to appropriately assign predictor weights based on regression coefficients; the remaining studies (83%) gave no information or used an incorrect method (such as simply assigning one point per variable). Time-to-event analysis (Cox model) was appropriately used in six (50%) studies.37,40,42,43,47,48 Eleven (92%) studies did not perform internal validation and thus failed to account for model overfitting and optimism in model performance; one study47 used a split sample approach which is not thought to be an adequate method. Five studies43–46,50 (42%) modified existing scores, e.g. by adding another variable, or changing a variable cut-off, but did not consider these as new models or perform internal validation. No analyses were performed of the added value of a modified model compared with the previous one.
Analysis—model evaluation studies
Twenty-eight studies (31 cohorts) externally validated a previously developed model. Fifteen cohorts (50%) had sample sizes with event rates of 100 or over, whilst 16 had smaller sample sizes and event rates (<100). Most cohorts (90%) appeared to evaluate models using the same variable cut-offs as specified in the model development study. An exception were studies relating to ALARMEc50,51 where variable cut-offs were changed.
Analysis—all studies
For most analyses (70%), there was insufficient information on data completeness. Most studies were based on retrospective analyses and eligibility criteria sometimes related to availability of model variable data and/or a minimum follow-up time but this was not always made explicit. Around 60% of analyses presented a measure of model discrimination (c-statistic). Only two studies44,47 additionally considered model calibration. Neither discrimination nor calibration measures were reported in 30% of analyses.
Model performance
ALARMEc
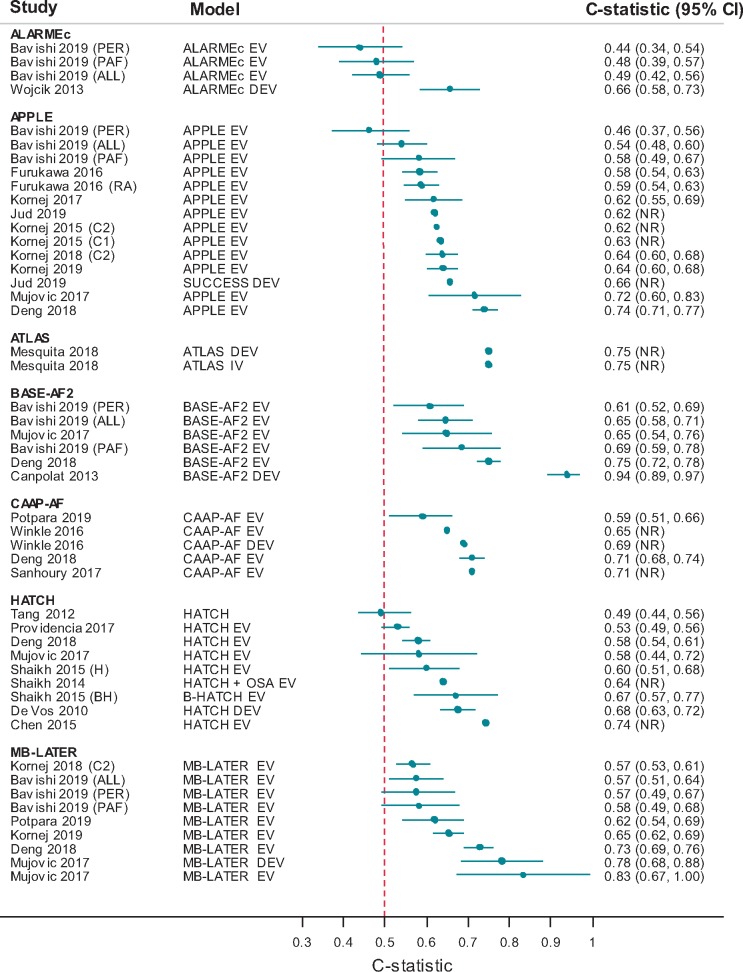
Five studies were identified. Berkowitsch et al.42 developed a risk score [variables: type of AF, metabolic syndrome, eGFR, and normalized left atrial area (NLA)], and applied this to patients undergoing first ablation (Supplementary material online, File S5). Subsequent studies added a further variable (cardiomyopathy)43 and externally validated the score43,50,51,67 in first and/or repeat ablation populations. There was inconsistency in terms of variable cut-off for NLA. Recurrence rates after a first procedure varied between 27% and 47% (Supplementary material online, File S6). Four studies found that recurrence increased with increasing risk scores. Two studies reported a c-statistic of 0.66 (95% CI 0.58–0.73)43 and 0.49 (95% CI 0.42–0.56),67 respectively (Figure 3). There was little difference in c-statistic for paroxysmal or persistent AF sub-groups.
Figure 3.
Model c-statistics (by model). ALL, all patients; BH, B-HATCH score; C1, cohort 1; C2, cohort 2; CI, confidence interval; DEV, model development; EV, external model validation; H, HATCH score; PAF, paroxysmal AF sub-group; PER, persistent AF sub-group; RA, repeat ablation sub-group.
APPLE
Ten studies35–37,44,52–55,66,67 evaluated the APPLE score [variables: age, type of AF, eGFR, left atrial diameter (LAD), and left ventricular ejection fraction] in first and/or repeat ablation populations (Supplementary material online, File S5). A model development study for this risk score was not identified. One study37 was specifically interested in very late prediction of recurrence (>12 months). One study (Jud et al.44) developed a new risk score by adding a variable (previous ablation) to the APPLE score; this new score (SUCCESS) was not internally validated and there was no attempt to quantify the added value of this score compared with APPLE.
Recurrence rates ranged from 16% to 64%. Eight studies reported c-statistics ranging from 0.46 to 0.74 (Figure 3) indicating very poor to good discriminative ability. The poorest discriminative ability was in a subgroup of patients with persistent AF.67 There was little difference in c-statistic between a repeat ablation subgroup and the total population,52 a paroxysmal AF subgroup and total population67 or between the APPLE score and the modified APPLE (SUCCESS) score.44
One study (Jud et al.44) reported a calibration measure and found no statistically significant difference between observed and expected events based on the Hosmer–Lemeshow test. This test has limited statistical power and is difficult to interpret as there is no indication of direction or magnitude of miscalibration.69 Other measures reported were proportions of recurrence for different scores and odds ratios (Supplementary material online, File S6).
ATLAS
One study (Mesquita et al.47) developed and validated this score in patients undergoing first ablation [variables: age, sex, type of AF, current smoking, and indexed left atrial volume]. The recurrence rate was 27%. The c-statistic was 0.75 in both the development and validation cohorts. The calibration-in-the-large-statistic was 0.077 (P = 0.272) and the calibration slope 0.93 indicating that observed events were only slightly higher than predicted.
BASE-AF2
This score was developed by Canpolat et al.48 and validated in a further three studies.37,66,67 Included variables were type of AF, LAD, body mass index, current smoking, AF history, and early recurrence. Two studies37,67 had mixed populations in terms of single and repeat ablation. Recurrence rates varied between 15% and 27%. Studies reported c-statistics ranging from 0.61 to 0.94 (Figure 3). Sub-group analysis in Bavishi et al.67 indicated slightly poorer discriminative ability in a persistent AF population [c-statistic 0.61 (95% CI 0.52–0.69)] compared with a paroxysmal AF population [c-statistic 0.69 (95% CI 0.59–0.78)]. A sensitivity of 80% and specificity of 91.6% (CI NR) were reported in the development study48 (threshold BASE-AF2 ≥ 3).
CAAP-AF
The score was developed and externally validated by Winkle et al.,40 and validated in a further three studies.56,64,66 Included variables were age, sex, type of AF, LAD, coronary artery disease, and number of antiarrhythmic drugs failed. Most patients were undergoing first ablation.
Recurrence rates varied between 8% and 59%. The studies reported a c-statistic between 0.59 and 0.71 suggesting weak to good discriminative ability (Figure 3). Sensitivity and specificity were reported in two studies: 64% and 68% (Sanhoury et al.56; threshold ≥ 5, CI NR) and 57.9% (95% CI 49.0–66.4) and 57% (95% CI 46.3–67.7) (Potpara et al.64; threshold ≥ 6), respectively.
HATCH
This score was developed for the prediction of progression from paroxysmal to persistent AF in patients who had not undergone ablation (de Vos et al.49). It has subsequently been applied in 12 studies to predict recurrence of AF in post-ablation cohorts. Included variables are age, heart failure, hypertension, chronic obstructive pulmonary disease, and stroke/transient ischaemic attack. Patients were undergoing first ablation in most studies; three studies37,58,60 had a proportion with repeat ablation. In two studies,61,62 ablation was performed for atrial flutter rather than AF; however, the model was applied to predict post-ablation AF. One study37 used the score to predict very late recurrence (>12 months post-ablation). Shaikh et al.45 applied a modified version of HATCH (with obstructive sleep apnoea added as variable), and Shaikh et al.46 evaluated both the HATCH score and a modified version (HATCH + BNP as added variable); neither study performed internal validation of the modified score.
Recurrence rates varied between 16% and 48%. Eight studies reported a c-statistic between 0.49 and 0.74 (Figure 3) indicating very poor to good discriminative ability. The remaining studies reported proportion of recurrence according to score and/or mean scores in those with and without recurrence (Supplementary material online, File S6). There was no clear trend towards increasing recurrence with higher scores. At a threshold ≥2, the sensitivities and specificities were 25.0% and 92.4% (Miao et al.68) and 51.8% and 84.7% (Chen et al.61), respectively.
MB-LATER
This score was developed by Mujovic et al.37 for the prediction of very late recurrence (>12 months) and validated in a very small cohort (n = 39). Another five studies55,64–67 applied the score to post-ablation cohorts, with one study65 predicting very late recurrence. Included variables are sex, type of AF, LAD, early recurrence, and bundle branch block. Three studies included a proportion of repeat ablations.37,64,67
Recurrence rates were between 15% and 64%. Five studies reported c-statistics (Figure 3) varying between 0.58 and 0.83 indicating very weak to very good discriminative ability. Little difference in c-statistic was reported between paroxysmal [0.58 (95% CI 0.49–0.68)] and persistent AF [0.58 (95% 0.49–0.67)] populations.67 Two studies reported sensitivity and specificity of 42.9% (95% CI 34.3–51.7) and 74.2% (95% CI 64.1–82.7) (Potpara et al.,64 threshold ≥ 2) and 75% and 72.6% (Mujovic et al.,37 threshold ≥ 2). No calibration measures were reported.
Other models
Two additional studies were identified that developed and externally validated a model in separate cohorts, the FER2CI score39 [variables: sex, coupling interval of atrial premature contraction, and early recurrence] and a ‘risk score’38 [variables: duration of persistent AF, eGFR, and presence of severe comorbidity]. Both studies were reported as a conference abstract only. Egami et al.39 aimed to predict very late recurrence. Jarman et al.38 included only patients with persistent AF. Recurrence rates were 21% in the development cohort in Egami et al.39 and not reported for the other cohorts. Both studies found an association between higher risk scores and recurrence but did not report model performance.
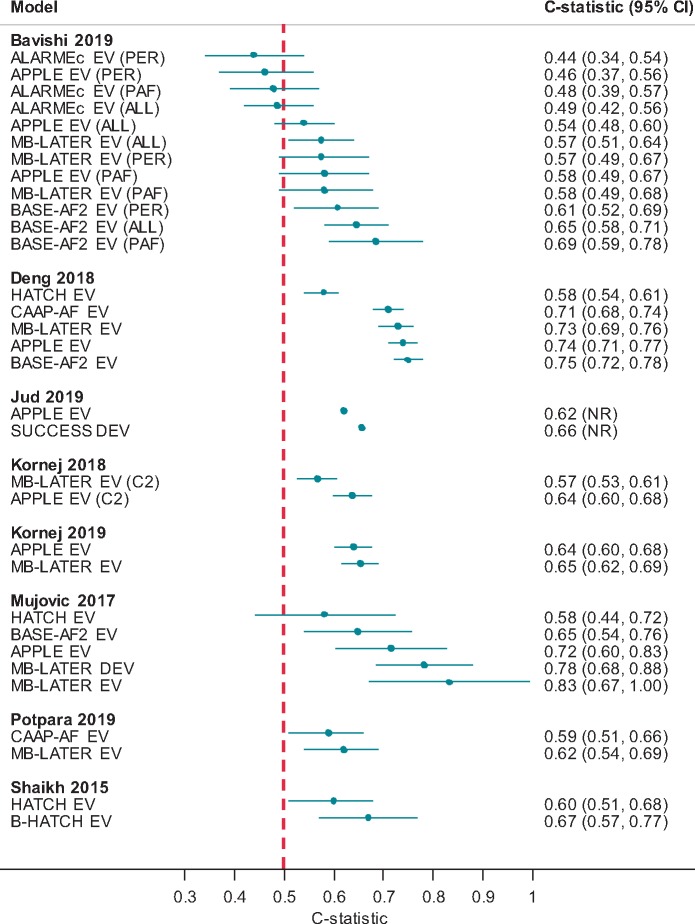
Studies comparing models
Eight studies36,37,44,46,55,64,66,67 compared two or more risk scores in the same population. There was no consistency across studies in terms of which models were compared, and no model consistently showed better discrimination based on the c-statistic (Figure 4).
Figure 4.
Model c-statistics (by study). ALL, all patients; C1, cohort 1; C2, cohort 2; CI, confidence interval; DEV, model development; EV, external model validation; PAF, paroxysmal AF sub-group; PER, persistent AF sub-group.
Four studies37,46,64,66 reported risk reclassification measures such as NRI or IDI, albeit without CIs, and/or undertook decision curve analysis (Supplementary material online, File S6). Findings suggested that (i) adding BNP as a variable (to HATCH) may improve the model,46 (ii) MB-LATER may be able to better predict recurrence compared with APPLE, ALARMEc, BASE-AF2, and HATCH,37 (iii) MB-LATER, BASE-AF2, APPLE, and CAAP-AF showed similar clinical usefulness but are more useful than HATCH,66 and (iv) MB-LATER showed greater clinical usefulness compared with CAAP-AF.64
Discussion
Main findings
This systematic review found 33 studies developing and/or validating 13 models to predict AF recurrence after ablation. Model discriminatory ability based on the c-statistic was reported for around 60% of analyses and was highly variable—from very poor to very good. No model had consistently poor or good discriminatory ability across studies. Eight studies compared two or more models in the same population, again with no model showing consistently better discrimination compared with others.
Model calibration was only reported by two studies, and assessment of overall model performance therefore remains incomplete. While our systematic review suggests that clinical risk prediction of recurrent AF after ablation has potential, there is a need for robust evaluation of risk factors and development of risk scores.
The most common model variables were left atrial parameters, type of AF and age, and to a lesser extent sex and eGFR. All model variables can be measured before ablation and therefore models could be used pre-procedurally to predict the likelihood of recurrence. The exception are those models (MB-LATER, BASE-AF2, and FER2CI) including early recurrence (within 3 months after ablation) as a variable; these scores can hence only be used to predict late recurrence. Given the inconsistent and sometimes poor performance of the models to date, it is possible that incorporating other variables may improve model performance. There may be a role for biomarkers in assessing AF risk, including serum biomarkers such as BNP70,71 or fibroblast growth factor 23,70 imaging of atrial function, ECG-based parameters, and genetic factors.19 Some as yet unvalidated models (Supplementary material online, File S3) include additional variables. A large ongoing study from South Korea (NCT02138695) plans to develop a simulation model to predict recurrence based on clinical, electrophysiological, anatomical, imaging, and serological characteristics. Clearly, these efforts would benefit from robust evaluation of clinical candidate predictors for recurrent AF after ablation.
Issues identified
A major risk of bias is that none of the development studies performed internal validation, which may result in overly optimistic and/or biased model performance estimates. This is reflected in Figure 3, which shows that c-statistics reported for development studies are often higher than those of validation studies. Overestimation of model performance is more likely to occur when the number of events per candidate predictor is low, model variables are dichotomized based on study data, variables are selected by univariate analyses and weights are incorrectly assigned to predictors. These were all commonly encountered issues. Whilst external validation studies mostly applied the models as originally developed and thus met this quality criterion, this does not mitigate the fact that models were often poorly developed in the first place. Furthermore, around half of studies undertaking external validation did not have a sufficiently large event rate to minimize bias in effect estimates. Risk of bias assessment was hampered by poor reporting, especially on completeness and handling of missing data, as well as predictor assessment. Poor reporting was not limited to conference abstracts but also seen across full-text studies; this is a recognized issue in prognostic research, despite the existence of reporting guidelines.72 For comparisons of models, we note that interpretation of both the NRI and the IDI are considered problematic in terms of magnitude and clinical applicability and thus any inferences regarding superior model performance should be regarded as uncertain.73
In addition to risk of bias, we also considered the GRADE criteria of imprecision, inconsistency, indirectness, and publication bias. There were concerns regarding indirectness as some models were not applied in the population they were developed in, or for the purpose they were developed for. So for example, HATCH was developed to predict progression to persistent AF but is commonly used to predict AF recurrence after ablation. MB-LATER was developed to predict very late recurrence (>12 months post-ablation) but has been applied in studies to predict recurrence after 3 months. In terms of precision, CIs around c-statistics were often wide, and many encompassed values that spanned weak to good model performance; seven (33%) studies reporting a c-statistic did not report a CI. Heterogeneity could not be quantified since we did not perform a meta-analysis, but inconsistency in discriminatory ability is evident within groups of studies for individual models. Variability may stem from differences in populations, ablation procedure, length of follow-up, and intensity of outcome ascertainment. Publication bias was not assessed as no meta-analysis was performed; it is however known to be an issue in prognosis research.74
Strengths of review and future directions
This systematic review used sensitive search strategies and identified more studies than reported in previous reviews. To the best of our knowledge, it is also the first systematic review in this area to conduct detailed risk of bias assessment using PROBAST. Whilst heterogeneity precluded meta-analysis, results have been presented where possible in forest plots. Screening of all references was performed by only one reviewer due to the large number of references retrieved; the potential for missed studies was mitigated by reference checking of relevant reviews and primary studies, searching in conference abstract databases and screening of a sub-set of references by more than one reviewer.
Impact studies quantify the effect of using a model on decision-making and patient outcome. No studies were identified that looked at the impact of using risk categories based on model scores to influence clinical practice. Given the performance of the models to date, an impact study would likely be premature. Equally, a focus on developing ever more models may not be helpful unless these are more rigorously developed or validated. Future research could focus on revalidating existing models using more methodologically sound approaches particularly with regard to internal validation, variable selection and weighting, assessment of model calibration, and reporting of methods used. Future model development and validation studies may also want to consider pre-specifying sub-groups, e.g. patients with persistent and paroxysmal AF, or first or repeat ablation. Prospective measurement of model variables and outcomes would ensure that patients are not selected based on availability of variable or outcome data, whilst continuous assessment of outcome using implanted devices would be more effective for detecting the outcome. It is recognized that AF is caused by different mechanisms which are currently not targeted by treatment strategies.75,76 Research is ongoing to identify clinical markers related to potential causal mechanisms and to integrate these into prediction models; this may ultimately allow development of more tailored approaches to prevention and therapy.76 Future research on model development and validation will likely need to consider differences in underlying causal mechanisms to ensure that models are an appropriate fit to different patient groups.
Conclusions
Whilst our systematic review suggests that clinical risk prediction of recurrent AF after ablation has potential, there is a need for robust evaluation of risk factors and further development of risk scores to achieve clinical utility.
Supplementary Material
Acknowledgements
The authors thank Dr Boliang Guo (University of Nottingham) for translating a Chinese paper.
Funding
This work was partially supported by European Union [grant agreement no. 633196 (CATCH ME) to P.K. and L.F.], European Union BigData@Heart (grant agreement EU IMI 116074), British Heart Foundation (FS/13/43/30324 to P.K. and L.F.; PG/17/30/32961 to P.K.; AA/18/2/34218 to P.K. and L.F.), German Centre for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK, via a grant to AFNET to P.K.), and Leducq Foundation to P.K. D.K. is funded by a UK National Institute for Health Research (NIHR) Career Development Fellowship (CDF-2015-08-074). Y.T. is funded by an NIHR Postdoctoral Fellowship (PDF-2017-10-059). J.J.D. is an NIHR Senior Investigator Emeritus. Both Y.T. and J.J.D. are supported by the NIHR Birmingham Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Conflict of interest: P.K. has received research support from several drug and device companies active in atrial fibrillation and has received honoraria from several such companies in the past. L.F. and P.K. are listed as inventors on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). L.F. has received institutional research grants from governmental and charity funding agencies (EU, DFG, MRC, and BHF). L.F. has received institutional research grants from industry in the past (Gilead). D.K. has received research support, grants and speaker fees on atrial fibrillation from the National Institute for Health Research, the British Heart Foundation, the EU Innovative Medicines Initiative, Bayer, Servier, Bioserenity, Menarini, and Atricure. All other authors have declared no conflict of interest.
References
- 1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ. et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation 2014;129:837–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lane DA, Skjoth F, Lip GYH, Larsen TB, Kotecha D.. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc 2017;6:e005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morillo CA, Banerjee A, Perel P, Wood D, Jouven X.. Atrial fibrillation: the current epidemic. J Geriatr Cardiol 2017;14:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sankaranarayanan R, Kirkwood G, Visweswariah R, Fox DJ.. How does chronic atrial fibrillation influence mortality in the modern treatment era? Curr Cardiol Rev 2015;11:190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78 [DOI] [PubMed] [Google Scholar]
- 6. Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M.. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol 2016;68:2217–28. [DOI] [PubMed] [Google Scholar]
- 7. Kotecha D, Piccini JP.. Atrial fibrillation in heart failure: what should we do? Eur Heart J 2015;36:3250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirchhof P, Breithardt G, Bax J, Benninger G, Blomstrom-Lundqvist C, Boriani G. et al. A roadmap to improve the quality of atrial fibrillation management: proceedings from the fifth Atrial Fibrillation Network/European Heart Rhythm Association consensus conference. Europace 2016;18:37–50. [DOI] [PubMed] [Google Scholar]
- 9. Kotecha D, Breithardt G, Camm AJ, Lip GYH, Schotten U, Ahlsson A. et al. Integrating new approaches to atrial fibrillation management: the 6th AFNET/EHRA Consensus Conference. Europace 2018;20:395–407. [DOI] [PubMed] [Google Scholar]
- 10. Kirchhof P, Andresen D, Bosch R, Borggrefe M, Meinertz T, Parade U. et al. Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (Flec-SL): a prospective, randomised, open-label, blinded endpoint assessment trial. Lancet 2012;380:238–46. [DOI] [PubMed] [Google Scholar]
- 11. Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL. et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med 2005;352:1861–72. [DOI] [PubMed] [Google Scholar]
- 12. Cosedis Nielsen J, Johannessen A, Raatikainen P, Hindricks G, Walfridsson H, Kongstad O. et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med 2012;367:1587–95. [DOI] [PubMed] [Google Scholar]
- 13. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE. et al. ; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Darby AE. Recurrent atrial fibrillation after catheter ablation: considerations for repeat ablation and strategies to optimize success. J Atr Fibrillation 2016;9:1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sultan A, Luker J, Andresen D, Kuck KH, Hoffmann E, Brachmann J. et al. Predictors of atrial fibrillation recurrence after catheter ablation: data from the German Ablation Registry. Sci Rep 2017;7:16678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Blomstrom-Lundqvist C, Gizurarson S, Schwieler J, Jensen SM, Bergfeldt L, Kenneback G. et al. Effect of catheter ablation vs antiarrhythmic medication on quality of life in patients with atrial fibrillation: the CAPTAF randomized clinical trial. JAMA 2019;321:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KH. et al. ; CABANA Investigators. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Willems S, Meyer C, de Bono J, Brandes A, Eckardt L, Elvan A. et al. Cabins, castles and constant hearts: rhythm control therapy in patients with atrial fibrillation. Eur Heart J 2019;40:3793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirchhof P, Lip GY, Van Gelder IC, Bax J, Hylek E, Kaab S. et al. Comprehensive risk reduction in patients with atrial fibrillation: emerging diagnostic and therapeutic options—a report from the 3rd Atrial Fibrillation Competence NETwork/European Heart Rhythm Association consensus conference. Europace 2012;14:8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brandes A, Smit MD, Nguyen BO, Rienstra M, Van Gelder IC.. Risk factor management in atrial fibrillation. Arrhythm Electrophysiol Rev 2018;7:118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balk EM, Garlitski AC, Alsheikh-Ali AA, Terasawa T, Chung M, Ip S.. Predictors of atrial fibrillation recurrence after radiofrequency catheter ablation: a systematic review. J Cardiovasc Electrophysiol 2010;21:1208–16. [DOI] [PubMed] [Google Scholar]
- 22. Lizewska-Springer A, Dabrowska-Kugacka A, Lewicka E, Drelich L, Krolak T, Raczak G.. Echocardiographic predictors of atrial fibrillation recurrence after catheter ablation: a literature review. Cardiol J 2018;doi:10.5603/CJ.a2018.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deng H, Bai Y, Shantsila A, Fauchier L, Potpara TS, Lip G.. Clinical scores for outcomes of rhythm control or arrhythmia progression in patients with atrial fibrillation: a systematic review. Clin Res Cardiol 2017;106:813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kosich F, Schumacher K, Potpara T, Lip GY, Hindricks G, Kornej J.. Clinical scores used for the prediction of negative events in patients undergoing catheter ablation for atrial fibrillation. Clin Cardiol 2019;42:320–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dretzke J, Chuchu N, Chua W, Fabritz L, Bayliss S, Kotecha D. et al. Prognostic models for predicting incident or recurrent atrial fibrillation: protocol for a systematic review. Syst Rev 2019;8:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steyerberg EW, Moons KG, van der Windt DA, Hayden JA, Perel P, Schroter S. et al. ; PROGRESS Group. Prognosis Research Strategy (PROGRESS) 3: prognostic model research. PLoS Med 2013;10:e1001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Debray TP, Damen JA, Snell KI, Ensor J, Hooft L, Reitsma JB. et al. A guide to systematic review and meta-analysis of prediction model performance. BMJ 2017;356:i6460. [DOI] [PubMed] [Google Scholar]
- 28. Geersing GJ, Bouwmeester W, Zuithoff P, Spijker R, Leeflang M, Moons KG.. Search filters for finding prognostic and diagnostic prediction studies in Medline to enhance systematic reviews. PLoS One 2012;7:e32844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moons KG, de Groot JA, Bouwmeester W, Vergouwe Y, Mallett S, Altman DG. et al. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med 2014;11:e1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, Reitsma JB. et al. ; PROBAST Group. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med 2019;170:51–8. [DOI] [PubMed] [Google Scholar]
- 31. Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HC. et al. Outcome parameters for trials in atrial fibrillation: executive summary: recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork (AFNET) and the European Heart Rhythm Association (EHRA). Eur Heart J 2007;28:2803–17. [DOI] [PubMed] [Google Scholar]
- 32. D’Agostino RB Sr, Pencina MJ, Massaro JM, Coady S.. Cardiovascular disease risk assessment: insights from Framingham. Glob Heart 2013;8:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldet G, Howick J.. Understanding GRADE: an introduction. J Evid Based Med 2013;6:50–4. [DOI] [PubMed] [Google Scholar]
- 34. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. [DOI] [PubMed] [Google Scholar]
- 35. Kornej J, Hindricks G, Shoemaker MB, Husser D, Arya A, Sommer P. et al. The APPLE score: a novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin Res Cardiol 2015;104: 871–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kornej J, Schumacher K, Dinov B, Kosich F, Sommer P, Arya A. et al. Prediction of electro-anatomical substrate and arrhythmia recurrences using APPLE, DR-FLASH and MB-LATER scores in patients with atrial fibrillation undergoing catheter ablation. Sci Rep 2018;8:12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener H-C. et al. Outcome parameters for trials in atrial fibrillation: Recommendations from a consensus conference organized by the German Atrial Fibrillation Competence NETwork and the European Heart Rhythm Association. Europace 2007;9:1006–23. [DOI] [PubMed] [Google Scholar]
- 38. Jarman JWE, Patel PJ, Lysitsas DN, Kontogeorgis A, Richardson JA, Wong T. et al. Intelligent patient selection for persistent atrial fibrillation ablation. J Interv Card Electrophysiol 2012;33:261–383. 11-2 Abstract 26–12.22430537 [Google Scholar]
- 39. Egami Y. Novel score for prediction of late recurrence in the patients without recurrence > 12 months after catheter ablation of atrial fibrillation. Circulation 2017;136(Suppl 1):A17592. [Google Scholar]
- 40. Winkle RA, Jarman JW, Mead RH, Engel G, Kong MH, Fleming W. et al. Predicting atrial fibrillation ablation outcome: the CAAP-AF score. Heart Rhythm 2016;13:2119–25. [DOI] [PubMed] [Google Scholar]
- 41. Kosiuk J, Dinov B, Kornej J, Acou WJ, Schonbauer R, Fiedler L. et al. Prospective, multicenter validation of a clinical risk score for left atrial arrhythmogenic substrate based on voltage analysis: DR-FLASH score. Heart Rhythm 2015;12:2207–12. [DOI] [PubMed] [Google Scholar]
- 42. Berkowitsch A, Kuniss M, Greiss H, Wojcik M, Zaltsberg S, Lehinant S. et al. Impact of impaired renal function and metabolic syndrome on the recurrence of atrial fibrillation after catheter ablation: a long term follow-up. Pacing Clin Electrophysiol 2012;35:532–43. [DOI] [PubMed] [Google Scholar]
- 43. Wojcik M, Berkowitsch A, Greiss H, Zaltsberg S, Pajitnev D, Deubner N. et al. Repeated catheter ablation of atrial fibrillation: how to predict outcome? Circ J 2013;77:2271–9. [DOI] [PubMed] [Google Scholar]
- 44. Jud FN, Obeid S, Duru F, Haegeli LM.. A novel score in the prediction of rhythm outcome after ablation of atrial fibrillation: the SUCCESS score. Anatol J Cardiol 2019;21:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shaikh AY, Maan A, Esa N, Kinno M, Floyd KC, Rosenthal LS. et al. Modified hatch score predicts 6-month recurrence of atrial fibrillation after pulmonary vein isolation: data from the University of Massachusetts Atrial Fibrillation Registry. Cardiology 2014;128(Suppl 1):1–527; A165. [Google Scholar]
- 46. Shaikh AY, Esa N, Martin-Doyle W, Kinno M, Nieto I, Floyd KC. et al. Addition of B-type natriuretic peptide to existing clinical risk scores enhances identification of patients at risk for atrial fibrillation recurrence after pulmonary vein isolation. Crit Path Cardiol 2015;14:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mesquita J, Ferreira AM, Cavaco D, Moscoso Costa F, Carmo P, Marques H. et al. Development and validation of a risk score for predicting atrial fibrillation recurrence after a first catheter ablation procedure—ATLAS score. Europace 2018;20:f428–35. [DOI] [PubMed] [Google Scholar]
- 48. Canpolat U, Aytemir K, Yorgun H, Şahiner L, Kaya EB, Oto A.. A proposal for a new scoring system in the prediction of catheter ablation outcomes: promising results from the Turkish Cryoablation Registry. Int J Cardiol 2013;169:201–6. [DOI] [PubMed] [Google Scholar]
- 49. de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen R-JS. et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–31. [DOI] [PubMed] [Google Scholar]
- 50. Wojcik M, Berkowitsch A, Zaltsberg S, Hamm CW, Pitschner HF, Kuniss M, Et L.. Score associated with the outcome after multiple ablation procedures in patients with atrial fibrillation. Pacing Clin Electrophysiol 2014;37:682–90. [DOI] [PubMed] [Google Scholar]
- 51. Wojcik M, Berkowitsch A, Zaltsberg S, Hamm CW, Pitschner HF, Kuniss M. et al. Cryoballoon ablation of atrial fibrillation: how important is the proper selection of patients? Cardiol J 2015;22:194–200. [DOI] [PubMed] [Google Scholar]
- 52. Furukawa Y, Yamada T, Morita T, Tamaki S, Iwasaki Y, Kawasaki M. et al. A novel and simple risk score for the prediction of recurrence of atrial fibrillation after radio frequency catheter ablation. Eur Heart J 2016;37:European Society of Cardiology P5348. [Google Scholar]
- 53. Kornej J, Hindricks G, Arya A, Sommer P, Husser D, Bollmann A.. The APPLE score—a novel score for the prediction of rhythm outcomes after repeat catheter ablation of atrial fibrillation. PLoS One 2017;12:e0169933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Miake J, Ogura K, Iitsuka K, Okamura A, Tomomori T, Tsujimoto D. et al. Pre-ablation levels of brain natriuretic peptide are independently associated with the recurrence of atrial fibrillation after radiofrequency catheter ablation in patients with nonvalvular atrial fibrillation. Heart Vessels 2019;34:517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kornej J, Schumacher K, Zeynalova S, Sommer P, Arya A, Weiß M. et al. Time-dependent prediction of arrhythmia recurrences during long-term follow-up in patients undergoing catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Sci Rep 2019;9:7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sanhoury M, Moltrasio M, Tundo F, Riva S, Dello Russo A, Casella M. et al. Predictors of arrhythmia recurrence after balloon cryoablation of atrial fibrillation: the value of CAAP-AF risk scoring system. J Interv Card Electrophysiol 2017;49:129–35. [DOI] [PubMed] [Google Scholar]
- 57. Tang R, Dong J, Liu X, Long D, Yu R, Ma C.. Can hatch score predict recurrence of atrial fibrillation after catheter ablation? Heart 2010;96:A176–7. [Google Scholar]
- 58. Tang RB, Dong JZ, Long DY, Yu RH, Ning M, Jiang CX. et al. Efficacy of catheter ablation of atrial fibrillation beyond HATCH score. Chin Med J 2012;125:3425–9. [PubMed] [Google Scholar]
- 59. Silva N, Valdigem B, Hollanda L, Luize C, Cirenza C, De Paola A. The hatch score predicts recurrence after catheter ablation of paroxysmal atrial fibrillation. J Interv Card Electrophysiol 2011;30:87–198. 11-5 Abstract 05–01. [Google Scholar]
- 60. Schmidt EU, Schneider R, Lauschke J, Wendig I, Bansch D.. The HATCH and CHA2DS2-VASc scores. Prognostic value in pulmonary vein isolation. Herz 2014;39:343–8. [DOI] [PubMed] [Google Scholar]
- 61. Chen K, Bai R, Deng W, Gao C, Zhang J, Wang X. et al. HATCH score in the prediction of new-onset atrial fibrillation after catheter ablation of typical atrial flutter. Heart Rhythm 2015;12:1483–9. [DOI] [PubMed] [Google Scholar]
- 62. Garcia-Seara J, Gude Sampedro F, M, Sande JL, Fernandez LX, Rodriguez MM, Gonzalez Melchor L. et al. Is HATCH score a reliable predictor of atrial fibrillation after cavotricuspid isthmus ablation for typical atrial flutter? Int J Cardiol Heart Vasc 2016;12:88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Providencia R, Albenque JP, Combes S, Vieira M, Khoueiry Z, Sousa P. et al. The HATCH score does not predict FREEDOM from atrial fibrillation relapse following catheter ablation: development of a novel stratifying tool. Europace 2017;19(Suppl 3):ppiii285. [Google Scholar]
- 64. Potpara TS, Mujovic N, Sivasambu B, Shantsila A, Marinkovic M, Calkins H. et al. Validation of the MB-LATER score for prediction of late recurrence after catheter-ablation of atrial fibrillation. Int J Cardiol 2019;276:130–5. [DOI] [PubMed] [Google Scholar]
- 65. Kaplan RM, Potpara TS, Vullaganti S, Liu M, Andrei AC, Bohn MM. et al. Prediction of very late recurrence of atrial fibrillation after cryoballoon ablation using the MB-later clinical score. Heart Rhythm 2018;15(5 Suppl 1):S329. [Google Scholar]
- 66. Deng H, Shantsila A, Xue Y, Potpara TS, Bai Y, Zhan X. et al. Using the MB-LATER score for predicting arrhythmia outcome after catheter ablation for atrial fibrillation: the Guangzhou atrial fibrillation project. Int J Clin Pract 2018;72:e13247. [DOI] [PubMed] [Google Scholar]
- 67. Bavishi AA, Kaplan RM, Peigh G, Diaz CL, Baman JR, Trivedi A. et al. Patient characteristics as predictors of recurrence of atrial fibrillation following cryoballoon ablation. Pacing Clin Electrophysiol 2019;42:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Miao DD, Zang XB, Zhang SL, Gao LJ, Xia YL, Yin XM. et al. Predictive value of HATCH score on atrial fibrillation recurrence post radiofrequency catheter ablation. Zhonghua Xin Xue Guan Bing Za Zhi 2012;40:821–4. [PubMed] [Google Scholar]
- 69. Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS. et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med 2019;170:W1–33. [DOI] [PubMed] [Google Scholar]
- 70. Chua W, Purmah Y, Cardoso VR, Gkoutos G, Tull SP, Neculau G. et al. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur Heart J 2019;40:1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schnabel RB, Larson MG, Yamamoto JF, Sullivan LM, Pencina MJ, Meigs JB. et al. Relations of biomarkers of distinct pathophysiological pathways and atrial fibrillation incidence in the community. Circulation 2010;121:200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW. et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1–73. [DOI] [PubMed] [Google Scholar]
- 73. Cook NR. Quantifying the added value of new biomarkers: how and how not. Diagn Progn Res 2018;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hemingway H, Riley RD, Altman DG.. Ten steps towards improving prognosis research. BMJ 2009;339:b4184. [DOI] [PubMed] [Google Scholar]
- 75.Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA et al EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace 2016;18:1455–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chua W, Easter CL, Guasch E, Sitch A, Casadei B, Crijns H. et al. Development and external validation of predictive models for prevalent and recurrent atrial fibrillation: a protocol for the analysis of the CATCH ME combined dataset. BMC Cardiovasc Disord 2019;19:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.