Abstract
Aims
Randomized trials suggest reductions in all-cause mortality and heart failure (HF) rehospitalizations with catheter ablation (CA) in patients with atrial fibrillation (AF) and HF. Whether these results can be replicated in a real-world population with long-term follow-up or varies over time is unknown. We sought to evaluate the long-term effectiveness of CA in reducing the incidence of all-cause mortality, HF hospitalizations, stroke, and major bleeding in AF–HF patients.
Methods and results
In a cohort of patients newly diagnosed with AF–HF in Quebec, Canada (2000–2017), CA patients were matched 1:2 to controls on time and frequency of hospitalizations. Confounders were controlled for using inverse probability of treatment weighting. Multivariable Cox models adjusted for the presence of cardiac electronic implantable devices and medication use during follow-up, and the effect of time since CA was modelled with B-splines. For non-fatal outcomes, the Lunn–McNeil approach was used to account for the competing risk of death. Among 101 933 AF–HF patients, 451 underwent CA and were matched to 899 controls. Over a median follow-up of 3.8 years, CA was associated with a statistically significant reduction in all-cause mortality [hazard ratio 0.4 (95% confidence interval 0.2–0.7)], but no difference in stroke or major bleeding. The hazard of HF rehospitalization for CA patients, relative to non-CA patients, varied with time since CA (P = 0.01), with a reduction in HF rehospitalizations until approximately 3 years post-CA.
Conclusion
Compared with matched non-CA patients, CA was associated with a long-term reduction in all-cause mortality and a reduction in HF rehospitalizations until 3 years post-CA.
Keywords: Catheter ablation, Atrial fibrillation, Heart failure
What’s new?
This real-world investigation of the effectiveness of catheter ablation (CA) in the atrial fibrillation (AF) population with comorbid heart failure (HF) included the largest cohort of AF-HF patients who underwent CA with the longest follow-up period.
This study complements the results of randomized trials by accounting for the competing risk of death, medication use during follow-up (antiarrhythmic drugs and anticoagulation), repeat CA, and the time-varying effect of CA.
Similar to the CASTLE-AF and AATAC trials, the present study demonstrated that CA is associated with a long-term reduction in all-cause mortality.
The protective effect of CA against HF re-hospitalizations, however, was limited to 3 years post-CA.
Introduction
Atrial fibrillation (AF) and heart failure (HF) frequently coexist1,2 and are associated with an increased risk of all-cause mortality, HF hospitalizations, and stroke.1,3,4 There is lack of consensus regarding optimal treatment for AF in patients with comorbid HF.1,3 As pharmacological rhythm control therapies have not demonstrated long-term effectiveness in this population,5–8 catheter ablation (CA) has been used to reduce AF burden and improve cardiac function.9–11
Current clinical guidelines recommend use of CA to treat AF in selected patients with HF, but specify that evidence supporting its use in this population is limited (Class IIb recommendation).12–14 Randomized trials, such as CASTLE-AF and AATAC, demonstrated that CA was associated with a reduction in HF rehospitalizations compared with medical therapy;9,10 however, only CASTLE-AF showed a statistically significant decrease in all-cause mortality.9 A recent sub-analysis from the CABANA trial suggested that among patients with HF, the risk of the combined endpoint of mortality, stroke, bleeding, and cardiac arrest may be reduced after CA compared with antiarrhythmic drugs (AADs).15 On the other hand, CA did not appear to have an effect on stroke risk.9,10 Furthermore, none of these trials assessed whether the benefit of CA may vary with time since CA.
While the results of randomized trials are encouraging, whether they are replicable in the real-world AF–HF population, persist in the long-term, or varies over time, remains to be determined. The objective of the present study was to evaluate the long-term effectiveness of CA in AF–HF patients in reducing the incidence of all-cause mortality, HF hospitalizations, and major morbidities [stroke/transient ischaemic attack (TIA) and major bleeding], in a real-life clinical context.
Methods
Data sources and population selection
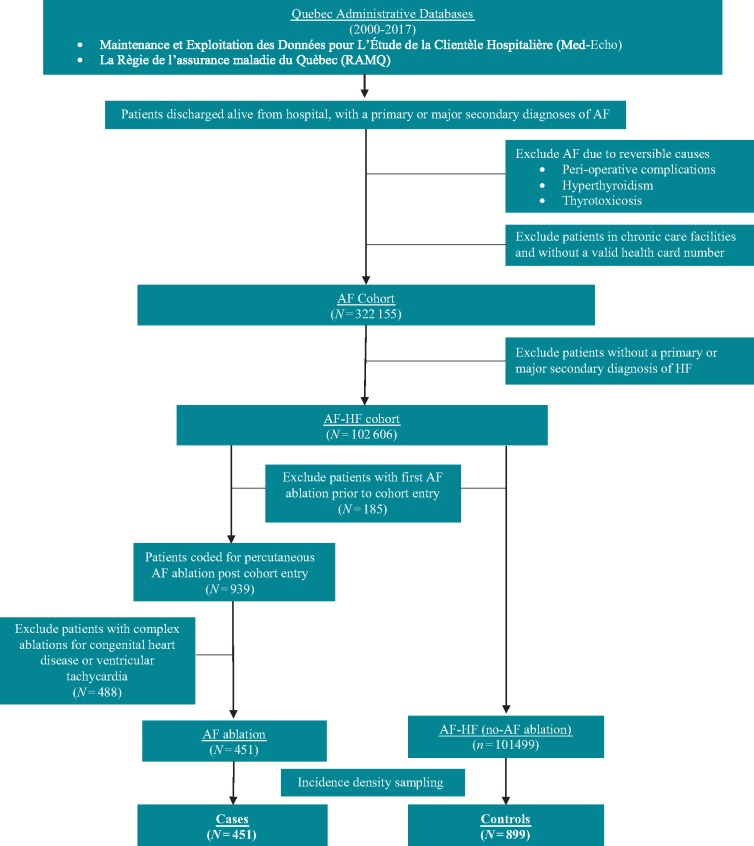
Maintenance et Exploitation des Données pour l’Étude de la Clientèle Hospitalière (Med-Echo) and la Régie de l’assurance maladie du Québec (RAMQ), hospitalization and physician claims databases from the province of Quebec, Canada, were used to create the AF–HF cohort. Methods for creation of a Quebec AF cohort (from 2000 to 2013) have been published.16–19 For the current AF–HF cohort, we extended follow-up to include patients with a primary or major secondary diagnosis of AF between 1 April 2000 and 31 March 2017. The AF–HF cohort was limited to patients with a hospitalization recorded in Med-Echo with HF listed as either primary or major secondary diagnosis at admission [International Classification of Disease-9th and 10th Revisions (ICD-9/10) codes: 428.0-4, 428.9/I50.1-4, I50.9]. Patients entered the AF–HF cohort at the date of their first HF admission. Patients with first CA prior to cohort entry were excluded. Only patients with available medication information were included (Quebec government prescription coverage includes all patients ≥65 years and all those without private prescription insurance). Cohort creation is described further in Figure 1. The study received institutional review board approval from the McGill University Faculty of Medicine (A05-M79-08B).
Figure 1.
Cohort creation flow chart. AF, atrial fibrillation; HF, heart failure.
Ascertainment of treatment with catheter ablation
Treatment and date of CA were identified by the billed procedure code for percutaneous AF ablation in RAMQ (code 291). To ensure the CA was for AF, the date of CA billed in RAMQ was matched to a hospital admission on the same date as CA (MED-Echo) (all patients undergoing CA in Quebec are admitted to a hospital). Only matched admissions with a primary or major secondary diagnosis of AF at hospital admission were included. To exclude complex ablations for congenital heart disease and ventricular tachycardia (also billed under RAMQ code 291), patients with a primary or major secondary diagnosis of ventricular tachycardia or any diagnosis of congenital heart disease at CA admission were excluded.
Incidence density sampling of matched non-catheter ablation controls
To avoid immortal time bias20,21 and ensure the comparability of the follow-up between CA and non-CA groups, incidence density sampling was used to select matched non-CA controls. Specifically, each patient who underwent CA was matched to two randomly selected patients. Eligible potential controls were selected based on (i) time at risk (since being diagnosed with both AF and HF) before date of CA, (ii) frequency of previous all-cause hospitalizations, and (iii) presence of a recent hospitalization (within 6 months prior to match date). Number of hospitalizations is an indicator for severity of disease and thus, its inclusion as a criterion for matching further created comparability between cases and controls.
The index date (beginning of follow-up) was the date of CA for CA patients and the matched date for the control group.
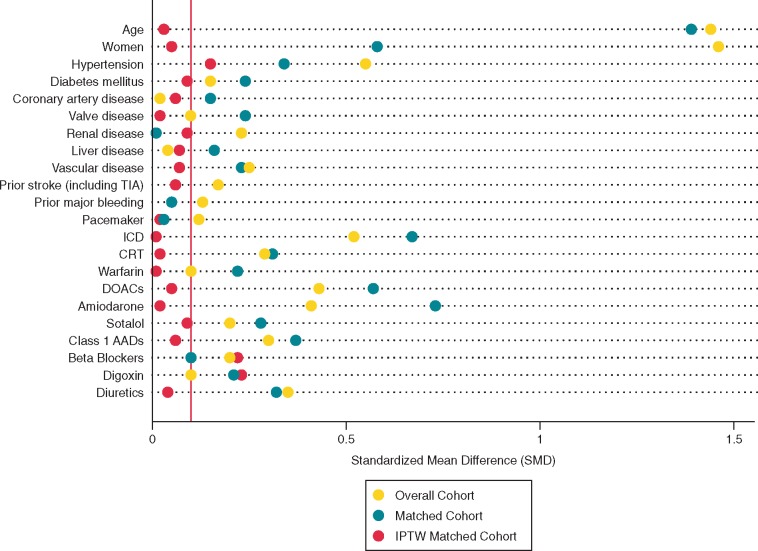
Inverse probability of treatment weighting
To control for measured confounders, inverse probability of treatment weightings (IPTWs) were calculated from the matched sample as the inverse propensity for receiving CA (logistic regression).22–24 Baseline variables incorporated into the propensity score model were predefined and listed in Figure 2.22
Figure 2.
Standardized mean differences comparing CA and non-CA patients. All SMDs <0.1 considered balanced. CA, catheter ablation; IPTW, inverse probability of treatment weighting; SMD, standardized mean difference.
Outcome ascertainment
Outcomes investigated were (i) all-cause mortality (primary endpoint), (ii) HF hospitalizations, (iii) stroke (including TIAs), and (iv) major bleeding (intracranial bleeding, or bleeding from the respiratory, gastrointestinal, or urinary tract) (ICD-9/10 codes listed in Supplementary material online, Table S1) and were analysed separately. Outcomes were identified from Med-Echo based on the primary diagnoses from hospitalizations and emergency department visits. Updated information on vital status until 31 March 2017 (end of cohort) and dates of death were obtained from both the Med-Echo and RAMQ databases.
Statistical analyses
Standardized mean differences (SMDs) were calculated to compare the balance of covariates between cases and the matched controls in the IPTW sample.25 For each covariate, an absolute SMD of <0.1 was considered as evidence of a balanced distribution.25 To ensure accurate adjustment for those variables that were not adequately balanced after IPTW, we included them as covariates in the final multivariable Cox model for the outcome.22,25
The associations of CA with the hazards of clinical endpoints were investigated using time-to-event analyses. In all analyses, Time 0 was defined as the index date, i.e. the date of the first CA for each CA case and his/her matched non-CA controls. Individual event time was defined as the time from the index date to the first event of interest and patients who had no event during the follow-up were right censored at the date of administrative end of the cohort (31 March 2017) or—for non-fatal outcomes—death of any cause, whichever came first. Crude cumulative incidence rates were calculated as the number of events per 100 person-years. In separate analyses, the associations of CA with each of the effectiveness outcomes was assessed with IPTW multivariable Cox models that additionally adjusted for the time-varying covariates, updated during the follow-up, indicating the current presence of implantable cardioverter-defibrillators (ICDs), cardiac resynchronization therapy (CRT), warfarin, direct-acting oral anticoagulants (DOACs), and AADs use during follow-up. As patients discontinued, restarted, and switched medications throughout the follow-up period, time-varying covariates accounted for current use of medications (separate time-varying covariates for warfarin, DOACs, and AADs). Current use was assumed from the time of the start of a prescription until 30 days after discontinuation. For the analyses of non-fatal outcomes, the Lunn–McNeil (cause-specific) approach was used to account for the competing risk of all-cause mortality.26 Results of the Cox and Lunn–McNeil analyses were presented in terms of adjusted hazard ratios (aHRs), with 95% confidence interval (CI).
When the proportional hazards assumption was violated (P < 0.05)27 for a given outcome we employed flexible B-spline modelling of the time-dependent effect to describe how the aHR for CA varied with increasing time since CA.28–30 The pointwise 95% CIs were calculated with 500 bootstrap resampling.30–33
R version 3.6.0 (RStudio, Inc. Boston, MA, USA) was employed for all analyses.
Sensitivity analyses
Sensitivity analyses were conducted to account for repeat CAs, shorter medication discontinuation periods, and confounding by indication.34–38
Results
The presence of both AF and HF were identified in 101 933 medication insured patients, of whom 451 (0.4%) who underwent CA were matched to 899 matched controls. Overall, the CA patients were younger and had fewer comorbidities compared with patients who had no CA (Table 1). However, after IPTW, the distributions of all potential confounders included in the propensity score model were balanced between cases and controls (age 65.5 11.0 vs. 61.6 11.6 years; 24% vs. 20% women; CHA2DS2-Vasc score 3.2 2.3 vs. 2.9 2.1; SMD < 0.1 for all covariates shown in Figure 2).
Table 1.
Baseline characteristics
| Before matching and IPTWb | After matching and IPTWb |
|||
|---|---|---|---|---|
| All AF–HF patients (N = 101 933) | Cases (N = 451) | Controls (N = 899) | Standardized mean differencec | |
| Age (years), mean (SD)a | 79.6 (9.4) | 65.6 (11.0) | 61.6 (11.6) | 0.03 |
| <65 | 6.7% | 38% | 55% | −0.52 |
| 65–75 | 21.7% | 11% | 12% | 0.01 |
| ≥75 | 71.6% | 44% | 28% | 0.35 |
| Womena | 51.4% | 24% | 22% | 0.05 |
| Hypertensiona | 31.9% | 72% | 65% | 0.15 |
| Diabetes mellitusa | 16.5% | 37% | 33% | 0.09 |
| Coronary artery diseasea | 26.8% | 55% | 52% | 0.06 |
| Prior myocardial infarction | 11.2% | 33% | 24% | 0.20 |
| Valvular diseasea | 27.3% | 25% | 26% | 0.02 |
| Valve replacement | 2.8% | 9% | 12% | 0.08 |
| Chronic obstructive pulmonary disease | 16.2% | 27% | 27% | 0.02 |
| Chronic renal failurea | 14.2% | 29% | 25% | 0.09 |
| Prior stroke (including TIA)a | 2.1% | 2% | 1% | 0.06 |
| Liver diseasea | 2.2% | 9% | 11% | 0.07 |
| Vascular diseasea | 11.8% | 19% | 17% | 0.07 |
| Prior major bleedinga | 4.1% | 11% | 7% | 0.13 |
| Pacemaker | 12.3% | 20% | 19% | 0.02 |
| Implantable cardioverter-defibrillatora | 2.5% | 29% | 29% | 0.01 |
| Cardiac resynchronization therapya | 9.4% | 34% | 33% | 0.02 |
| CHA2DS2-Vasc score, mean (SD) | 3.8 (1.3) | 3.2 (2.3) | 2.8 (2.0) | 0.04 |
| HAS-BLED score, mean (SD) | 1.5 (0.9) | 1.8 (1.4) | 1.5 (1.6) | 0.07 |
| Medications | ||||
| Oral anticoagulation | 54.5% | 90% | 93% | 0.10 |
| Warfarina | 47.6% | 65% | 66% | 0.01 |
| DOACsa | 8.4% | 38% | 40% | 0.05 |
| Dabigatran | 2.9% | 15% | 16% | 0.03 |
| Rivaroxaban | 3.0% | 18% | 18% | 0.01 |
| Apixaban | 3.0% | 12% | 8% | 0.14 |
| Amiodaronea | 10.0% | 58% | 59% | 0.02 |
| Sotalola | 3.3% | 16% | 13% | 0.09 |
| Class 1 antiarrhythmicsa | 2.4% | 19% | 20% | 0.06 |
| Digoxina | 24.7% | 28% | 39% | 0.23 |
| Beta blockersa | 49.8% | 81% | 72% | 0.22 |
| Angiotensin-converting enzyme inhibitors | 39.7% | 59% | 63% | 0.09 |
| Angiotension II receptor blockers | 17.9% | 20% | 22% | 0.05 |
| Calcium channel blockers | 17.3% | 23% | 19% | 0.10 |
| Diureticsa | 69.5% | 72% | 70% | 0.04 |
AF, atrial fibrillation; DOACs, direct oral anticoagulants; HF, heart failure; IPTW, inverse probability of treatment weighting; SD, standard deviation; TIA, transient ischaemic attack.
Predefined variables included in the propensity score model.
The prevalence of covariates for the overall cohort at measured at cohort entry and the prevalence of covariates in the matched and IPTW cohorts are measured on the date of AF ablation (cases) or matched date (controls).
Standardized mean difference <0.10 denotes balance for baseline characteristics between AF ablation and no AF ablation patients.
All-cause mortality
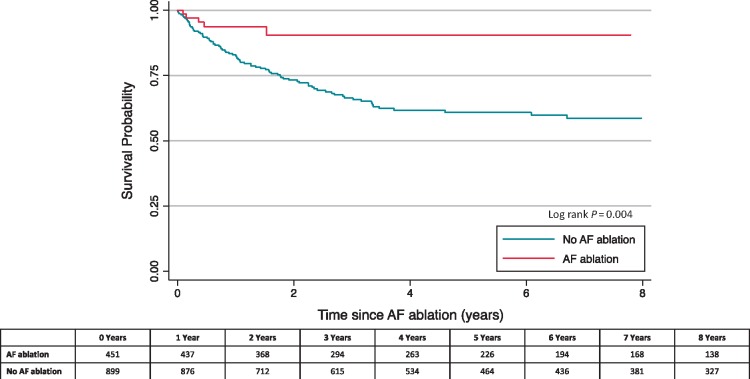
Over a median follow-up time of 3.8 years [interquartile range (IQR) 1.7–7.7], 21 (4.7%) CA patients and 171 (19.0%) non-CA patients died (Table 2). In multivariable IPTW Cox model, with adjustment for additional time-varying covariates, CA was associated with a statistically significant reduction in the hazard of all-cause mortality [aHR 0.4 (95% CI 0.2–0.7)] compared with non-CA patients (Table 2). Consistent with these results, comparison of the IPTW Kaplan–Meier curves showed the sustained reduction in mortality over the follow-up period (log-rank P-value = 0.004; Figure 3).
Table 2.
Incidence of outcomes
| N | Event rate, N (%) | Incidence rate per 100 person-years | Adjusted hazard ratio | 95% confidence interval | |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| Cases | 431 | 21 (4.6) | 1.2 | 0.4a | 0.2–0.7 |
| Controls | 899 | 171 (19.0) | 3.5 | ||
| Heart failure hospitalizations | |||||
| Cases | 431 | 70 (15.5) | 4.6 | 1.2a,b | 0.8–1.6 |
| Controls | 899 | 272 (30.3) | 6.8 | ||
| Stroke (including TIA) | |||||
| Cases | 431 | 9 (2.0) | 0.5 | 1.4a,b | 0.56–3.7 |
| Controls | 899 | 41 (4.6) | 0.9 | ||
| Major bleeding | |||||
| Cases | 431 | 10 (2.2) | 0.6 | 1.8a,b | 0.7–4.7 |
| Controls | 899 | 46 (5.1) | 1.0 | ||
ATT, average treatment effect in the treated; CRT, Cardiac resynchronization therapy; DOACs, direct oral anticoagulants; ICD, implantable cardioverter-defibrillator; IPTW, inverse probability of treatment weighting; TIA, transient ischaemic attack.
Hazard ratio adjusted baseline covariates of hypertension, prior major bleeding, and antiarrhythmic medications (amiodarone, sotalol, and Class I antiarrhythmic medications) and time-varying covariates of warfarin, DOACs, and antiarrhythmic medications as well as the presence of an ICD or CRT. Hazard ratios were IPTW with stabilized ATT weights.
Hazard ratios were also adjusted for the competing risk of all-cause mortality.
Figure 3.
Kaplan–Meier curve for all-cause mortality. Kaplan–Meier curve was IPTW and adjusted for hypertension, prior bleeding, beta blockers, and digoxin at baseline. AF, atrial fibrillation; IPTW, inverse probability of treatment weighting.
Heart failure hospitalizations
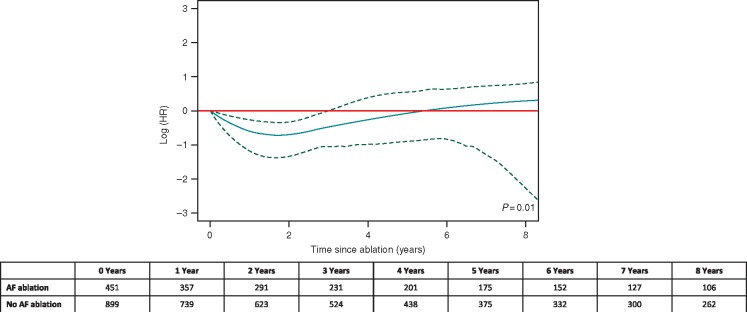
A total of 70 (15.5%) CA patients had an HF hospitalization compared with 272 (30.3%) of non-CA patients, with an incidence of 4.6 and 6.8 HF hospitalizations per 100 person-years (P = 0.002 for log-rank), respectively (Table 2). Within 30 days of CA, 3 (0.6%) of CA patients died (two patients died during CA hospitalization and one patient died post-discharge).39 In multivariable analyses, with an additional adjustment for the competing risk of all-cause mortality, there was no statistically significant difference in HF hospitalizations between CA and non-CA patients across the follow-up period [aHR 1.2 (95% CI 0.8–1.6)]. However, Figure 4 shows how the aHR associated with CA (black curve) varies with time elapsed since the date of CA (horizontal axis) and indicates a statistically significant reduction in HF hospitalizations during the first 3 years after CA, when the 95% CI (dashed curves) remain below 1.0 (P = 0.01; Figure 4). The time-dependent HR estimate (black curve in Figure 4) suggests reduced hazard of HF hospitalization starts shortly after CA, reaches about 50% risk reduction in 1–2 years after CA, but then becomes gradually weaker and disappears after approximately 5 years (Figure 4).
Figure 4.
Time-dependent effect of CA for HF hospitalizations. Adjusted for time-varying covariates and the competing risk of all-cause mortality, as well as IPTW. Time at which the upper boundary of the 95% CI (dotted line) crossed 1.0 (red line; corresponding to no effect) was used to approximate the duration of the statistically significant protective effect of CA for HF hospitalizations. CA, catheter ablation; CI, confidence interval; HF, heart failure; IPTW, inverse probability of treatment weighting.
Stroke and major bleeding
Nine (2.0%) CA patients had a stroke (including TIA) compared with 41 (4.6%) of non-CA patients. Major bleeding events occurred in 10 (2.2%) of CA patients and 46 (5.1%) of non-CA patients. Over the follow-up period, no statistically significant difference was detected for the hazards of either stroke/TIA or major bleeding in CA and non-CA patients (Table 2). The hazards of both outcomes appeared to vary over time since CA (P < 0.05), however, due to the low event rate, the time-dependent effect could not be modelled (Supplementary material online, Figures S4 and S5).
Sensitivity analyses
More than half (58.9%) of CA patients had a repeat CA within a median of 0.8 years (IQR 0.3–2.02). Results and conclusions were consistent with the main results presented above, after (i) accounting for repeat CAs and (ii) adjustment for a 14-day discontinuation period for medications (all sensitivity analyses are presented in the Supplementary material online).
Discussion
The present study provides very long-term follow-up to evaluate the effectiveness of CA to reduce all-cause mortality, HF hospitalizations, stroke/TIA, and major bleeding among patients with both AF and HF. The main findings were that (i) CA was associated with a long-term reduction in all-cause mortality and (ii) CA was protective against HF hospitalizations for only 3-year post-procedure.
All-cause mortality
The present study extends the findings of CASTLE-AF by demonstrating a statistically significant decrease in all-cause mortality with CA over the long-term.9 Furthermore, estimates and precision for the mortality reduction were also very similar between studies [HR 0.5 (95% CI 0.3–0.8) for CASTLE-AF vs. aHR 0.4 (95% CI 0.2–0.7) for the present study].9 The AATAC trial also trended towards a protective effect of CA for all-cause mortality with a comparable effect estimate [HR 0.4 (95% CI 0.2–1.0)].10 The mortality reduction may be as a result of an improvement in the complex interplay between AF and HF, measured indirectly via a reduction in AF burden, N-terminal pro b-type natriuretic peptide, and improvements in left ventricular ejection fraction (LVEF), 6-min walk distance, and quality of life measures.9,10,40–43 As described in subgroup analyses of the AFFIRM and AF-CHF trials, maintenance of sinus rhythm is associated with a lower risk of mortality5,6,44 and specifically for CA, Ullah et al.45 found that recurrent AF after CA strongly predicted mortality in AF–HF patients.
In the present study, the large sample size and the length of follow-up increased power to detect a significant difference. In CASTLE-AF, the mortality benefit of CA only emerged at 3 years of follow-up.9 In addition, randomization only balances baseline confounding, however, the present study also accounted for time-varying confounders during follow-up, including ICD, CRT, oral anticoagulant, and AAD therapies, all of which could affect the association between CA and all-cause mortality.
Heart failure hospitalizations
Both the CASTLE-AF and AATAC trials showed that HF hospitalizations reduced in CA patients compared with those on medical therapy9,10; however, the results of the present study suggest that this protective effect of CA does not persist in the long-term. The end of follow-up was 2 years for AATAC and 3 years for CASTLE,9,10 which is within the protective period of at least 3 years as identified in the present study [3.8 years (IQR 1.7–7.7)]. Perhaps with a longer follow-up, the trials would have also detected an increasing number of HF hospitalizations among CA patients. Regardless, the hazards for HF hospitalizations was similar during the protective period for the present study (lowest point, HR ∼0.5) and CASTLE-AF [HR 0.6 (95% CI 0.4–0.8)]. In addition to the time-dependent effect, our results further enhance those of randomized trials by accounting for the competing risk of death and medication, ICD, and CRT use over the follow-up which may have prevented the outcome.46
The reduction in HF hospitalizations has been attributed to decreased AF recurrence after CA in AF–HF patients. A stratified pooled analysis of randomized trial results showed that CA led to a mean relative reduction of 96% in AF/atrial tachycardia recurrence.44 Although the present study was not able to investigate AF recurrences, almost 60% of patients underwent a repeat CA which may indicate AF recurrence necessitating a repeat procedure.
Stroke/transient ischaemic attack and major bleeding
Similar to the present study, there was also a low incidence stroke/TIA in randomized trials [stratified pooled analysis of randomized trials: 2.8% vs. 4.7%; our study: 2.0% vs. 4.6%; CA vs. non-CA, respectively]45 and no statistically significant difference for stroke risk between treatment groups.9,40,43,47 This is similar to results in AF patients with and without HF, in which the reduction in stroke risk after CA was not statistically significant.15,16
The present study is the first to investigate association between CA and major bleeding in AF–HF patients and found no statistically significant difference. This is also similar to the results of studies of AF patients who underwent CA, regardless of comorbid HF.15,16 Although no statistical difference was detected between CA and non-CA patients in all studies, the estimates for both stroke and bleeding trended in opposite direction from randomized trials. Analogous to HF hospitalizations, the difference in effect may be due to randomized trials having shorter follow-up, not accounting for the competing risk of mortality, and a potential time-dependent effect.
Limitations
Important immeasurable factors that mark severity of disease including type of AF (paroxysmal, persistent, or permanent), AF burden, New York Heart Association class, and LVEF were not contained in the database. To account for severity, we adjusted for diuretic use and presence of cardiac implantable electronic devices. In addition, we conducted a confounding by indication sensitivity analysis. Also, procedural information such as technologies and ablation strategies used were not contained in Quebec administrative databases and could not be adjusted for in analyses.
Medication information is only present for a subset of the population (90% of AF–HF population and 75% of CA patients). Therefore, the results for the medication cohort may be less generalizable to the typical population referred to for the CA procedure as they are older and may have differing effects from treatments and medications.
For both stroke and bleeding events, wide CIs, due to low number of events, make the comparisons less conclusive, and the time-dependent effect of CA could not be accurately tested and modelled.
Conclusion
In a large cohort of patients with AF and HF, treatment with CA was associated with a long-term reduction in all-cause mortality. Although CA was also associated with a reduction in HF hospitalizations, the protective effect lasted for approximately 3 years after the procedure. The results of the present study suggest that CA may be particularly beneficial in the select AF–HF patients referred for CA; however, it remains to be investigated if the protective effect persists among patients with more advanced HF.
Funding
This study was supported by an operating grant from the Canadian Institutes of Health Research (CIHR), a Clinical Research Scholar Award to V.E. from Fonds de recherché du Quebec-Santé (FRQS), as well as Doctoral Training award to M.S. from FRQS. L.P. and M.A. hold a James McGill Chairs of, respectively, Medicine and Biostatistics, at McGill University.
Conflict of interest: V.E. has received honoraria from Biosense Webster Inc., St. Jude Medical, Medtronic Inc., Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, and Servier. All other authors have nothing to disclose.
Supplementary Material
References
- 1. Skanes AC, Tang A.. Atrial fibrillation and heart failure: untangling a modern Gordian knot. Can J Cardiol 2018;34:1437–48. [DOI] [PubMed] [Google Scholar]
- 2. Anter E, Jessup M, Callans DJ.. Atrial fibrillation and heart failure: treatment considerations for a dual epidemic. Circulation 2009;119:2516–25. [DOI] [PubMed] [Google Scholar]
- 3. Richter S, Di Biase L, Hindricks G.. Atrial fibrillation ablation in heart failure. Eur Heart J 2019;40:663–71. [DOI] [PubMed] [Google Scholar]
- 4. Siller-Matula JM, Pecen L, Patti G, Lucerna M, Kirchhof P, Lesiak M. et al. Heart failure subtypes and thromboembolic risk in patients with atrial fibrillation: the PREFER in AF-HF substudy. Int J Cardiol 2018;265:141–7. [DOI] [PubMed] [Google Scholar]
- 5. Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL. et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667–77. [DOI] [PubMed] [Google Scholar]
- 6. Freudenberger RS, Wilson AC, Kostis JBAFFIRM Investigators and Committees. Comparison of rate versus rhythm control for atrial fibrillation in patients with left ventricular dysfunction (from the AFFIRM study). Am J Cardiol 2007;100:247–52. [DOI] [PubMed] [Google Scholar]
- 7. Hagens VE, Crijns HJ, Van Veldhuisen DJ, Van Den Berg MP, Rienstra M, Ranchor AV. et al. Rate control versus rhythm control for patients with persistent atrial fibrillation with mild to moderate heart failure: results from the RAte Control versus Electrical cardioversion (RACE) study. Am Heart J 2005;149:1106–11. [DOI] [PubMed] [Google Scholar]
- 8. Shelton RJ, Clark AL, Goode K, Rigby AS, Houghton T, Kaye GC. et al. A randomised, controlled study of rate versus rhythm control in patients with chronic atrial fibrillation and heart failure: (CAFE-II study). Heart 2009;95:924–30. [DOI] [PubMed] [Google Scholar]
- 9. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L. et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 10. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D. et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation 2016;133:1637–44. [DOI] [PubMed] [Google Scholar]
- 11. AlTurki A, Proietti R, Dawas A, Alturki H, Huynh T, Essebag V.. Catheter ablation for atrial fibrillation in heart failure with reduced ejection fraction: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovasc Disord 2019;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andrade JG, Verma A, Mitchell LB, Parkash R, Leblanc K, Atzema C. et al. 2018 focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol 2018;34:1371–92. [DOI] [PubMed] [Google Scholar]
- 13. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr. et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2019;74:104–32. [DOI] [PubMed] [Google Scholar]
- 14. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L et al. ; Document Reviewers. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE. et al. ; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA 2019;321:1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joza J, Samuel M, Jackevicius CA, Behlouli H, Jia J, Koh M. et al. Long-term risk of stroke and bleeding post-atrial fibrillation ablation. J Cardiovasc Electrophysiol 2018;29:1355–62. [DOI] [PubMed] [Google Scholar]
- 17. Samuel M, Almohammadi M, Tsadok MA, Joza J, Jackevicius CA, Koh M. et al. Population-based evaluation of major adverse events after catheter ablation for atrial fibrillation. JACC Clin Electrophysiol 2017;3:1425–33. [DOI] [PubMed] [Google Scholar]
- 18. Samuel M, Avgil Tsadok M, Joza J, Behlouli H, Verma A, Essebag V. et al. Catheter ablation for the treatment of atrial fibrillation is associated with a reduction in health care resource utilization. J Cardiovasc Electrophysiol 2017;28:733–41. [DOI] [PubMed] [Google Scholar]
- 19. Avgil Tsadok M, Gagnon J, Joza J, Behlouli H, Verma A, Essebag V. et al. Temporal trends and sex differences in pulmonary vein isolation for patients with atrial fibrillation. Heart Rhythm 2015;12:1979–86. [DOI] [PubMed] [Google Scholar]
- 20. Renoux C, Suissa S.. Immortal time bias in the study of effectiveness of interferon-beta in multiple sclerosis. Ann Neurol 2008;64:109–10; author reply 110. [DOI] [PubMed] [Google Scholar]
- 21. Karim ME, Gustafson P, Petkau J, Tremlett H, Long-Term B; Long-Term Benefits and Adverse Effects of Beta-Interferon for Multiple Sclerosis (BeAMS) Study Group. Comparison of statistical approaches for dealing with immortal time bias in drug effectiveness studies. Am J Epidemiol 2016;184:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosenbaum PR, Rubin DB.. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41–55. [Google Scholar]
- 24. Rosenbaum PR, Rubin DB.. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc 1984;79:516–24. [Google Scholar]
- 25. Austin PC, Stuart EA.. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lunn M, McNeil D.. Applying Cox regression to competing risks. Biometrics 1995;51:524–32. [PubMed] [Google Scholar]
- 27. Therneau T, Crowson C, Atkinson E.. Using time dependent covariates and time dependent coefficients in the Cox model. Red 2013;2:1. [Google Scholar]
- 28. O’Quigley J, Pessione F.. The problem of a covariate-time qualitative interaction in a survival study. Biometrics 1991;47:101–15. [PubMed] [Google Scholar]
- 29. Quantin C, Abrahamowicz M, Moreau T, Bartlett G, MacKenzie T, Tazi MA. et al. Variation over time of the effects of prognostic factors in a population-based study of colon cancer: comparison of statistical models. Am J Epidemiol 1999;150:1188–200. [DOI] [PubMed] [Google Scholar]
- 30. Abrahamowicz M, Mackenzie T, Esdaile JM.. Time-dependent hazard ratio: modeling and hypothesis testing with application in lupus nephritis. J Am Stat Assoc 1996;91:1432–9. [Google Scholar]
- 31. Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med 2016;35:5642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wynant W, Abrahamowicz M.. Flexible estimation of survival curves conditional on non-linear and time-dependent predictor effects. Stat Med 2016;35:553–65. [DOI] [PubMed] [Google Scholar]
- 33. Esdaile JM, Abrahamowicz M, Mackenzie T, Hayslett JP, Kashgarian M.. The time-dependence of long-term prediction in lupus nephritis. Arthritis Rheum 1994;37:359–68. [DOI] [PubMed] [Google Scholar]
- 34. Abrahamowicz M, Bjerre LM, Beauchamp ME, LeLorier J, Burne R.. The missing cause approach to unmeasured confounding in pharmacoepidemiology. Stat Med 2016;35:1001–16. [DOI] [PubMed] [Google Scholar]
- 35. Lin DY, Psaty BM, Kronmal RA.. Assessing the sensitivity of regression results to unmeasured confounders in observational studies. Biometrics 1998;54:948–63. [PubMed] [Google Scholar]
- 36. Joffe MM. Confounding by indication: the case of calcium channel blockers. Pharmacoepidemiol Drug Saf 2000;9:37–41. [DOI] [PubMed] [Google Scholar]
- 37. Greenland S. Basic methods for sensitivity analysis of biases. Int J Epidemiol 1996;25:1107–16. [PubMed] [Google Scholar]
- 38. Pilote L, Abrahamowicz M, Rodrigues E, Eisenberg MJ, Rahme E.. Mortality rates in elderly patients who take different angiotensin-converting enzyme inhibitors after acute myocardial infarction: a class effect? Ann Intern Med 2004;141:102–12. [DOI] [PubMed] [Google Scholar]
- 39. Samuel M, Abrahamowicz M, Joza J, Pilote L, Essebag V.. Population-level evaluation of complications after catheter ablation in patients with atrial fibrillation and heart failure. J Cardiovasc Electrophysiol 2019;30:2678–85. [DOI] [PubMed] [Google Scholar]
- 40. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V. et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol 2014;7:31–8. [DOI] [PubMed] [Google Scholar]
- 41. Khan MN, Jais P, Cummings J, Di Biase L, Sanders P, Martin DO. et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med 2008;359:1778–85. [DOI] [PubMed] [Google Scholar]
- 42. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A. et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol 2017;70:1949–61. [DOI] [PubMed] [Google Scholar]
- 43. MacDonald MR, Connelly DT, Hawkins NM, Steedman T, Payne J, Shaw M. et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: a randomised controlled trial. Heart 2011;97:740–7. [DOI] [PubMed] [Google Scholar]
- 44. Chen S, Purerfellner H, Meyer C, Acou WJ, Schratter A, Ling Z. et al. Rhythm control for patients with atrial fibrillation complicated with heart failure in the contemporary era of catheter ablation: a stratified pooled analysis of randomized data. Eur Heart J 2019; doi:10.1093/eurheartj/ehz443. [DOI] [PubMed] [Google Scholar]
- 45. Ullah W, Ling LH, Prabhu S, Lee G, Kistler P, Finlay MC. et al. Catheter ablation of atrial fibrillation in patients with heart failure: impact of maintaining sinus rhythm on heart failure status and long-term rates of stroke and death. Europace 2016;18:679–86. [DOI] [PubMed] [Google Scholar]
- 46. Pocock SJ, Collier TJ.. Statistical appraisal of 6 recent clinical trials in cardiology: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2740–55. [DOI] [PubMed] [Google Scholar]
- 47. Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL. et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol 2013;61:1894–903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.