Abstract
Aims
Atrial electrical remodelling (AER) is a transitional period associated with the progression and long-term maintenance of atrial fibrillation (AF). We aimed to study the progression of AER in individual patients with implantable devices and AF episodes.
Methods and results
Observational multicentre study (51 centres) including 4618 patients with implantable cardioverter-defibrillator +/−resynchronization therapy (ICD/CRT-D) and 352 patients (2 centres) with pacemakers (median follow-up: 3.4 years). Atrial activation rate (AAR) was quantified as the frequency of the dominant peak in the signal spectrum of AF episodes with atrial bipolar electrograms. Patients with complete progression of AER, from paroxysmal AF episodes to electrically remodelled persistent AF, were used to depict patient-specific AER slopes. A total of 34 712 AF tracings from 830 patients (87 with pacemakers) were suitable for the study. Complete progression of AER was documented in 216 patients (16 with pacemakers). Patients with persistent AF after completion of AER showed ∼30% faster AAR than patients with paroxysmal AF. The slope of AAR changes during AF progression revealed patient-specific patterns that correlated with the time-to-completion of AER (R2 = 0.85). Pacemaker patients were older than patients with ICD/CRT-Ds (78.3 vs. 67.2 year olds, respectively, P < 0.001) and had a shorter median time-to-completion of AER (24.9 vs. 93.5 days, respectively, P = 0.016). Remote transmissions in patients with ICD/CRT-D devices enabled the estimation of the time-to-completion of AER using the predicted slope of AAR changes from initiation to completion of electrical remodelling (R2 = 0.45).
Conclusion
The AF progression shows patient-specific patterns of AER, which can be estimated using available remote-monitoring technology.
Keywords: Atrial fibrillation, Atrial fibrillation progression, Electrical remodeling, Implantable cardioverter-defibrillator, eHealth, Telemedicine, Mobile health
What’s new?
Current remote-monitoring technology in implantable devices enables the identification of patient-specific slopes of atrial electrical remodelling (AER) during atrial fibrillation (AF) progression.
Time-to-completion of AER can be estimated using the computed patient-specific slope of atrial activation rate changes and the underlying cardiac condition.
The results represent a step forward in monitoring the progression of AER, which may further assist physicians in the stratification and personalized care of patients with AF.
Introduction
Atrial fibrillation (AF) is currently considered a growing and inevitable worldwide epidemic with important economic and social burden.1,2 Atrial fibrillation diagnosis is simple when using non-invasive surface electrocardiographic (ECG) recordings,3 although can also be obtained from intracardiac atrial recordings in patients with implantable devices and atrial leads.4 Characteristically, both surface and intracardiac AF tracings will show fast atrial activation rate (AAR) and irregular activity.3,4
Atrial fibrillation is often a progressive cardiac arrhythmia from short-lasting episodes to long-standing persistent episodes,3 which increases the risk of hospitalization and adverse cardiovascular events.5 Atrial fibrillation itself induces progressive functional and structural changes in the atrial myocardium that facilitate long-term perpetuation of the arrhythmia.6 Concomitant genetic and cardiovascular conditions also establish an underlying substrate favouring AF initiation and perpetuation,2 although they do not provide information about individualized progression of AF remodelling as the arrhythmia persists.
Initially, AF provokes ion channel changes leading to shortening of the action potential duration and progressively faster AAR until reaching a complete electrically remodelled state.6,7 As AF evolves other mechanical and structural changes also progressively develop,6 which further decrease the probability of achieving a successful rhythm control strategy.8 Interestingly, in translational animal models of persistent AF, the time-course of atrial electrical remodelling (AER) has been shown to present distinct progression from animal to animal,6 whose remodelling slopes are sensitive to the effect of pharmacological treatment.7 Although monitoring AER may have diagnostic and therapeutic implications in the clinic, it has not been demonstrated that AER also follows patient-specific patterns.
We tested the hypothesis that AAR changes during AF progression show patient-specific patterns that can be estimated using clinical variables and remote-monitoring tracings from implantable devices. We aimed to study AARs and the time-course of AER progression using bipolar AF recordings obtained from two populations of patients with implantable cardioverter-defibrillator+/−resynchronization therapy (ICD/CRT-D) and pacemaker devices. We also aimed to study the effect of the underlying clinical conditions on the AER process of AF.
Methods
Study design
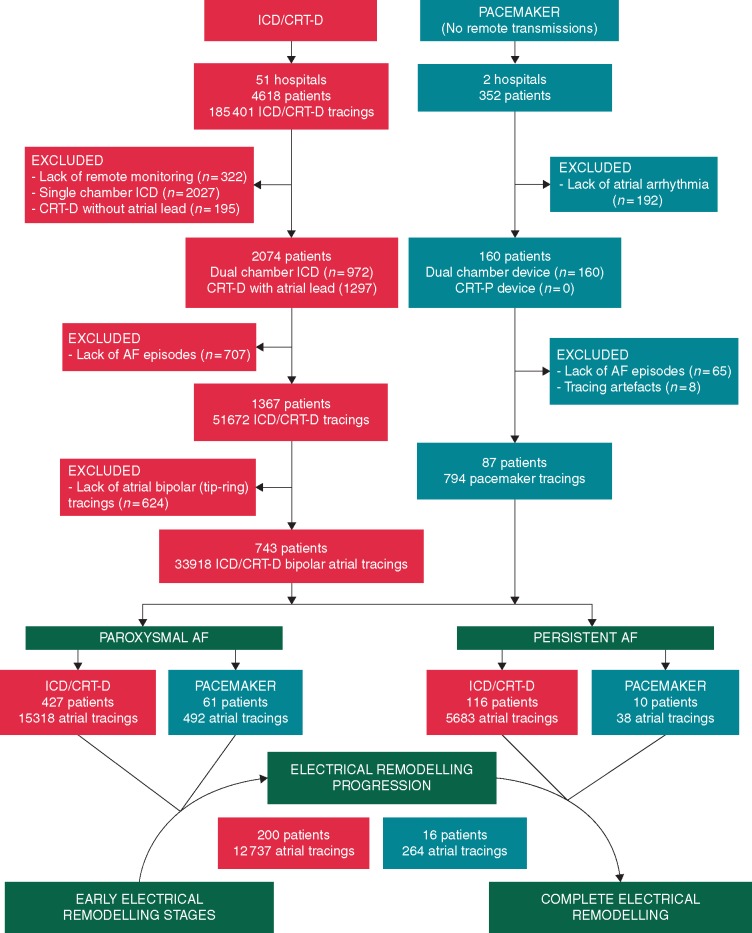
The ICD/CRT-D data were obtained from an observational multicentre registry from 51 Spanish centres included in the UMBRELLA study (NCT01561144 at clinicaltrials.gov). This database is supported by the scientific co-operation platform, which is a cloud-based big-data tool that enables automatic and non-invasive data transmission with digitally structured storage. The database consisted of 4618 patients undergoing a Medtronic (Tolochenaz, Switzerland) ICD/CRT-D implantation from August 2011 to November 2017. A total of 2074 patients with dual-chamber ICD/CRT-D devices and remote-monitoring transmissions were considered for the study. Pacemaker data were obtained from a retrospective observational study in two Spanish centres. All patients undergoing a dual-chamber Microport (Clamart, France) pacemaker implantation from December 2009 to December 2018 were considered for the study. Atrial leads were routinely implanted in the right atrial appendage. Figure 1 shows the study flow chart.
Figure 1.
Study flow chart. AF, atrial fibrillation; CRT-D/P, cardiac resynchronization therapy plus defibrillator/pacemaker; ICD, implantable cardioverter-defibrillator.
We obtained ethics committee approval for both ICD/CRT-D and pacemaker series in accordance with the Helsinki Declaration. All patients signed an informed consent. In patients with ICD/CRT-D devices, clinical baseline and demographic data were retrospectively collected at the time of device implantation using the official data collection sheet from the Spanish Society of Cardiology (see also Supplementary material online). In cases with device replacement during follow-up, the baseline data were selected using the data collection that was closest to the initiation of AF history. In pacemaker patients, clinical data and pharmacological history were obtained from electronic medical records during the follow-up period. In these patients, left atrial (LA) diameter was also measured using transthoracic echocardiography and a parasternal long-axis view.
Data selection and rhythm classification
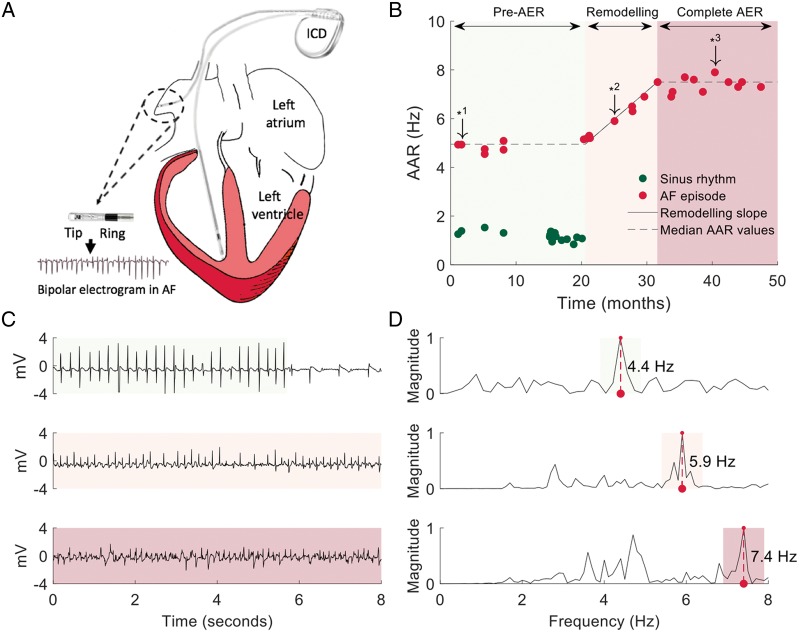
The UMBRELLA scientific committee initially reviewed and classified 27461 ICD/CRT-D stored episodes registered in a digital two-channel electrogram format. Three independent investigators used a custom-made Java-based software tool (Standard Edition 8, Oracle, Redwood, CA, USA) to further review and classify 31 672 tracings obtained from remote-monitoring transmissions. Stored pacemaker tracings obtained at the time of regular device interrogations were reviewed using the same software tool. Poor signal quality tracings were excluded from the study during the review process. Then, all AF tracings with a bipolar lead configuration (tip-ring; Figure 2A), overt irregular and fast activation rates >3 Hz, and ≥3-s long recordings were included in the study. Manual selection of AF segments was performed in tracings with documented AF termination at the end of the tracing. Signal artefacts were manually excluded if necessary.
Figure 2.
Sample signal processing and representation of the time-course of AER. (A) Schematic representation of a dual-chamber implantable cardioverter-defibrillator to obtain atrial bipolar electrograms during AF episodes. (B) Sample time-course of AAR during AF progression from early stages of AER (light green background colour) with paroxysmal AF episodes to completion of AER in persistent AF (red light background colour). (C) Sample tracings from downward arrows in B that represent different stages of AER. (D) Power spectra and DF values associated with the sample tracings in C. AAR, atrial activation rate; AER, atrial electrical remodelling; AF, atrial fibrillation; DF, dominant frequency.
Selected AF tracings were further classified as paroxysmal or persistent AF based on the recorded episode duration. In pacemaker patients, further confirmation of AF classification was obtained from clinical records. Atrial fibrillation episodes lasting <7 days were classified as paroxysmal AF and episodes lasting ≥7 days as persistent AF.3 In ICD/CRT-D patients, AF tracings without episode duration were classified using subsequent recordings in a way that two or more consecutive remote transmissions documenting AF, at least 7 days apart, were considered as persistent AF.
Computation of atrial activation rates
Atrial signals from stored episodes (ICD/CRT-Ds and pacemakers) were obtained at 128 Hz and data from remote transmissions (ICD/CRT-Ds only) were obtained at 256 Hz. Signals were high-pass filtered with cut-off frequency of 0.5 Hz. The median duration of AF tracings per patient was 9.6 [5.03, 10.00] s in ICD/CRT-D devices and 14.6 [10.6, 19.7] s in pacemakers. Local right AAR was computed using spectral analysis and the fast Fourier transform to convert the tracing into the frequency domain as reported elsewhere.6 Specifically, we used the non-parametric Welch method for power spectrum estimation with a median spectral resolution per patient of 0.10 [0.06, 0.24] Hz and 0.07 [0.05, 0.09] Hz in ICD/CRT-Ds and pacemakers, respectively. Spectral information below 10 Hz was normalized to the amplitude of the highest spectral peak within this band. The dominant frequency (DF) was defined as the frequency with the highest power within this band. The DF value for each AF tracing was reviewed to detect potential harmonic peaks, and if needed, it was manually corrected to the estimated average local AAR using a custom-made Java tool. Thus, the DF peak provided a robust and rapid calculation of the reciprocal average local activation intervals [estimated average cycle length (ms) = [(1/DF) × 1000]; Figure 2B–D].
Time-course analysis of atrial electrical remodelling
The DF-derived AARs were ordered by date to depict any changes during the follow-up period (Figure 2B). Then, patients with progression of AER from paroxysmal AF episodes (AER remodelling not present or incomplete) to persistent AF episodes with complete electrical remodelling were used to plot remodelling slopes. Pre- and post-electrical remodelling segments were manually selected using a custom-made Java tool. Because atrial activations during AF are irregular and DF values may slightly vary during consecutive tracings, we used median DF values during the pre- and post-remodelling phases to compute the difference between the two stages. Then, the slope of AER was computed automatically by fitting the DF values to a linear regression function dependent on both the median DF values and the documented date of initiation and completion of AER (Figure 2B).
Statistical analysis
All data are presented as median and inter-quartile range [25th, 75th percentiles], except where noted. Normal distribution of variables was assessed with the Shapiro–Wilk test. Statistical significance was assessed by the T-test or the Mann–Whitney–Wilcoxon test, as appropriate. The ANOVA or the Kruskal–Wallis test was used for three- to four-group comparisons according to data distribution. Categorical variables were compared using the χ2 test. The log(·) function was used to transform the exponential nature of the time-to-completion of AER distribution to a normally distributed variable. Pearson’s correlation coefficient (R2) was used for correlation analysis between continuous variables and DF values during persistent AF. A P-value of <0.05 was considered statistically significant.
The large series of patients with ICD/CRT-D devices was used to study the predictive value of clinical variables to establish the time-to-completion of AER, by means of univariate analysis and a multivariate stepwise linear regression model. Then, we developed a computational method using remote-monitoring tracings to estimate patient-specific times-to-completion of AER. First, all variables statistically associated with DF values during persistent AF were included in a multivariate stepwise linear regression model to predict AAR during persistent AF after completion of AER. Then, for each patient, the time-to-completion of AER was estimated using the predicted values of AAR after completion of the AER process and the computed slope at 25% of the expected change in AAR from paroxysmal AF to complete electrical remodelling in persistent AF. The analyses were carried out using SPSS v21 (IBM Corp, New York, NY, USA) and custom Matlab (MathWorks Inc., Natick, MA, USA) scripts for mathematical assistance.
Results
A total of 34 712 AF tracings from 830 patients (87 with pacemakers) were suitable for the study. Patients with pacemakers showed a similar follow-up period to ICD/CRT-D patients (3.4 [1.7, 5.3] and 3.4 [1.9, 4.3] years, respectively), although the former were significantly older (78.3 [73.1, 85.3] vs. 67.2 [59.3, 73.7] year old, P < 0.001, respectively) and had a predominant left ventricular ejection fraction (LVEF) >35%. Table 1 shows baseline clinical characteristics and comparisons between pacemaker and ICD/CRT-D populations. Complete progression of AER from pre-remodelling to complete remodelling in persistent AF was documented in 216 patients (16 with pacemakers). Other 543 patients in the ICD/CRT-D population and 71 patients in the pacemaker population were classified as paroxysmal (no progression to persistent AF during the follow-up) or persistent AF (repeated transmissions in AF during the follow-up; Figure 1). The ICD/CRT-D indication was for primary prevention in the majority of patients (71.7%) and the underlying myocardial substrates were mainly ischaemic cardiomyopathy (ICM, 50.6%) and non-ischaemic dilated cardiomyopathy (DCM, 33.2%), with a LVEF ≤35% in 73.4% of patients (Table 2).
Table 1.
Baseline clinical and demographic characteristics of implantable cardioverter-defibrillator +/−resynchronization therapy and pacemaker populations
| Clinical characteristics | ICD/CRT-D (n = 743) | Pacemaker (n = 87) | P-value |
|---|---|---|---|
| Age (years), n (median [IQR]) | 67.2 [59.3, 73.7] | 78.3 [73.1, 85.3] | <0.001 |
| Male, n (%) | 609 (82.0) | 51 (58.6) | <0.001 |
| Cardiomyopathy, n (%) | <0.001 | ||
| ICM | 376 (50.6) | 19 (21.8) | |
| DCM | 247 (33.2) | 1 (1.1) | |
| Other SCM: HCM, ARVC, VHD, or CHD | 97 (13.1) | 3 (3.4) | |
| Non-structural arrhythmogenic disease | 23 (3.1) | 0 (0.0) | |
| Non-cardiomyopathy | 0 (0) | 64 (73.6) | |
| LBBB, n (%) | 334 (45.6) | 12 (13.8) | <0.001 |
| LVEF (≤35%), n (%) | 544 (73.4) | 4 (4.6) | <0.001 |
| Functional class, n (%) | <0.001 | ||
| NYHA I | 119 (18.2) | 34 (39.5) | |
| NYHA II | 288 (44.0) | 41 (47.7) | |
| NYHA III | 235 (35.9) | 10 (11.6) | |
| NYHA IV | 12 (1.8) | 1 (1.2) | |
| Clinical history, n (%) | |||
| Hypertension | 434 (59.9) | 77 (88.5) | <0.001 |
| Diabetes mellitus | 227 (31.2) | 25 (28.7) | 0.641 |
| Hyperlipidaemia | 388 (54.6) | 50 (57.5) | 0.608 |
| Current smoking | 206 (30.2) | 5 (5.7) | <0.001 |
| Chronic renal failure | 143 (20.0) | 12 (13.8) | 0.168 |
| Previous stroke or TIA | 50 (7.5) | 6 (6.9) | 0.852 |
| Medications during the AF period, n (%) | |||
| β-blocker | – | 40 (46.0) | |
| ACE-inhibitor/ARB | – | 61 (70.1) | |
| Verapamil/diltiazem | – | 6 (6.9) | |
| Mineralocorticoid receptor antagonist | – | 9 (10.3) | |
| Statins | – | 52 (59.8) | |
| Class I antiarrhythmic drug | – | 8 (9.2) | |
| Class III antiarrhythmic drug | – | 9 (10.3) | |
| Anticoagulant | – | 58 (66.7) |
Bold values highlight statistical significance. ACE, angiotensin-converting-enzyme; AF, atrial fibrillation; ARB, angiotensin-receptor blocker; ARVC, arrhythmogenic right ventricular cardiomyopathy; CHD, congenital heart disease; DCM, non-ischaemic dilated cardiomyopathy; ICD/CRT-D, implantable cardioverter-defibrillator+/−resynchronization therapy; ICM, ischaemic cardiomyopathy; HCM, hypertrophic cardiomyopathy; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; SCM, structural cardiomyopathy; TIA, transient ischaemic attack; VHD, valvular heart disease.
Table 2.
Baseline clinical and demographic characteristics of patients with implantable cardioverter-defibrillator +/−resynchronization therapy devices in different atrial fibrillation groups
| Clinical characteristics | Paroxysmal AF (n = 427) | Complete AER progression (n = 200) | Persistent AF (n = 116) | P-value |
|---|---|---|---|---|
| Age (years), median [IQR] | 66.2 [58.1, 72.9] | 67.9 [58.1, 74.5] | 69.3 [62.7, 75.3] | 0.005 |
| Male, n (%) | 334 (78.2) | 178 (89.0) | 97 (83.6) | 0.004 |
| Cardiomyopathy, n (%) | 0.575 | |||
| ICM | 214 (50.1) | 101 (50.5) | 61 (52.6) | |
| DCM | 141 (33.0) | 68 (34.0) | 38 (32.8) | |
| Other SCM: HCM, ARVC, VHD, or CHD | 55 (12.9) | 29 (14.5) | 13 (11.2) | |
| Non-structural arrhythmogenic disease | 17 (4.0) | 2 (1.0) | 4 (3.4) | |
| LBBB, n (%) | 195 (46.5) | 84 (42.0) | 55 (48.7) | 0.444 |
| LVEF (≤35%), n (%) | 303 (71.1) | 147 (73.5) | 94 (81.7) | 0.073 |
| Functional class, n (%) | 0.073 | |||
| NYHA I | 79 (21.6) | 30 (16.8) | 10 (9.1) | |
| NYHA II | 156 (42.7) | 80 (44.7) | 52 (47.3) | |
| NYHA III | 124 (34.0) | 67 (37.4) | 44 (40.0) | |
| NYHA IV | 6 (1.6) | 2 (1.1) | 4 (3.6) | |
| Clinical history, n (%) | ||||
| Hypertension | 244 (59.2) | 120 (61.2) | 70 (60.3) | 0.891 |
| Diabetes mellitus | 134 (32.5) | 57 (28.5) | 36 (31.0) | 0.601 |
| Hyperlipidaemia | 220 (55.1) | 110 (55.0) | 58 (51.8) | 0.812 |
| Current smoking | 127 (32.5) | 53 (28.8) | 26 (24.5) | 0.253 |
| Chronic renal failure | 73 (18.0) | 44 (22.6) | 26 (22.6) | 0.312 |
| Previous stroke or TIA | 24 (6.3) | 16 (8.7) | 10 (9.1) | 0.464 |
| Clinical presentation, n (%) | ||||
| Asymptomatic | 184 (44.0) | 92 (46.5) | 44 (39.3) | 0.473 |
| Syncope | 68 (16.3) | 32 (16.2) | 18 (16.1) | 0.999 |
| Sudden cardiac death | 40 (9.6) | 15 (7.6) | 7 (6.3) | 0.459 |
| Primary prevention, n (%) | 305 (71.4) | 143 (71.5) | 85 (73.3) | 0.923 |
| Device type (ICD), n (%) | 229 (53.6) | 91 (45.5) | 41 (35.3) | <0.001 |
ACE, angiotensin-converting-enzyme; AF, atrial fibrillation; ARB, angiotensin-receptor blocker; ARVC, arrhythmogenic right ventricular cardiomyopathy; CHD, congenital heart disease; DCM, non-ischaemic dilated cardiomyopathy; ICD/CRT-D, implantable cardioverter-defibrillator+/−resynchronization therapy; ICM, ischaemic cardiomyopathy; HCM, hypertrophic cardiomyopathy; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; SCM, structural cardiomyopathy; TIA, transient ischaemic attack; VHD, valvular heart disease.
Atrial fibrillation stage determines atrial activation rates and structural changes
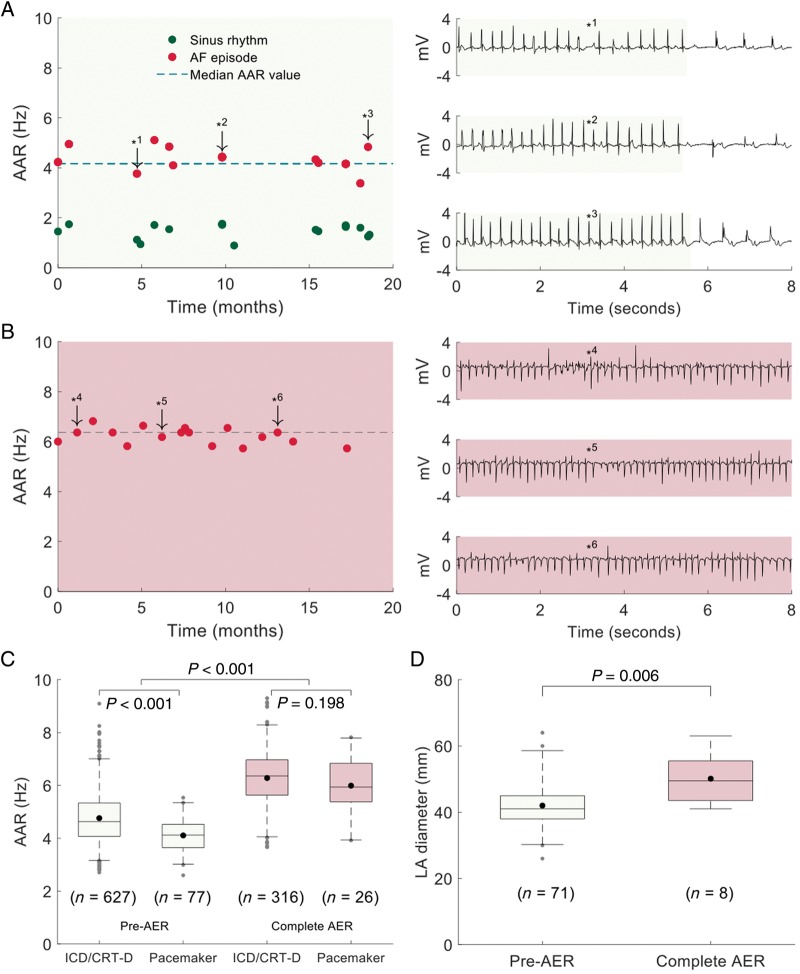
Patients with paroxysmal AF showed slower AAR than patients with persistent AF. Two representative cases are shown in Figure 3A, B. Overall, AAR was ∼30% higher after completion of AER compared with pre-remodelling stages in paroxysmal AF (Figure 3C). The data reflect the presence of two electrical remodelling stages with an intermediate period between them. This increase in AAR was present in both pacemaker and ICD/CRT-D populations, although pacemaker patients showed slower AAR during paroxysmal AF episodes compared with ICD/CRT-D patients (4.12 [3.64, 4.53] Hz vs. 4.63 [4.07, 5.34] Hz, respectively, P < 0.001). These differences were not documented after completion of AER in persistent AF (Figure 3C). Moreover, in the pacemaker population, echocardiography data showed a progression in structural remodelling reflected by a significantly larger LA diameter after completion of AER compared with early electrical remodelling stages (50 [40, 56] mm vs. 38 [41, 45] mm, P = 0.006; Figure 3D).
Figure 3.
Atrial activation rates during paroxysmal and persistent AF and associated structural changes. (A, B) Left panels show the time-course of AAR during paroxysmal and persistent AF. Right panels show sample AF tracings at downward arrows. Light green and red background colours indicate early and complete AER stages, respectively. (C) Box plots and comparisons of AAR during paroxysmal (pre-AER) and persistent AF (complete AER) between patients with ICD/CRT-D and pacemaker devices. (D) Box plots and comparison of left atrial diameters between pre-AER and complete AER stages. Box plot data show median and inter-quartile ranges. Central dots inside the box plots show the mean. AAR, atrial activation rate; AER, atrial electrical remodelling; AF, atrial fibrillation; ICD/CRT-D, implantable cardioverter-defibrillator+/−resynchronization therapy.
More specific analysis of AARs in patients with an ICD or CRT-D device and complete monitoring of AER (n = 200) showed that AARs during paroxysmal AF episodes were significantly slower in CRT-D patients compared with ICD patients (4.15 [3.48, 4.80] Hz vs. 4.45 [3.81, 5.25] Hz, respectively, P = 0.005). However, these differences were not statistically significant after completion of AER in persistent AF (Supplementary material online, Figure S1). Biventricular capture in CRT-D patients was 95.4 [89.3, 99.0] %. In the pacemaker population, we also documented a trend to slower AARs under Class I/III antiarrhythmic drugs (Supplementary material online, Figure S2).
Prediction of atrial activation rates after completion of atrial electrical remodelling
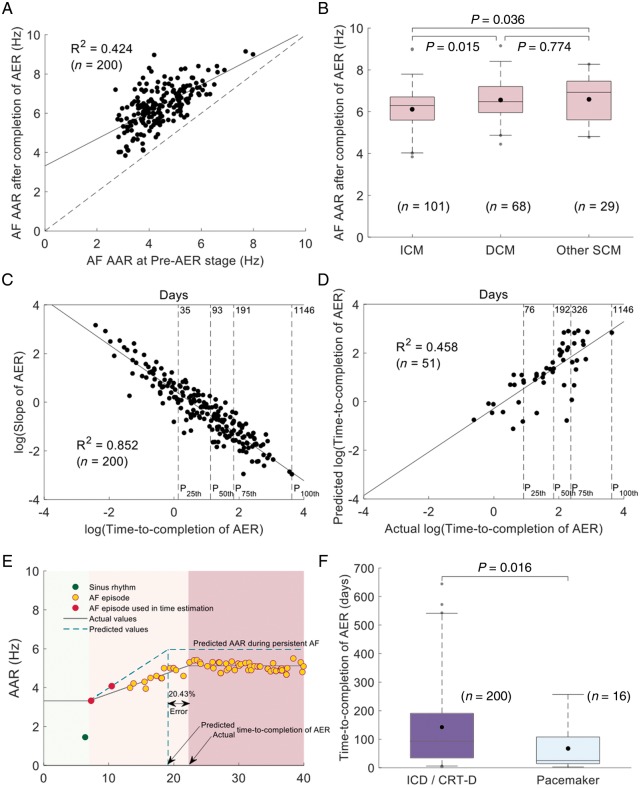
In ICD/CRT-D patients with complete monitoring of AER, there was a statistically significant correlation between AARs during paroxysmal AF and at the completion of AER in persistent AF (R2 = 0.424, P < 0.001; Figure 4A). Thus, the slower the AAR during paroxysmal AF, the slower the expected AAR after completion of AER. Moreover, AARs after completion of AER were significantly lower in patients with ICM compared with patients with DCM or other structural cardiomyopathies (6.30 [5.60, 6.70] Hz vs. 6.48 [5.96, 7.20] Hz, and vs. 6.93 [5.61, 7.45] Hz, respectively, P = 0.015 and P = 0.036, respectively; Figure 4B). Left ventricular ejection fraction ≤35% was also associated with lower AARs compared with LVEF >35% (6.30 [5.61, 6.79] Hz vs. 6.84 [5.72, 7.38] Hz, respectively, P = 0.013). Table 3 shows the univariate analysis of baseline clinical characteristics and any statistically significant association with AAR values during persistent AF after completion of AER. Importantly, a predictive model using the underlying cardiac condition and the AAR during early stages of AER in paroxysmal AF significantly correlated with AAR values after completion of AER in persistent AF (Table 4; R2 = 0.460).
Figure 4.
Monitoring and prediction of time-to-completion of AER. (A) Scatter-plot of AAR during paroxysmal (pre-AER stage) AF and after completion of AER in persistent AF. Each black dot represents an individual patient. The diagonal dashed line represents the line of equality. (B) Box plots and comparisons of AAR during persistent AF among patients with ICM, DCM, and other SCM. (C) Log(slope) correlation with log(time-to-completion AER). (D) Correlation of predicted and actual log(time-to-completion of AER). (E) Sample case with the predicted and actual times-to-completion of AER. (F) Box plots and comparison of time-to-completion of AER between the ICD/CRT-D and pacemaker populations. All panels, but F shows data from the ICD/CRT-D population. P25th, P50th, P75th, P100th in panels C, D indicate percentiles. AAR, atrial activation rate; AER, atrial electrical remodelling; AF, atrial fibrillation; DCM, non-ischaemic dilated cardiomyopathy; ICD/CRT-D, implantable cardioverter-defibrillator+/−resynchronization therapy; ICM, ischaemic cardiomyopathy; SCM, structural cardiomyopathies.
Table 3.
Univariate analysis of variables potentially associated with atrial activation rates during persistent atrial fibrillation after completion of atrial electrical redemolling
| Clinical characteristics | No | Yes | P-value/R2 |
|---|---|---|---|
| Age | 0.002/0.047 | ||
| Male, n (Hz) | 22 (6.29) | 178 (6.40) | 0.925/− |
| Cardiomyopathy, n (Hz) | 0.008/− | ||
| ICM | 101 (6.30) | ||
| DCM | 68 (6.48) | ||
| Other SCM: HCM, ARVC, VHD, or CHD | 29 (6.93) | ||
| Non-structural arrhythmogenic disease | 2 (7.76) | ||
| LBBB, n (Hz) | 116 (6.40) | 84 (6.28) | 0.536/– |
| LVEF (≤35%), n (Hz) | 53 (6.84) | 147 (6.30) | 0.013/– |
| Functional class, n (Hz) | 0.194/– | ||
| NYHA I | 30 (6.60) | ||
| NYHA II | 80 (6.39) | ||
| NYHA III | 67 (6.30) | ||
| NYHA IV | 2 (5.26) | ||
| Clinical history, n (Hz) | |||
| Hypertension | 76 (6.31) | 120 (6.39) | 0.930/– |
| Diabetes mellitus | 143 (6.40) | 57 (6.30) | 0.314/– |
| Hyperlipidaemia | 90 (6.47) | 110 (6.28) | 0.136/– |
| Current smoking | 131 (6.28) | 53 (6.50) | 0.169/– |
| Chronic renal failure | 151 (6.30) | 44 (6.43) | 0.671/– |
| Previous stroke or TIA | 167 (6.30) | 16 (6.86) | 0.242/– |
| Clinical presentation, n (Hz) | |||
| Asymptomatic | 106 (6.27) | 92 (6.42) | 0.263/– |
| Syncope | 166 (6.40) | 32 (6.30) | 0.900/– |
| Sudden cardiac death | 183 (6.36) | 15 (6.50) | 0.787/– |
| Primary prevention, n (Hz) | 57 (6.32) | 143 (6.40) | 0.926/– |
| Device type (ICD), n (Hz) | 109 (6.30) | 91 (6.44) | 0.118/– |
| AAR during paroxysmal AF | <0.001/0.424 |
Data are shown as the number of patients (n) and the median AAR during persistent AF after completion of AER (in Hz).
AAR, atrial activation rate; ACE, angiotensin-converting-enzyme; AER, atrial electrical remodelling; AF, atrial fibrillation; ARB, angiotensin-receptor blocker; ARVC, arrhythmogenic right ventricular cardiomyopathy; CHD, congenital heart disease; DCM, non-ischaemic dilated cardiomyopathy; ICD/CRT-D, implantable cardioverter-defibrillator +/−resynchronization therapy; ICM, ischaemic cardiomyopathy; HCM, hypertrophic cardiomyopathy; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; SCM, structural cardiomyopathy; TIA, transient ischaemic attack; VHD, valvular heart disease.
Table 4.
Predictive model of atrial activation rates during persistent atrial fibrillation after completion of atrial electrical remodelling
| Estimate | Standard error | t-statistic | P-value | |
|---|---|---|---|---|
| Intercept | 2.947 | 0.270 | 10.923 | <0.0001 |
| Cardiomyopathy | 0.258 | 0.071 | 3.6311 | <0.0001 |
| AAR during paroxysmal AF | 0.675 | 0.056 | 12.150 | <0.0001 |
| R 2 | 0.460 |
AAR, atrial activation rate; AF, atrial fibrillation.
Atrial fibrillation progression shows patient-specific patterns of electrical remodelling
The log(slope) of AARs during AF progression showed patient-specific patterns that strongly correlated with the log(time-to-completion of AER), both in the ICD/CRT-D and pacemaker populations (R2 = 0.852 and R2 = 0.853, respectively; P < 0.001; Figure 4C and Supplementary material online, Figure S3). Using the large population of patients with ICD/CRT-D devices, univariate analysis of baseline clinical variables showed that only the presence of left bundle branch block (P = 0.036) was associated with the time-to-completion of AER (Supplementary material online, Table S1). However, multivariate analysis did not identify any statistically significant association of clinical variables with the time-to-completion of AER.
Importantly, patient-specific time-to-completion of AER could be estimated in a subset of the ICD/CRT-D population (n = 51) with remote-monitored AF tracings. Thus, the predicted value of AARs at the completion of the AER process and the computed slope at 25% of the expected change in AARs, from paroxysmal AF to complete electrical remodelling in persistent AF, correlated with the time-to-completion of AER (R2 = 0.458; Figure 4D, E and Supplementary material online, Figure S4). Error quantification of the estimated log(time-to-completion of AER) showed a median value of 33.56% [12.72, 57.06].
Further comparisons between the pacemaker population and patients with ICD/CRT-D devices showed that the time-to-completion of AER was significantly shorter in patients with pacemakers (24.9 [14.3, 108.2] d vs. 93.5 [36.6, 190.5] d, respectively; P = 0.016; Figure 4F). Despite limitations to identify the underlying causes of such differences, older ages in the pacemaker population may be involved (Table 1).
Discussion
We studied the time-course of AER in two populations of patients with implantable devices during AF progression. Patients with paroxysmal and persistent AF showed significantly different AARs, with faster activation rates during persistent AF after completion of AER. We have identified that AAR after completion of AER could be predicted using the underlying cardiac condition and the AAR during paroxysmal AF. Moreover, we have documented patient-specific patterns of time-to-completion AER, which were significantly faster in the older population of patients with pacemakers compared with the ICD/CRT-D population. Individual patterns of AER progression could be estimated using the predicted values of AAR at the completion of the AER process and the computed slope at 25% of the expected change in AAR from the initiation to complete electrical remodelling in persistent AF.
The influence of the underlying cardiac condition and therapies (e.g. CRT-D) on AARs may represent different predominant mechanisms affecting atrial electrophysiology. Yoshida et al.9 have reported a direct relationship between LA pressure and the DF of AF at the LA appendage. Thus, atrial stretch in the presence of increased intracavitary pressures may lead to faster AARs as we have documented in patients with DCM. Slower AARs during paroxysmal AF episodes in patients with CRT-D compared with ICD patients may also reflect an improvement in cardiac haemodynamic status upon resynchronization therapy. Interestingly, the majority of patients with other structural cardiomyopathies had hypertrophic cardiomyopathy, which is also associated with atrial diastolic dysfunction and increased LA pressures.10 Therefore, atrial stretch may represent the cause of faster AARs in patients with other structural cardiomyopathies compared with patients with ICM. Although patients with ICM can also have increased intracavitary pressures,11 infarct-related scar tissue may affect atrial activation and potentially decrease AAR. The latter would be consistent with the study by Swartz et al.12 showing an inverse correlation between LA fibrosis and LA activation frequencies. This is also consistent with our data from patients with pacemakers; a more advanced age in this population justifies higher levels of underlying atrial fibrosis and significantly slower AAR during paroxysmal AF compared with the ICD/CRT-D population. At the cellular level, the myofibroblast/myocyte ratio in two-dimensional cardiac monolayers has also shown an inverse relationship with activation frequencies of re-entrant activity.13
The data demonstrate the potential of personalized monitoring of AARs as a marker of electrical remodelling during AF progression. Moreover, regardless of the current classification of paroxysmal vs. persistent AF, which does not provide accurate information about the underlying remodelling stage, our approach to assess the progression and stage of AER may have important therapeutic and prognostic implications. Lankveld et al.14 have documented that slower AARs on single surface ECG recordings of patients with persistent AF undergoing electrical cardioversion were associated with sinus rhythm maintenance after 1 year of follow-up. Interestingly, the mean episode duration was 3 months in patients with and without AF recurrences, which suggests that within the same time period slower progression of AER will lead to slower AARs and higher probability of sinus rhythm maintenance. Bollman et al.15 have also shown that single ECG-derived atrial fibrillatory frequencies of 5.9 Hz in patients with persistent AF were associated with successful pharmacological cardioversion. Conversely, mean atrial fibrillatory frequencies of 6.4 Hz did associate with unsuccessful pharmacological cardioversion, while mean episode duration (4 to 6 months) was not significantly different between the two groups. Data from ablation procedures have also documented the role of ECG-based parameters to predict AF termination during the procedure and long-term freedom from AF. Using a retrospective cohort of patients undergoing persistent AF ablation, Lankveld et al.16 could document that DF values <6.0 Hz were frequently associated with AF termination during the ablation procedure, along with long-term freedom from the arrhythmia. Such DF values were mainly present in AF episodes with duration <12 months. The latter is consistent with our data in which the majority of patients in the ICD/CRT-D population (n = 150, 75th percentile) showed complete electrical remodelling after 191 days of remodelling initiation.
Remote monitoring of different cardiac conditions has already demonstrated an impact on clinical outcomes, health care utilization and expenditures.17 Continuous ECG monitoring with implantable cardiac monitors has also demonstrated that AF detection can be substantially improved in patients at high risk of both AF and stroke.18 However, despite the potential impact of early AF detection on clinical outcomes as rhythm control or stroke, data from trials with early therapeutic interventions are warranted. Our data represent one step forward in AF monitoring to detect both the electrical remodelling stage during AF episodes and the progression rate of AER as AF evolves. These two aspects may potentially assist physicians in personalized care of patients with AF and implantable devices; e.g. adjusting medical therapy and further monitoring its effects or proposing an early interventional ablation procedure before completion of AER.
We have obtained AARs from intracardiac bipolar tracings, which provided local activation rates from the right atrium. However, remodelling progression could also be monitored using single-lead ECG tracings after QRS-T subtraction as it has been performed in animal models of long-standing persistent AF.6 The latter opens new diagnostic options for monitoring AF progression using non-invasive long-term recording devices such as smart watches or wearable Holter ECG, which would require implementing appropriate algorithms for QRS-T subtraction and optimization of remote transmissions once AF is detected. Importantly, AF is an irregular rhythm with variations in local AAR within specific time windows.4 Therefore, a single ECG tracing or DF value may not represent an accurate measurement of AAR compared with repeating measurements for the same day. In patients with complete monitoring of AER in the ICD/CRT-D population, we have used a median of 64 [34, 122] AF tracings per patient, which minimized this potential limitation. Moreover, estimated slope calculations were performed using median DF values for repeating tracings from the same day. The latter could be further improved using algorithms that can store reliable DF values or average atrial activation cycle lengths using repeating samples from the same day. The DF values will have the advantage of providing a rapid measurement with no need for detecting individual activations, which may be challenging in the presence of fragmented electrograms or low amplitude signals.19,20 In fact, low fibrillatory wave amplitudes are commonly present in long-standing AF episodes.16 Conversely, DF values may be affected by harmonic peaks, which may require improved algorithms or additional revision by the physician using representative tracings that could be transmitted to a cloud-based data tool and avoid compromising devices’ battery longevity.
Limitations
The design of the study did not enable us to obtain remote-monitoring transmissions using specific criteria, which could have improved the estimation of AER slopes. This limitation was more relevant in pacemaker devices, in which remote transmissions were not available and AF tracings were only obtained from stored AF episodes. Representative cases are shown in Supplementary material online, Figure S5.
The study was focused on monitoring AER using a large number of tracings during a long follow-up. However, other remodelling changes as structural remodelling were not thoroughly evaluated because of study design limitations to obtain such data in ICD/CRT-D patients. Pharmacological history was not included in the official datasheet at the time of ICD/CRT-D implantation, which precluded us from obtaining more meaningful data about specific drug effects on both AAR and the progression of AER. Slower progression of AER in patients with ICD/CRT-D devices may be related to a high percentage of these patients taking upstream therapies (based on historical series with ICD/CRT-D devices) compared with pacemaker patients (Supplementary material online, Figure S2). However, this study shows that regardless the underlying pharmacological effects on remodelling slopes, AER could be efficiently monitored and estimated using current technology. The methodology used in this study is not available in clinical practice and would require further implementation in implantable devices or remote-monitoring platforms to validate the impact in prospective series.
Conclusion
Atrial fibrillation progression shows patient-specific patterns of AER, which can be estimated using the computed individual slope of AAR changes and the underlying cardiac condition.
Supplementary Material
Acknowledegments
We thank the UMBRELLA study (NCT01561144 at clinicaltrials.gov) and the scientific co-operation platform for providing a valuable source of data to independent investigators in Spain. We thank Stuart Pocock for his statistical advice.
Conflict of interest: none declared.
Funding
The Centro Nacional de Investigaciones Cardiovasculares (CNIC) is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia, Innovación y Universidades (MCNU), and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (SEV-2015–0505). This study was supported by grants from the Fondo Europeo de Desarrollo Regional (CB16/11/00458) and the Spanish MCNU (SAF2016-80324-R). The study was also partially supported by the Fundación Interhospitalaria para la Investigación Cardiovascular (FIC, Madrid, Spain) and the Heart Rhythm Association of the Spanish Society of Cardiology (D.F.-R., J.J.G.-F.). J.J. is supported by R01 Grant HL122352 from the National Heart Lung and Blood Institute, USA National Institutes of Health.
References
- 1. Kim MH, Johnston SS, Chu BC, Dalal MR, Schulman KL.. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011;4:313–20. [DOI] [PubMed] [Google Scholar]
- 2. Weng LC, Preis SR, Hulme OL, Larson MG, Choi SH, Wang B. et al. Genetic predisposition, clinical risk factor burden, and lifetime risk of atrial fibrillation. Circulation 2018;137:1027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace 2016;18:1609–78. [DOI] [PubMed] [Google Scholar]
- 4. Isa R, Villacastin J, Moreno J, Perez-Castellano N, Salinas J, Doblado M. et al. Differentiating between atrial flutter and atrial fibrillation using right atrial bipolar endocardial signals. Rev Esp Cardiol 2007;60:104–9. [PubMed] [Google Scholar]
- 5. de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJ. et al. Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–31. [DOI] [PubMed] [Google Scholar]
- 6. Martins RP, Kaur K, Hwang E, Ramirez RJ, Willis BC, Filgueiras-Rama D. et al. Dominant frequency increase rate predicts transition from paroxysmal to long-term persistent atrial fibrillation. Circulation 2014;129:1472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takemoto Y, Ramirez RJ, Yokokawa M, Kaur K, Ponce-Balbuena D, Sinno MC. et al. Galectin-3 regulates atrial fibrillation remodeling and predicts catheter ablation outcomes. JACC Basic Transl Sci 2016;1:143–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quintanilla JG, Alfonso-Almazan JM, Perez-Castellano N, Pandit SV, Jalife J, Perez-Villacastin J. et al. Instantaneous amplitude and frequency modulations detect the footprint of rotational activity and reveal stable driver regions as targets for persistent atrial fibrillation ablation. Circ Res 2019;125:609–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoshida K, Ulfarsson M, Oral H, Crawford T, Good E, Jongnarangsin K. et al. Left atrial pressure and dominant frequency of atrial fibrillation in humans. Heart Rhythm 2011;8:181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Geske JB, Sorajja P, Nishimura RA, Ommen SR.. Evaluation of left ventricular filling pressures by Doppler echocardiography in patients with hypertrophic cardiomyopathy: correlation with direct left atrial pressure measurement at cardiac catheterization. Circulation 2007;116:2702–8. [DOI] [PubMed] [Google Scholar]
- 11. Kyhl K, Vejlstrup N, Lonborg J, Treiman M, Ahtarovski KA, Helqvist S. et al. Predictors and prognostic value of left atrial remodelling after acute myocardial infarction. Open Heart 2015;2:e000223.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swartz MF, Fink GW, Lutz CJ, Taffet SM, Berenfeld O, Vikstrom KL. et al. Left versus right atrial difference in dominant frequency, K(+) channel transcripts, and fibrosis in patients developing atrial fibrillation after cardiac surgery. Heart Rhythm 2009;6:1415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zlochiver S, Munoz V, Vikstrom KL, Taffet SM, Berenfeld O, Jalife J.. Electrotonic myofibroblast-to-myocyte coupling increases propensity to reentrant arrhythmias in two-dimensional cardiac monolayers. Biophys J 2008;95:4469–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lankveld T, de Vos CB, Limantoro I, Zeemering S, Dudink E, Crijns HJ. et al. Systematic analysis of ECG predictors of sinus rhythm maintenance after electrical cardioversion for persistent atrial fibrillation. Heart Rhythm 2016;13:1020–7. [DOI] [PubMed] [Google Scholar]
- 15. Bollmann A, Binias KH, Toepffer I, Molling J, Geller C, Klein HU.. Importance of left atrial diameter and atrial fibrillatory frequency for conversion of persistent atrial fibrillation with oral flecainide. Am J Cardiol 2002;90:1011–4. [DOI] [PubMed] [Google Scholar]
- 16. Lankveld T, Zeemering S, Scherr D, Kuklik P, Hoffmann BA, Willems S. et al. Atrial fibrillation complexity parameters derived from surface ECGs predict procedural outcome and long-term follow-up of stepwise catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol 2016;9:e003354.. [DOI] [PubMed] [Google Scholar]
- 17. Piccini JP, Mittal S, Snell J, Prillinger JB, Dalal N, Varma N.. Impact of remote monitoring on clinical events and associated health care utilization: a nationwide assessment. Heart Rhythm 2016;13:2279–86. [DOI] [PubMed] [Google Scholar]
- 18. Reiffel JA, Verma A, Kowey PR, Halperin JL, Gersh BJ, Wachter R. et al. Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high-risk population: the REVEAL AF study. JAMA Cardiol 2017;2:1120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oral H, Chugh A, Good E, Wimmer A, Dey S, Gadeela N. et al. Radiofrequency catheter ablation of chronic atrial fibrillation guided by complex electrograms. Circulation 2007;115:2606–12. [DOI] [PubMed] [Google Scholar]
- 20. Kuklik P, Zeemering S, Maesen B, Maessen J, Crijns HJ, Verheule S. et al. Reconstruction of instantaneous phase of unipolar atrial contact electrogram using a concept of sinusoidal recomposition and Hilbert transform. IEEE Trans Biomed Eng 2015;62:296–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.