1.
The human BCS1L gene encodes a homolog of Saccharomyces cerevisiae bcs1 protein involved in the assembly of complex III of the mitochondrial respiratory chain. Phenotypes associated with pathogenic biallelic BCS1L variants range from growth retardation, aminoaciduria, cholestasis, iron overload, lactic acidosis and early death (GRACILE) syndrome; MIM#603388) to Bjornstad syndrome (MIM#262000), characterized by abnormal flattening and twisting of hair shafts (pili torti) and sensori‐neural hearing loss without intellectual impairment. No causal treatment for this mitochondrial disease is available.
Ketogenic diets stimulate mitochondrial biogenesis, improve mitochondrial function and decrease oxidative stress1, 2, 3, 4 and have been implemented in some patients with mitochondrial disease and epilepsy.5, 6, 7
We report on a 7‐year‐old female (BCS1L, NM_001079866.2, c.232A>G; p.Ser78Gly, c.794G>A; p.Arg265Gln) with very sparse and brittle hair since birth (Figure 1A,B), with progressive sensori‐neural hearing loss noted from age 18 months onwards. A 3‐day dietary history indicated an adequate diet with no protein deficiency but aminoaciduria, as a possible contributing cause for alopecia, was not confirmed or excluded. We initiated a mild ketogenic diet (modified Atkins diet [MAD] with 30‐40 g/day carbohydrates [1500 kcal/day], 10% of energy from carbohydrates, 25% from protein and 65% from fat). This was well tolerated and blood ketone levels of 2‐4 mmol/L were achieved.8 Hair growth improved during treatment (Figure 1C‐ E). Despite this, the family decided to go back to a regular diet after 4 months of diet because of the child craving for carbohydrates, and lack of improvement of hearing. Figure 1F shows that the hair was lost again 6 months after cessation of MAD.
Figure 1.
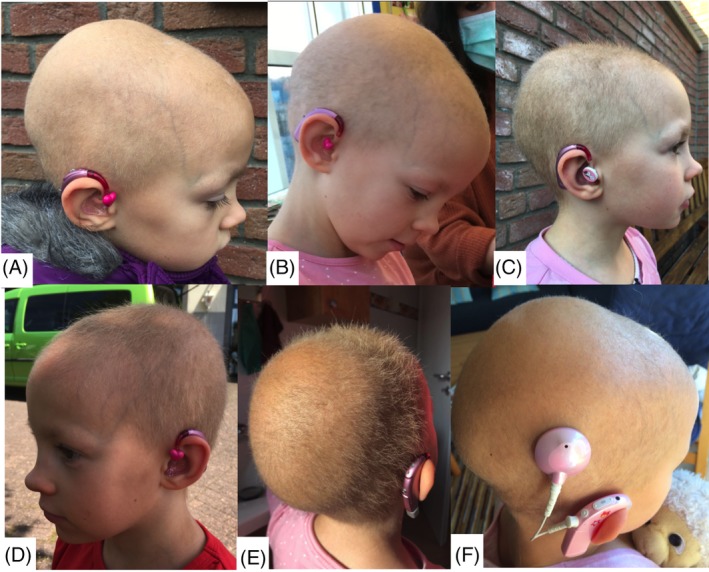
Patient with BCS1L related mitochondrial disease (Bjornstad syndrome) 2 months before (A), at day of starting (B) and after three (C) and four (D, E) months of modified Atkins diet. (F) Shows the patient 6 months after cessation of modified Atkins diet
There was improved hair growth during MAD in this individual with BCS1l related mitochondrial disease, We can only speculate if continuation of MAD would have further increased the hair growth or would have influenced the hearing loss and what effects an earlier initiation of MAD would have had.
Della Marina A, Leiendecker B, Roesch S, Wortmann SB. Ketogenic diet for treating alopecia in BCS1l‐related mitochondrial disease (Bjornstad syndrome). JIMD Reports. 2020;53:10–11. 10.1002/jmd2.12109
Communicating Editor: Avihu Boneh
REFERENCES
- 1. Stafford P, Abdelwahab MG, Kim do Y, Preul MC, Rho JM, Scheck AC. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutr Metab. 2010;7:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahola‐Erkkilä S, Carroll CJ, Peltola‐Mjösund K, et al. Ketogenic diet slows down mitochondrial myopathy progression in mice. Hum Mol Genet. 2010;19:1974‐1984. [DOI] [PubMed] [Google Scholar]
- 3. Bough KJ, Wetherington J, Hassel B, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223‐235. [DOI] [PubMed] [Google Scholar]
- 4. Danial NN, Hartman AL, Stafstrom CE, Thio LL. How does the ketogenic diet work? Four potential mechanisms. J Child Neurol. 2013;28:1027‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Santra S, Gilkerson RW, Davidson M, Schon EA. Ketogenic treatment reduces deleted mitochondrial DNAs in cultured human cells. Ann Neurol. 2004;56:662‐669. [DOI] [PubMed] [Google Scholar]
- 6. Joshi CN, Greenberg CR, Mhanni AA, Salman MS. Ketogenic diet in Alpers‐Huttenlocher syndrome. Pediatr Neurol. 2009;40:314‐316. [DOI] [PubMed] [Google Scholar]
- 7. Kang HC, Lee YM, Kim HD, Lee JS, Slama A. Safe and effective use of the ketogenic diet in children with epilepsy and mitochondrial respiratory chain complex defects. Epilepsia. 2007;48:82‐88. [DOI] [PubMed] [Google Scholar]
- 8. Kossoff EH, Zupec‐Kania BA, Auvin S, et al. Optimal clinical management of children receiving dietary therapies for epilepsy: updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018;3(2):175‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]


