Key Points
Question
What would the reduction be in all-cause mortality for the US population if all eligible patients with heart failure with reduced ejection fraction were prescribed sodium-glucose cotransporter 2 inhibitor therapy?
Findings
This decision analytical model found that optimal implementation of sodium-glucose cotransporter 2 inhibitor therapy for patients with heart failure with reduced ejection fraction would be estimated to result in as many as 34 125 lives saved per year (range, 21 840-49 140 lives saved per year).
Meaning
The timely addition of sodium-glucose cotransporter 2 inhibitor therapy to standard guideline-directed medical therapy, even if not fully deployed, has the potential to substantially reduce all-cause mortality among patients with heart failure in the United States.
Abstract
Importance
Sodium-glucose cotransporter 2 inhibitor (SGLT2-i) therapy provided incremental survival benefit to patients with heart failure and reduced ejection fraction (HFrEF) who received guideline-directed medical therapy regardless of type 2 diabetes status in a recent clinical trial. To date, estimation of the potential benefits that could be gained from optimal implementation of SGLT2-i therapy at the population level has not been quantified.
Objective
To quantify the projected gains for deaths prevented or postponed with comprehensive implementation of SGLT2-i therapy for patients with HFrEF in the United States.
Design, Setting, and Participants
This decision analytical model, performed from September 25 to October 20, 2019, used published sources to estimate the US population of patients with HFrEF eligible for SGLT2-i therapy and the numbers needed to treat to prevent or postpone overt death. Sensitivity analyses were performed to account for the range of potential benefits.
Main Outcomes and Measures
All-cause mortality.
Results
Of the 3.1 million patients with HFrEF in the United States, 2 132 800 (69%) were projected to be candidates for SGLT2-i therapy. Optimal implementation of SGLT2-i therapy was empirically estimated to prevent up to 34 125 deaths per year (range 21 840-49 140 deaths per year). A secondary analysis excluding patients on the basis of N-terminal–pro brain natriuretic peptide levels and other trial entry criteria would yield a potential benefit of 25 594 deaths per year prevented (range, 16 380-36 855 deaths per year prevented).
Conclusions and Relevance
This study suggests that a substantial number of deaths in the United States could be prevented by optimal implementation of SGLT2-i therapy. These data support implementation of the current evidence into practice in a timely manner to achieve important public health benefits and to reduce the mortality burden of HFrEF.
This decision analytical model quantifies the projected gains for deaths prevented or postponed with comprehensive implementation of sodium-glucose cotransporter 2 inhibitor (SGLT2-i) therapy for patients with heart failure with reduced ejection fraction in the United States.
Introduction
Sodium-glucose cotransporter 2 inhibitor (SGLT2-i) therapy was initially directed toward the management of type 2 diabetes. The first cardiovascular clinical outcome trial of SGLT2-i therapy, the Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME), demonstrated an unexpected and substantial reduction in all-cause mortality, cardiovascular death, and heart failure (HF) hospitalizations among patients with type 2 diabetes.1 Significant reductions in HF hospitalizations were also demonstrated with the SGLT2-i canagliflozin in the Canagliflozin Cardiovascular Assessment Study2 and with dapagliflozin in the Dapagliflozin Effect on Cardiovascular Events trial,3 thus confirming the EMPA-REG OUTCOME findings. Sodium-glucose cotransporter 2 inhibitors are now established as among the most effective therapies to prevent HF in patients with type 2 diabetes.4
More recently, the SGLT2-i dapagliflozin has been demonstrated to reduce HF events, cardiovascular death, and all-cause mortality among patients with HF with reduced ejection fraction (HFrEF) irrespective of whether or not they had type 2 diabetes in the Dapagliflozin and Prevention of Adverse outcomes in Heart Failure (DAPA-HF) trial.5 These landmark data argue for the addition of the SGLT2-i class to the therapeutic armamentarium for HFrEF. Our study aimed to estimate the potential magnitude of benefit with optimal implementation of SGLT2-i therapy at the population level.
Methods
This decision analytical model was performed from September 25 to October 20, 2019. Eligibility for SGLT2-i therapy was based on the population of patients with HF in the American Heart Association Heart Disease and Stroke Statistics 2019 Update.6
The magnitude of mortality reduction for SGLT2-i was determined from the DAPA-HF trial.5 The number needed to treat at 12 months was calculated to estimate the potential lives saved per year with SGLT2-i therapy, as previously described.7 We evaluated the range of benefits using a multilevel analysis-of-extremes method.8 This approach assigns a lower value and an upper value using ±20% relative differences for the number of patients eligible for treatment and risk reduction variables. We further estimated population-level benefits in HF hospitalizations and whether treatment with SGLT2-i therapy was confined to only patients meeting the N-terminal–pro brain natriuretic peptide cutpoints in the DAPA-HF trial.
Results
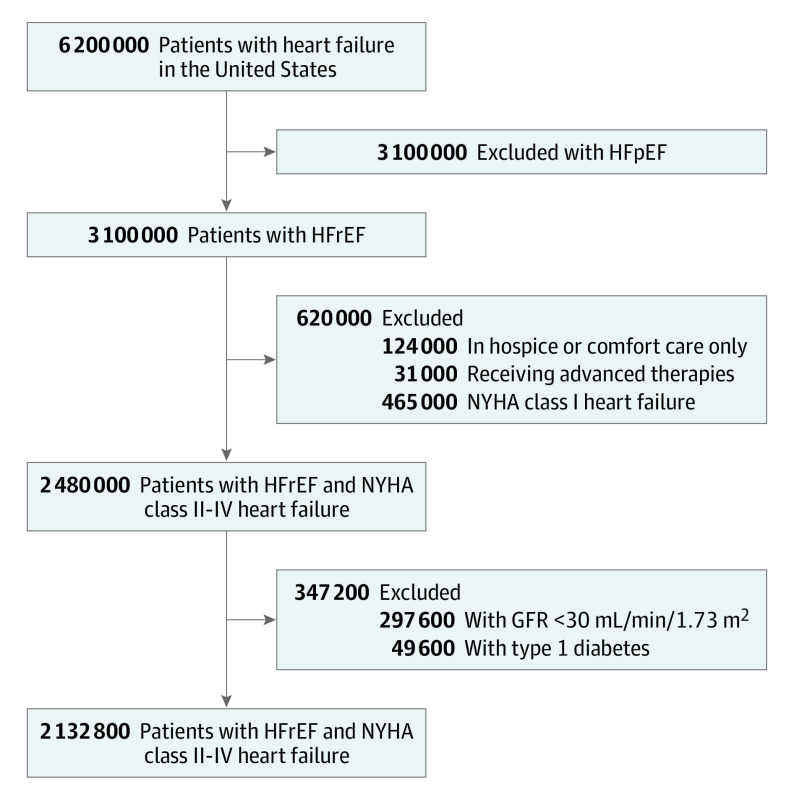
The prevalence of HF is 6 200 000 cases per the entire US population,6 and 50% of these patients have a left ventricular ejection fraction of 40% or less.9 Of the 3 100 000 patients with HFrEF, additional exclusions were applied for those receiving hospice or comfort care–only measures (124 000 [4%]); for those receiving continuous inotropic agents, requiring ventricular assist devices, or requiring urgent heart transplantation (31 000 [1%])7; and for those with New York Heart Association class I HF (465 000 [15%]) (Figure).10 For the remaining 2 480 000 patients with HFrEF, 297 600 (12%)11 were excluded on the basis of having a glomerular filtration rate less than 30 mL/min/1.73 m2, and 49 600 (2%) were excluded on the basis of having type 1 diabetes, leaving 2 132 800 patients as candidates for SGLT2-i therapy. The magnitude of benefit demonstrated with SGLT2-i therapy incremental to guideline-directed medical therapy is shown in Table 1 and Table 2. Based on the DAPA-HF trial, the number needed to treat to prevent 1 death, standardized to 12 months, was calculated to be 62.5 (based on a mortality rate of 7.9 per 100 patient-years with dapagliflozin vs 9.5 per 100 patient-years with placebo added to standard background therapy).5 The number of deaths prevented each year with optimal implementation of SGLT2-i therapy was calculated as 34 125; multiple-way sensitivity analyses using the analysis-of-extremes method yields a range of 21 840 to 49 140 estimated deaths prevented per year with SGLT2-i therapy.
Figure. Sodium-Glucose Cotransporter 2 Inhibitor (SGLT2-i) Therapy Eligibility Flow Diagram.
Derivation of the population of patients with heart failure and reduced ejection fraction eligible for SGLT2-i therapy. GFR indicates glomerular filtration rate; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; and NYHA, New York Heart Association.
Table 1. Demonstrated Benefits of Evidence-Based Therapies for Patients With Heart Failure and Reduced Ejection Fractiona.
| Evidence-based therapy | Relative risk reduction in all-cause mortality in pivotal randomized clinical trial(s), % | NNT to prevent all-cause mortality over timeb | NNT for all-cause mortality | |
|---|---|---|---|---|
| Standardized to 12 mo | Standardized to 36 mo | |||
| ACEI or ARB | 17 | 22 for 42 mo | 77 | 26 |
| ARNIc | 16 | 36 for 27 mo | 80 | 27 |
| β-Blocker | 34 | 28 for 12 mo | 28 | 9 |
| Aldosterone antagonist | 30 | 9 for 24 mo | 18 | 6 |
| Hydralazine and nitrated | 43 | 25 for 10 mo | 21 | 7 |
| CRT | 36 | 12 for 24 mo | 24 | 8 |
| ICD | 23 | 14 for 60 mo | 70 | 23 |
| Transcatheter MVR | 38 | 6 for 24 mo | 12 | 4 |
| SGLT2-i | 17 | 43 for 18 mo | 63 | 22 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; CRT cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; MVR, mitral value repair; NNT, number needed to treat; SGLT2-i, sodium-glucose cotransporter 2 inhibitor.
See the eTable in the Supplement for randomized clinical trials used.
Median duration of follow-up in the respective clinical trial.
Benefit of ARNI therapy incremental to that achieved with ACEI therapy. For the other medications shown, the benefits are based on comparisons with placebo control.
Benefit of hydralazine and nitrate combination therapy was limited to African Americans in this trial.
Table 2. Estimated Cumulative Benefits for All-Cause Mortality of Evidence-Based Medical Therapies for Patients With Heart Failure and Reduced Ejection Fractiona.
| Evidence-based therapy | Relative risk reduction in all-cause mortality, % | Mortality at 24 mo, % | Absolute reduction in all-cause mortality, % |
|---|---|---|---|
| None | NA | 35 | NA |
| ARNI (vs imputed placebo) | 28 | 25 | 10 |
| β-Blocker | 35 | 16 | 9 |
| Aldosterone antagonist | 30 | 12 | 5 |
| SGLT2-i | 17 | 10 | 2 |
| Quadruple therapyb | 73 | 10 | 26 |
Abbreviations: ARNI, angiotensin receptor neprilysin inhibitor; NA, not applicable; SGLT2-i, sodium-glucose cotransporter 2 inhibitor.
The number needed to treat for 24 months with all 4 medical therapies to prevent 1 death = 3.9. This number is based on the premise that the efficacy demonstrated for each successive therapy is cumulative, without overlap or attenuation, and each therapy is optimally dosed, monitored, and adhered to.
ARNI, β-blocker, aldosterone antagonist, and SGLT2-i therapy.
In the sensitivity analysis that accounts for N-terminal–pro brain natriuretic peptide cutoffs and other exclusions in the DAPA-HF trial, an additional 25% of all eligible patients were excluded. Doing so would yield a total number of 1 599 600 eligible patients and a potential to prevent death in 25 594 patients (range, 16 380-36 855 patients).
Discussion
Heart failure is a reported comorbid condition for 1 in every 9 deaths in the United States.12 The prevention of incident HF is the most effective population health strategy through lifestyle interventions, including a heart-healthy diet, abstaining from smoking, and daily physical exercise.13 Second, intensive medical management of modifiable risk factors, such as hypertension, dyslipidemia, and type 2 diabetes, is critical in the prevention of HF. Although there have been many therapeutic advances for patients with HFrEF, there remains substantial residual risk for mortality and rehospitalization. This study defines the population-level projected gains for deaths prevented or postponed with optimal implementation of SGLT2-i therapy for patients with HFrEF.
With the introduction of SGLT2-I therapy, a new class of medications is now available that demonstrates reductions in incident HF, in exacerbations of chronic HF, in cardiovascular death, and in all-cause mortality. More important, for patients with HFrEF in the DAPA-HF trial, the benefits were in addition to the high rates of guideline-directed medical therapy: 96% of patients were receiving β-blockers; 95% were receiving angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or angiotensin receptor neprilysin inhibitors; and 71% were receiving mineralocorticoid receptor antagonists.5 Based on the findings of our study, which is the first, to our knowledge, to quantify the magnitude of survival benefit in the United States, if SGLT2-i therapy were comprehensively applied to eligible patients with HFrEF, then up to 34 125 deaths per year could be prevented or postponed.
Establishing the potential population-level benefits with optimal implementation of a new HF therapy may be informative when considering the commitment of resources and national quality improvement efforts toward implementation of the new therapy. The fact that tens of thousands of lives could be saved with appropriate adoption of SGLT2-i therapy for eligible patients suggests that there may be downsides to delaying implementation of this new therapy in clinical practice. Although this study focused on mortality, SGLT2-i therapy has been shown to have other clinical benefits, including improved health status and reduced HF hospitalizations.5 Given the variable and slow uptake of other evidence-based HF therapies, compelling guideline recommendations as well as other effective performance improvement interventions will be required for meaningful population-level implementation, as previously described.11
The underlying premise of this analysis is that the magnitude of efficacy demonstrated in the DAPA-HF trial will be similar for patients with HFrEF encountered in clinical practice. This premise may or may not be the case, particularly for patient subgroups not adequately represented in the trial. In addition, the expected survival benefits of implementation of SGLT2-i therapy is contingent on being able to apply this therapy with similar levels of safety, tolerability, and dosing as achieved in clinical trials. This study does not account for a potential increase in adverse events at the population level, including diabetic ketoacidosis, infections, fracture, and Fournier gangrene, although these rates did not differ from those seen with placebo in the DAPA-HF trial. The DAPA-HF trial demonstrated substantial benefits to patients regardless of whether the patients had type 2 diabetes or not. There was no heterogeneity for the primary composite end point or for mortality reduction by diabetes status in the DAPA-HF trial. If subsequent trial data reveal different magnitudes of survival benefits by diabetes status, the population-wide estimations may need to be further refined.
Limitations
There are important limitations to our analysis with respect to inclusion and exclusion criteria, cost, access, and adherence. The DAPA-HF trial studied 4744 carefully selected patients for a median of 18.2 months and excluded many patients with HF seen in daily practice with the following attributes: New York Heart Association class I HF, advanced-stage chronic kidney disease, and type 1 diabetes. The exact proportion of eligible patients with contraindications for SGLT2-i therapy may deviate from the estimates used in this study. Second, SGLT2-i therapy represents an additional cost burden to patients with HF and health systems. Although formal cost-effectiveness analyses are warranted, the financial implications of a population-level implementation are likely to be prohibitive at current price levels. Third, dapagliflozin was provided to patients enrolled in the DAPA-HF trial, and adherence was supported and evaluated by a team of health care professionals—2 key attributes that may not be available to many patients in clinical practice. Fourth, as with any novel and efficacious therapy representing a paradigm shift, widespread adoption of SGLT2-i therapy into clinical practice will be a challenge. Given the fact that many outpatient registry patients with HFrEF are not receiving optimal therapy with the current armamentarium of guideline-directed medical therapy (ie, β-blockers, mineralocorticoid receptor antagonists, or angiotensin receptor neprilysin inhibitors),14 it represents an extra challenge for the prescriber and patient to add yet another medication to the medication list. Yet our analysis demonstrates that this is an effort worth undertaking given the substantial increase in potential lives saved.
The DAPA-HF trial is the first trial to demonstrate substantial benefit in patients with HFrEF without type 2 diabetes. The results from the Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction,15 which will study the use of the SGLT2-i empagliflozin in the treatment of patients with HFrEF, half of whom do not have type 2 diabetes, as well as other HF outcome trials, are upcoming.
Conclusions
This study suggests that a substantial mortality benefit can be achieved with the implementation of SGLT2-i therapy on a population-wide basis for appropriately selected patients with HFrEF. Given the substantial HF burden and potential benefits of implementation of SGLT2-i therapy for preventing deaths, new and potentially disruptive efforts to ensure comprehensive implementation of SGLT2-i therapy should be considered.
eTable. Randomized Clinical Trials
References
- 1.Zinman B, Wanner C, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 2.Neal B, Perkovic V, Mahaffey KW, et al. ; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Raz I, Bonaca MP, et al. ; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 4.Vardeny O, Vaduganathan M. Practical guide to prescribing sodium-glucose cotransporter 2 inhibitors for cardiologists. JACC Heart Fail. 2019;7(2):169-172. doi: 10.1016/j.jchf.2018.11.013 [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJV, Solomon SD, Inzucchi SE, et al. ; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995-2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 6.Benjamin EJ, Muntner P, Alonso A, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56-e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence-based heart failure therapies on mortality. Am Heart J. 2011;161(6):1024-1030. doi: 10.1016/j.ahj.2011.01.027 [DOI] [PubMed] [Google Scholar]
- 8.Chew DP, Huynh LT, Liew D, Astley C, Soman A, Brieger D. Potential survival gains in the treatment of myocardial infarction. Heart. 2009;95(22):1844-1850. doi: 10.1136/hrt.2009.174276 [DOI] [PubMed] [Google Scholar]
- 9.Steinberg BA, Zhao X, Heidenreich PA, et al. ; Get With the Guidelines Scientific Advisory Committee and Investigators . Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126(1):65-75. doi: 10.1161/CIRCULATIONAHA.111.080770 [DOI] [PubMed] [Google Scholar]
- 10.Braunschweig F, Linde C, Benson L, Ståhlberg M, Dahlström U, Lund LH. New York Heart Association functional class, QRS duration, and survival in heart failure with reduced ejection fraction: implications for cardiac resychronization therapy. Eur J Heart Fail. 2017;19(3):366-376. doi: 10.1002/ejhf.563 [DOI] [PubMed] [Google Scholar]
- 11.Fonarow GC, Albert NM, Curtis AB, et al. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122(6):585-596. doi: 10.1161/CIRCULATIONAHA.109.934471 [DOI] [PubMed] [Google Scholar]
- 12.Yancy CW, Jessup M, Bozkurt B, et al. ; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines . 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-e239. doi: 10.1016/j.jacc.2013.05.019 [DOI] [PubMed] [Google Scholar]
- 13.Larsson SC, Tektonidis TG, Gigante B, Åkesson A, Wolk A. Healthy lifestyle and risk of heart failure: results from 2 prospective cohort studies. Circ Heart Fail. 2016;9(4):e002855. doi: 10.1161/CIRCHEARTFAILURE.115.002855 [DOI] [PubMed] [Google Scholar]
- 14.Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018;72(4):351-366. doi: 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 15.Packer M, Butler J, Filippatos GS, et al. ; EMPEROR-Reduced Trial Committees and Investigators . Evaluation of the effect of sodium-glucose co-transporter 2 inhibition with empagliflozin on morbidity and mortality of patients with chronic heart failure and a reduced ejection fraction: rationale for and design of the EMPEROR-Reduced trial. Eur J Heart Fail. 2019;21(10):1270-1278. doi: 10.1002/ejhf.1536 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Randomized Clinical Trials