Key Points
Question
Has association between genetic factors and autism spectrum disorders (ASDs) changed over time?
Findings
In this study, data were available from 2 twin cohorts, one born between 1982 and 2008 (n = 22 678 pairs) and the other between 1992 and 2008 (n = 15 279 pairs). Genetic factors were associated with ASD and autistic traits and the relative importance of these factors was consistent over time, whereas environmental factors played a smaller role.
Meaning
Environmental factors associated with ASD have not increased in importance over time and are unlikely to explain the apparent increase in the prevalence of ASD.
Abstract
Importance
The frequency with which autism spectrum disorders (ASDs) are diagnosed has shown a marked increase in recent years. One suggestion is that this is partly because of secular changes in the environment, yet to our knowledge this hypothesis lacks evidence.
Objective
To assess whether the relative importance of genetic and environmental associations with ASD and autistic traits has changed over a 16-year and 26-year period.
Design, Setting, and Participants
A twin design was used to assess whether the heritability of ASD and autistic traits has changed over time. Data from 2 nationwide Swedish twin cohorts was used: the Swedish Twin Registry (STR; participants born between January 1982 and December 2008) and the Child and Adolescent Twin Study in Sweden (CATSS; participants born between January 1992 and December 2008). Autism spectrum disorder diagnoses were identified for twins in the STR, with follow-up to 2013. Questionnaires assigned screening diagnoses of ASD to CATSS participants and assessed autistic traits. Analyses were performed from September 1, 2018, to March 31, 2019.
Exposures
Each sample was divided into several birth cohorts covering 1982 to 1991 (for the STR only), 1992-1995, 1996-1999, 2000-2003, and 2004-2008.
Outcomes
We assessed whether the genetric and environment variance underlying autistic traits changed across birth cohorts and examined whether the relative contribution of genetics and environment to liability for autism changed across birth cohorts.
Results
Data were available for 22 678 twin pairs (5922 female same-sex pairs [26.1%], 5563 male same-sex pairs [24.5%], and 11193 opposite-sex pairs [49.4%]) in the STR and 15 280 pairs (4880 female same-sex pairs [31.9%], 5092 male same-sex pairs [33.3%], and 5308 opposite-sex pairs [34.7%]) in CATSS. The heritability of ASD diagnoses in the STR ranged from 0.88 (95% CI, 0.74-0.96) to 0.97 (95% CI, 0.89-0.99). The heritability of screening diagnoses in CATSS varied from 0.75 (95% CI, 0.58-0.87) to 0.93 (95% CI, 0.84-0.98). Autistic traits showed a modest variance increase over time that was associated with increases in genetic and environmental variance, with the total variance increasing from 0.95 (95% CI, 0.92-0.98) to 1.17 (95% CI, 1.13-1.21) over time.
Conclusions and Relevance
Weak evidence was found for changes in the genetic and environmental factors underlying ASD and autistic traits over time. Genetic factors played a consistently larger role than environmental factors. Environmental factors are thus unlikely to explain the increase in the prevalence of ASD.
This cohort study assesses changes in the genetic and environmental variance underlying autism spectrum disorder and autistic traits over time in Sweden.
Introduction
Autism spectrum disorders (ASDs) are neurodevelopmental disorders that affect social communication and behavioral routines.1 While previously estimated to affect as few as 1 in 10 000 individuals in the 1960s,2 prevalence studies from the 1980s suggested that as many as 72 in 10 000 individuals had ASD, rising to 1% in the 2000s.2,3 More recent studies report prevalence rates of more than 2%.4,5,6
Debate has focused on the reasons for this trend. One argument is that it arises from secular changes in diagnostic practices over time. A Swedish study showed that although ASD diagnoses became more common over time among individuals born between 1992 and 2002, the underlying level of autistic traits did not.7 Subsequent studies indicated decreasing differences between individuals with and without ASD on symptom measures and cognitive test results.8,9 However, only a third of ASD diagnoses in Denmark could be explained by broadened diagnostic criteria in a recent study, emphasizing the need to consider alternative explanations.10 One possibility is that environmental factors associated with ASD have become more common over time, leading to a genuine increase in the prevalence of ASD.11 Environmental exposures associated with ASD tend to occur during the prenatal period, such as air pollution exposure, paternal age, and maternal psychotropic medication use during pregnancy.12,13
Twin methods provide a tool for testing this hypothesis because they can compare the magnitude of genetic and environmental contributions with a trait over different groups, such as individuals born during different periods. If changes in the environment over time account for the changing prevalence of a trait, then one hypothesis is that the environmental variance would be expected to increase over time. If the underlying genetic variance showed little or no change, then one expectation is that the heritability of a given trait would also decrease. To our knowledge, no prior twin studies have investigated whether this is true for ASD.
We used data from 2 twin cohorts born over 16-year and 26-year periods to compare the degree with which ASD was associated with genetic and environmental factors over time, including clinical and broader screening diagnoses, to replicate results across differing definitions of ASD. We further used a continuous measure of autistic traits, which enabled us to examine whether the genetic and environmental variance associated with autistic traits has fluctuated over time (eAppendix 1 in the Supplement).
Methods
Participants
Swedish Twin Registry
This study and both of the samples and their linkage with registries have ethical approval from the Regional Ethical Review Board in Stockholm, Sweden. When twins are born in Sweden, their families are invited to register in the Swedish Twin Registry (STR).14 The STR was linked with the Medical Birth Register (MBR), which records births in Sweden since 1973, to identify twins born between January 1982 and December 2008. We identified additional opposite-sex twins from the MBR, defined as individuals of opposite sex born on the same day to the same mother. We identified 22 678 twin pairs who were divided into 5 birth cohort groups: 1982 to 1991, 1992 to 1995, 1996 to 1999, 2000 to 2003, and 2004 to 2008. The sample sizes are in Table 1. Families registered in the STR provide written informed consent before registration, although the requirement for informed consent is waived for the registry linkage.
Table 1. Descriptive Statistics.
| Characteristic | No. (%) | |||||
|---|---|---|---|---|---|---|
| Overall | 1982-1991 | 1992-1995 | 1996-1999 | 2000-2003 | 2004-2008 | |
| Swedish Twin Registry | ||||||
| No. | 23 639 | 6079 | 4829 | 4656 | 4404 | 3671 |
| Sex/zygosity | ||||||
| MZF | 3091 (13.1) | 1100 (18.1) | 655 (13.6) | 521 (11.2) | 513 (11.6) | 302 (8.2) |
| DZF | 2831 (12.0) | 643 (10.6) | 618 (12.8) | 643 (13.8) | 609 (13.8) | 318 (8.7) |
| MZM | 2604 (11.0) | 742 (12.2) | 593 (12.3) | 488 (10.5) | 468 (10.6) | 313 (8.5) |
| DZM | 2959 (12.5) | 466 (7.7) | 739 (15.3) | 720 (15.5) | 705 (16.0) | 329 (9.0) |
| DZOS | 11 193 (47.3) | 2983 (49.1) | 1914 (39.6) | 1918 (41.2) | 2036 (46.2) | 2342 (63.8) |
| Unknown | 961 (4.1) | 145 (2.4) | 310 (6.4) | 366 (7.9) | 73 (1.7) | 67 (1.8) |
| ASD descriptives | ||||||
| Clinical ASDa | 715 (1.5) | 140 (1.2) | 157 (1.6) | 176 (1.9) | 160 (1.8) | 82 (1.1) |
| Child and Adolescent Twin Study in Sweden | ||||||
| No. | 15 524 | NA | 3777 | 3932 | 3825 | 3990 |
| Sex/zygosity | ||||||
| MZF | 2377 (15.3) | 612 (16.2) | 542 (13.8) | 529 (13.8) | 694 (17.4) | |
| DZF | 2503 (16.1) | 597 (15.8) | 670 (17.0) | 626 (16.4) | 610 (15.3) | |
| MZM | 2201 (14.2) | 571 (15.1) | 512 (13.0) | 481 (12.6) | 637 (16.0) | |
| DZM | 2891 (18.6) | 745 (19.7) | 754 (19.2) | 720 (18.8) | 672 (16.8) | |
| DZOS | 5308 (34.2) | 1208 (32.0) | 1401 (35.6) | 1409 (36.8) | 1290 (32.3) | |
| Unknown | 244 (1.6) | 44 (1.2) | 53 (1.3) | 60 (1.6) | 87 (2.2) | |
| ASD descriptives | ||||||
| A-TAC ASD, mean (SD) | 0.85 (1.65) | 0.80 (1.57) | 0.75 (1.54) | 0.79 (1.58) | 1.03 (1.85) | |
| Broad ASD | 1244 (4.0) | 274 (3.6) | 247 (3.1) | 284 (3.7) | 439 (5.5) | |
| Clinical ASDa | 434 (1.4) | 127 (1.7) | 139 (1.8) | 126 (1.6) | 42 (0.5) | |
Abbreviations: ASD, autism spectrum disorder; A-TAC, Autism, Tics, Attention-Deficit/Hyperactivity Disorder, and Other Comorbidities Inventory; DZF, dizygotic female; DZM, dizygotic male; DZOS, dizygotic opposite sex; MZF, monozygotic female; MZM, monozygotic male; NA, not applicable.
Clinical ASD, recorded ASD diagnosis in the National Patient Register; Broad ASD, screening diagnosis of ASD, based on scoring 4.5 or more on the A-TAC.
Child and Adolescent Twin Study in Sweden
Families of Swedish twins born since 1992 are invited to participate in the Child and Adolescent Twin Study in Sweden (CATSS) in connection with the twins’ ninth or 12th birthdays.15 A new cohort is recruited each year; data were collected from individuals born in every year between 1992 and 2008. The sample of 15 280 twin pairs was divided into 4 birth cohort groups: 1992 to 1995, 1996 to 1999, 2000 to 2003, and 2004 to 2008. The sample sizes are in Table 1. CATSS is based on identifying twins using the STR, meaning that there was some overlap in participants, with 70.7% of individuals in the STR born from 1992 to 2008 participating in CATSS. CATSS participants provided written informed consent.
Measures
Diagnoses of ASD
We identified ASD diagnoses by linking the STR with the National Patient Register, which records inpatient and outpatient diagnoses,16 with follow-up to December 31, 2014. The ASD diagnoses were defined based on International Classification of Diseases, Ninth Revision (ICD-9) code 299A and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code F84 (excluding Rett Syndrome, other childhood disintegrative disorders, and overactive disorder associated with intellectual disability and stereotyped movements). The National Patient Register is continuously updated and validated. The ASD diagnoses were specifically validated for Stockholm Country, with 96% of diagnoses confirmed through an independent review of case notes.17 Table 1 shows the number of individuals in the STR with ASD.
Screening diagnoses of ASD were assigned to CATSS participants using the Autism-Tics, Attention-Deficit/Hyperactivity Disorder, and Other Comorbidities Inventory (A-TAC), a structured, parental telephone interview.18 Seventeen questions assess ASD and can be answered yes (scored as 1), yes, to a certain degree (0.5), or no (0). Scores range from 0 to 17. Scores of 4.5 or more denote a broad screening diagnosis of ASD, which has a sensitivity of 0.71 and specificity of 0.96.19 Similar validity was replicated in a longitudinal follow-up.20 We used a broad definition of ASD to increase power and identify individuals with elevated ASD symptoms who may not have received a diagnosis. The number of individuals with broad ASD is in Table 1.
Autistic Traits
The A-TAC ASD scale was also used as a continuous measure of autistic traits. Descriptive statistics for the scale are in Table 1.
Data Analysis
All twin analyses were conducted using the OpenMx package of R (R Foundation).21 Statistical significance was set at P < .05.
The Twin Design
The twin design estimates the relative contribution of genetic and environmental factors to variation in a trait and assumes that such variation comprises 3 components: additive genetic (A), nonshared environment (E), which includes measurement errors, and either non-A (D) or shared environments (C). Only 1 of the latter 2 components can be estimated because they confound one another in the classical twin design. These components are estimated based on comparing the phenotypic resemblance of monozygotic (MZ) twins, who share all their segregating DNA code, with dizygotic (DZ) twins, who share 50% on average. Increased MZ resemblance compared with DZ resemblance signifies that a trait is associated with genetic factors. A more detailed description of the twin design is provided elsewhere.22
Analysis of ASD Diagnoses
We analyzed categorical variables using liability threshold models, which assume a continuous distribution of liability underlies such variables. The proportions of liability that were genetic and environmental and a liability threshold were estimated. Univariate models were used to gain an indication of the degree to which ASD diagnoses were associated with genetic and environmental factors. We then used a quantitative model to test whether the relative importance of these associations changed over time. This model allowed each variance component and thresholds to differ across birth cohorts. The total variance was constrained to 1 in these models to ensure identification. We then equated the thresholds and variance components to be equal across groups. These constrained models were compared with the quantitative model using the likelihood-ratio test. Statistically significant results suggest that the constrained model is a poorer fit to the data then the quantitative model, thus suggesting statistically significant changes in the variance components over time. We only tested ACE models rather than ADE models because univariate analyses did not suggest D and to increase power. Heritability estimates should thus be interpreted as broad-sense heritability. We performed these analyses for ASD diagnoses in the STR and broad screening diagnoses of ASD in CATSS.
Analysis of Autistic Traits
All analyses were repeated for autistic traits in CATSS. However, in these analyses twin models decomposed continuous variation in autistic traits into genetic and environmental components and estimated the mean level of and variance in autistic traits for each cohort. We examined whether the total variance underlying autistic traits had changed over time as well as the underlying total genetic and environmental variance. These estimates were then used to calculate the proportion of total variance explained by genetic and environmental factors in each birth cohort.
Sample Characteristics
To ascertain whether the STR and CATSS are representative of the Swedish population across time, we identified information about parental education, parental age, birth weight, and gestational age. We linked the STR with the Longitudinal Integrated Database for Labor Market and Sickness Insurance Research and the MBR to identify these variables for each cohort. In CATSS, parental reports were used for all variables except for birth weight and gestational age, which were identified from the MBR. We also identified this information for all individuals born in Sweden from 1982 to 2008 (n = 2 700 244). We used regression models to test associations between the birth cohorts and each variable.
Results
The univariate results are in eTables 1, 2, 3, and 4 in the Supplement. Such analyses did not suggest qualitative sex differences, hence opposite-sex twins were included and assumed equivalent to same-sex twins. Twin correlations and model estimates for each birth cohort are in eTables 5 and 6 in the Supplement and Figure 1 and Figure 2.
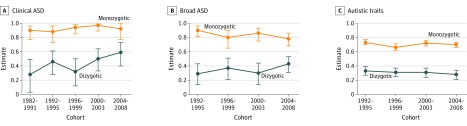
Figure 1. Twin Correlations by Birth Cohort.
A, Clinical autism spectrum disorder (ASD): recorded diagnosis of ASD in the National Patient Register. B, Broad ASD: screening diagnosis of ASD defined by a score of 4.5 or more on the Autism-Tics, Attention-Deficit/Hyperactivity Disorder, and Other Comorbidities Inventory.
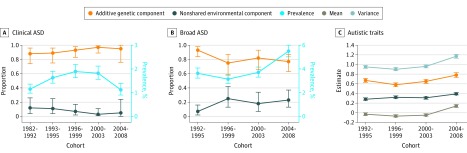
Figure 2. Twin Model Estimates by Birth Cohort.
A, Clinical autism spectrum disorder (ASD): recorded diagnosis of ASD in the National Patient Register. B, Broad ASD: screening diagnosis of ASD defined by a score of 4.5 or more on the Autism-Tics, Attention-Deficit/Hyperactivity Disorder, and Other Comorbidities Inventory. The prevalence of each ASD diagnosis is given in place of thresholds here for ease of interpretation. The analyses of autistic traits show the variance components as the standardized total amounts of variance as opposed to proportions.
Analysis of ASD Diagnoses
Clinical Diagnoses
Across the birth cohorts, the heritability of ASD was 0.93 (95% CI, 0.93-0.96) (eTable 4 in the Supplement). The twin correlations were higher for MZ than DZ twins in all cohorts, indicating that ASD was consistently associated with genetic factors over time (Figure 1). The prevalence of ASD increased across cohorts (Figure 2), and thus thresholds in the twin models could not be equated over birth cohorts (see Table 2 for model fit statistics). C could be dropped. As shown in Figure 2, the heritability of ASD ranged from 0.88 (95% CI, 0.74-0.96) to 0.97 (95% CI, 0.89-0.99) and could be equated over cohorts (Table 2). The eFigure in the Supplement shows the statistical power of this model, calculated using R functions published by Verhulst.23
Table 2. Twin Model Fit Statistics.
| Model | −2LLa | Parameters | df | AICb | BICc | Comparison model | Δχ2d | Δdfe | P value |
|---|---|---|---|---|---|---|---|---|---|
| Clinical ASD | |||||||||
| Fully saturatedf | 6693.49 | 30 | 45326 | −83958.51 | −447887.78 | NA | NA | NA | NA |
| ACE | 6753.43 | 20 | 45346 | −83938.57 | −448028.43 | Fully saturated | 59.93 | 20 | <.001 |
| AE | 6755.15 | 15 | 45351 | −83946.85 | −448076.86 | ACE | 1.72 | 5 | .89 |
| CE | 6843.25 | 15 | 45351 | −83858.75 | −447988.76 | ACE | 89.82 | 5 | <.001 |
| E | 7110.52 | 10 | 45356 | −83601.48 | −447771.63 | ACE | 357.1 | 10 | <.001 |
| Eq threshg | 6783.64 | 11 | 45355 | −83926.36 | −448088.48 | AE | 28.49 | 4 | <.001 |
| Hom | 6758.70 | 7 | 45359 | −83959.30 | −448153.54 | AE | 3.55 | 8 | .90 |
| Broad ASD | |||||||||
| Fully saturated | 7028.33 | 24 | 24188 | −41347.67 | −220420.01 | NA | NA | NA | NA |
| ACE | 7101.09 | 16 | 24204 | −41306.91 | −220497.71 | Fully saturated | 72.76 | 16 | <.001 |
| AE | 7101.44 | 12 | 24208 | −41314.56 | −220534.97 | ACE | 0.35 | 4 | .99 |
| CE | 7182.24 | 12 | 24208 | −41233.76 | −220454.16 | ACE | 81.16 | 4 | <.001 |
| E | 7383.26 | 8 | 24212 | −41040.74 | −220290.77 | ACE | 282.17 | 8 | <.001 |
| Eq thresh | 7113.87 | 9 | 24211 | −41308.13 | −220550.75 | AE | 12.43 | 3 | .01 |
| Hom | 7108.42 | 6 | 24214 | −41319.58 | −220584.41 | AE | 6.98 | 6 | .32 |
| Autistic traits | |||||||||
| Fully saturated | 81972.81 | 40 | 30432 | 21108.81 | −211218.21 | NA | NA | NA | NA |
| ACE | 82190.13 | 16 | 30456 | 21278.13 | −211232.11 | Fully saturated | 217.32 | 24 | <.001 |
| AE | 82191.24 | 12 | 30460 | 21271.23 | −211269.54 | ACE | 1.10 | 4 | .89 |
| CE | 83005.32 | 12 | 30460 | 22085.32 | −210455.46 | ACE | 815.19 | 4 | <.001 |
| E | 85986.30 | 8 | 30464 | 25058.30 | −207513.02 | ACE | 3796.16 | 8 | <.001 |
| Eq meanh | 82324.13 | 9 | 30463 | 21398.13 | −211165.56 | AE | 132.89 | 3 | <.001 |
| Homi | 82361.15 | 6 | 30466 | 21429.15 | −211157.44 | AE | 169.91 | 6 | <.001 |
Abbreviations: −2LL, −2*log-likelihood; A, additive genetic component; AIC, Akaike Information Criteria; BIC, Bayesian Information Criteria; C, shared environmental component; E, nonshared environmental component; Eq, equate; Hom, homogeneity; NA, not applicable.
−2LL: fit statistic, which is −2*log-likelihood of the data.
AIC, an alternative fit index. Lower values denote better model fits.
BIC, an alternative fit index. Lower values denote better model fits.
Δχ2: difference in −2LL between two models, distributed χ2.
Δdf: difference in degrees of freedom between 2 models, which is equal to the difference in number of parameters.
Fully saturated: a baseline model of the observed data, which models the thresholds separately for each twin in a pair and across zygosity (or variances and means for autistic traits).
Eq thresh: constrained model in which the thresholds were equated across birth cohorts.
Eq mean: constrained model in which the mean was equated across birth cohorts.
Hom: homogeneity model, in which the variance components (and total variance for autistic traits) were equated across birth cohorts.
Screening Diagnoses
The heritability of broad screening diagnoses of ASD was 0.82 (eTable 4 in the Supplement). Twin correlations were higher in MZ twins than DZ twins over time ( Figure 1), indicating consistently high heritability. The thresholds in the twin model could not be equated over time (Table 2). As for clinical diagnoses, C could be dropped. As shown in Figure 2, the heritability of these diagnoses varied from 0.77 to 0.93 over time, yet these estimates could be equated across cohorts (Table 2).
Analysis of Autistic Traits
The heritability of autistic traits was 0.61 to 0.73 (eTable 4 in the Supplement). The MZ correlations exceeded the DZ correlations in all cohorts (Figure 1), indicating consistently strong heritability of autistic traits. The mean level of autistic traits showed a modest increase that could not be equated over cohorts without a statistically significant deterioration of model fit (Table 2). C was dropped. The total variance in autistic traits increased over time (Figure 2). This could not be equated across birth cohorts (Table 2). The underlying genetic and environmental variance also increased between the first and last cohorts. The proportion of the total variance explained by each component was relatively consistent over time, with heritability estimates from 0.64 to 0.70.
Sample Characteristics
Comparisons of the characteristics of each cohort are shown in eTables 7 through 10 in the Supplement. More parents had a college education among later cohorts in the STR and CATSS. Mean parental ages increased over time. This pattern was reflected in the Swedish population.
Post Hoc Analyses
To test whether the increasing means and variances in autistic traits might account for the increase in diagnoses, we calculated the expected proportion of individuals above a hypothetical threshold for ASD in each cohort given the varying means and variances. Full details are in eAppendix 2 and eTable 11 in the Supplement. More individuals were expected to be above a diagnostic threshold for ASD over time based on these means and variances. This was more pronounced when the variance was permitted to differ over time, whereas means were assumed equal over time.
Discussion
Increasing rates of ASD diagnoses in recent years have triggered debate around the reasons for this increase. We assessed changes in the genetic and environmental variance underlying ASD and autistic traits over time. There was an increase in the environmental variance underlying autistic traits, which was accompanied by a similar small increase in genetic variance. We observed minor fluctuations in the heritability of ASD and associated autistic traits. Despite these fluctuations, genetic factors consistently played a stronger role than environmental factors in ASD. Our results thus do not provide strong evidence that the etiology of ASD and autistic traits has changed over time.
The degree to which environmental factors contributed to ASD remained relatively constant over time despite its increased prevalence. This aligns with prior twin studies of ASD that have consistently reported a high heritability of clinical diagnoses24,25,26 and autistic traits.27,28,29,30,31,32 An emerging emphasis on environmental factors may stem from 3 contradictory studies, suggesting that environmental factors were of at least equal importance as genetic factors in ASD.33,34,35 It is important to note some caveats about these studies when reconciling our results, as well as those of other twin studies, with these studies. A review article highlighted that a model that estimated a higher heritability of ASD was not a poorer fit with the observed data than the reported model in one study.33,36 In another study, a reanalysis of the same data yielded a much larger heritability estimate.34,37 The overall sum of the evidence is that ASD is strongly heritable. Our results concur with a meta-analysis of twin studies that reported strong heritability and a modest role for environment across studies.38
Our results add further robustness to the notion that ASD is strongly heritable, in that more studies have replicated this result in different contexts. Most earlier twin studies of ASD were conducted in northern Europe and the US; subsequent studies show that ASD is heritable outside of these countries, including Japan,39 and more recently Israel and Australia.40 Autistic traits are also heritable across multiple developmental periods.41,42 Our results add further to these replications by extending them over time.
However, our results do not dismiss a role for environmental factors in ASD and concur with prior studies suggesting that these factors are likely to be nonshared rather than shared. Nonshared environmental factors contribute toward differences between relatives sharing the same home (eg, birth weight in association with ASD).43 This could also include measurement error and a change in measurement error over time could also be associated with the nonshared environmental variance components underlying ASD and autistic traits. The relatively modest nonshared environmental variance, combined with the consistency of the estimates of nonshared environment over time, also indicates that gene by nonshared environmental interaction is unlikely to play a large role in ASD and autistic traits. Such effects would be expected to inflate nonshared environmental variance components and produce changes in these components across birth cohorts.44
However, our study did show a small increase in the mean level of and variance in autistic traits, specifically among individuals born between 2004 and 2008. Notably, a similar increase has been shown for traits of attention-deficit/hyperactivity disorder within CATSS.45 Several factors could have contributed to this increase. First, increased awareness and reporting of ASD could have led to a wider spread of autistic trait scores. Second, we also found that the genetic and environmental variance underlying autistic traits had shown small increases over time. There could thus be modest changes to the environmental factors associated with autistic traits. Alternatively, we cannot rule out the possibility that measurement errors contributed to the seeming increase in environmental variance underlying autistic traits. However, these arguments should be tempered with reference to the fact that the increases in means and variances over time were generally small and likely only statistically significant because of the large sample. Our results thus do not suggest that it is likely that environmental factors can explain the rising prevalence of ASD. Nonetheless, follow-up of subsequent birth cohort, and the replication of our results in independent samples would help to further clarify the variance changes in autistic traits.
Strengths and Limitations
Our study had several strengths. We used data from 2 of the largest twin cohorts in the world, whose participants were born over multiple decades. Registry linkage and questionnaire data also enabled us to use multiple informants (parents and clinicians) and allowed the replication of our results for multiple definitions of ASD. However, we are unable to reach strong conclusions around whether specific environmental factors of relevance to ASD have become more common over time. Therefore, we cannot rule out the possibility that our results are driven by some factors becoming more common while others have become less common. Our results should also be replicated outside of Sweden because the frequency of certain environmental exposures could differ across countries. One may question whether our results generalize beyond twins, albeit the mean level of autistic traits does not seem to differ between twins and singletons.46 While we did not detect statistically significant changes in the heritability of diagnoses of ASD over time, it would have been informative to be able to examine the raw variance underlying these diagnoses, which was not possible because of the use of a binary variable in the analyses. Because of the etiological consistency between ASD and autistic traits,24,47 we believe such analyses would yield similar results to autistic traits, but future studies should seek to replicate our results using ordinal definitions of ASD. There was also overlap in participants in the STR and CATSS, albeit we focused on different variables in these cohorts. Furthermore, the STR comprised an additional cohort of individuals born from 1982 to 1991, introducing some additional independent participants to the sample.
Conclusions
This study suggests that the relative importance of genetic and environmental factors in association with ASD has not changed markedly over time. This is despite an increase in the rate of clinical diagnoses of ASD and a slight increase in the environmental variance underlying autistic traits. Our results emphasize the enduring importance of genetic factors. These results thus do not suggest that environmental factors explain the increasing prevalence of ASD.
eAppendix 1. Description of measures
eAppendix 2. Posthoc analyses
eTable 1. Twin correlations
eTable 2. Assumption testing
eTable 3. Model fit statistics
eTable 4. Model estimates
eTable 5. Twin correlations by birth cohort
eTable 6. Model estimates by birth cohort
eTable 7. Descriptive statistics for the Swedish Twin Registry
eTable 8. Descriptive statistics for CATSS
eTable 9. Descriptive statistics for the total Swedish population born 1982-2008
eTable 10. Regression models of the association between birth cohort and each characteristic
eTable 11. Twin correlations by birth cohort
eFigure. Power for the liability threshold model of the STR
References
- 1.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013. [Google Scholar]
- 2.Elsabbagh M, Divan G, Koh Y-J, et al. . Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012;5(3):160-179. doi: 10.1002/aur.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baird G, Simonoff E, Pickles A, et al. . Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: the Special Needs and Autism Project (SNAP). Lancet. 2006;368(9531):210-215. doi: 10.1016/S0140-6736(06)69041-7 [DOI] [PubMed] [Google Scholar]
- 4.Baio J, Wiggins L, Christensen DL, et al. . Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 2018;67(6):1-23. doi: 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schendel DE, Thorsteinsson E. Cumulative incidence of autism into adulthood for birth cohorts in Denmark, 1980-2012. JAMA. 2018;320(17):1811-1813. doi: 10.1001/jama.2018.11328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim YS, Leventhal BL, Koh Y-J, et al. . Prevalence of autism spectrum disorders in a total population sample. Am J Psychiatry. 2011;168(9):904-912. doi: 10.1176/appi.ajp.2011.10101532 [DOI] [PubMed] [Google Scholar]
- 7.Lundström S, Reichenberg A, Anckarsäter H, Lichtenstein P, Gillberg C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. BMJ. 2015;350(2):h1961-h1961. doi: 10.1136/bmj.h1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arvidsson O, Gillberg C, Lichtenstein P, Lundström S. Secular changes in the symptom level of clinically diagnosed autism. J Child Psychol Psychiatry. 2018;59(7):744-751. doi: 10.1111/jcpp.12864 [DOI] [PubMed] [Google Scholar]
- 9.Rødgaard E-M, Jensen K, Vergnes J-N, Soulières I, Mottron L. Temporal changes in effect sizes of studies comparing individuals with and without autism: a meta-analysis. JAMA Psychiatry. 2019. doi: 10.1001/jamapsychiatry.2019.1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen SN, Schendel DE, Parner ET. Explaining the increase in the prevalence of autism spectrum disorders: the proportion attributable to changes in reporting practices. JAMA Pediatr. 2015;169(1):56-62. doi: 10.1001/jamapediatrics.2014.1893 [DOI] [PubMed] [Google Scholar]
- 11.Hertz-Picciotto I, Schmidt RJ, Krakowiak P. Understanding environmental contributions to autism: causal concepts and the state of science. Autism Res. 2018;11(4):554-586. doi: 10.1002/aur.1938 [DOI] [PubMed] [Google Scholar]
- 12.Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. 2017;8(1):13. doi: 10.1186/s13229-017-0121-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bölte S, Girdler S, Marschik PB. The contribution of environmental exposure to the etiology of autism spectrum disorder. Cell Mol Life Sci. 2019;76(7):1275-1297. doi: 10.1007/s00018-018-2988-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252(3):184-205. doi: 10.1046/j.1365-2796.2002.01032.x [DOI] [PubMed] [Google Scholar]
- 15.Anckarsäter H, Lundström S, Kollberg L, et al. . The Child and Adolescent Twin Study in Sweden (CATSS). Twin Res Hum Genet. 2011;14(6):495-508. doi: 10.1375/twin.14.6.495 [DOI] [PubMed] [Google Scholar]
- 16.Ludvigsson JF, Andersson E, Ekbom A, et al. . External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):450. doi: 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idring S, Rai D, Dal H, et al. . Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PLoS One. 2012;7(7):e41280. doi: 10.1371/journal.pone.0041280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansson SL, Svanström Röjvall A, Rastam M, Gillberg C, Gillberg C, Anckarsäter H. Psychiatric telephone interview with parents for screening of childhood autism—tics, attention-deficit hyperactivity disorder and other comorbidities (A-TAC): preliminary reliability and validity. Br J Psychiatry. 2005;187(3):262-267. doi: 10.1192/bjp.187.3.262 [DOI] [PubMed] [Google Scholar]
- 19.Halleröd SLH, Larson T, Ståhlberg O, et al. . The Autism–Tics, AD/HD and Other Comorbidities (A-TAC) telephone interview: convergence with the Child Behavior Checklist (CBCL). Nord J Psychiatry. 2010;64(3):218-224. doi: 10.3109/08039480903514443 [DOI] [PubMed] [Google Scholar]
- 20.Larson T, Lundström S, Nilsson T, et al. . Predictive properties of the A-TAC inventory when screening for childhood-onset neurodevelopmental problems in a population-based sample. BMC Psychiatry. 2013;13(1):233. doi: 10.1186/1471-244X-13-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neale MC, Hunter MD, Pritikin JN, et al. . OpenMx 2.0: extended structural equation and statistical modeling. Psychometrika. 2016;81(2):535-549. doi: 10.1007/s11336-014-9435-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Brief Bioinform. 2002;3(2):119-133. doi: 10.1093/bib/3.2.119 [DOI] [PubMed] [Google Scholar]
- 23.Verhulst B. A power calculator for the classical twin design. Behav Genet. 2017;47(2):255-261. doi: 10.1007/s10519-016-9828-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colvert E, Tick B, McEwen F, et al. . Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry. 2015;72(5):415-423. doi: 10.1001/jamapsychiatry.2014.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey A, Le Couteur A, Gottesman I, et al. . Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25(1):63-77. doi: 10.1017/S0033291700028099 [DOI] [PubMed] [Google Scholar]
- 26.Lichtenstein P, Carlström E, Råstam M, Gillberg C, Anckarsäter H. The genetics of autism spectrum disorders and related neuropsychiatric disorders in childhood. Am J Psychiatry. 2010;167(11):1357-1363. doi: 10.1176/appi.ajp.2010.10020223 [DOI] [PubMed] [Google Scholar]
- 27.Constantino JN, Todd RD. Genetic structure of reciprocal social behavior. Am J Psychiatry. 2000;157(12):2043-2045. doi: 10.1176/appi.ajp.157.12.2043 [DOI] [PubMed] [Google Scholar]
- 28.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60(5):524-530. doi: 10.1001/archpsyc.60.5.524 [DOI] [PubMed] [Google Scholar]
- 29.Ronald A, Happé F, Bolton P, et al. . Genetic heterogeneity between the three components of the autism spectrum: a twin study. J Am Acad Child Adolesc Psychiatry. 2006;45(6):691-699. doi: 10.1097/01.chi.0000215325.13058.9d [DOI] [PubMed] [Google Scholar]
- 30.Hoekstra RA, Bartels M, Verweij CJH, Boomsma DI. Heritability of autistic traits in the general population. Arch Pediatr Adolesc Med. 2007;161(4):372-377. doi: 10.1001/archpedi.161.4.372 [DOI] [PubMed] [Google Scholar]
- 31.Robinson EB, Koenen KC, McCormick MC, et al. . Evidence that autistic traits show the same etiology in the general population and at the quantitative extremes (5%, 2.5%, and 1%). Arch Gen Psychiatry. 2011;68(11):1113-1121. doi: 10.1001/archgenpsychiatry.2011.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lundström S, Chang Z, Råstam M, et al. . Autism spectrum disorders and autistic like traits: similar etiology in the extreme end and the normal variation. Arch Gen Psychiatry. 2012;69(1):46-52. doi: 10.1001/archgenpsychiatry.2011.144 [DOI] [PubMed] [Google Scholar]
- 33.Hallmayer J, Cleveland S, Torres A, et al. . Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095-1102. doi: 10.1001/archgenpsychiatry.2011.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The familial risk of autism. JAMA. 2014;311(17):1770-1777. doi: 10.1001/jama.2014.4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frazier TW, Thompson L, Youngstrom EA, et al. . A twin study of heritable and shared environmental contributions to autism. J Autism Dev Disord. 2014;44(8):2013-2025. doi: 10.1007/s10803-014-2081-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Posthuma D, Polderman TJC. What have we learned from recent twin studies about the etiology of neurodevelopmental disorders? Curr Opin Neurol. 2013;26(2):111-121. doi: 10.1097/WCO.0b013e32835f19c3 [DOI] [PubMed] [Google Scholar]
- 37.Sandin S, Lichtenstein P, Kuja-Halkola R, Hultman C, Larsson H, Reichenberg A. The heritability of autism spectrum disorder. JAMA. 2017;318(12):1182-1184. doi: 10.1001/jama.2017.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tick B, Bolton P, Happé F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry. 2016;57(5):585-595. doi: 10.1111/jcpp.12499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taniai H, Nishiyama T, Miyachi T, Imaeda M, Sumi S. Genetic influences on the broad spectrum of autism: study of proband-ascertained twins. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):844-849. doi: 10.1002/ajmg.b.30740 [DOI] [PubMed] [Google Scholar]
- 40.Bai D, Yip BHK, Windham GC, et al. . Association of genetic and environmental factors with autism in a 5-country cohort. JAMA Psychiatry. 2019. doi: 10.1001/jamapsychiatry.2019.1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Zeeuw EL, van Beijsterveldt CEM, Hoekstra RA, Bartels M, Boomsma DI. The etiology of autistic traits in preschoolers: a population-based twin study. J Child Psychol Psychiatry. 2017;58(8):893-901. doi: 10.1111/jcpp.12741 [DOI] [PubMed] [Google Scholar]
- 42.Taylor MJ, Gillberg C, Lichtenstein P, Lundström S. Etiological influences on the stability of autistic traits from childhood to early adulthood: evidence from a twin study. Mol Autism. 2017;8(1):5. doi: 10.1186/s13229-017-0120-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Class QA, Rickert ME, Larsson H, Lichtenstein P, D’Onofrio BM. Fetal growth and psychiatric and socioeconomic problems: population-based sibling comparison. Br J Psychiatry. 2014;205(5):355-361. doi: 10.1192/bjp.bp.113.143693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res. 2002;5(6):554-571. doi: 10.1375/136905202762342026 [DOI] [PubMed] [Google Scholar]
- 45.Rydell M, Lundström S, Gillberg C, Lichtenstein P, Larsson H. Has the attention deficit hyperactivity disorder phenotype become more common in children between 2004 and 2014? trends over 10 years from a Swedish general population sample. J Child Psychol Psychiatry. 2018;59(8):863-871. doi: 10.1111/jcpp.12882 [DOI] [PubMed] [Google Scholar]
- 46.Curran S, Dworzynski K, Happé F, et al. . No major effect of twinning on autistic traits. Autism Res. 2011;4(5):377-382. doi: 10.1002/aur.207 [DOI] [PubMed] [Google Scholar]
- 47.Taylor MJ, Martin J, Lu Y, et al. . Association of genetic risk factors for psychiatric disorders and traits of these disorders in a Swedish population twin sample. JAMA Psychiatry. 2019;76(3):280-289. doi: 10.1001/jamapsychiatry.2018.3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Description of measures
eAppendix 2. Posthoc analyses
eTable 1. Twin correlations
eTable 2. Assumption testing
eTable 3. Model fit statistics
eTable 4. Model estimates
eTable 5. Twin correlations by birth cohort
eTable 6. Model estimates by birth cohort
eTable 7. Descriptive statistics for the Swedish Twin Registry
eTable 8. Descriptive statistics for CATSS
eTable 9. Descriptive statistics for the total Swedish population born 1982-2008
eTable 10. Regression models of the association between birth cohort and each characteristic
eTable 11. Twin correlations by birth cohort
eFigure. Power for the liability threshold model of the STR