Abstract
Tartary buckwheat tea (TBT) is the most popular and widely consumed buckwheat product in many countries. However, the perfect quality control standards for TBT were still lacking, and the content of heavy metals in TBT and their health risks to consumers were still unknown. In this research, the total phenolic content, total flavonoid content, and antioxidant capacity as well as six metal contents and their health risks in TBT were detected. The results showed that the total phenolic content, total flavonoid content, and antioxidant capacity varied significantly among different types of TBT. Meanwhile, six metal concentrations in TBT leaves and infusions decreased in the order of Zn, Cu, Cr, Ni, Pb, and Cd. Health risk assessment indicated that the heavy metal intake only from TBT would not cause a noncarcinogenic risk to consumers. However, a strong carcinogenic risk of Cr in TBT for consumers should be paid more attention.
Introduction
Tartary buckwheat (Fagopyrum tataricum), recognized as a health food, is widely consumed in many countries, including China, Japan, and Korea.1 It contains a variety of bioactive substances with antioxidant, hypoglycemic, bacteriostatic, and anticancer properties.2−4 For example, quercetin extracted from Tartary buckwheat was found to have the efficacy of alleviating imiquimod-induced psoriasis-like skin lesions in mice.5 As a raw material for food, Tartary buckwheat has been processed into various products, such as noodles, breads, biscuits, and tea.1 Among them, Tartary buckwheat tea (TBT) is increasingly welcomed due to its unique malt fragrance, beneficial effects, and convenience.1 Based on the differences of raw materials, TBT in the global consumer market could generally be divided into three types: whole plant tea (WPT), whole bran tea (WBT), and whole embryo tea (WET).6 WPT is processed from the mixture of stems, leaves, and flowers of Tartary buckwheat. WBT is processed from the bran of the Tartary buckwheat seed, while whole embryo tea is the product processed from the embryo of the Tartary buckwheat seed. Due to the varied chemical composition in different parts of the Tartary buckwheat plant,7 the content of flavonoids and phenolic in different types of TBT are different, which has resulted to the different antioxidant capacity (AC) among different types of TBT.
With the increasing soil pollution, many reports have raised concerns about the health issues of food products containing high concentrations of metals, including copper (Cu), zinc (Zn), lead (Pb), cadmium (Cd), chromium (Cr), and nickel (Ni).8−12 Additionally, due to the cumulative properties of metals, the dose of metals in the human body could reach a threshold level that seriously endangers human health.10 Therefore, to guide people’s healthy diet and avoid excessive metal intake, the metal content and their health risks of many foods, including rice, bread, and tea, have been extensively studied before.8,13,14 Due to the different ability of plant organs for metal accumulation, the content of metal elements and their health risks in foods processed from these plant organs were also different.10,15,16 Meanwhile, the content of various mineral elements in Tartary buckwheat organs has been reported;17−20 however, metal contents in TBT and their health risk for consumers are still poorly understood.
In this study, the total phenolic content (TPC), total flavonoid content (TFC), and antioxidant capacity (AC) of 27 different TBT samples, which could cover the mainstream merchandise of buckwheat tea in the commercial market in China, were evaluated and compared. In addition, the concentration of six metal elements (Cd, Pb, Ni, Cr, Cu, and Zn) in different types of TBT leaves and infusions were also analyzed, and the health risks of metals were evaluated based on their concentration in the TBT infusions. These results would develop the understanding of the commercial TBT, offering useful reference for the optimization of industrial production of buckwheat tea and providing a theoretical basis for completing the quality control standards of healthy Tartary buckwheat foods.
Results and Discussion
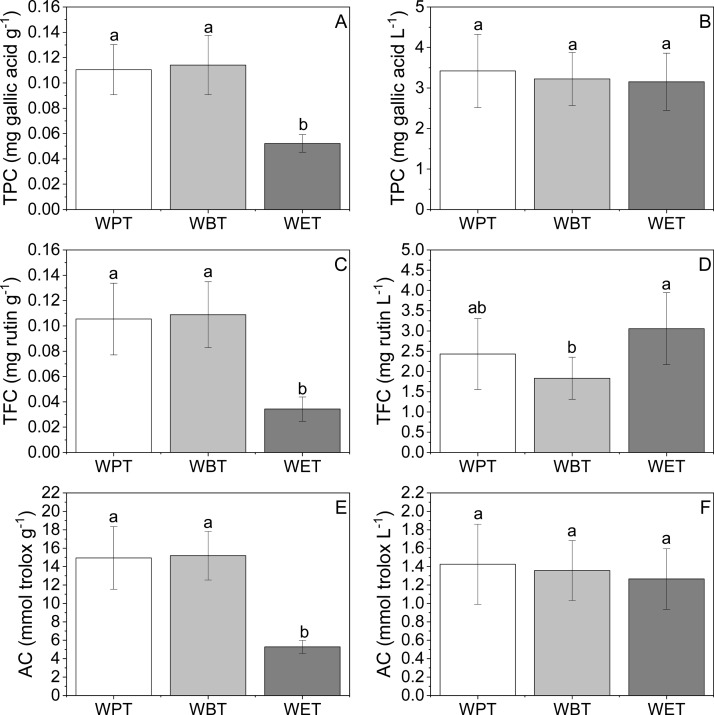
In this research, the TPC, TFC, and AC in different TBT are evaluated, which are illustrated in the Supporting Information, and the average values of TPC, TFC, and AC that came from three types, including WPT, WBT, and WET, are illustrated in Figure 1 below. Subsequently, the variations of TPC, TFC, and AC were observed in three types of TBT leaves and infusions (Figure 1). It was shown that TPC, TFC, and AC in WPT and WBT leaves were similar (Figure 1A,C,E), and compared with those in WET leaves, they were all significantly higher. However, there was no significant difference in TPC and AC among the three types of TBT infusions (Figure 1B,F); meanwhile, the TFC of WET infusion and WBT infusion showed the highest and lowest value, respectively (Figure 1D). Fu et al. reported TFC and FRAP values of 51 kinds of commercial herbal and tea infusions made in China.21 When compared to these commercial herbal and tea infusions, the TPC of WPT, WBT, and WET infusions were higher and the FRAP values of WPT, WBT, and WET infusions were higher than Ping An Tang mao gen zhu zhe shui, Ping An Tang suan mei tang, and Nian ci an run, but the FRAP values of WPT, WBT, and WET infusions were lower than Qi Lin cha wu, Qi Lin sheng cha, and Ya Tian bing hong cha.
Figure 1.
TPC, TFC, and AC of TBT methanol extracts and infusions. (A, C, E) Methanol extracts of the three types of TBT. (B, D, F) Infusions of the three types of TBT. The bars marked with different lowercase letters indicated that the difference was significant at the 0.05 level.
The previous studies have demonstrated that the chemical composition of Tartary buckwheat products were affected by raw materials and different processing methods, which were the two critical factors for the determination of TBT nutritional and biological characteristics.22 In our research, all results indicated that the raw materials and dissolution characteristics of bioactive ingredients could affect the TPC, TFC, and AC in different commercial TBT. More importantly, the samples came from different companies, which have different raw materials that came from different places of origin, indicating that the materials and its locations of TBT could influence the TPC, TFC, and AC and also played an important role in the quality determination of TBT.
Additionally, the different manufacturing process of TBT could be inferred that it has correlation of the differences in TPC, TFC, and AC properties. Three types of TBT also showed the differences in TPC, TFC, and AC, which were caused by the different processing methods. Especially, the rutin-degrading enzyme would get in contact with rutin during the grounding and mixing with water in the processing of commercial TBT, and it will induce the concentration of rutin and cause it to largely convert to quercetin; after that, the TPC, TFC, and AC will be influenced in the end. Subsequently, previous studies have found that the distribution of flavonoids in buckwheat has tissue-specific characteristics, and it was reported that the flavonoids were more highly concentrated in hull and bran than those in buckwheat embryo.23 The different types of TBT used different plant organs for their materials, which also cause the differences between the three types of TBT in TPC, TFC, and AC properties, and it also illustrated the causes of the differences between WPT, WBT, and the WET.
Peng et al. described that the biological activities in the tea soup of TBT were significantly reduced compared to the alcohol extracts, which suggested that the biological activities that were decreasing were caused by the flavonoids dissolved in water.1 Our research also showed the same trends; the TPC, TFC, and AC in water infusions of TBT did not correspond to those in TBT leaves. It revealed that the bioactive ingredients such as flavonoids dissolved in water, and we also suggest that the differences between water infusions and leaves mainly result from the concentration of the bioactive substance in water infusion affected by the leaching rat. On the other hand, TFC, TPC, and AC in TBT leaves and infusions showed different distribution trends; this showed that the TPC, TFC, and AC in TBT infusion were not only affected by the corresponding concentration in the TBT leaves but also related to their dissolution characteristics.
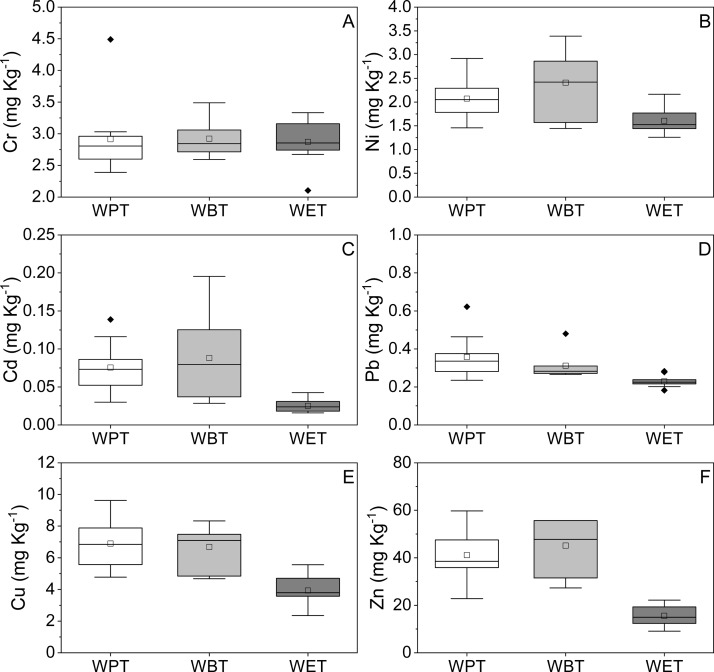
Figure 2 illustrates the average concentration of six metal elements in TBT leaves. It showed that the three types of TBT leaves had different characteristics of metal composition, and the average concentration of Zn was the highest among all metals followed by Cu, Ni, Cr, Pb, and Cd. However, the average concentration of a certain metal in the three types of TBT was different; WET had the lowest average concentration of all metals, WBT had the highest average concentration of all metal elements except Pb, and WPT had the highest average concentration of Pb.
Figure 2.
Metal contents in TBT. (A–F) Concentrations of Cr, Ni, Cd, Pb, Cu, and Zn of the three types of TBT, respectively. The black solid diamond in the figure represented the outlier.
On the other hand, regulatory limits for metals in tea leaves or herbal materials had been developed in different countries (Table 1). Cr in all types of TBT samples had exceeded the standard limit value, which was set to be 2.0 mg kg–1 in Canada. Additionally, concentrations of the other five studied metals were lower than the corresponding standard limit value. These results suggested that it was highly necessary to assess health risks of metals in TBT to the human body.
Table 1. Regulatory Limits (mg kg–1) for Metals in Tea or Herbal Materials from Different Countries.
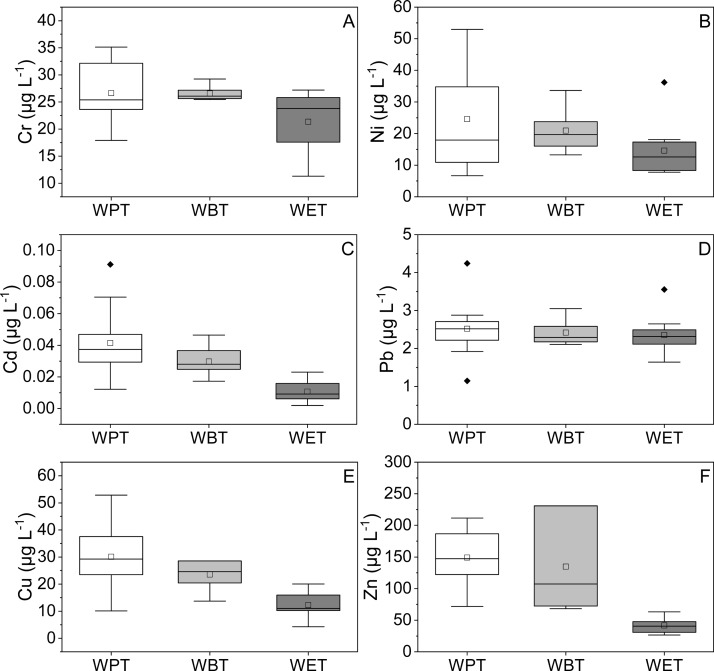
The distribution of metals in TBT infusions was different with TBT leaves (Figure 3). The average concentration of Zn was the highest in TBT infusions followed by Cu, Cr, Ni, Pb, and Cd. All average metal concentrations were lowest in WET infusion. WBT infusion had the highest Cr and Ni average concentration, and WPT had the highest Cu, Cd, Zn, and Pb average concentration.
Figure 3.
Metal contents in TBT infusions. (A–F) Concentrations of Cr, Ni, Cd, Pb, Cu, and Zn of the three types of TBT infusions, respectively. The black solid diamond in the figure represented the outlier.
In this study, the standards for drinking water quality in China (GB 5749-2006) were used to evaluate the metal content in TBT infusions due to the fact that there was lack of standards for tea infusion quality. All metal content in the three types of TBT infusions were below the corresponding standard limit values except for Ni. Concentrations of Ni in 40% of WPT samples, 43% of WBT samples, and 10% of WET samples exceeded the standard limit value of Ni (20 μg L–1) for drinking water quality in China.
Meanwhile, the significantly positive correlation was observed among TFC, TPC, and AC in TBT leaves, which suggested that flavonoid was the main antioxidant in TBT. In addition, Ni and Zn were observed to be significantly positively correlated with TPC, TFC, and AC in tea leaves, and Cu was observed to have a significantly positive correlation with TPC. This reminds us that, when evaluating the quality of TBT, the content of bioactive substances should not be the only indicator; harmful substance contents and their relationship with bioactive substances should be also fully considered. The correlation analysis of heavy metals and TFC, TPC, and AC is presented in Figure 4, which shows the positive/negative correlations between the two elements. When the value is close to 1.0, it was indicated that there is a strong correlation between the two elements. From the results, Cd and Ni, Cu and Zn showed significant positive correlations, implying that these metals in the three types of TBT might come from the same source.
Figure 4.
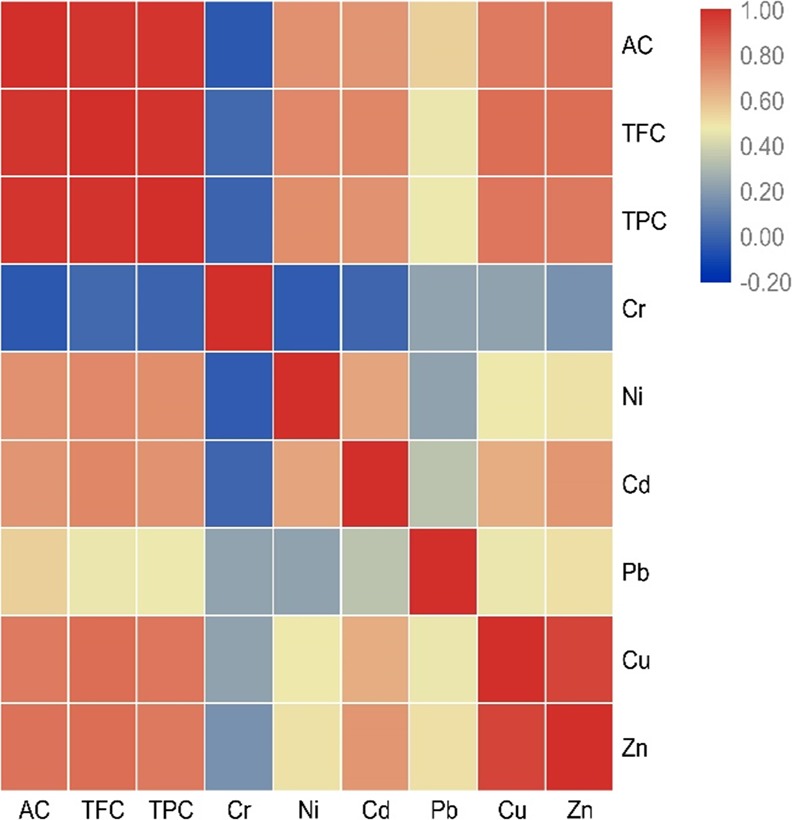
Correlation among TFC, TPC, AC, and heavy metal contents in TBT.
All results indicated that the concentration of different metals in TBT and its infusions varied with the different raw materials, and they were contaminated by certain heavy metals. It is necessary and important to further explore the daily intake and health risks of heavy metals from TBT. Nowadays, TBT has become more and more popular all around the world, and the herbal tisane made from Tartary buckwheat is the fashion and health beverage in Southeast Asia, Japan, and Europe, which could provide lots of vitamins, minerals, and flavonoids with beneficial effects and a unique flavor. At the same time, previous research has reported that the buckwheat accumulated high aluminum (Al) and tolerance to Al toxicity.28 However, there was no report about the heavy metal enrichment and distribution characteristics of TBT, and this lacking information would impede the quality evaluation of commercial TBT, even hindering the development of the buckwheat industry in the future.
Here, the heavy metal contents in different types of TBT and its correlation with TPC, TFC, and AC were processed. The results indicated that the heavy metal concentrations were significantly different among the three types of TBT whether in the leaves or infusions, which revealed that the heavy metals accumulated in buckwheat, and the average concentration of Cr in the three types of TBT leaves were close as well as the average concentration of Pb in TBT infusions were basically the same. The heavy metal accumulation showed that the tissue-specific distribution such as the Al was easily accumulated in the leaves of buckwheat. This explains the differential concentration of heavy metals in different types of TBT. On the other hand, based on the correlation between heavy metal contents and TPC, TFC, and AC, our results suggested that the Ni and Zn in TBT could be present in combination with flavonoids, and Cu may be present in combination with phenolic compounds. Other studies also revealed that the flavonoids could bind with metal ions effectively. Meanwhile, the experimental evidence proved that the flavonoid metal complexes could act as the mimics of superoxide dismutase.29 All in all, our research demonstrated the previous theories and provided direct evidence that link heavy metal contents and TFC and AC.
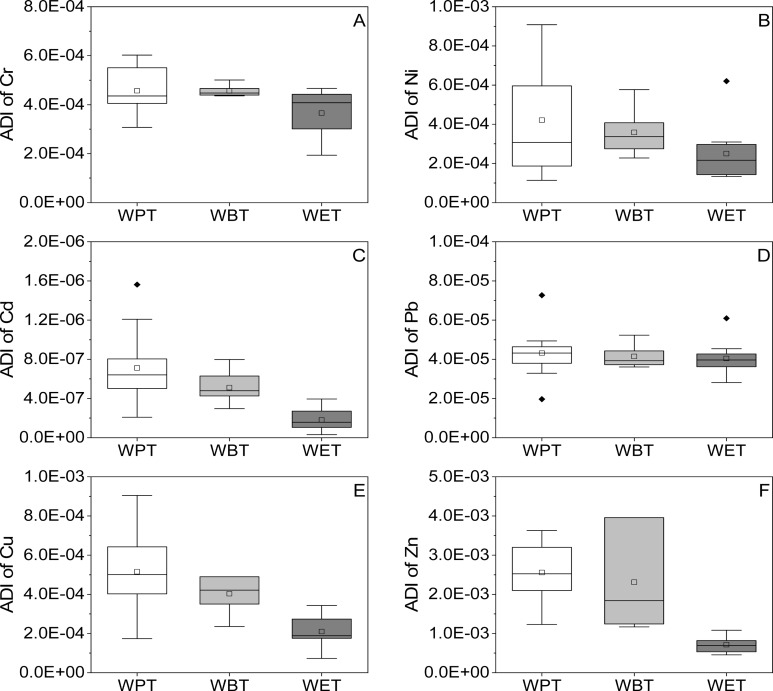
The ADI values of metals from the three types of TBT are shown in Figure 5. The highest ADI value of metals from the three types of TBT was Zn followed by Cu, Ni, Cr, Pb, and Cd. For the three types of TBT, WBT had the highest ADI values of Cr and Ni; WPT had the highest ADI values of Cd, Pb, Cu, and Zn; and WET had the lowest ADI values of six metals. RfD values for six metals were used to assess the ADI value of six metals from the three types of TBT.30,31 The ADI values of six metals from the three types of TBT were all lower than their corresponding RfD values, meaning that TBT consumption might not pose health risks to consumers.
Figure 5.
ADI values of metals in TBT. (A–F) ADI of Cr, Ni, Cd, Pb, Cu, and Zn of the three types of TBT, respectively. The black solid diamond in the figure represented the outlier.
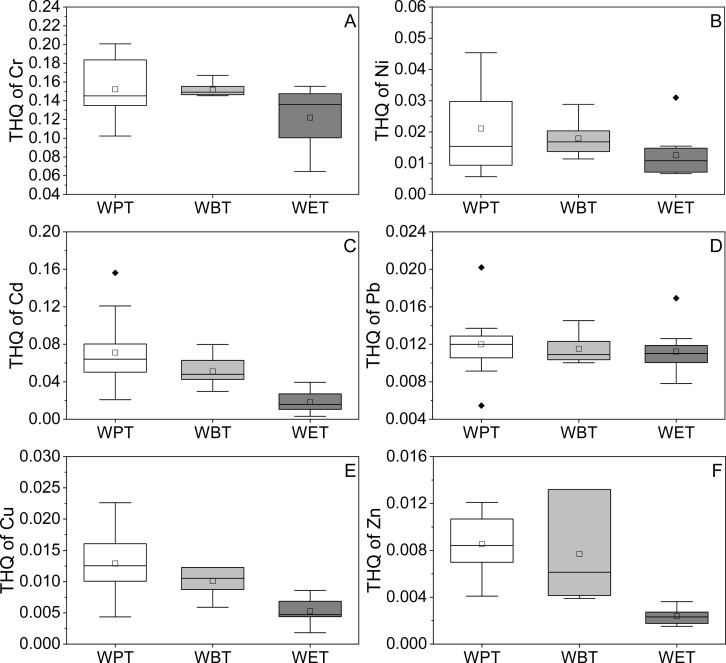
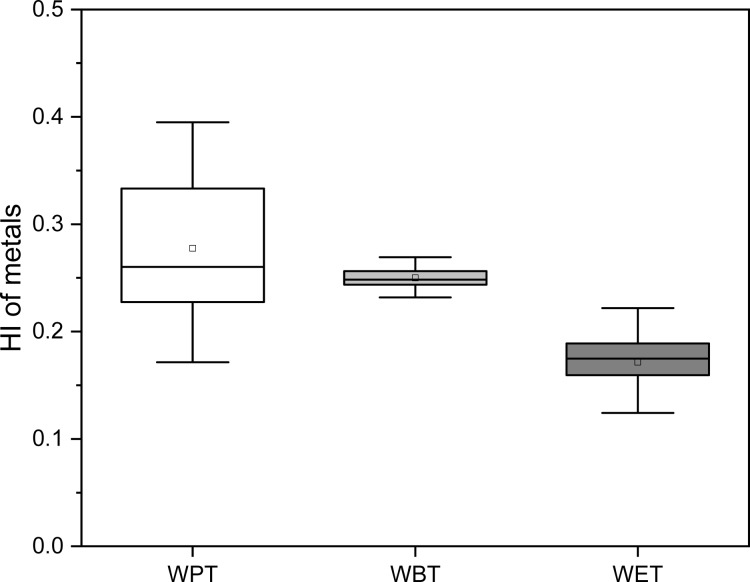
The THQ values of different metal elements in the same type of TBT were obviously different, and the THQ value of Cr in the three types of TBT was the highest followed by Cd, Ni, Pb, Cu, and Zn (Figure 6). Meanwhile, the THQ and HI values of the same metal element in the three types of TBT were also different. WPT has the highest THQ and HI of heavy metals followed by WBT and WET (Figures 6 and 7). Besides, THQ and HI values of metal elements in the three types of TBT are all less than 1.0, indicating that metal elements in the three types of TBT had no significant risk of noncarcinogenic effects for the exposed consumers.
Figure 6.
THQ value of heavy metals in TBT. (A–F) THQ of Cr, Ni, Cd, Pb, Cu, and Zn of three types of TBT, respectively. The black solid diamond in the figure represented the outlier.
Figure 7.
HI value of metals in TBT.
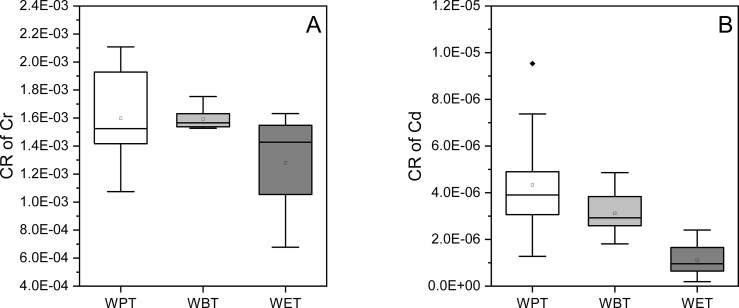
To evaluate the carcinogenic risk of the heavy metals in TBT, CR values of carcinogenic heavy metals including Cr and Cd were calculated. As seen clearly from Figure 7, CR values of Cr in all TBTs were higher than Cd. In addition, WBT had the highest CR value of Cr followed by WPT and WET (Figure 8A), and WPT had the highest CR value of Cd followed by WBT and WET (Figure 8B). Meanwhile, CR values of Cd in the three types of TBT were all between 10–7–10–4, suggesting that their carcinogenic risks were acceptable. However, CR values of Cr in all TBTs were higher than 10–4, implying the strong risk of developing cancer for the exposed consumers.
Figure 8.
CR values of Cr and Cd in TBT. (A, B) CR of Cr and Cd of the three types of TBT, respectively. The black solid diamond in the figure represented the outlier.
The health risk assessment has been used in a number of scientific researches,32 like food safety,33 environmental pollution,34 and so on. The heavy metals have been detected in the commercial TBT products, and the concentration of Cr was relatively high, which is close to the acceptable daily intake value of Cr.35 Therefore, health risk assessment of metals in TBT is necessary from this point. Based on the health risk assessment, the ADI and THQ of heavy metals of TBT were illustrated, and most of the heavy metals in TBT were suitable for health food standards, which indicated that the buckwheat tea was still safe to drink. However, the CR of Cr in TBT still had potential, and the Cr intake from Tartary buckwheat tea infusions would induce a strong carcinogenic risk for consumers, which reminds us that, when evaluating the quality of TBT, the content of bioactive substances should not be the only indicator; harmful substance contents and their relationship with bioactive substances should be also fully considered.
Conclusions
Our research revealed that the heavy metals were accumulated in TBT, and the concentrations of heavy metals were significantly diverse in different types of TBT due to the fact that raw materials came from various tissues of buckwheat. It caused the significant difference of TPC, TFC, and AC in three different types of TBT. Finally, we demonstrated the potential risk of TBT and that the safety issue of TBT like heavy metals should be taken into consideration when evaluating the quality. It also revealed that the place of origin, raw materials, and the processing methods of TBT affected the concentrations of the heavy metals.
In this way, the environmental safety of origin of place and quality control in manufacturing should be taken as a serious concern. Moreover, the quality control standards for Tartary buckwheat foods should also be drafted and completed in the future, and it could contribute to the comprehensive evaluation of Tartary buckwheat foods. Last but not least, the cultivation of buckwheat with heavy metal low-accumulation properties by molecular breeding should be given special attention, which is the major challenge in the buckwheat food industry.
Materials and Methods
A total of 27 samples (Table 2) representing the three types of TBT were collected from commercial markets in China, including 10 WPT, 7 bran teas WBT, and 10 WET. These samples were dried at 80 °C to constant weight and ground into fine powder to obtain a representative sample. Then, TBT leaf extracts and infusions were prepared based on the previous methods of Velioglu et al. and de Oliveira et al., respectively.8,36 In short, 0.1 g of TBT leaf was added to 10 mL of 80% methanol aqueous solution, and the mixture was shaken at 65 °C for 2 h at a rate of 160 r/min; then, the mixture was filtered, and the TBT leaf extracts were collected. The TBT leaf (1.0 g) was added to 50 mL of boiling water and kept boiling for 5 min, and then, the mixture was filtered and the TBT infusions were collected.
Table 2. Types and Brands of Tartary Buckwheat Tea Samples Collected from Commercial Markets.
| numbers | category | name of product |
|---|---|---|
| 1 | whole plant teas | Luojishan |
| 2 | Daliangshan | |
| 3 | Sanjiang | |
| 4 | Hangfei | |
| 5 | Hongqiaodi | |
| 6 | Qiaoxiangrenjia | |
| 7 | Yijiale | |
| 8 | Qiliba | |
| 9 | Aimeili | |
| 10 | Yangguangshangpin | |
| 11 | whole embryo teas | Siqixiang |
| 12 | Hongqiaodi | |
| 13 | Yixiangren | |
| 14 | Huiqiao | |
| 15 | Gaoshanyiren | |
| 16 | Sanjiang | |
| 17 | Wucailiangshan | |
| 18 | Xiangzi | |
| 19 | Jintiankang | |
| 20 | Shuxiangyinxiang | |
| 21 | whole bran teas | Sanjiang |
| 22 | Gaoshanyiren | |
| 23 | Yizhai | |
| 24 | Wucailiang | |
| 25 | Caohaiyangguang | |
| 26 | Yuchamengmo | |
| 27 | Kuake |
TPC in TBT leaves and infusions were determined using the Folin–Ciocalteu reagent.37 TFC in TBT leaves and infusions were measured by the aluminum nitrate colorimetry method.38 Briefly, 5.0 mL of TBT leaf extracts or TBT infusions was added to 10 mL of a plastic centrifuge tube. Then, 1 mL of ultrapure water and 2.5 mL of aluminum chloride solution (26.6 mg of aluminum trichloride hexahydrate and 80 mg of sodium acetate in 20 mL of ultrapure water) were added to the centrifuge tube. After standing at room temperature for 30 min, the absorbance of the solution at a wavelength of 430 nm was measured. Antioxidant capacity of TBT leaves and infusions were tested by the ferric ion reducing antioxidant power method.39
The TBT sample (0.25 g) or TBT infusions (25 mL) were microwave-digested with 65% HNO3.40 Then, the digestion solutions were diluted with ultrapure water to a final volume of 25 mL and analyzed for Pb, Zn, Cu, Ni, Cd, and Cr by a inductively coupled plasma mass spectrometer (ICP-MS; Agilent 7800).
All reagents used in this study including HNO3 were suprapure reagents. All glassware and equipment used in this study were soaked with 20% HNO3 overnight and rinsed three times with ultrapure water prior to use. All samples were measured in triplicate. Two certified reference materials [GBW 10019 and GBW 07602 (GSV-1)] were used to test the accuracy of the method. The recoveries for total metals in the two certified reference materials ranged from 89 to 114%.
All data were expressed as the average value of three repetitions with standard error. One-way ANOVA tests (P < 0.05) by Duncan’s multiple grouping were used to determine significance differences among different teas. The Pearson correlation coefficient used to determine significance positive/negative correlation among TFC, TPC, AC, and metal concentrations was calculated with the SPSS 22.0 version. Figures were drawn by Origin Pro 2018.
ADI of metals is a basic index in the chronic health risk assessments. ADI of metals from TBT depends on both the metal concentration in TBT infusions and the amount of TBT infusion consumption, which was calculated using eq 1: in this equation, the ADI was the average daily intake (mg kg–1 day–1); C was the metal concentration in TBT infusions (mg L–1); DI was the average daily intake rate of TBT infusions (L day–1); BW was the body weight (kg); EF was the exposure frequency (day year–1); ED was the exposure duration (year); and AT was the average exposure time (day).
| 1 |
In this research, we by default show that BW was 70 kg,41 DI was 1.2 L day–1,42 EF was 365 day year–143 (EPA, 1989), ED was 50 years,42 and AT was 50 × 365 days for carcinogenic and noncarcinogenic risk because people who drink tea were typically adults, and it was widely believed that adults started drinking tea at the age of 20.40
The potential noncarcinogenic effects of each metal were evaluated by the target hazard quotients (THQ) using eq 2.27,30 A THQ value of less than 1.0 indicates no significant risk of noncarcinogenic effects for the exposed consumers. The probability of noncarcinogenic effects increases with the increasing THQ value. In eq 2, ADI was the average daily intake (mg kg–1 day–1); RfD was the oral reference dose; the RfD values for Cd, Cr(VI), Cu, Ni, Pb, and Zn were set to be 1.0 × 10–5, 3.0 × 10–3, 4.0 × 10–2, 2.0 × 10–2, 3.6 × 10–3, and 3.0 × 10–1 mg kg–1 day–1, respectiveley;12,30,33,44 and the RfD value of Cr was set to the value of Cr(VI) based on the principle of protecting health protection assumptions.40
| 2 |
The overall potential risk impacts may be caused by the exposure of multiple contaminants. The hazard index (HI) was generally used to assess the total noncarcinogenic health hazard caused by exposure to multiple metals.30 It was the sum of the hazard quotients of all the studied contaminants and calculated using eq 3
| 3 |
The THQi was the THQ value of element i; if HI was less than 1, then the exposure dose was lower than the adverse reaction threshold without noncarcinogenic risk; if HI was greater than 1, then the exposure dose was greater than the adverse reaction threshold, and there would be very likely to have a negative impact on human health; when the HI value was greater than 10.0, there is a chronic toxic effect on human health.40
Carcinogenic risk referred to the possibility that individuals would suffer from any type of cancer during their whole lifetime due to exposure to carcinogenic hazards.32 The acceptable carcinogenic risk level was 10–7–10–4.40 If the carcinogenic risk level was higher than 10–4, then it meant that the risk of developing cancer was strong. The carcinogenic risk level of less than 10–7 meant that the risk of developing cancer could be ignored. The aggregate carcinogenic risk (CR) was calculated by eq 4
| 4 |
In this equation, ADI was the average daily intake (mg kg–1 day–1); SF0 was risk factor for carcinogen. SF0 of Cr(VI) and Cd were 3.5 and 6.1 (mg kg–1 day–1)−1, respectively. Based on the principle of protecting health protection assumptions, it was assumed here that all Cr were Cr(VI).40
Acknowledgments
This work was financially supported by the National Key R&D Program of China (2018YFD1000706/2018YFD1000700) and the Key Project of Science and Technology of Sichuan, China (grant no. 04NG001–015).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.9b04007.
Raw data of TFC, TPC, AC, and six metal contents (PDF)
Author Contributions
C.W., J.S., and X.Z. conceived the experiments; Z.L., H.L., Y.H., Y.J., Y.L., and W.W. collected the samples; and C.W. and Z.L. wrote the manuscript. All authors reviewed the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Peng L. X.; Zou L.; Wang J. B.; Zhao J. L.; Xiang D. B.; Zhao G. Flavonoids, Antioxidant Activity and Aroma Compounds Analysis from Different Kinds of Tartary Buckwheat Tea. Indian J. Pharm. Sci. 2015, 77, 661–667. 10.4103/0250-474x.174972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft I.; Fabjan N.; Yasumoto K. Rutin content in buckwheat (Fagopyrum esculentum Moench) food materials and products. Food Chem. 2006, 98, 508–512. 10.1016/j.foodchem.2005.05.081. [DOI] [Google Scholar]
- Li F.; Zhang X.; Zheng S.; Lu K.; Zhao G.; Ming J. The composition, antioxidant and antiproliferative capacities of phenolic compounds extracted from tartary buckwheat bran [Fagopyrum tartaricum (L.) Gaerth]. J. Funct. Foods 2016, 22, 145–155. 10.1016/j.jff.2016.01.027. [DOI] [Google Scholar]
- Wang L.; Yang X.; Qin P.; Shan F.; Ren G. Flavonoid composition, antibacterial and antioxidant properties of tartary buckwheat bran extract. Ind. Crops Prod. 2013, 49, 312–317. 10.1016/j.indcrop.2013.04.039. [DOI] [Google Scholar]
- Bischoff S. C. Quercetin: potentials in the prevention and therapy of disease. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 733–740. 10.1097/MCO.0b013e32831394b8. [DOI] [PubMed] [Google Scholar]
- Qin P.; Wu L.; Yao Y.; Ren G. Changes in phytochemical compositions, antioxidant and α-glucosidase inhibitory activities during the processing of tartary buckwheat tea. Food Res. Int. 2013, 50, 562–567. 10.1016/j.foodres.2011.03.028. [DOI] [Google Scholar]
- Liu B.; Zhu Y. Extraction of flavonoids from flavonoid-rich parts in tartary buckwheat and identification of the main flavonoids. J. Food Eng. 2007, 78, 584–587. 10.1016/j.jfoodeng.2005.11.001. [DOI] [Google Scholar]
- de Oliveira L. M.; Das S.; da Silva E. B.; Gao P.; Gress J.; Liu Y.; Ma L. Q. Metal concentrations in traditional and herbal teas and their potential risks to human health. Sci. Total Environ. 2018, 633, 649–657. 10.1016/j.scitotenv.2018.03.215. [DOI] [PubMed] [Google Scholar]
- Koch W.; Kukula-Koch W.; Komsta L̷.; Marzec Z.; Szwerc W.; Gl̷owniak K. Green Tea Quality Evaluation Based on Its Catechins and Metals Composition in Combination with Chemometric Analysis. Molecules 2018, 23, 1689. 10.3390/molecules23071689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Domingo M. C.; Pla A.; Hernández A. F.; Olmedo P.; Navas-Acien A.; Lozano-Paniagua D.; Gil F. Determination of metalloid, metallic and mineral elements in herbal teas. Risk assessment for the consumers. J. Food Compos. Anal. 2017, 60, 81–89. 10.1016/j.jfca.2017.03.009. [DOI] [Google Scholar]
- Peng C.-y.; Zhu X.-h.; Hou R.-y.; Ge G.-f.; Hua R.-m.; Wan X.-c.; Cai H.-m. Aluminum and Heavy Metal Accumulation in Tea Leaves: An Interplay of Environmental and Plant Factors and an Assessment of Exposure Risks to Consumers. J. Food Sci. 2018, 83, 1165–1172. 10.1111/1750-3841.14093. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Yang R.; Chen R.; Peng Y.; Wen X.; Gao L. Accumulation of Heavy Metals in Tea Leaves and Potential Health Risk Assessment: A Case Study from Puan County, Guizhou Province, China. Int. J. Environ. Res. Public Health 2018, 15, 133. 10.3390/ijerph15010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshref A. M. S.; Moselhy W. A.; Hassan N. E.-H. Y. Heavy metals and trace elements levels in milk and milk products. J. Food Meas. Charact. 2014, 8, 381–388. 10.1007/s11694-014-9203-6. [DOI] [Google Scholar]
- Zeng F.; Wei W.; Li M.; Huang R.; Yang F.; Duan Y. Heavy Metal Contamination in Rice-Producing Soils of Hunan Province, China and Potential Health Risks. Int. J. Environ. Res. Public Health 2015, 12, 15584–15593. 10.3390/ijerph121215005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider S.; Naithani V.; Barthwal J.; Kakkar P. Heavy Metal Content in Some Therapeutically Important Medicinal Plants. Bull. Environ. Contam. Toxicol. 2004, 72, 119–127. 10.1007/s00128-003-0249-0. [DOI] [PubMed] [Google Scholar]
- Sarma H.; Deka S.; Deka H.; Saikia R. R. Accumulation of heavy metals in selected medicinal plants. Rev. Environ. Contam. Toxicol. 2011, 214, 63–86. 10.1007/978-1-4614-0668-6_4. [DOI] [PubMed] [Google Scholar]
- Ahmed A.; Khalid N.; Ahmad A.; Abbasi N. A.; Latif M. S. Z.; Randhawa M. A. Phytochemicals and biofunctional properties of buckwheat: a review. J. Agric. Sci. 2014, 152, 349–369. 10.1017/S0021859613000166. [DOI] [Google Scholar]
- Bonafaccia G.; Gambelli L.; Fabjan N.; Kreft I. Trace elements in flour and bran from common and tartary buckwheat. Food Chem. 2003, 83, 1–5. 10.1016/S0308-8146(03)00228-0. [DOI] [Google Scholar]
- Huang X.-Y.; Zeller F. J.; Huang K.-F.; Shi T.-X.; Chen Q.-F. Variation of major minerals and trace elements in seeds of tartary buckwheat (Fagopyrum tataricum Gaertn.). Genet. Resour. Crop Evol. 2013, 61, 567–577. 10.1007/s10722-013-0057-2. [DOI] [Google Scholar]
- Steadman K. J.; Burgoon M. S.; Lewis B. A.; Edwardson S. E.; Obendorf R. L. Minerals, phytic acid, tannin and rutin in buckwheat seed milling fractions. J. Sci. Food Agric. 2001, 81, 1094–1100. 10.1002/jsfa.914. [DOI] [PubMed] [Google Scholar]
- Fu L.; Xu B. T.; Gan R. Y.; Zhang Y.; Xu X. R.; Xia E. Q.; Li H. B. Total phenolic contents and antioxidant capacities of herbal and tea infusions. Int. J. Mol. Sci. 2011, 12, 2112–2124. 10.3390/ijms12042112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H.; Yang X.; Zhou H.; Luo X.; Qin P.; Li J.; Ren G. Comparison of Nutritional Composition, Aroma Compounds, and Biological Activities of Two Kinds of Tartary Buckwheat Tea. J. Food Sci. 2017, 82, 1735–1741. 10.1111/1750-3841.13772. [DOI] [PubMed] [Google Scholar]
- Guo X.-D.; Wu C.-S.; Ma Y.-J.; Parry J.; Xu Y.-Y.; Liu H.; Wang M. Comparison of milling fractions of tartary buckwheat for their phenolics and antioxidant properties. Food Res. Int. 2012, 49, 53–59. 10.1016/j.foodres.2012.07.019. [DOI] [Google Scholar]
- Nejatolahi M.; Mortazavi S.; Ildoromi A. Levels of Cu, Zn, Pb, and Cd in the leaves of the tea plant (Camellia sinensis) and in the soil of Gilan and Mazandaran farms of Iran. J. Food Meas. Charact. 2014, 8, 277–282. 10.1007/s11694-014-9186-3. [DOI] [Google Scholar]
- Street R.; Száková J.; Drabek O.; Mládková L. The status of micronutrients (Cu, Fe, Mn, Zn) in tea and tea infusions in selected samples imported to the Czech Republic. Czech J. Food Sci. 2006, 24, 62–71. [Google Scholar]
- Karimi G.; Hasanzadeh M.; Nili A.; Khashayarmanesh Z.; Samiei Z.; Nazari F.; Teimuri M. Concentrations and health risk of heavy metals in tea samples marketed in Iran. Pharmacology 2008, 3, 164–174. [Google Scholar]
- Gasser U.; Klier B.; Kühn A. V.; Steinhoff B. Current findings on the heavy metal content in herbal drugs. Pharmeur. Sci. Notes 2009, 2009, 37–50. [PubMed] [Google Scholar]
- Lei G. J.; Yokosho K.; Yamaji N.; Fujii-Kashino M.; Ma J. F. Functional characterization of two half-size ABC transporter genes in aluminium-accumulating buckwheat. New Phytol. 2017, 215, 1080–1089. 10.1111/nph.14648. [DOI] [PubMed] [Google Scholar]
- Agati G.; Tattini M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. 10.1111/j.1469-8137.2010.03269.x. [DOI] [PubMed] [Google Scholar]
- EPA . Concepts, Methods and Data Sources for Cumulative Health Risk Assessment of Multiple Chemicals, Exposures and Effects, A Resource Document; United States Environmental Protection Agency, 2007.
- Nkansah M. A.; Opoku F.; Ackumey A. A. Risk assessment of mineral and heavy metal content of selected tea products from the Ghanaian market. Environ. Monit. Assess. 2016, 188, 332. 10.1007/s10661-016-5343-y. [DOI] [PubMed] [Google Scholar]
- Li Z.; Ma Z.; van der Kuijp T. J.; Yuan Z.; Huang L. A review of soil heavy metal pollution from mines in China: pollution and health risk assessment. Sci. Total Environ. 2014, 468-469, 843–853. 10.1016/j.scitotenv.2013.08.090. [DOI] [PubMed] [Google Scholar]
- Li L.; Fu Q. L.; Achal V.; Liu Y. A comparison of the potential health risk of aluminum and heavy metals in tea leaves and tea infusion of commercially available green tea in Jiangxi, China. Environ. Monit. Assess. 2015, 187, 228. 10.1007/s10661-015-4445-2. [DOI] [PubMed] [Google Scholar]
- Cao S.; Duan X.; Zhao X.; Wang B.; Ma J.; Fan D.; Sun C.; He B.; Wei F.; Jiang G. Health risk assessment of various metal(loid)s via multiple exposure pathways on children living near a typical lead-acid battery plant, China. Environ. Pollut. 2015, 200, 16–23. 10.1016/j.envpol.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Llobet J. M.; Falcó G.; Casas C.; Teixidó A.; Domingo J. L. Concentrations of Arsenic, Cadmium, Mercury, and Lead in Common Foods and Estimated Daily Intake by Children, Adolescents, Adults, and Seniors of Catalonia, Spain. J. Agric. Food Chem. 2003, 51, 838–842. 10.1021/jf020734q. [DOI] [PubMed] [Google Scholar]
- Velioglu Y. S.; Mazza G.; Gao L.; Oomah B. D. Antioxidant Activity and Total Phenolics in Selected Fruits, Vegetables, and Grain Products. J. Agric. Food Chem. 1998, 46, 4113–4117. 10.1021/jf9801973. [DOI] [Google Scholar]
- Singleton V. L.; Orthofer R.; Lamuela-Raventós R. M.. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology: Academic Press: 1999; Vol. 299, pp 152–178, 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Sedej I.; Sakač M.; Mandić A.; Mišan A.; Tumbas V.; Čanadanović-Brunet J. Buckwheat (Fagopyrum esculentumMoench) Grain and Fractions: Antioxidant Compounds and Activities. J. Food Sci. 2012, 77, C954–C959. 10.1111/j.1750-3841.2012.02867.x. [DOI] [PubMed] [Google Scholar]
- Lee L.-S.; Choi E.-J.; Kim C.-H.; Sung J.-M.; Kim Y.-B.; Seo D.-H.; Choi H.-W.; Choi Y.-S.; Kum J.-S.; Park J.-D. Contribution of flavonoids to the antioxidant properties of common and tartary buckwheat. J. Cereal Sci. 2016, 68, 181–186. 10.1016/j.jcs.2015.07.005. [DOI] [Google Scholar]
- Sun J.; Hu G.; Liu K.; Yu R.; Lu Q.; Zhang Y. Potential exposure to metals and health risks of metal intake from Tieguanyin tea production in Anxi, China. Environ. Geochem. Health 2019, 41, 1291–1302. 10.1007/s10653-018-0212-y. [DOI] [PubMed] [Google Scholar]
- Supplemental guidance for developing soil screening levels for superfund sites. Peer Review Draft, OSWER. 2002, 2002, 4–24. [Google Scholar]
- Shen F. M.; Chen H. W. Element composition of tea leaves and tea infusions and its impact on health. Bull. Environ. Contam. Toxicol. 2008, 80, 300–304. 10.1007/s00128-008-9367-z. [DOI] [PubMed] [Google Scholar]
- EPA . Risk assessment guidance for superfund. Volume I: human health evaluation manual (part a); In: EPA/540/1–89/002.United States Environmental Protection Agency, 1989.
- Gruszecka-Kosowska A.; Mazur-Kajta K. Potential health risk of selected metals for Polish consumers of oolong tea from the Fujian Province, China. Hum. Ecol. Risk Assess.: Int. J. 2016, 22, 1147–1165. 10.1080/10807039.2016.1146572. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.