Abstract
Objective
Children with epilepsy experience cognitive deficits and well-being issues that have detrimental effects on their development. Pharmacotherapy is the standard of care in epilepsy, however, few interventions exist to promote cognitive development and to mitigate disease burden. We aimed to examine the impact of two different modalities of neurofeedback (NFB) on cognitive functioning and quality of life (QOL) measurements in children and adolescents with controlled focal epilepsy. The study also explored the effects of NFB on clinical outcomes and electroencephalography (EEG) quantitative analysis.
Methods
Participants (n = 44) with controlled focal epilepsy were randomized to one of three arms: sensorimotor rhythm (SMR) NFB (n = 15), slow cortical potentials (SCP) NFB (n= 16), or sham NFB (n = 13). All participants received 25 sessions of intervention. The attention switching task (AST), Liverpool Seizure Severity Scale (LSSS), seizure frequency (SF), EEG power spectrum and coherence were measured at baseline, post intervention, and at 3 months’ follow up.
Results
In children and adolescents with controlled focal epilepsy, SMR training significantly reduced reaction time in the AST (p= 0.006), and this was correlated with the difference of change for theta power on EEG (p= 0.03); only the SMR group showed a significant decrease in beta coherence (p= 0.03). All groups exhibited improvement in QOL (p= <0.05).
Conclusions
This study provides the first data on two NFB modalities (SMR and SCP) including cognitive, neurophysiological, and clinical outcomes in pediatric epilepsy. SMR NFB improved cognitive functioning, while all the interventions showed improvements in QOL, demonstrating a powerful placebo effect in the sham group.
Keywords: Neurofeedback, cognition, quality of life, pediatric focal epilepsy, placebo effect
1. Introduction
Epilepsy is often comorbid with other cognitive and behavioral issues that are more disabling than seizures [1]. Community-based studies document the prevalence of such neurobehavioral comorbidities in epilepsy, which are divided into psychiatric, cognitive, and social categories [2]. A significant potential complication of epilepsy is impairment in some aspect of objectively assessed cognition including intelligence, language, visuoperception, learning and memory, executive function, and/or processing speed [3]. Pediatric epilepsy is particularly challenging due to neurodevelopmental comorbidities associated with the underlying pathology which are then compounded by antiepileptic drug (AED) side effects. Quality of life (QOL) in pediatric epilepsy is often more affected by issues associated with social and family life, academic performance and achievement, than by the occurrence of seizures [4]. Yet, AEDs only target seizure occurrence and may worsen other QOL factors.
An option for cognitive and QOL issues is neurofeedback (NFB), or electroencephalographic (EEG) biofeedback, a form of brain-computer interface whereby electrical activity from cortical areas is registered and processed using signal analysis methods. The aggregated neuronal activity is measured and transformed in real-time to visual and auditory feedback presented to patients, enabling them to self-regulate the activity of specific brain areas associated with behaviors or symptoms in a relatively individualized way. Theoretical NFB mechanisms have emerged from learning theories, especially associative learning theory, which includes principles of classical and operant conditioning. The use of NFB to treat epilepsy dates back to the early 1970s [5], with subsequent studies showing that seizure frequency could be reduced in most patients who were trained to decrease slow frequencies in the 3 to 8Hz range while rewarding those contained within the 9 to 18Hz range [6]. Low side effects in existing studies and clinical experience suggest that NFB is safe, though safety has not been systematically investigated [7].
Sensorimotor rhythm (SMR or μ rhythm) and slow cortical potentials (SCPs) are two NFB techniques with demonstrated efficacy in epilepsy [8–11]. SMR, a specific archiform rhythm of the sensorimotor cortex (corresponding to C3-Cz-C4) in awake participants which is supressed by thinking about or performing movement in the contralateral hand, has a frequency of 12– 20Hz with a spectral peak of 12–15Hz. SMR seems to originate from inhibitory thalamocortical circuits [12], hence its potential to regulate hyperexcitability.
SCPs are slow event-related direct-current shifts of the EEG. When large cortical neuron assemblies depolarize, they lead to SCPs in the electrical negative direction and reduced excitation thresholds; when they repolarize, they lead to SCPs in the positive direction and a rise in excitation thresholds [13, 14]. Hence negative SCP shifts increase the firing probabilities of a cell assembly, whereas positive SCP shifts inhibit this activity [15]. The slow electrical shifts between positive and negative phases may help regulate attention [16] and other neurological functions as the cortical assemblies have a prominent role in cortico-striato-thalamo-cortical feedback loops[14, 17]. For example, frontocentral contingent negative variation (CNV) is a well-studied SCP reflecting cognitive activation and preparation; it is typically reduced in ADHD children compared with healthy controls [13], and children with ADHD who received SCP-NFB training had an increase in CNV and improved ADHD symptoms [18]. SCP NFB has also shown long-term effectiveness in epilepsy [19], demonstrating that SCP training can reduce seizure frequency and severity [20, 21]. In epilepsy the most accepted hypothesis refers to the ability to generate cortical positivity (elevating excitation thresholds), and to carry over this skill into conditions without continuous feedback can serve as a seizure-controlling factor [22].
Neuropsychological-based treatments to reduce seizures and improve QOL in epilepsy have not been validated, although one review reported cognitive and motor improvements in patients who had the greatest seizure reduction following NFB [23]. However, importantly, most of the literature to date has been in adult populations. Little is known regarding the efficacy of NFB in pediatric epilepsy, as no randomized controlled study of NFB for pediatric epilepsy has been published to date [7].
A recent review on behavioral interventions in epilepsy included neurofeedback as a behavioral modality for seizure control and improvements in multiple QOL and cognitive domains [24]. It has been speculated that hyperexcitable cortical circuits leading to worsened QOL and cognition could be reorganized by NFB-based EEG entrainment, thereby leading to symptom control; both SMR and SCP may alter information processing in such networks by enabling inhibitory mechanisms. SMR intervention led to improvements in both QOL and attention measures in fibromyalgia, a disorder with impaired intracortical inhibition [25] highlighting the role of an inhibitory EEG frequency on symptom modulation [26].
We hypothesized that SMR and SCP neurofeedback protocols (30-minute sessions, 5 consecutive days/week over five weeks) would improve cognitive performance and QOL in patients with controlled focal epilepsy compared to sham NFB in a within-group design. Our secondary aim was to explore neurophysiological changes as well as seizure frequency and severity.
2. Methods
2.1. Trial design
This was an exploratory randomized, double-blinded, sham-controlled, 3-arm parallel-group (SMR, SCP, and sham NFB) clinical trial. This study was approved by the Bioethics and Research Committees of the Biomedical Research Institute (Aguascalientes, Mexico), and the Ethics Committee of De Montfort University (Leicester, United Kingdom). Written informed consent from each participant’s parent or legal guardian was obtained; all participants assented to participate.
2.2. Participants
Study subjects were recruited from local epilepsy clinics and referrals from neurological centers at the Neuromodulation Center NEOCEMOD in Aguascalientes City, Mexico. Eligibility criteria for inclusion were (1) aged 10–18 years old; (2) proven focal epilepsy with seizures originating within networks usually limited to one hemisphere (defined as clinical seizures plus presence of unilateral interictal EEG findings in accordance with the ILAE classification system [27]); (3) pharmacoresponsive focal epilepsy as defined by no seizures in the last 3 months and no more than one seizure in the last 6 months; (4) AED treatment during the last 12 months; (5) cognitive difficulties in school as reported by parents and/or poor school performance; (6) Mini-Mental Status score >23; (7) normal vision and hearing function (as assessed by standardized testing) and ability to receive and understand visual and auditory feedback stimuli.
Exclusion criteria were (1) presence of psychogenic nonepileptic seizures; (2) idiopathic focal or generalized epilepsy; (3) previous epilepsy surgery or craniotomy; (4) history of recent stupor or coma; (5) active intracranial infection; or (6) neurodegenerative diseases. Participants were required to stay on a stable AED regimen during intervention and follow-up periods. While the inclusion criteria limited seizure frequency for enrollment, once patients were consented they were not dropped out if they had more seizures during the trial.
2.3. Interventions
Each of the three interventions were given for 5 consecutive days per week over 5 weeks for a total of 25 sessions.
The SMR NFB protocol was implemented using the ProComp Infiniti Encoder, an 8-channel, battery-powered system for real-time data acquisition (Thought Technology, Montreal). The signal was amplified using a pre-amplified EEG-Z sensor, with a recording electrode placed at Cz with reference to linked earlobes. The pre-set feedback parameters were as follows: inhibit theta (4–7Hz) at least 20% below participant’s automated calculated threshold, reinforce SMR (12–15Hz) 80% of the time and inhibit high beta (25–35Hz) to at least 20% below their threshold. The automatic threshold calculation is based on a moving 30-second window average; the threshold moves to maintain the required average percentage of success for inhibition/excitation within that period. Based on the changing window average, the threshold may adjust once every 2 seconds by 0.2 microvolt increments to the value maintaining success 80% of the time.
SMR training sessions consisted of 8 trials of 3 minutes each, inter-trial interval of 30 seconds, and the whole SMR NFB session lasted approximately 30 minutes. The visual display for participants included a puzzle with three bars representing each frequency band. One piece of the puzzle was open, and bars turned green whenever the participant achieved the parameters for 0.5 seconds, indicating a positive reward. This was reinforced by an auditory stimulus presented as a bell. In addition, by opening subsequent puzzles the participant could see a numerical reward of the points he or she earned.
The SCP NFB protocol followed previously described methods. Negative/positive SCP trials were equally presented in random order. Each trial lasted for 8 seconds (2-second baseline period, 6-second feedback period), and inter-trial interval was set to 5 ± 1 seconds [18]. Feedback was calculated from Cz, with reference placed on both earlobes with the EEG-Z3™ pre-amplified sensor for low frequency (0.01Hz – 1 kHz) and DC (0Hz – 1kHz) modes. To identify muscle artifact from flickering, a second electrode was placed on the temporal area. Finally, as a reference, the third electrode was placed on Pz. Vertical eye movements, recorded from electrodes above and below the left eye, were corrected online using regression-based algorithms [28]. Artifact thresholds were set to ± 100μV in the EEG channel and ± 200μV in the electrooculogram (EOG) channel. The first 15 of the 75 trials were at a 1:1 channel that inhibited 50% of the stimuli and rewarded 50% of the rest. The objective of this exercise was to allow a learning opportunity for participants to control their inhibition and reward cortical shifts. The channel changed to 3:1 after 15 trials inhibiting 66% and rewarding 33%. Thereafter, the participant learned to inhibit negativity. Each session consisted of 75 trials. The visual display for participants included a balloon moving up the screen when the goal was to activate the stimulus, or a submarine moving down when the goal was to inhibit the stimulus.
The sham NFB was identical to the SMR technique, but consisted of a pre-recorded NFB session not contingent to the participant’s EEG activity.
2.4. Outcomes
Primary efficacy outcome measures were changes in performance on the attention switching task (AST) and changes in QOL on the Impact of Pediatric Epilepsy Scale (IPES) from baseline to post-intervention. Secondary outcomes were seizure frequency, seizure Severity, and EEG metrics (defined below). Feasibility was evaluated by recruitment and trial completion rates; safety was monitored by a side effects checklist. All outcomes were recorded at baseline, at the end of the 5-week intervention period, and at the 3-month follow-up.
2.4.1. Attention switching task (AST)
The AST is a computerized task applying principles of the Dimensional Change Card Sort (DCCS) task, which measures the ability to adjust behavior in accordance with changing task goals [29]. We used a modified version of the attention switching task (AST), consisting of 64 trials. The parameters measured are: commission and omission errors, accuracy, as well as switch cost and reaction time for each of the categories – number of letters (even vs. odd numbers) and letter category (vowel vs. consonants). Switch costs are calculated by subtracting reaction time of repeat trials from reaction time of switch trials [30].
Each trial started with a cue presented on the computer screen for 500ms, which was then replaced by a 2000ms target. The cue could be either a “+” or a “Δ” sign. If the cue was “+”, participants were asked to respond by pressing the “L” key if the subsequent target (i.e. group of letters) consisted of an odd number of letters (i.e. 3 or 5), or by pressing the “A” key if an even number (i.e. 2 or 4 letters). If the cue presented before the target was “Δ”, participants should instead respond to vowels by pressing the “A” key, and to consonants by pressing the “L” key. If the cue remained the same for two consecutive trials, it was counted as a repeat trial; if not, it was considered a switch trial. Each cue had a 50% probability of being presented.
Children’s performances in an attention switching task indicate increased flexibility in their attentional control during development, whereas selective attention processes may contribute to flexible attention switching. Therefore, the AST can be considered a measurement of both attentional functions [31].
2.4.2. Quality of Life (QOL)
We used the Impact of Pediatric Epilepsy Scale (IPES) [32], a validated standardized instrument comprised of 11-items typically used in clinical trials for AEDs. The questionnaire was administerd to the subject’s parent or guardian. Each item featured a severity score of 0 (not at all) to 3 (a lot) and included the subdomains of academic improvement, social adaptation and self-esteem.
2.4.3. Seizure Frequency (SF)
A seizure diary was used to evaluate SF. The same parent or guardian recorded detailed seizure- related information over the duration of the study period. The diary registered date, time of day, percieved intensity, duration, and time of any seizure. SF extracted from diaries was compared between the post-intervention and follow up timepoints. Baseline SF was not available.
2.4.4. Liverpool Seizure Severity Scale (LSSS)
The LSSS was used to quantify participant perceptions of changed seizure severity patterns during the study period. The LSSS consists of 20 items in two categories: perception of control subscale (8 items) and perceived ictal/postictal severity subscale (12 items). Each item is scored on a 1 to 4-point Likert response scale, depending on the response category (e.g., Item 5 titled Perceived control over attacks response category is very good, fairly good, little control, no control; Item 19 Time to full recovery response category is <1min, 1–5min, 6–60min, >60min) [33].
2.4.5. EEG metrics
Awake EEGs (10 minutes each for eyes open and closed conditions) were recorded at each time point. Standard EEGs were recorded according to the American Clinical Neurophysiology Society recommendations using the 10/20 International System [34]. Data were sampled at a rate of 256 Hz, amplified and filtered using a bandpass of 0.3–50 Hz. EEG tracings were treated as continuous data and offline analysis included bandpass filtering with a low-pass cut-off of 40Hz and high-pass of 1Hz, and manual artifact detection and rejection. Fast Fourier transformation (averaged windows of 5s with 50% overlap) was used to calculate power (μV2) for the EEG bands: delta (0.5–4Hz), theta (4–8Hz), alpha (8–13Hz), SMR (12–15Hz), and beta (15–30Hz) rhythms. As a measure of connectivity, we used pair-wise coherence for C3-Cz, and C4-Cz electrodes. Welch’s averaged modified periodogram method was used to find the estimated coherence of signal x and y, representing each electrode site.
2.5. Sampling and Blinding
Participants were randomly assigned to the intervention groups using a computerized random number generator. The evaluating physician (D.M.) was blinded and independent from the investigator testing the patients. The technician applying NFB was not blinded but did not collect outcome data. Data collectors, as well as investigators performing data analysis (L.M.Q., D.M., M.E.), were blinded. The EEGs were manually cleaned by D.M., who was blinded. 2.6. Statistical Analysis
Statistical analysis was performed with SPSS v.20 software (IBM Corp, New York, NY, 2012). The statistical significance level was defined with two-tailed p< 0.05. Confidence intervals were defined at the 95% confidence level. Descriptive statistics (mean, frequency, range, and percentage) were used to describe socio-demographic variables. A mixed 3×3 and 3×2 analysis of variance (ANOVA) was used for AST, SF, LSSS, and EEG metrics with treatment group as a between-participant variable with three levels (SMR, SCP, and control), and time as a withinparticipants variable with either two or three levels depending on the measure (baseline, posttreatment, and follow up). The AST was divided into four variables: letter category reaction time, letter category error rate, letter counting reaction time, and letter counting error rate. A 3×3 mixed ANOVA was conducted on each of the AST variables. The Wilcoxon-signed rank test was used to compare the IPES from baseline to post-intervention and follow-up, and the Friedman test for the LSSS ordinal scale to compare results across the three treatment timepoints (baseline, post-intervention, and follow-up). To evaluate changes in brain activity and cognitive outcomes, a regression analysis was used to examine SMR NFB responders as a dependent variable, with mean absolute power per EEG frequency band and reaction time in the AST as dependent variables. Pearson correlation was applied to assess association between those frequencies showing positive changes as a result of NFB and each of the four AST reaction time variables.
3. Results
In total, 107 patients were assessed for eligibility, and 44 were enrolled and randomized to the experimental interventions and follow-up, with no dropouts and with 100% adherence (Figure 1 - CONSORT flow diagram). No participants were excluded by changes in their AEDs or dosage during the clinical trial. All participants tolerated the interventions well; there were no side effects in the NFB or sham interventions reported on the session checklists. Table 1 and 2 details baseline demographics and disease characteristics. None of the enrolled participants presented with the diagnosis of childhood epilepsy with centrotemporal spikes (CECTS), nor did localization include central regions (Table 2).
Figure 1.
CONSORT flow diagram. SMR: sensorimotor rhythm; SCP: slow cortical potentials; NFB: neurofeedback. 30min training × 25 sessions.
Table 1.
Demographic characteristics: by sex, mean age in years (SD), localization of epileptic foci (unknown or localized), and antiepileptic drug (AED) management (monotherapy or polytherapy).
| SMR | SCP | Control | |
|---|---|---|---|
| Male/female | 7/8 | 9/7 | 6/7 |
| Age, years (SD) | 14.8 (2.33) | 14.8 (2.3) | 15 (2.3) |
| Foci unknown/localized | 8/7 | 9/7 | 6/7 |
| AED mono/poly | 9/6 | 13/3 | 8/5 |
Table 2.
Individual type of seizures and total AED’s dosage per day. Abbreviations: ST: seizure type; CPS: complex partial seizures; TCS: tonic–clonic seizures; AEDs: antiepileptic drugs; CBZ: carbamazepine; OX: oxcarbamazepine; VA: valproic acid; LM: lamotrigine; LV: levetiracetam; DZ: diazepam; PH: phenobarbital; PH: phenobarbital; UNK: unknown; TL: temporal left; TR temporal right; T: temporal; P: parietal.
| No. | Condition | Gender | Age | Localization | ST | AED 1 | D/24H | AED2 | D/24H | AED3 | D/24H |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SMR | M | 18 | UNK | CPS | NA | |||||
| 2 | SMR | F | 15 | TL | CPS | CBZ | 600 | LM | 200 | ||
| 3 | SMR | M | 13 | TL | TCS | OX | 600 | ||||
| 4 | SMR | F | 17 | UNK | CPS/TCS | VA | 1200 | CBZ | 900 | ||
| 5 | SCP | F | 12 | UNK | CPS | VA | 1500 | ||||
| 6 | SCP | F | 13 | UNK | CPS/TCS | VA | 900 | LV | 2000 | TP | 300 |
| 7 | Control | M | 12 | TL | CPS | VA | 600 | LM | 200 | CBZ | 200 |
| 8 | Control | F | 13 | UNK | TCS | VA | 800 | ||||
| 9 | Control | M | 16 | T/P | CPS/TCS | CBZ | 200 | LV | 1000 | DZ | 10 |
| 10 | SMR | F | 14 | T | CPS | PH | 100 | LM | 100 | ||
| 11 | SCP | M | 18 | UNK | TCS | LV | 1000 | ||||
| 12 | SMR | F | 12 | P | TCS | VA | 800 | ||||
| 13 | SCP | M | 15 | T | CPS | VA | 1200 | ||||
| 14 | Control | M | 12 | P | TCS | LM | 200 | ||||
| 15 | SCP | M | 12 | UNK | CPS | NA | |||||
| 16 | SCP | M | 12 | T | CPS | PH | 800 | ||||
| 17 | SMR | M | 12 | TL | CPS | LV | 1000 | DZ | 10 | VA | 600 |
| 18 | SCP | F | 12 | UNK | TCS | VA | 600 | ||||
| 19 | Control | F | 13 | UNK | CPS | VA | 800 | ||||
| 20 | SMR | M | 18 | UNK | TCS | VA | 800 | ||||
| 21 | SMR | F | 17 | UNK | CPS/TCS | VA | 600 | ||||
| 22 | SCP | M | 14 | UNK | TCS | PH | 200 | ||||
| 23 | SCP | F | 15 | UNK | CPS | VA | 500 | ||||
| 24 | SMR | M | 16 | TL | CPS | VA | 1400 | ||||
| 25 | Control | F | 18 | UNK | CPS | VA | 400 | ||||
| 26 | Control | F | 18 | UNK | TCS | VA | 800 | ||||
| 27 | Control | F | 14 | T | CPS/TCS | VA | 600 | OX | 600 | LV | 1000 |
| 28 | SMR | F | 18 | UNK | CPS | VA | 600 | LV | 1000 | ||
| 29 | SCP | M | 18 | TL | CPS | LV | 1000 | ||||
| 30 | Control | M | 15 | T | CPS | VA | 800 | PHY | 200 | ||
| 31 | SMR | M | 13 | UNK | CPS | VA | 400 | ||||
| 32 | SCP | M | 16 | T | CPS | CBZ | 800 | DZ | 5 | ||
| 33 | SCP | F | 14 | T | CPS | NA | |||||
| 34 | SMR | F | 14 | TL | CPS | VA | 600 | CBZ | 100 | ||
| 35 | Control | M | 15 | T | CPS | LV | 1000 | ||||
| 36 | SCP | M | 16 | P | TCS | VA | 1200 | CBZ | 400 | ||
| 37 | SMR | M | 13 | UNK | CPS | PH | 200 | ||||
| 38 | SCP | M | 15 | T | CPS | VA | 400 | ||||
| 39 | Control | F | 13 | TR | TCS | CBZ | 200 | ||||
| 40 | SCP | F | 18 | UNK | CPS | VA | 800 | ||||
| 41 | SCP | F | 18 | UNK | CPS | VA | 1200 | ||||
| 42 | SMR | F | 12 | UNK | CPS | CBZ | 250 | ||||
| 43 | Control | F | 18 | UNK | CPS | VA | 600 | CBZ | 200 | ||
| 44 | Control | M | 18 | UNK | CPS | CBZ | 300 |
3.1. Effects of SMR and SCP on Cognitive Function
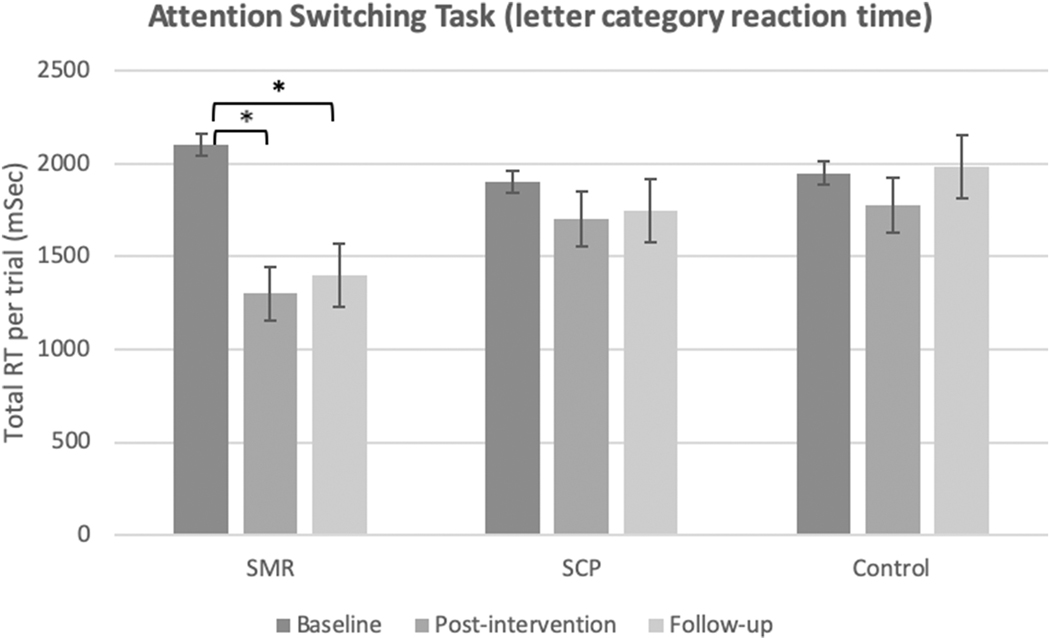
For the AST letter category reaction time, within-group analysis showed a significant main effect of time for SMR [F (2,82)= 7.043, MSE= 4.5, p= 0.006, ηp2= 0.147], and for intervention [F (4,82)= 3.319, MSE= 0.652, p= 0.046, ηp2= 0.139]. There was no significant interaction effect [F (2,82) =1.35 MSe= 0.352, p = 0.269]. Pairwise within group comparisons of SMR showed significant improvement in the letter category reaction time from baseline to post-intervention (757 msec, p= 0.015) and from baseline to follow-up (- 644 msec, p= 0.04) (Figure 2).
Figure 2.
Total Attention Switching Task reaction time per trial by group, for the letter category variable. Bars indicate standard error of the means. *statistically significant p<0.05.
No significant changes were observed for the letter counting reaction time, letter category error rate, letter counting error rate, or switching time (p >0.05).
3.2. Effects of SMR and SCP on Quality of Life
Significant improvements were observed in the QOL (IPES) scores for SMR (Z= −3.035, p= 0.002), and this represented a 1.5 point change in the QOL score. The analysis also revealed a significant improvement in the QOL score for SCP (Z = −3.416, p= 0.001), with a change of 1.9 points. For the sham group, the analysis showed a significant improvement in the QOL score (Z= −2.762, p= 0.006), and a difference of 1.3 points. The follow-up had the maximum improvement from baseline for all 3 groups. Figure 3 illustrates the mean IPES by group baseline/follow-up.
Figure 3.
Mean Impact of Pediatric Epilepsy Scale (IPES) global score from baseline to followup by group. Bars indicate standard error of the means, *statistically significant p<0.05.
3.3. Effects of SMR and SCP on Clinical Outcomes
There was no significant effect of time (p= 0.18) or group (p= 0.61), and no significant interaction effect (p= 0.57) for any clinical outcome.
There was a trend in the SMR and SCP groups for seizure reduction over time. SF difference from post-intervention to follow-up showed that SF after SMR dropped by 34.7% from 3.20 (SD= 1.75) to 2.10 (SD= 0.75); SCP group SF decreased 21.4% from 2.75 (SD= 1.21) to 2.16 (SD= 1.41); the sham group showed an increase of 11.6% from 2.3 (SD= 1.87) to 3 (SD= 1.26). In average numbers, the SMR group SF dropped from 3.2 at the end of intervention period to 2.1 by the end of the follow-up; the SCP group from 2.75 to 2.16, and the sham group SF increased from 2.65 to 3.
There were no significant differences in the LSSS in any of the intervention groups: SMR (χ2 = 2.8, p= 0.247), SCP (χ2=2.0, p= 0.368) and sham group (χ2= 2.0, p= 0.370).
3.4. Effects of SMR and SCP on EEG metrics
There was no group or time effect on power for any EEG band (all ps> 0.05).
Regarding interhemispheric central coherence (C3-C4), only SMR differed significantly from the other groups, showing a decreased coherence in the beta band for time (p= 0.04), and intervention (p= 0.035) across all time points (Figure 4).
Figure 4.
Mean change in EEG beta coherence (coherence ± SD) per group. Bars indicate standard error of the means, *statistically significant p<0.05.
3.5. Associations Between Neurofeedback Learning and Cognitive Function
We performed a post-hoc exploratory analysis in the SMR group examining the association between NFB learning and cognitive function (letter category reaction time). We found a correlation between the mean difference per EEG bandwidths between baseline and follow-up using the predefined threshold (theta and beta inhibition, and SMR reinforcement) and the mean difference in reaction time between baseline and follow-up for the subjects who learned how to succesfully achieve these thresholds.
Exploratory analysis was done for participants in the SMR group who succesfuly learned how to modulate the theta band. There was a positive correlation between the difference in theta power changes from baseline to follow-up, and improvements in reaction time (r2= 0.629, p= 0.033), i.e. the lower the theta power, the shorter the reaction time (Figure 5). However, the two subjects on the right (outliers who performed extremely well) were driving the results; by removing those two subjects the correlation was still positive (in the same direction) but did not reach significance.
Figure 5.
Scatterplot of positive correlation between the difference of average AST reaction time in msec versus difference in EEG theta power.
4. Discussion
This study showed that (i) SMR has positive effects on reaction time in an attentional task, and that the greater the theta band modulation, the greater the change in cognitive performance (improved reaction times); (ii) both active and sham NFB interventions showed significant improvements in the QOL (IPES) assessment; (iii) SMR and SCP showed a trend for seizure frequency reduction; and (iv) SMR training over the vertex reduced EEG beta coherence.
These findings suggest three important conclusions: 1. SMR can improve cognitive performance in participants with pharmacoresponsive focal epilepsy (specifically attention-related), and these changes may be associated with decreased theta band power over sensorimotor cortices; 2. Expectations for the treatment (with elaborate interactions embedded in the trial) and positive motivation generated strong placebo effects on subjective measurements, as seen particularly in QOL measures; and 3. SMR training decreased beta connectivity.
4.1. Neurofeedback, cognitive performance, and neurophysiology
SMR significantly reduced AST reaction time, and this effect was maintained through the follow-up period. This change was not seen in the other groups. While learning effect can be problematic in interpreting cognitive behavioral tests, this effect would be difficult to aquire for AST due to its psychometric properties [35]. The use of NFB as an operant conditioning paradigm facilitates cognitive functioning [36–38]. SMR training has been associated with enhanced cued recall performance [38], and focused attention in healthy individuals [39]. Both cognitive properties are relevant when performing under AST conditions. For SMR training, recording electrode was placed over the sensorimotor cortex; imaging studies have shown the sensorimotor cortex active during movement, understanding, anticipation, organization, planning, motor response, and inhibition [40]. All these functions relate to attention switching paradigm performance. These results are relevant as children with epilepsy can have problems with reaction time under attention-demanding conditions [41]. Moreover, these functional abnormalities have been associated with the presence of slow EEG activity [42]. SMR not only improved reaction time, but this was associated with changes in the theta band, demonstrating an interesting association between behavioral response and neurophysiological modulation – specifically, cognitive function improved with decreased theta slowing. As slowing in the theta and delta range reflect encephalopathy (when generalized) or focal dysfunction (when focal), this finding is consistent with clinical practice.
Another interesting finding was the decrease in interhemispheric beta coherence over the sensorimotor cortices (C3-Cz-C4) in the SMR group. Increased beta connectivity can be considered the result of persistent disruptive cortical activity that impacts some cognitive functions, as observed in adults with histories of poor academic performance and delayed recall [43]. Moreover, a recent meta-analysis showed that cognitive dysfunction is associated with alterations of functional connectivity within and between neurocognitive networks, particularly hyperconnectivity between the default mode network (DMN), the frontoparietal network (FPN), and the salience network (SN) [44]. By understanding the proposed mechanism of action behind SMR, one can postulate the decrease observed in beta hyperconnectivity may be the result of enhanced thalamocortical inhibitory transmission and concurrent cortical SMR generation.
4.2. Placebo effect and quality of life
Participants in each group showed significant improvements in QOL as measured by the IPES scale. Perhaps most interesting is the robust effect on QOL in those receiving sham NFB, suggesting the role of non-specific “placebo effects”. Placebo effects have been long recognized in AEDs trials [45] as well as medical device and technology trials [46]. Placebo is often considered the effect of an “inert substance,” or “sham intervention” but our study suggests that this characterization is misleading. In a broad sense, placebo effects are improvements in patients’ symptoms that are attributable to their participation in the therapeutic encounter (or experimental setting), with its rituals, symbols, and interactions [47]. The influence of the experimental environment, the symbols associated with the intervention, and personal interactions can dramatically enhance the effectiveness of sham interventions in a controlled trial. In fact, this effect has been described to extend to family members, whereby their feelings and perceptions about the experimental intervention may influence their judgements about effectiveness, and anticipation, excitement, and behavioral changes of family members favor a positive patient response independent of any placebo effect on the patient [48]. Unfortunately, we did not conduct post-trial interviews exploring the parents’ and children’s perceptions of the trial.
The fact that no participant was lost to follow-up suggests that participants (and parents) might have developed a positive expectancy to the intervention, and an attachment to the staff and technicians involved in the experiment as the same technician provided all training sessions. Converging evidence substantiates the view that placebo responses are complex psychoneurobiological phenomena involving the activation of distinct brain areas, particularly those associated with reward, expectations, and anxiety regulation [49]. Associative learning (conditioning) and conscious and non-conscious expectancy formation are the two main psychological mechanisms that contribute to the placebo effect [50]. In this study, it could be argued that both NFB interventions and the sham group carried an especially elaborate ritualistic experience (e.g., electrode placement, EEG traces being displayed, and video game-like interactions). There is evidence suggesting the existence of an enhanced placebo effect for devices and medical procedures; this phenomenon cannot be ignored because of its relevance for the appropriate interpretation of medical technology trials [51].
Surprisingly, participants in the sham group reported improvements in QOL despite no changes in clinical outcomes. This type of in-trial behavior might represent a phenomenon called “experimental subordination” [52] which is driven by the subject’s desire to please investigator expectancies. On the other hand, it might also suggest that support groups or group activities with ritualistic elements might improve QOL for patients with epilepsy in broad ways not captured by specific clinical outcomes.
That said, our study showed a trend toward seizure frequency improvement (which is more objective than QOL measures) after the treatment ended toward the follow-up period, where both NFB groups’ seizure frequencies somewhat improved while the sham group worsened. This may point to placebo’s time-limited effects on neuroplasticity and seizure frequency compared to NFB, although our study may have been underpowered to detect significant changes.
4.3. Limitations
The main limiltation of the study is a small sample size which means the trial is best understood as an underpowered exploration, and results should be interpreted cautiously. The lack of clinical efficacy observed in seizure frequency and intensity might be attributable to lack of power due to this limited number of participants and the use of a Likert scale (LSSS) for intensity, especially as a trend for seizure frequency reduction was observed in the SMR group but was less evident for the SCP intervention. Also important to consider is that these subjects had AED-controlled epilepsy, which might obscure the potential benefits of both NFB techniques in reducing ictal activity. Another limitation was the number of sessions in the SCP group. Previous SCP studies averaged 125 trials [53]. In the present study, the training period was 75 trials per session, a modification for feasibility and acceptability based on the expected attention-span in a pediatric population. However, the SCP group may not have had enough training trials to develop the necessary learning and consolidation for an operant conditioned effect on cortical inhibition.
Despite these limitations, this study provides the first data on two NFB modalities (SMR and SCP) including cognitive, neurophysiological, and clinical outcomes in pediatric epilepsy. While all previous NFB investigations in epilepsy focused on adults, our study provides valuable information specifically in children with controlled focal epilepsy. NFB may be a safe, noninvasive intervention that might be used in conjunction with pharmacotherapy in this population. The results from this trial may be used for power/sample size calculations in future studies; an adequately powered clinical trial might help to elucidate the real impact of NFB on seizure control. NFB should also be explored further as an integrative treatment for comorbidities associated with pediatric epilepsy.
Highlights.
Neurofeedback (NFB) can improve cognitive performance in pediatric participants with pharmacoresponsive focal epilepsy.
Sensorimotor rhythm (SMR) NFB modulates theta (4–8Hz) activity, and this is associated with improvements in reaction time. SMR neurofeedback decreases beta (15–30Hz) hyperconnectivity over the sensorimotor cortex.
Treatment expectations and positive motivation generate strong placebo effects on subjective QOL measurements.
Aknowledgements
The authors thank all the families and the participants in this study. This work is based on the PhD thesis from D.M. (Comparison of the Effects of Sensorimotor Rhythm and Slow Cortical Potential Neurofeedback in Epilepsy, Leicester, 2018). This research was supported by Thought Technology. L.M.Q. was funded by the Institutional National Research Service Award from the National Center for Complementary and Integrative Health (NCCIH grant number T32AT000051); D.M. Received support from the Mexican National Council of Science and Technology (CONACyT); G.Y. was supported by NIH NCCIH K24AT009465.
Abbreviations
- NFB
neurofeedback
- SMR
sensorymotor rhythm
- SCP
slow cortical potentials
- QOL
quality of life
- AST
attention switching task
- LSSS
Liverpool Seizure Severity Sclae
- SF
Seizure Frequency
Footnotes
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Doyle DA, Structural changes during ion channel gating. Trends in Neurosciences, 2004. 27(6): p. 298–302. [DOI] [PubMed] [Google Scholar]
- 2.Josephson CB, et al. , The impact of seizures on epilepsy outcomes: A national, communitybased survey. Epilepsia, 2017. 58(5): p. 764–771. [DOI] [PubMed] [Google Scholar]
- 3.Lin JJ, Mula M, and Hermann BP, Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet, 2012. 380(9848): p. 1180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keezer MR, Sisodiya SM, and Sander JW, Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol, 2016. 15(1): p. 106–15. [DOI] [PubMed] [Google Scholar]
- 5.Sterman MB, Basic concepts and clinical findings in the treatment of seizure disorders with EEG operant conditioning. Clin Electroencephalogr, 2000. 31(1): p. 45–55. [DOI] [PubMed] [Google Scholar]
- 6.Walker JE and Kozlowski GP, Neurofeedback treatment of epilepsy. Child Adolesc Psychiatr Clin N Am, 2005. 14(1): p. 163–76. [DOI] [PubMed] [Google Scholar]
- 7.Hurt E, Arnold LE, and Lofthouse N, Quantitative EEG neurofeedback for the treatment of pediatric attention-deficit/hyperactivity disorder, autism spectrum disorders, learning disorders, and epilepsy. Child Adolesc Psychiatr Clin N Am, 2014. 23(3): p. 465–86. [DOI] [PubMed] [Google Scholar]
- 8.Birbaumer N, et al. , Neurofeedback and brain-computer interface clinical applications. Int Rev Neurobiol, 2009. 86: p. 107–17. [DOI] [PubMed] [Google Scholar]
- 9.Monderer RS, Harrison DM, and Haut SR, Neurofeedback and epilepsy. Epilepsy Behav, 2002. 3(3): p. 214–218. [DOI] [PubMed] [Google Scholar]
- 10.Sterman MB and Egner T, Foundation and Practice of Neurofeedback for the Treatment of Epilepsy. Appl Psychophysiol Biofeedback, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Birbaumer N, et al. , Biofeedback of slow cortical potentials in epilepsy, in Clinical applied psychophysiology. Plenum series in behavioral psychophysiology and medicine., A.R.S.N.B. Carlson John G., Editor. 1994, Plenum Press, New York, NY, US: p. 29–42. [Google Scholar]
- 12.Sterman MB, Macdonald LR, and Stone RK, Biofeedback training of the sensorimotor electroencephalogram rhythm in man: effects on epilepsy. Epilepsia, 1974. 15(3): p. 395–416. [DOI] [PubMed] [Google Scholar]
- 13.Strehl U, et al. , Self-regulation of slow cortical potentials: a new treatment for children with attention-deficit/hyperactivity disorder. Pediatrics, 2006. 118(5): p. e1530–40. [DOI] [PubMed] [Google Scholar]
- 14.Albrecht JS, et al. , Effects of a structured 20-session slow-cortical-potential-based neurofeedback program on attentional performance in children and adolescents with attentiondeficit hyperactivity disorder: retrospective analysis of an open-label pilot-approach and 6-month follow-up. Neuropsychiatr Dis Treat, 2017. 13: p. 667–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birbaumer N, et al. , Slow potentials of the cerebral cortex and behavior. Physiol Rev, 1990. 70(1): p. 1–41. [DOI] [PubMed] [Google Scholar]
- 16.Rockstroh B, et al. , Biofeedback: Evaluation and therapy in children with attentional dysfunctions, in Brain and behavior in child psychiatry. 1990, Springer; p. 345–357. [Google Scholar]
- 17.Rockstroh B, et al. , “Probing” the nature of the CNV. Electroencephalogr Clin Neurophysiol, 1993. 87(4): p. 235–41. [DOI] [PubMed] [Google Scholar]
- 18.Gevensleben H, et al. , Neurofeedback of slow cortical potentials: neural mechanisms and feasibility of a placebo-controlled design in healthy adults. Front Hum Neurosci, 2014. 8: p. 990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strehl U, et al. , Sustained reduction of seizures in patients with intractable epilepsy after selfregulation training of slow cortical potentials - 10 years after. Front Hum Neurosci, 2014. 8: p. 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotchoubey B, et al. , Self-regulation of slow cortical potentials in epilepsy: a retrial with analysis of influencing factors. Epilepsy Res, 1996. 25(3): p. 269–76. [DOI] [PubMed] [Google Scholar]
- 21.Tan G, et al. , Meta-analysis of EEG biofeedback in treating epilepsy. Clin EEG Neurosci, 2009. 40(3): p. 173–9. [DOI] [PubMed] [Google Scholar]
- 22.Kotchoubey B, et al. , Stability of cortical self-regulation in epilepsy patients. Neuroreport., 1997. 8(8): p. 1867–70. doi: 10.1097/00001756-199705260-00015. [DOI] [PubMed] [Google Scholar]
- 23.Michaelis R, et al. , Psychological treatments for people with epilepsy. Cochrane Database Syst Rev, 2017. 10: p. CD012081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haut SR, Gursky JM, and Privitera M, Behavioral interventions in epilepsy. Curr Opin Neurol., 2019. 32(2): p. 227–236. doi: 10.1097/WCO.0000000000000661. [DOI] [PubMed] [Google Scholar]
- 25.Cardinal TM, et al. , Differential Neuroplastic Changes in Fibromyalgia and Depression Indexed by Up-Regulation of Motor Cortex Inhibition and Disinhibition of the Descending Pain System: An Exploratory Study. Front Hum Neurosci, 2019. 13: p. 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbosa-Torres C, et al. , [Neurofeedback to improve attention, chronic pain, and quality of life in patients with fibromyalgia]. Aten Primaria., 2019. 51(5): p. 316–317. doi: 10.1016/j.aprim.2019.01.004. Epub 2019 Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheffer IE, et al. , ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia, 2017. 58(4): p. 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotchoubey B, et al. , A new method for self-regulation of slow cortical potentials in a timed paradigm. Appl Psychophysiol Biofeedback, 1997. 22(2): p. 77–93. [DOI] [PubMed] [Google Scholar]
- 29.Hanania R and Smith LB, Selective attention and attention switching: towards a unified developmental approach. Dev Sci, 2010. 13(4): p. 622–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morales-Quezada L, et al. , Behavioral effects of transcranial pulsed current stimulation (tPCS): Speed-accuracy tradeoff in attention switching task. Neurosci Res, 2016. 109: p. 48–53. [DOI] [PubMed] [Google Scholar]
- 31.Hanania R, Ionescu T, and Smith LB. Dimension word knowledge and flexible attention shifting. in Proceedings of the Annual Meeting of the Cognitive Science Society 2010. [Google Scholar]
- 32.Camfield C, Breau L, and Camfield P, Impact of pediatric epilepsy on the family: a new scale for clinical and research use. Epilepsia, 2001. 42(1): p. 104–12. [DOI] [PubMed] [Google Scholar]
- 33.Baker GA, et al. , Liverpool Seizure Severity Scale revisited. Seizure, 1998. 7(3): p. 201–5. [DOI] [PubMed] [Google Scholar]
- 34.Guideline 1: minimum technical requirements for performing clinical electroencephalography. Am J Electroneurodiagnostic Technol, 2006. 46(3): p. 198–204. [PubMed] [Google Scholar]
- 35.Sanchez-Cubillo I, et al. , Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc, 2009. 15(3): p. 438–50. [DOI] [PubMed] [Google Scholar]
- 36.Keizer AW, Verment RS, and Hommel B, Enhancing cognitive control through neurofeedback: A role of gamma-band activity in managing episodic retrieval. Neuroimage, 2010. 49(4): p. 34043413. [DOI] [PubMed] [Google Scholar]
- 37.Plerou A, Vlamos P, and Triantafillidis C, The Effectiveness of Neurofeedback Training in Algorithmic Thinking Skills Enhancement. Adv Exp Med Biol, 2017. 988: p. 181–191. [DOI] [PubMed] [Google Scholar]
- 38.Vernon D, et al. , The effect of training distinct neurofeedback protocols on aspects of cognitive performance. Int J Psychophysiol, 2003. 47(1): p. 75–85. [DOI] [PubMed] [Google Scholar]
- 39.Ros T, et al. , Optimizing microsurgical skills with EEG neurofeedback. BMC Neurosci, 2009. 10: p. 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Binkofski F, et al. , Neural activity in human primary motor cortex areas 4a and 4p is modulated differentially by attention to action. J Neurophysiol, 2002. 88(1): p. 514–9. [DOI] [PubMed] [Google Scholar]
- 41.Reuner G, et al. , Attention and executive functions in the early course of pediatric epilepsy. Epilepsy Behav, 2016. 60: p. 42–49. [DOI] [PubMed] [Google Scholar]
- 42.Gazzellini S, et al. , Time-frequency analyses of reaction times and theta/beta EEG ratio in pediatric patients with traumatic brain injury: A preliminary study. Dev Neurorehabil, 2017. 20(7): p. 393–407. [DOI] [PubMed] [Google Scholar]
- 43.Tedrus GM, et al. , Correlations Between Cognitive Aspects and Quantitative EEG in Adults With Epilepsy. Clin EEG Neurosci, 2018: p. 1550059418793553. [DOI] [PubMed] [Google Scholar]
- 44.Sha Z, et al. , Common Dysfunction of Large-Scale Neurocognitive Networks Across Psychiatric Disorders. Biol Psychiatry, 2019. 85(5): p. 379–388. [DOI] [PubMed] [Google Scholar]
- 45.Zaccara G, Giovannelli F, and Schmidt D, Placebo and nocebo responses in drug trials of epilepsy. Epilepsy Behav, 2015. 43: p. 128–34. [DOI] [PubMed] [Google Scholar]
- 46.Kaptchuk TJ, et al. , Sham device v inert pill: randomised controlled trial of two placebo treatments. BMJ, 2006. 332(7538): p. 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaptchuk TJ and Miller FG, Placebo effects in medicine. New England Journal of Medicine, 2015. 373(1): p. 8–9. [DOI] [PubMed] [Google Scholar]
- 48.Grelotti DJ and Kaptchuk TJ, Placebo by proxy. BMJ, 2011. 343: p. d4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrovic P, et al. , Placebo in emotional processing--induced expectations of anxiety relief activate a generalized modulatory network. Neuron, 2005. 46(6): p. 957–69. [DOI] [PubMed] [Google Scholar]
- 50.Price DD, Finniss DG, and Benedetti F, A comprehensive review of the placebo effect: recent advances and current thought. Annu. Rev. Psychol, 2008. 59: p. 565–590. [DOI] [PubMed] [Google Scholar]
- 51.Kaptchuk TJ, et al. , Do medical devices have enhanced placebo effects? J Clin Epidemiol, 2000. 53(8): p. 786–792. [DOI] [PubMed] [Google Scholar]
- 52.Kienle GS and Kiene H, The powerful placebo effect: fact or fiction? J Clin Epidemiol, 1997. 50(12): p. 1311–8. [DOI] [PubMed] [Google Scholar]
- 53.Kotchoubey B, et al. , Modification of slow cortical potentials in patients with refractory epilepsy: a controlled outcome study. Epilepsia, 2001. 42(3): p. 406–16. [DOI] [PubMed] [Google Scholar]